The Kisspeptin System in Male Reproduction
Abstract
1. Introduction
2. Kiss1 and Kiss1R in the Central Control of Reproduction
3. Kiss1 and Kiss1R in Testis and Spermatozoa
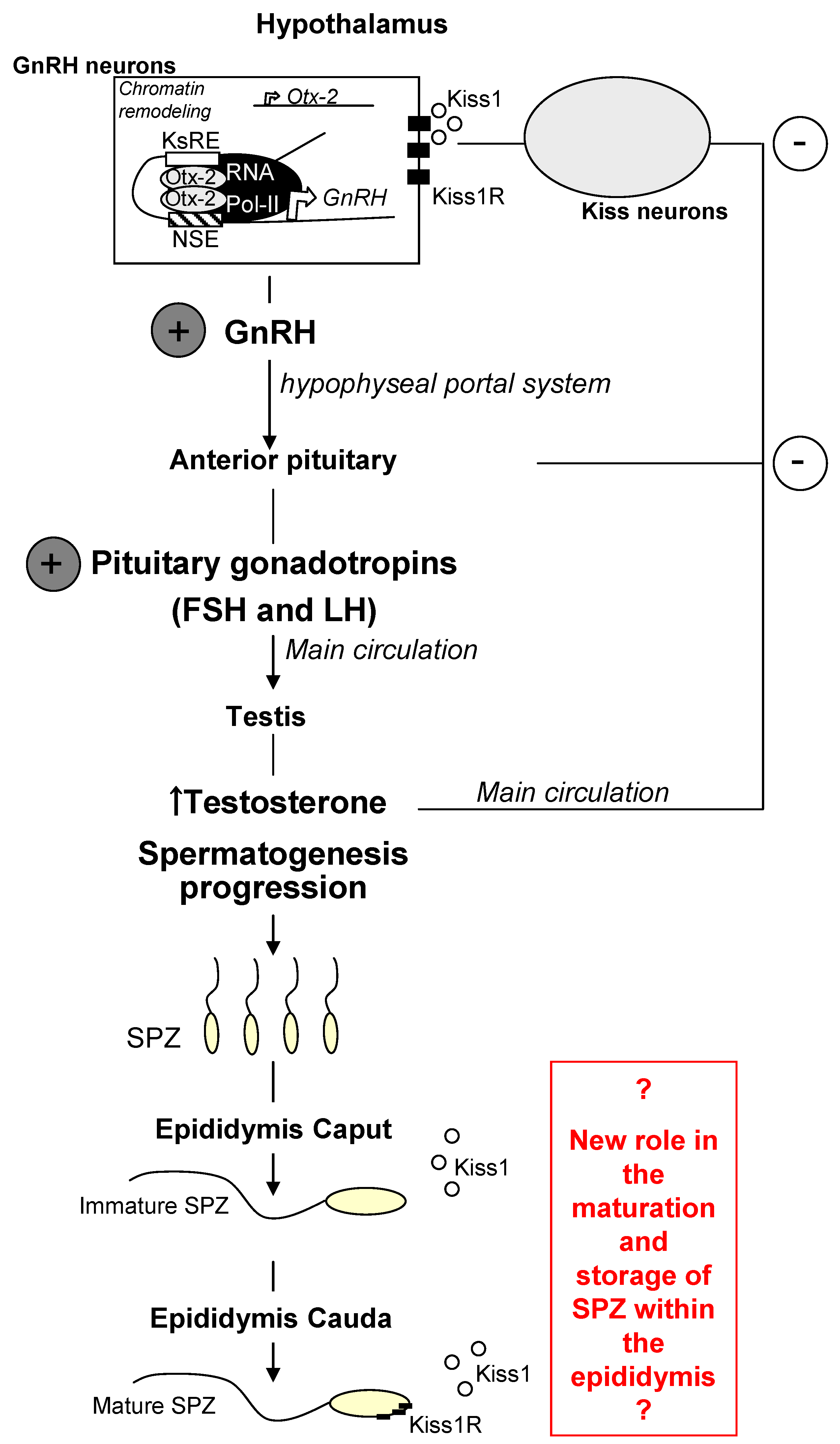
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chianese, R.; Colledge, W.H.; Fasano, S.; Meccariello, R. Editorial: The multiple facets of Kisspeptin activity in biological systems. Front. Endocrinol. 2018, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.-M.; LePoul, E.; Brezillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor geneKiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptorGPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.I.; Chamberlain, L.; Elshourbagy, N.A.; Michalovich, D.; Moore, D.J.; Calamari, A.; Szekeres, P.G.; Sarau, H.M.; Chambers, J.K.; Murdock, P.; et al. AXOR12, a novelhuman G protein-coupled receptor, activated by the peptide KiSS-1. J. Biol. Chem. 2001, 276, 28969–28975. [Google Scholar] [CrossRef] [PubMed]
- Ciaramella, V.; Della Corte, C.M.; Ciardiello, F.; Morgillo, F. Kisspeptin and Cancer: Molecular Interaction, Biological Functions, and Future Perspectives. Front. Endocrinol. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.; Brackstone, M.; Radovick, S.; Babwah, A.V.; Bhattacharya, M.M. KISS1/KISS1R in Cancer: Friend or Foe? Front. Endocrinol. 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- de Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic Hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef]
- Oakley, A.E.; Clifton, D.K.; Steiner, R.A. Kisspeptin signaling in the brain. Endocr. Rev. 2009, 30, 713–743. [Google Scholar] [CrossRef]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141. [Google Scholar]
- d’Anglemont de Tassigny, X.; Fagg, L.A.; Dixon, J.P.; Day, K.; Leitch, H.G.; Hendrick, A.G.; Zahn, D.; Franceschini, I.; Caraty, A.; Carlton, M.B.; et al. Hypogonadotropic Hypogonadism in mice lacking a functional Kiss1. Gene. Proc. Natl. Acad. Sci. USA 2007, 104, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.M.; Montagna, E.; de Oliveira, R.; Christofolini, D.M.; Barbosa, C.P.; Crandall, K.A.; Bianco, B. Kisspeptin/GPR54 System: What Do We Know About Its Role in Human Reproduction? Cell Physiol. Biochem. 2018, 49, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Lapatto, R.; Pallais, J.C.; Zhang, D.; Chan, Y.M.; Mahan, A.; Cerrato, F.; Le, W.W.; Hoffman, G.E.; Seminara, S.B. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007, 148, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and Reproduction: Physiological Roles and Regulatory Mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef] [PubMed]
- Novaira, H.J.; Fadoju, D.; Diaczok, D.; Radovick, S. Genetic mechanisms mediating kisspeptin regulation of GnRH gene expression. J. Neurosci. 2012, 32, 17391–17400. [Google Scholar] [CrossRef]
- Novaira, H.J.; Sonko, M.L.; Radovick, S. Kisspeptin Induces Dynamic Chromatin Modifications to Control GnRH Gene Expression. Mol. Neurobiol. 2016, 53, 3315–3325. [Google Scholar] [CrossRef]
- Motti, M.L.; Meccariello, R. Minireview: The epigenetic modulation of KISS1 in reproduction and cancer. Int. J. Environ. Res. Public Health 2019, 16, 2607. [Google Scholar] [CrossRef]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef]
- Wyatt, A.K.; Zavodna, M.; Viljoen, J.L.; Stanton, J.A.; Gemmell, N.J.; Jasoni, C.L. Changes in methylation patterns of kiss1 and kiss1r gene promoters across puberty. Genet. Epigenet. 2013, 5, 51–62. [Google Scholar] [CrossRef]
- Vazquez, M.J.; Toro, C.A.; Castellano, J.M.; Ruiz-Pino, F.; Roa, J.; Beiroa, D.; Heras, V.; Velasco, I.; Dieguez, C.; Pinilla, L.; et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun. 2018, 9, 4194. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 activity in the brain: Simultaneous effects on energy homeostasis and reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, estrogens and male fertility. Curr. Med. Chem. 2016, 23, 4070–4091. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thaventhiran, T.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Kisspeptin and testicular function-is it necessary? Int. J. Mol. Sci. 2020, 21, 2958. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Fasano, S.; Pierantoni, R. Kisspeptins, new local modulators of male reproduction: A comparative overview. Gen. Comp. Endocrinol. 2020, 299, 113618. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.H.; Ramzan, M.; Ramzan, F.; Wahab, F.; Jelani, M.; Khan, M.A.; Shah, M. Insight into the serum kisspeptin levels in infertile males. Arch. Iran Med. 2015, 18, 12–17. [Google Scholar] [PubMed]
- Kotani, M.; Katagiri, F.; Hirai, T.; Kagawa, J. Plasma kisspeptin levels in male cases with hypogonadism. Endocr. J. 2014, 61, 1137–1140. [Google Scholar] [CrossRef]
- Semple, R.K.; Achermann, J.C.; Ellery, J.; Farooqi, I.S.; Karet, F.E.; Stanhope, R.G.; O’Rahilly, S.; Aparicio, S.A. Two novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 2005, 90, 1849–1855. [Google Scholar] [CrossRef]
- Nimri, R.; Lebenthal, Y.; Lazar, L.; Chevrier, L.; Phillip, M.; Bar, M.; Hernandez-Mora, E.; De Roux, N.; Gat-Yablonski, G. A novel loss-of-function mutation in GPR54/KISS1R leads to hypogonadotropic hypogonadism in a highly consanguineous family. J. Clin. Endocrinol. Metab. 2011, 96, E536–E545. [Google Scholar] [CrossRef]
- León, S.; Barroso, A.; Vazquez, M.J.; Garcia-Galiano, D.; Manfredi-Lozano, M.; Ruiz-Pino, F.; Heras, V.; Romero-Ruiz, A.; Roa, J.; Schutz, G.; et al. Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Sci. Rep. 2016, 6, 19206. [Google Scholar] [CrossRef]
- Goto, T.; Hirabayashi, M.; Watanabe, Y.; Sanbo, M.; Tomita, K.; Inoue, N.; Tsukamura, H.; Uenoyama, Y. Testosterone Supplementation Rescues Spermatogenesis and In Vitro Fertilizing Ability of Sperm in Kiss1 Knockout Mice. Endocrinology 2020, 161, bqaa092. [Google Scholar] [CrossRef]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptinreceptor, GPR54, as a candidate for the regulation of testicular activity in the frog, Rana esculenta. Biol. Reprod. 2013, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin drives germ cell progression in the anuran amphibian Pelophylax esculentus: A study carried out in ex vivo testes. Gen. Comp. Endocrinol. 2015, 211, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, N.; Firat, T.; Kukner, A.; Ozoğul, C.; Tore, F.; Kandirali, İ.E.; Yilmaz, B. Theeffect of kisspeptin on spermatogenesis and apoptosis in rats. Turk. J. Med. Sci. 2017, 47, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, F.; Qureshi, I.Z. Intraperitoneal kisspeptin-10 administration inducesdose-dependent degenerative changes in maturing rat testes. Life Sci. 2011, 88, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.; Di Pietro, M.J.; Ramaswami, S.; Crowly, W.F.; Plant, T.M. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): A finding with therapeutic implications. Endocrinology 2006, 147, 2122–2126. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hsu, M.C.; Tseng, T.H.; Wu, L.S.; Yang, K.T.; Chiu, C.H. Kisspeptin expression in mouse Leydig cells correlates with age. J. Chin. Med. Assoc. 2015, 78, 249–257. [Google Scholar] [CrossRef][Green Version]
- Chiang, C.M.; Chiu, H.Y.; Jong, D.S.; Wu, L.S.; Lee, Y.J.; Chiu, C.H.J. Role of the Kisspeptin/KISS1 Receptor System in the Testicular Development of Mice. J. Chin. Med. Assoc. 2021, 84, 203–211. [Google Scholar] [CrossRef]
- Petrucci, L.; Maranesi, M.; VeriniSupplizi, A.; Dall’Aglio, C.; Mandara, M.T.; Quassinti, L.; Bramucci, M.; Miano, A.; Gobbetti, A.; Catone, G.; et al. Kisspeptin/GnRH1 system in Leydig cells of horse (Equus caballus): Presence andfunction. Theriogenology 2020, 152, 1–7. [Google Scholar] [CrossRef]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptinregulates steroidogenesis and spermiation in the anuran amphibian. Reproduction 2017, 154, 1–12. [Google Scholar] [CrossRef]
- Toolee, H.; Rastegar, T.; Solhjoo, S.; Mortezaee, K.; Mohammadipour, M.; Kashani, I.R.; Akbari, M. Roles for Kisspeptin in proliferation and differentiation of spermatogonial cells isolated from mice offspring when the cells are cocultured with somatic cells. J. Cell. Biochem. 2019, 120, 5042–5054. [Google Scholar] [CrossRef]
- Mele, E.; D’Auria, R.; Scafuro, M.; Marino, M.; Fasano, S.; Viggiano, A.; Pierantoni, R.; Santoro, A.; Meccariello, R. Differential Expression of Kisspeptin System and Kisspeptin Receptor Trafficking During Spermatozoa Transit in the Epididymis. Genes 2022, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.M.; Cejudo-Román, A.; Ravina, C.G.; Fernández-Sánchez, M.; Martín-Lozano, D.; Illanes, M.; Tena-Sempere, M.; Candenas, M.L. Characterization of the kisspeptin system in human spermatozoa. Int. J. Androl. 2012, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.C.; Wang, J.Y.; Lee, Y.J.; Jong, D.S.; Tsui, K.H.; Chiu, C.H. Kisspeptin modulates fertilization capacity of mouse spermatozoa. Reproduction 2014, 147, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Contri, A.; Mele, E.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin Receptor on the Sperm Surface Reflects Epididymal Maturation in the Dog. Int. J. Mol. Sci. 2021, 22, 10120. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Wang, X.; Chen, Q.; Yang, H.; Zhou, N.; Sun, L.; Chen, H.; Liu, J.; Ao, L.; Cui, Z. Kisspeptin Protein in Seminal Plasma Is Positively Associated with Semen Quality: Results from the MARHCS Study in Chongqing, China. Biomed. Res. Int. 2019, 2019, 5129263. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meccariello, R. The Kisspeptin System in Male Reproduction. Endocrines 2022, 3, 168-174. https://doi.org/10.3390/endocrines3020015
Meccariello R. The Kisspeptin System in Male Reproduction. Endocrines. 2022; 3(2):168-174. https://doi.org/10.3390/endocrines3020015
Chicago/Turabian StyleMeccariello, Rosaria. 2022. "The Kisspeptin System in Male Reproduction" Endocrines 3, no. 2: 168-174. https://doi.org/10.3390/endocrines3020015
APA StyleMeccariello, R. (2022). The Kisspeptin System in Male Reproduction. Endocrines, 3(2), 168-174. https://doi.org/10.3390/endocrines3020015





