Impact of Cu-Site Dopants on Thermoelectric Power Factor for Famatinite (Cu3SbS4) Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Famatinite Nanoparticles
2.3. Densification
2.4. Characterization Methods
3. Results and Discussion
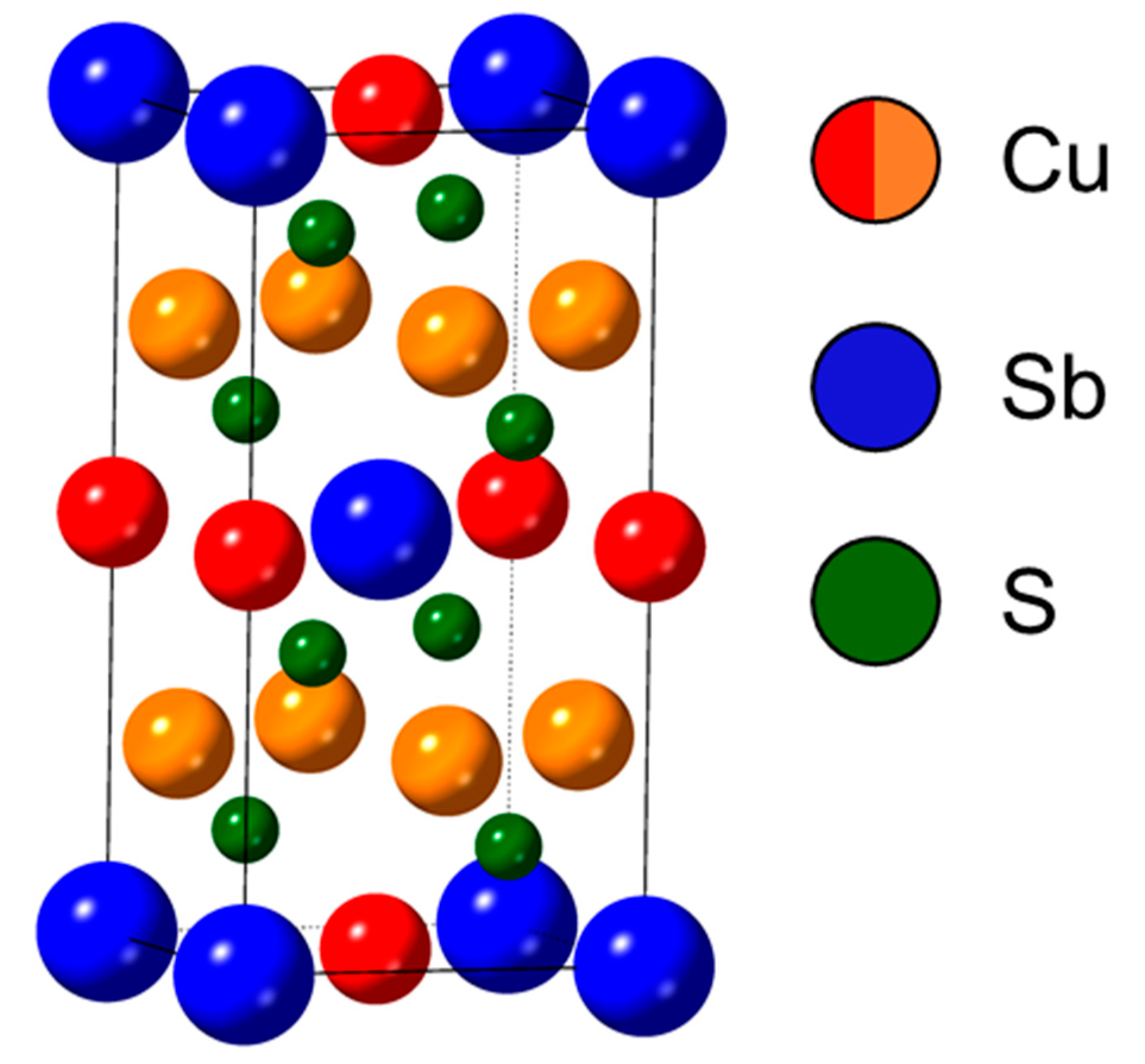
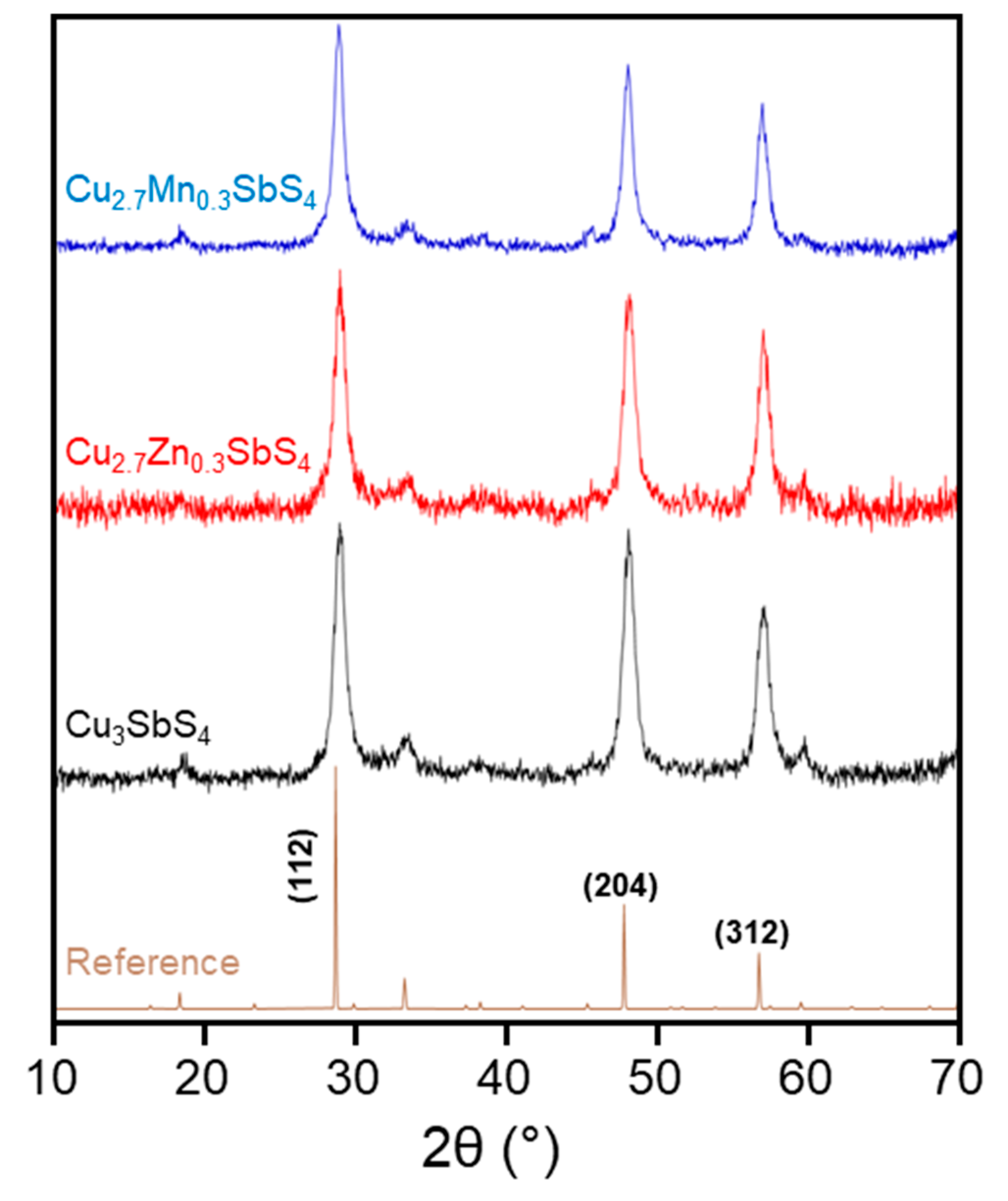
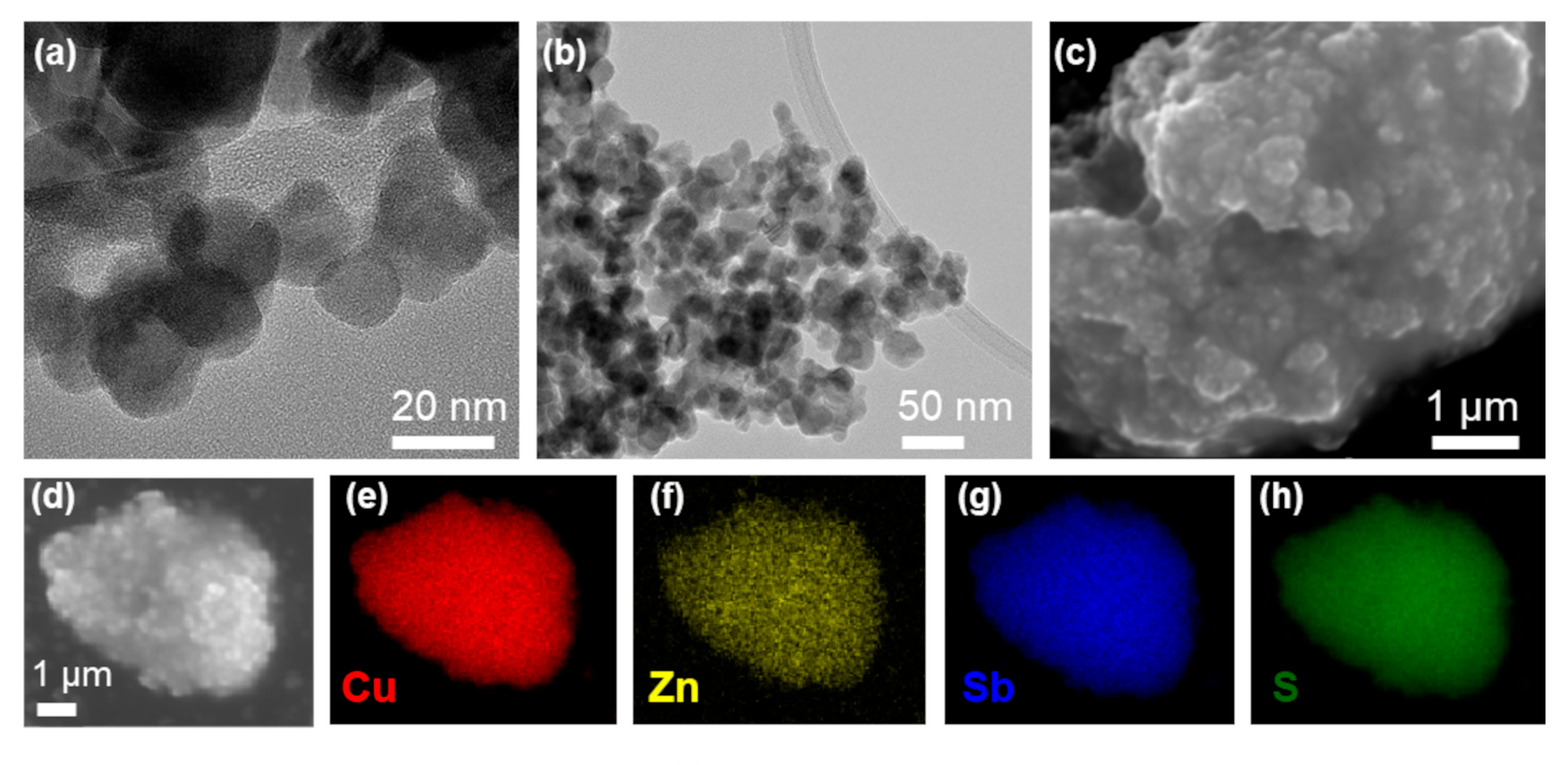
3.1. Structural, Morphological, and Compositional Analysis of As-Synthesized Nanomaterials
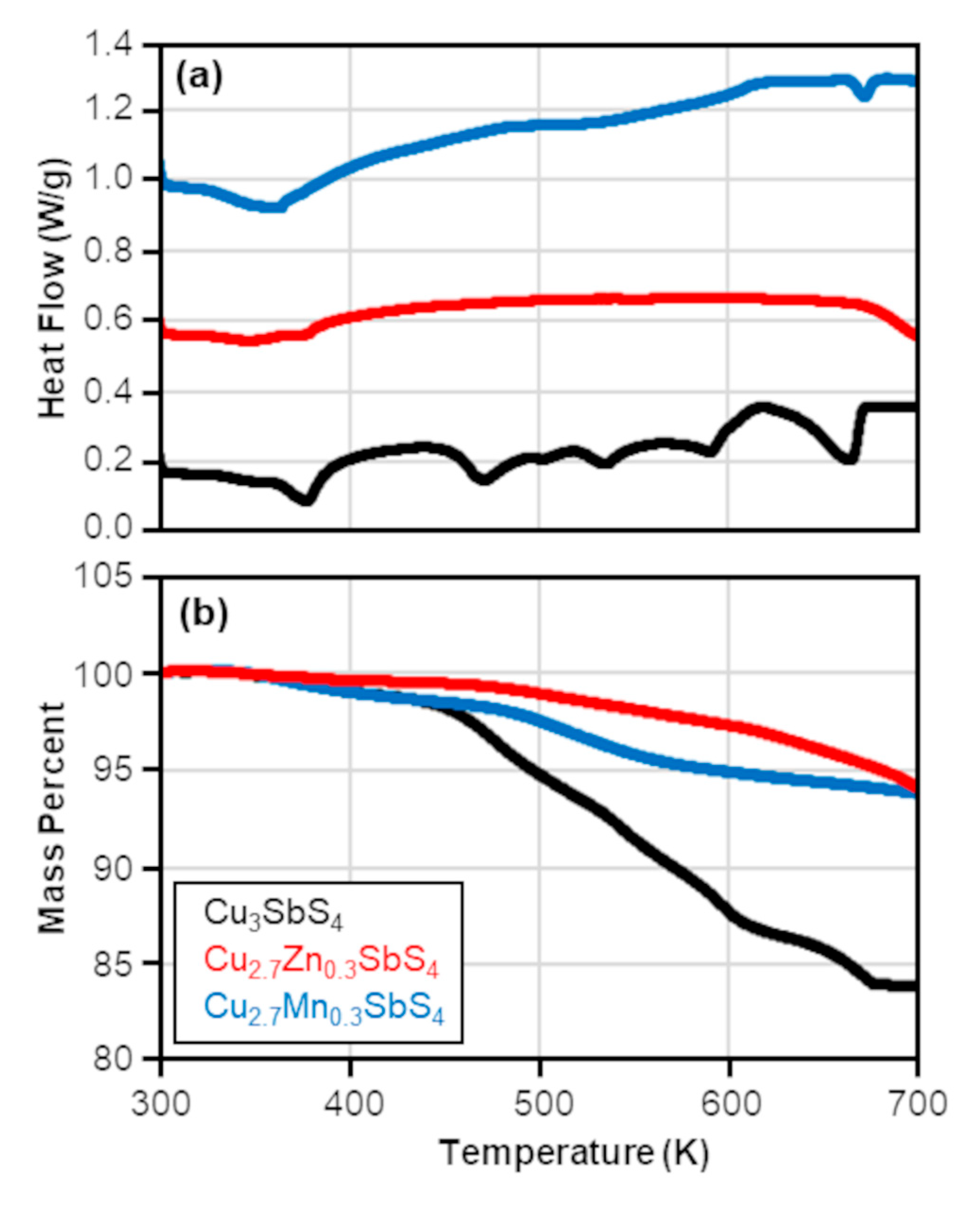
3.2. Densification Process and Pellet Analysis
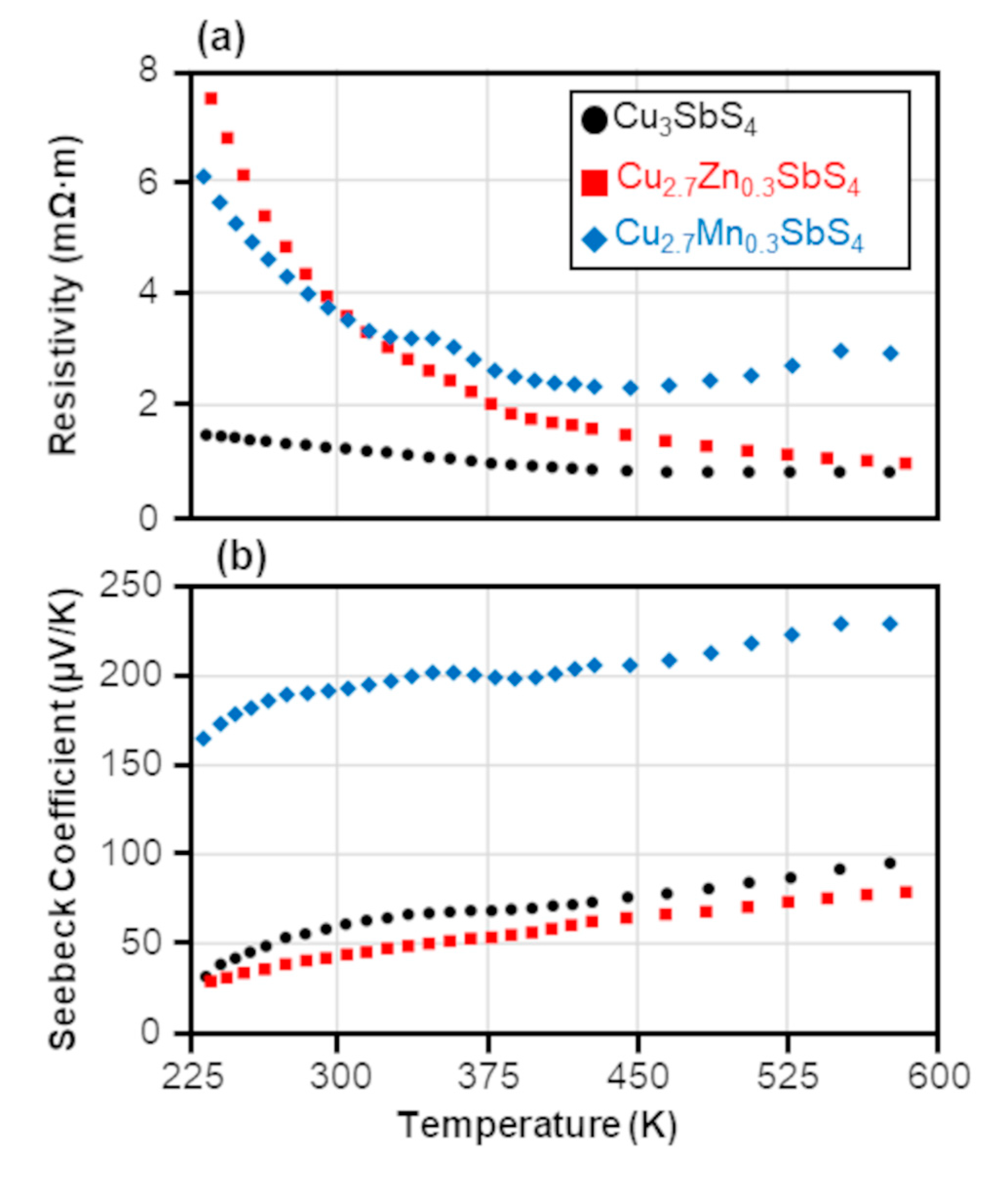
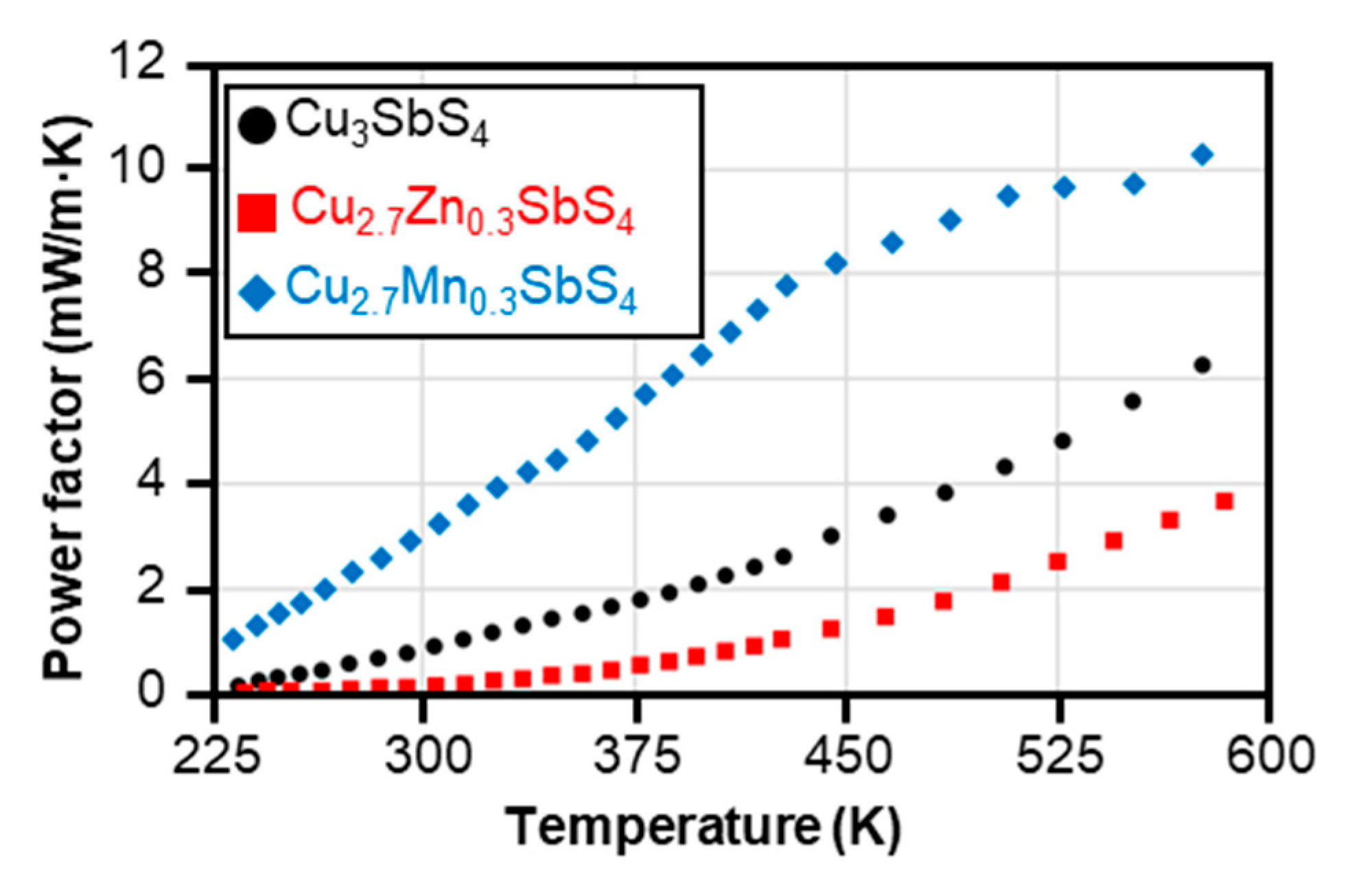
3.3. Electronic Transport Properties and Power Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Energy Transformation: A Roadmap to 2050. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Apr/IRENA_Report_GET_2018.pdf (accessed on 3 April 2024).
- Nyquist, S. Insights from the Ground Up. Available online: https://www.mckinsey.com/industries/oil-and-gas/our-insights/energy-2050-insights-from-the-ground-up (accessed on 3 April 2024).
- Outlook for Electricity. Available online: https://www.iea.org/reports/world-energy-outlook-2022/outlook-for-electricity (accessed on 3 April 2024).
- Tritt, T.M. Thermoelectric Phenomena, Materials, and Applications. Annu. Rev. Mater. Res. 2011, 41, 433–448. [Google Scholar] [CrossRef]
- Shi, X.; Chen, L. Thermoelectric Materials Step Up. Nat. Mater. 2016, 15, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Finn, P.A.; Asker, C.; Wan, K.; Bilotti, E.; Fenwick, O.; Nielsen, C.B. Thermoelectric Materials: Current Status and Future Challenges. Front. Electron. Mater. 2021, 1, 677845. [Google Scholar] [CrossRef]
- Mamur, H.; Dilmaç, Ö.F.; Begum, J.; Bhuiyan, M.R.A. Thermoelectric Generators Act as Renewable Energy Sources. Clean. Mater. 2021, 2, 100030. [Google Scholar] [CrossRef]
- Olabi, A.G.; Al-Murisi, M.; Maghrabie, H.M.; Yousef, B.A.; Sayed, E.T.; Alami, A.H.; Abdelkareem, M.A. Potential Applications of Thermoelectric Generators (TEGs) in Various Waste Heat Recovery Systems. Int. J. Thermofluids 2022, 16, 100249. [Google Scholar] [CrossRef]
- Kumar, A.; Bano, S.; Govind, B.; Bhardwaj, A.; Bhatt, K.; Misra, D.K. A Review on Fundamentals, Design and Optimization to High ZT of Thermoelectric Materials for Application to Thermoelectric Technology. J. Electron. Mater. 2021, 50, 6037–6059. [Google Scholar] [CrossRef]
- Giri, K.; Wang, Y.-L.; Chen, T.-H.; Chen, C.-H. Challenges and Strategies to Optimize the Figure of Merit: Keeping Eyes on Thermoelectric Metamaterials. Mater. Sci. Semicond. Process. 2022, 150, 106944. [Google Scholar] [CrossRef]
- Yan, Q.; Kanatzidis, M.G. High-Performance Thermoelectrics and Challenges for Practical Devices. Nat. Mater. 2022, 21, 503–513. [Google Scholar] [CrossRef]
- Rowe, D.M.; Min, G. Multiple Potential Barriers as a Possible Mechanism to Increase the Seebeck Coefficient and Electrical Power Factor. AIP Conf. Proc. 1994, 316, 339–342. [Google Scholar] [CrossRef]
- Puneet, P.; Podila, R.; Karakaya, M.; Zhu, S.; He, J.; Tritt, T.M.; Dresselhaus, M.S.; Rao, A.M. Preferential Scattering by Interfacial Charged Defects for Enhanced Thermoelectric Performance in Few-Layered n-Type Bi2Te3. Sci. Rep. 2013, 3, 3212. [Google Scholar] [CrossRef]
- Mehdizadeh Dehkordi, A.; Zebarjadi, M.; He, J.; Tritt, T.M. Thermoelectric Power Factor: Enhancement Mechanisms and Strategies for Higher Performance Thermoelectric Materials. Mater. Sci. Eng. R Rep. 2015, 97, 1–22. [Google Scholar] [CrossRef]
- Jaldurgam, F.F.; Ahmad, Z.; Touati, F. Low-Toxic, Earth-Abundant Nanostructured Materials for Thermoelectric Applications. Nanomaterials 2021, 11, 895. [Google Scholar] [CrossRef]
- Powell, A.V. Recent Developments in Earth-Abundant Copper-Sulfide Thermoelectric Materials. J. Appl. Phys. 2019, 126, 100901. [Google Scholar] [CrossRef]
- Mulla, R.; Rabinal, M.H.K. Copper Sulfides: Earth-Abundant and Low-Cost Thermoelectric Materials. Energy Technol. 2019, 7, 1800850. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef]
- Ramasamy, K.; Sims, H.; Butler, W.H.; Gupta, A. Selective Nanocrystal Synthesis and Calculated Electronic Structure of All Four Phases of Copper–Antimony–Sulfide. Chem. Mater. 2014, 26, 2891–2899. [Google Scholar] [CrossRef]
- Suehiro, S.; Horita, K.; Yuasa, M.; Tanaka, T.; Fujita, K.; Ishiwata, Y.; Shimanoe, K.; Kida, T. Synthesis of Copper–Antimony-Sulfide Nanocrystals for Solution-Processed Solar Cells. Inorg. Chem. 2015, 54, 7840–7845. [Google Scholar] [CrossRef]
- Suzumura, A.; Watanabe, M.; Nagasako, N.; Asahi, R. Improvement in Thermoelectric Properties of Se-Free Cu3SbS4 Compound. J. Electron. Mater. 2014, 43, 2356–2361. [Google Scholar] [CrossRef]
- Weller, D.P.; Morelli, D.T. Tetrahedrite Thermoelectrics: From Fundamental Science to Facile Synthesis. Front. Electron. Mater. 2022, 2, 913280. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.; Uher, C. High Performance Thermoelectricity in Earth-Abundant Compounds Based on Natural Mineral Tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, X.; Sun, Y.; Zhang, J.; Wang, Y.; Yi, L. Elastic Anisotropy and Anisotropic Transport Properties of Cu3SbSe4 and Cu3SbS4. J. Phys. Soc. Jpn. 2014, 83, 094606. [Google Scholar] [CrossRef]
- Chen, K.; Di Paola, C.; Du, B.; Zhang, R.; Laricchia, S.; Bonini, N.; Weber, C.; Abrahams, I.; Yan, H.; Reece, M. Enhanced Thermoelectric Performance of Sn-Doped Cu3SbS4. J. Mater. Chem. C 2018, 6, 8546–8552. [Google Scholar] [CrossRef]
- Garin, J.; Parthé, E. The Crystal Structure of Cu3PSe4 and Other Ternary Normal Tetrahedral Structure Compounds with Composition 13564. Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 1972, 28, 3672–3674. [Google Scholar] [CrossRef]
- Crespo, C.T. Microscopic Optical Absorption, Analysis, and Applications of Famatinite Cu3SbS4. J. Phys. Chem. C 2016, 120, 7959–7965. [Google Scholar] [CrossRef]
- Van Embden, J.; Latham, K.; Duffy, N.W.; Tachibana, Y. Near-Infrared Absorbing Cu12Sb4S13 and Cu3SbS4 Nanocrystals: Synthesis, Characterization, and Photoelectrochemistry. J. Am. Chem. Soc. 2013, 135, 11562–11571. [Google Scholar] [CrossRef] [PubMed]
- Tanishita, T.; Suekuni, K.; Nishiate, H.; Lee, C.-H.; Ohtaki, M. A Strategy for Boosting the Thermoelectric Performance of Famatinite Cu3SbS4. Phys. Chem. Chem. Phys. 2020, 22, 2081–2086. [Google Scholar] [CrossRef]
- Jensen, M.S.; Plass, K.E.; Anderson, M.E. Synthetic Strategies, Thermal Stability, and Optical Properties for Nanostructured Famatinite with Cu-Site Doping. Chem. Mater. 2022, 34, 9086–9097. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Cheng, N.; Zhang, Y.; Yang, J.; Uher, C.; Shi, X.; Chen, L.; Zhang, W. High-Performance Pseudocubic Thermoelectric Materials from Non-cubic Chalcopyrite Compounds. Adv. Mater. 2014, 26, 3848–3853. [Google Scholar] [CrossRef]
- Dutková, E.; Kováč, J.; Kováč, J.; Hejtmánek, J.; Levinský, P.; Kashimbetova, A.; Sayagués, M.J.; Fabián, M.; Lukáčová Bujňáková, Z.; Baláž, M.; et al. Properties of Mechanochemically Synthesized Famatinite Cu3SbS4 Nanocrystals. Micro 2023, 3, 458–470. [Google Scholar] [CrossRef]
- Neophytou, N.; Zianni, X.; Kosina, H.; Frabboni, S.; Lorenzi, B.; Narducci, D. Simultaneous Increase in Electrical Conductivity and Seebeck Coefficient in Highly Boron-Doped Nanocrystalline Si. Nanotechnology 2013, 24, 205402. [Google Scholar] [CrossRef]
- Lee, G.-E.; Pi, J.-H.; Kim, I.-H. Preparation and Thermoelectric Properties of Famatinite Cu3SbS4. J. Electron. Mater. 2020, 49, 2781–2788. [Google Scholar] [CrossRef]
- Chen, K.; Du, B.; Bonini, N.; Weber, C.; Yan, H.; Reece, M.J. Theory-Guided Synthesis of an Eco-Friendly and Low-Cost Copper Based Sulfide Thermoelectric Material. J. Phys. Chem. C 2016, 120, 27135–27140. [Google Scholar] [CrossRef]
- Lu, B.; Wang, M.; Yang, J.; Hou, H.; Zhang, X.; Shi, Z.; Liu, J.; Qiao, G.; Liu, G. Dense Twin and Domain Boundaries Lead to High Thermoelectric Performance in Sn-Doped Cu3SbS4. Appl. Phys. Lett. 2022, 120, 173901. [Google Scholar] [CrossRef]
- Pi, J.-H.; Lee, G.-E.; Kim, I.-H. Charge Transport and Thermoelectric Properties of Ge-Doped Famatinites Cu3Sb1−yGeyS4. Electron. Mater. Lett. 2021, 17, 427–435. [Google Scholar] [CrossRef]
- Pi, J.-H.; Lee, G.-E.; Kim, I.-H. Effects of Sn-Doping on the Thermoelectric Properties of Famatinite. J. Electron. Mater. 2020, 49, 2755–2761. [Google Scholar] [CrossRef]
- Shen, M.; Lu, S.; Zhang, Z.; Liu, H.; Shen, W.; Fang, C.; Wang, Q.; Chen, L.; Zhang, Y.; Jia, X. Bi and Sn Co-Doping Enhanced Thermoelectric Properties of Cu3SbS4 Materials with Excellent Thermal Stability. ACS Appl. Mater. Interfaces 2020, 12, 8271–8279. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Y.; Chen, Y.; Lu, T.; Wang, Y.; Zhou, J.; Liang, Z. Thermoelectric Enhancement of Ternary Copper Chalcogenide Nanocrystals by Magnetic Nickel Doping. Adv. Electron. Mater. 2016, 2, 1500473. [Google Scholar] [CrossRef]
- Heo, J.; Laurita, G.; Muir, S.; Subramanian, M.A.; Keszler, D.A. Enhanced Thermoelectric Performance of Synthetic Tetrahedrites. Chem. Mater. 2014, 26, 2047–2051. [Google Scholar] [CrossRef]
- Tippireddy, S.; Chetty, R.; Naik, M.H.; Jain, M.; Chattopadhyay, K.; Mallik, R.C. Electronic and Thermoelectric Properties of Transition Metal Substituted Tetrahedrites. J. Phys. Chem. C 2018, 122, 8735–8749. [Google Scholar] [CrossRef]
- Weller, D.P.; Stevens, D.L.; Kunkel, G.E.; Ochs, A.M.; Holder, C.F.; Morelli, D.T.; Anderson, M.E. Thermoelectric Performance of Tetrahedrite Synthesized by a Modified Polyol Process. Chem. Mater. 2017, 29, 1656–1664. [Google Scholar] [CrossRef]
- Weller, D.P.; Kunkel, G.E.; Ochs, A.M.; Morelli, D.T.; Anderson, M.E. Observation of N-Type Behavior in Fe-Doped Tetrahedrite at Low Temperature. Mater. Today Phys. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Li, J. Enhanced Thermoelectric Performance of Cu3SbS4 Flower-like Hierarchical Architectures Composed of Cl Doped Nanoflakes via an in Situ Generated CuS Template. Phys. Chem. Chem. Phys. 2018, 20, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Bella, M.; Rivero, C.; Blayac, S.; Basti, H.; Record, M.C.; Boulet, P. Oleylamine-Assisted Solvothermal Synthesis of Copper Antimony Sulfide Nanocrystals: Morphology and Phase Control. Mater. Res. Bull. 2017, 90, 188–194. [Google Scholar] [CrossRef]
- Anderson, M.E.; Bharadwaya, S.S.N.; Schaak, R.E. Modified Polyol Synthesis of Bulk-Scale Nanostructured Bismuth Antimony Telluride. J. Mater. Chem. 2010, 20, 8362–8367. [Google Scholar] [CrossRef]
- Lee, G.-E.; Kim, I.-H. Preparation and Investigation of Thermoelectric Properties of Cu3SbS4-Cu3SbSe4 Solid Solutions. Korean J. Met. Mater. 2022, 60, 384–390. [Google Scholar] [CrossRef]
- Tritt, T.M.; Browning, V.M. Chapter 2 Overview of Measurement and Characterization Techniques for Thermoelectric Materials. In Semiconductors and Semimetals; Tritt, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 69, pp. 25–49. ISBN 9780127521787. [Google Scholar]
- Nandihalli, N. A Short Account of Thermoelectric Film Characterization Techniques. Mater. Today Phys. 2023, 36, 101173. [Google Scholar] [CrossRef]
- Sachin; Pandey, B.K.; Jaiswal, R.L. Electrical Conductivity of Semiconducting Nanoparticles. Phys. B Condens. Matter 2022, 646, 414279. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Thanh, T.V.; Chen, Y.-F.; Lee, J.-K.; Lin, J.-J. Free-Electronlike Diffusive Thermopower of Indium Tin Oxide Thin Films. J. Appl. Phys. 2010, 108, 123708. [Google Scholar] [CrossRef]
- Daniel, J.E.; Weaver, S.I.; Matthias, B.R.; Golden, R.; George, G.M.; Kerpal, C.; Donley, C.L.; Jarocha, L.E.; Anderson, M.E. Investigating Cu-Site Doped Cu–Sb–S Nanoparticles Using Photoelectron and Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. C 2024, 128, 13888–13899. [Google Scholar] [CrossRef]
| Target | a (Å) | c (Å) | Grain Size (Å) | Chi2 * |
|---|---|---|---|---|
| Cu3SbS4 | 5.381 (2) | 10.715 (5) | 76.1 (3) | 1.1688 |
| Cu2.7Zn0.3SbS4 | 5.379 (2) | 10.707 (7) | 59.9 (3) | 0.9347 |
| Cu2.7Mn0.3SbS4 | 5.383 (1) | 10.734 (4) | 67.8 (4) | 1.2658 |
| Target Composition | Cu | Dopant | Sb | S |
|---|---|---|---|---|
| Cu3SbS4 | 3.23 ± 0.05 | - | 1.05 ± 0.01 | 4.00 ± 0.06 |
| Cu2.7Zn0.3SbS4 | 2.66 ± 0.03 | 0.284 ± 0.005 | 0.875 ± 0.008 | 4.00 ± 0.04 |
| Cu2.7Mn0.3SbS4 | 2.71 ± 0.02 | 0.28 ± 0.02 | 0.98 ± 0.01 | 4.00 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, J.E.; Watkins, E.; Jensen, M.S.; Benton, A., III; Rao, A.; Bhattacharya, S.; Anderson, M.E. Impact of Cu-Site Dopants on Thermoelectric Power Factor for Famatinite (Cu3SbS4) Nanomaterials. Electron. Mater. 2025, 6, 10. https://doi.org/10.3390/electronicmat6030010
Daniel JE, Watkins E, Jensen MS, Benton A III, Rao A, Bhattacharya S, Anderson ME. Impact of Cu-Site Dopants on Thermoelectric Power Factor for Famatinite (Cu3SbS4) Nanomaterials. Electronic Materials. 2025; 6(3):10. https://doi.org/10.3390/electronicmat6030010
Chicago/Turabian StyleDaniel, Jacob E., Evan Watkins, Mitchel S. Jensen, Allen Benton, III, Apparao Rao, Sriparna Bhattacharya, and Mary E. Anderson. 2025. "Impact of Cu-Site Dopants on Thermoelectric Power Factor for Famatinite (Cu3SbS4) Nanomaterials" Electronic Materials 6, no. 3: 10. https://doi.org/10.3390/electronicmat6030010
APA StyleDaniel, J. E., Watkins, E., Jensen, M. S., Benton, A., III, Rao, A., Bhattacharya, S., & Anderson, M. E. (2025). Impact of Cu-Site Dopants on Thermoelectric Power Factor for Famatinite (Cu3SbS4) Nanomaterials. Electronic Materials, 6(3), 10. https://doi.org/10.3390/electronicmat6030010






