Abstract
In this review, we describe genetic mutations affecting metabolic pathways of calcium and phosphorus homeostasis. Calcium and phosphorus homeostasis has tight hormonal regulation by three major hormones: vitamin D, parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). We describe the physiology and pathophysiology of disorders, their biochemical profile, clinical characteristics, diagnostics, and treatments.
1. Physiology
1.1. Calcium-Phosphorus Metabolism
Calcium and phosphorus minerals play a major role in bone mineralization. Both minerals are present in bones, and soluble in serum or intracellular in soft tissues. Calcium and phosphorus are stored as a crystalline structure in bone called hydroxyapatite and serve as storage and the basis of bone structure to carry body weight. In serum, calcium and phosphorus are either ionized, bound to albumin, or found in other ion complexes [1,2]. Calcium also plays a role in muscle contraction, hormone and neurotransmitter release, and enzyme activation and coagulation pathways [3]. Soluble calcium and phosphorus in serum play important roles in homeostasis and function of nervous, muscular, and other systems and therefore have tight hormonal regulation through intestinal absorption, bone resorption, renal excretion, and reabsorption.
Serum levels of calcium are tightly controlled and monitored by calcium sensing receptors (CaSR) [4]. The CaSRs are G-protein coupled receptors found in the kidneys and parathyroid glands. In excess, calcium binds to CaSRs resulting in decreased parathyroid hormone synthesis and secretion, as well as reduced renal calcium reabsorption [4].
In the form of hydroxyapatite, phosphorus is a structural component of bone. The main functions of phosphorus are to provide cellular energy and aid metabolism as a component of ATP [5]. Serum phosphorus homeostasis is maintained by continuous mineralization and resorption of bone, by a balance between osteoblasts, which are osteocytes that form bone, and osteoclasts that reabsorb bone [3,5].
Receptor Activator of Nuclear Factor-K (RANK) binding to its ligand called RANKL activates the osteoclast precursor and increases osteoclast activity. The ligand RANKL is produced by osteoblasts [6] and, under the influence of macrophage colony stimulating factor (M-CSF), it stimulates osteoclasts thereby increasing bone resorption [6]. To negate this process, osteoprotegerin, also produced by osteoblasts, will bind to RANKL and prevent downstream osteoclast activity [7]. This balance between RANK expression and osteoprotegerin secretion is affected by several different cytokines and hormones. This balance regulates the process of bone turnover, thereby aiding in the maintenance of homeostasis of intracellular phosphorus and calcium [8].
Physiologically, calcium and phosphorus are regulated by three main hormones: vitamin D 25(OH), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) [9].
1.2. Regulation by Vitamin D
Different forms of vitamin D are biochemically converted to an active form of vitamin D1,25(OH)2 or calcitriol (Figure 1). Vitamin D3 (cholecalciferol) in the skin of animals is initially synthesized from 7-dehydrocholesterol by exposure to UVB radiation and heat [10]. Vitamin D2 (ergocalciferol) is sourced from plants and fungi. Other sources include fish liver, oily fish, and is added to milk and orange juice [7]. In the liver, D2 and D3 are hydroxylated into calcidiol (vitamin D25(OH)) [7]. This intermediate metabolite functions mainly as the storage form of vitamin D [11,12]. In the kidneys, calcidiol is further hydroxylated by the enzyme 1α-hydroxylase to form active calcitriol, resulting in increased absorption of intestinal calcium and phosphorus [4,9,13,14,15,16].
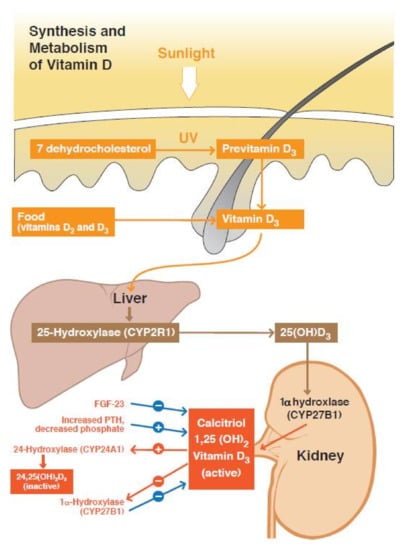
Figure 1.
Summary of the vitamin D pathway. Vitamin D activation involves the action of 25-hydroxylase (liver) and 1α-hydroxylase (kidney). Calcitriol or vitamin D1,25(OH)2 can bind its receptor to exert its biological action. Vitamin D inactivation involves 24-hydroxylase. This enzyme can metabolize vitamin D25(OH) to the intermediate metabolite vitamin D24,25(OH)2 and vitamin D1,25(OH)2 to vitamin D1,24,25(OH)3. The activity of 1α-hydroxylase and 24-hydroxylase is regulated mainly by PTH and FGF23. FGF23 = fibroblast growth factor 23.
1.3. Regulation by PTH
PTH produced by the four parathyroid glands, plays an important role in maintenance of calcium and phosphorus homeostasis in bone, the gut and the kidneys [17] (Figure 2). In the renal tubules, PTH increases reabsorption of calcium while increasing excretion of phosphorus [17]. In the gut, PTH promotes reabsorption of calcium and phosphorus indirectly via increased 1α-hydroxylase [3,17,18,19]. In bone, PTH stimulates osteoblasts and osteoclasts to increase bone turnover and ultimately bone resorption [19,20,21]. Bone resorption increases levels of both phosphorus and calcium; however, as PTH also increases the excretion of phosphorus in the kidneys, the net effect is an increase in serum calcium and a decrease in serum phosphorus [2,22]. The effects of PTH on the kidney are more immediate and occur within minutes, while in bone, this takes effect in about 1–2 h [8].
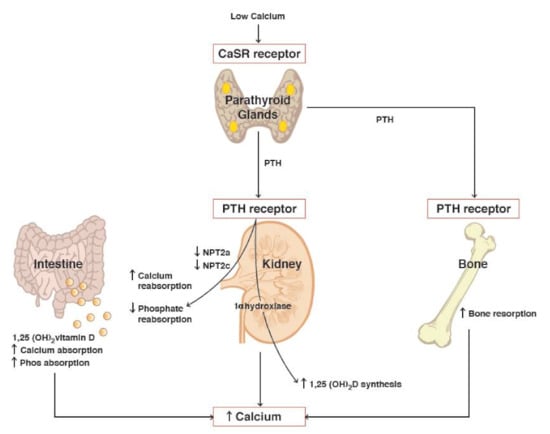
Figure 2.
Regulation of calcium-phosphate homeostasis by the parathyroid glands, bone, kidneys, and intestinal tract. Low serum Ca2+ stimulates PTH release and binding to receptors found on renal tubules and bone. Increased vitamin D1,25(OH)2 synthesis also leads to increased intestinal absorption of calcium and phosphorus. CaSR = calcium sensing receptor, PTH = parathyroid hormone, 1,25(OH)2 vitamin D = active form of vitamin D, NPT2a, NPT2c = sodium phosphorus co-transporters.
1.4. Regulation by FGF23
FGF23 is a major hormone of phosphorus regulation, a phosphatonin that promotes phosphaturia [18,23] (Figure 3). This hormone is believed to act on the proximal renal tubule and binds to its receptor FGFR1, along with co-receptor klotho [18]. In conjunction with this receptor, FGF23 acts as an inhibitor of 1α-hydroxylase and decreases levels of calcitriol (vitaminD1,25(OH)2) [4,23]. An increase in serum phosphate levels stimulates skeletal production of FGF23, which in turn depresses renal tubular reabsorption of phosphate and the synthesis of calcitriol. The FGF23 also increases 24α-hydroxylase, the enzyme which inactivates vitamin D25(OH) to vitaminD24,25(OH)2, an inactive metabolite [18,23].
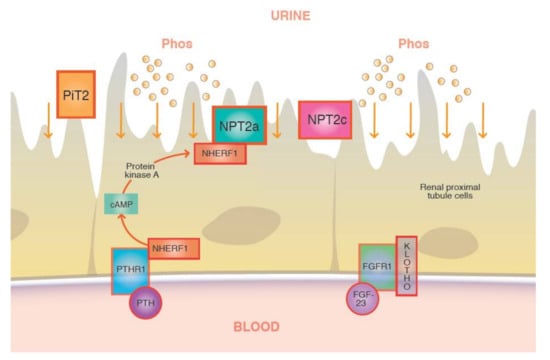
Figure 3.
Binding of FGF23 to its receptor and coreceptor will downregulate the luminal sodium phosphate cotransporters (NPT2a/c) at the renal tubules through which phosphate is reabsorbed from the glomerular filtrate.
2. Pathophysiology of Calcium/Phosphorus Disorders
Vitamin D pathway mutations can lead to disorders of hypocalcemia and hypercalcemia [24].
2.1. Vitamin D Pathway Mutations-Hypercalcemia
Idiopathic Infantile Hypercalcemia (IIH)
Idiopathic infantile hypercalcemia is an autosomal recessive condition. There are two variants. Biallelic mutations in the CYP24A1 gene and the SLC34A1 gene [24,25,26] are responsible for an increased level of calcitriol caused by hyperactivity of 1α-hydroxylase [3,27]. Inactivating mutations of SLC34A1 that encode a renal sodium-phosphate co-transporter (NaPi2a) in the renal proximal tubule leads to renal phosphorus loss, phosphaturia, and hypophosphatemia [14,28]. Hypophosphatemia leads to low levels of FGF23, which means decreased inhibition of 1α-hydroxlase and decreased 24-hydroxylase activity [14,26]. Hypercalcemia is the consequence of these pathways causing elevated calcitriol.
Monoallelic CYP24A1 gene mutations are rare [25]. Loss of function mutations results in reduced activity of enzyme 24-hydroxylase. The 24-hydroxylase enzyme inactivates vitamin D25(OH) and vitamin D1,25(OH)2 to inactive metabolites, vitamin D24,25(OH)2, and vitamin D1,24,25(OH)3 respectively [25,29]. Impaired degradation leads to accumulation of vitamin D1,25(OH)2 and consequently, causes hypercalcemia and hypercalciuria [13,25,29]. Monoallelic mutation presents with incomplete penetrance, leading to a broad range of calcium levels and various clinical presentations [14].
Historically, clinical cases of IIH were brought to attention during the middle of the last century after reports of hypercalcemia in infants receiving Vitamin D fortified milk [26]. Infants presented with symptoms of severe hypercalcemia, including acute presentation as anorexia, vomiting, polyuria, dehydration, and febrile episodes [26]. Chronic hypercalcemia also presented as failure to thrive, psychomotor delay, hypotonia, and nephrocalcinosis [26]. Symptoms usually improve in childhood [14].
Clinically, both variants of IIH are very similar and may display great variability by severity and age of presentation [13,25,26,29,30].
2.2. Vitamin D Pathway Mutations–Hypocalcemia
Vitamin D mutations leading to insufficient production of vitamin D or end organ resistance to vitamin D cause hypocalcemia and rickets [4]. Calcipenic rickets is a disorder of insufficient mineralization of the bone, which affects the growth plate/metaphyseal region [31]. Insufficient intake of calcium and vitamin D can cause nutritional rickets, with clinical presentations similar to genetic mutations affecting vitamin D production or responsiveness [32,33]. Vitamin D pathway is shown in detail in Figure 1. Specific subtypes of rickets and corresponding electrolyte abnormalities are presented in Table 1.

Table 1.
Genetic mutations in Vitamin D pathway leading to hypocalcemia.
Genetic defects resulting in decreased levels of calcitriol include Vitamin D-dependent rickets type 1A (VDDR1A) and Vitamin D–dependent rickets type 1B (VDDR1B) (Table 1).
- Vitamin D-dependent rickets type 1A is due to failure to synthesize calcitriol due to a defect in CYP27B1 encoding 1α-hydroxylase [16].
- Vitamin D-dependent rickets type 1B is the result of failure to synthesize vitamin D25(OH) due to mutations in CYP2R1 that encodes 25-hydroxylase [16,34].
In contrast, genetic defects of end-organ resistance to vitamin D result in the following:
- Vitamin D–dependent rickets type 2 is the result of an impaired vitamin D receptor VDR gene (VDDR2A), or post-receptor errors (hormone response element-binding protein HNRNPC gene), that interferes with vitamin D receptor (VDDR2B) function [35]. As the defect is at the receptor level, vitamin D25(OH) levels are normal and calcitriol levels are markedly elevated. Alopecia is found in VDDR2A [34,35].
- Vitamin D-dependent rickets type 3 is due to the autosomal dominant mutation of CYP3A4, which causes elevated serum 4,25-dihydroxyvitamin D, and low vitamin D25(OH) and vitamin D1,25(OH)2. Patients require higher than usual doses of ergocalciferol or calcitriol supplementation [12,34].
Although vitamin D25(OH) and vitamin D1,25(OH)2 levels are varied, electrolyte abnormalities of calcium, phosphorus, PTH, and alkaline phosphatase (ALP) are similar for all types [3,12,34]. Typically, decreased serum calcium levels lead to secondary hyperparathyroidism, which causes rapid removal of sodium-dependent phosphate co-transporter proteins (NaPi2a and NaPi2c) in the proximal renal tubules. Ultimately, this leads to renal phosphate excretion and low serum phosphate [10,36].
Nutritional vitamin D deficiency similarly presents with low calcium and phosphorus, secondary hyperparathyroidism, and compensatory increased renal calcitriol production. However, the description of nutritional rickets is out of the scope of this review [18,36].
Calcipenic rickets characteristically presents with growth plate and metaphyseal bone changes. Specific clinical features include symptoms of acute hypocalcemia affecting excitability of neurons (paresthesia, laryngospasm, muscle cramps, numbness, tetany, seizures, positive Chvostek and Trousseau signs, and ECG changes such as QT prolongation) [18]. Chronic hypocalcemia impacts muscle hypotonia and weakness, delayed tooth eruption, and motor developmental delay [12,18]. Characteristic skeletal findings of rickets are well known, including craniotabes, delayed closure of fontanelles, frontal bossing, deformities such as bowed legs or knock-knee appearance in the lower extremities, widened wrists and ankles with the medial malleoli, rachitic rosary and Harrison grooves [12,18].
2.3. Parathyroid Hormone-Hyperparathyroidism
Primary hyperparathyroidism is caused by excessive PTH production from overactive parathyroids with targets receptors in the bones, guts, and kidneys, finally leading to hypercalcemia and hypophosphatemia [37,38]. This can be isolated or part of a genetic syndrome [38,39] (Table 2).

Table 2.
Genetic mutations causing Hyperparathyroidism.
The PTH concentration is determined by the CaSR sensing extracellular serum concentration of calcium [38,39]. When the extracellular serum calcium level is at the lower limit of the normal range, CaSR signals for more PTH production and release [40]. When the extracellular serum calcium level is at the upper limit of the normal range, CaSR lowers the level of PTH secretion [40]. Serum calcium concentration sensing by CaSR resulting in half-maximal PTH secretion is called a ‘set point’ [19,41,42] (Figure 4). The ability of CaSR to trigger PTH secretion depending on set point is crucial in the pathophysiology of hyper or hypoparathyroidism [41,42].
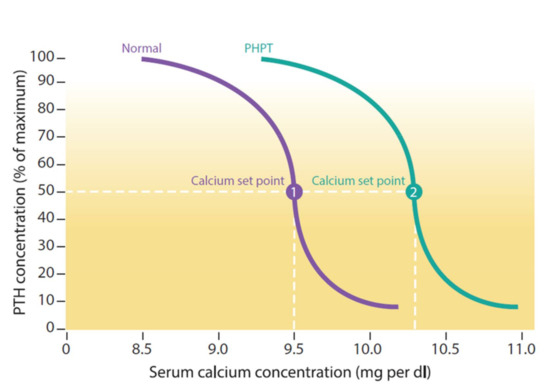
Figure 4.
The purple line shows the normal relationship between calcium and parathyroid hormone concentrations and the green primary hyperparathyroidism (PHPT) curve is shifted to the right (higher levels of PTH at each calcium). The calcium set point (dotted line) corresponds to the concentration of calcium at which PTH secretion is at 50%. In primary hyperparathyroidism (PHPT), PTH is less sensitive to calcium, leading to higher levels of calcium [19,41,42].
The CaSR is located on the membrane of the parathyroid chief cells, where it controls the release of PTH [42,43], as well in the thick ascending loop in the renal tubules. A loss-of-function mutation in CaSR results in an increased set point for serum calcium, leads to hypercalcemia [12,19,44]. Calcium is unable to activate the receptor in the renal tubules, which leads to an increase of renal reabsorption of calcium into the blood from the tubular fluid and hypocalciuria [19,20].
- A.
- Familial Hypocalciuric Hypercalcemia and Neonatal Severe Hyperparathyroidism
- FHH type 1, due to loss-of-function mutations of CaSR, presents with mild hypercalcemia, inappropriately normal or elevated PTH levels and urine calcium/creatinine clearance ratio < 0.01 or 24 h urine calcium < 6.25 mmol. Typically, patients are asymptomatic [38,45].
- FHH type 2 is due to loss-of-function mutations of GNA11 (guanine nucleotide-binding protein, alpha-11). It encodes the downstream G-protein signal of CaSR to intracellular signal transduction pathways, and its inactivation leads to hypercalcemia [46].
- FHH type 3 is due to loss-of-function mutations in AP2S1 (adaptor related protein complex 2, sigma1 subunit). Additionally, inactivating mutations lead to defective adaptor-related protein complex 2 interactions with β-arrestin (ARRB1), with a consequent defect in endocytosis of the CaSR from the cell surface. The clinical presentation comprises of hypercalcemia, low bone mineralization, and impaired cognition [46].
- Neonatal severe hyperparathyroidism (NSHPT) is a result of inactivating mutations of CaSR, however, it has a more severe presentation than FHH [47]. The NSHPT condition results from the inheritance of two abnormal CaSR alleles in either the homozygous or the compound heterozygous state, leading to severe hypercalcemia at birth. An autosomal recessive condition, it is a potentially lethal form of hyperparathyroidism that presents with life-threatening signs of acute hypercalcemia such as short QT, dysrhythmias, respiratory distress, and chronic hypercalcemia as demineralization and fractures [38,39]. Though hypercalcemia can lead to short QT intervals on ECG, arrhythmias are rare [20,37]. The time of initial presentation of NSHPT depends on the degree of hypercalcemia and varies from the first few days to several months of age [20].
- B.
- Isolated Non-Syndromic—Mutations on CDC73, CaSR, GNA11, and AP2S1 Genes Familial Isolated Hyperparathyroidism
- Type 1 is the benign familial form [3,45,46,47].
- Type 2, known as hyperparathyroidism jaw-tumour syndrome (HPT-JT), presents as severe malignant ossifying fibromas of the maxilla and/or mandible, renal tumours, uterine tumours and an increased risk of parathyroid carcinoma [38,48]. Interestingly, these tumours are not the brown tumours typically associated with hyperparathyroidism. Both subtypes are caused by germline mutations in CDC73 on chromosome 1q31.2, encoding parafibromin, a protein intrinsic to the cell division cycle. Penetrance of this mutation is 80% [38,49].
- C.
- Multiple Endocrine Neoplasia (MEN) syndromes
- MEN1 comprises a constellation of tumours in specific locations: hyperplasia of the parathyroid glands, tumours of the adenohypophysis (prolactinoma), and pancreatic tumours (gastrinoma, insulinoma, etc) [35,41,43]. MEN1 is due to an inactivating mutation of the MEN1 or menin tumour suppressor gene, located on chromosome 11q13.1 [41]. The syndrome MEN1 rarely presents before the age of 10, and usually occurs in the second decade of life [41,50]. Hyperparathyroidism is the initial presentation [20].
- MEN2A results from activating germline mutations of RET proto-oncogene, encoding a transmembrane receptor tyrosine kinase responsible for cell growth, differentiation, and survival [2,50]. Specifically, it is associated with parathyroid adenoma, medullary thyroid carcinoma, and pheochromocytoma [2,35,50]. In addition to hypercalcemia, high calcitonin levels produced by medullary thyroid carcinoma with elevated plasma or urine catecholamines produced by pheochromocytoma are characteristic [2,50].
- MEN4 is clinically similar to MEN1. However, it is caused by an inactivating mutation of CDKN1B (cyclin-dependent kinase inhibitor 1B) on chromosome 12p13.1 [2,35]. The tumour suppressor gene CDKN1B encodes p27 which blocks the cell cycle at G0/G1 phase, regulates apoptosis and cell motility [2,50]. Similar to MEN1, MEN4 typically comprises parathyroid adenoma and pituitary adenoma, and can be associated with gastrinomas, insulinomas, or malignancy in the genitourinary tracts [2,50].
- D.
- Diagnosis
Clinical signs of hyperparathyroidism are associated hypercalcemia and hypophosphatemia with non-suppressed PTH level [4,39]. Symptoms are milder, ranging from completely asymptomatic to non-specific behavioural changes including depression, headache, malaise, proximal muscle weakness, anorexia, abdominal cramping, nausea and vomiting, constipation, polydipsia and polyuria [4,20]. Rarely, complications such as nephrolithiasis, pancreatitis and pathological fractures due to osteopenia or lesions of osteitis fibrosa cystica may be part of the initial presentation.
Metabolic findings include normal-elevated serum intact PTH, hypercalcemia, hypophosphatemia, hypermagnesemia, elevated calcitriol values, and low renal tubular reabsorption of phosphate [38].
Imaging studies should be undertaken to evaluate for any evidence of parathyroid adenoma(s) (single vs. multigland disease). Sonogram, MRI, computed tomography or radionuclide scans (99 m technetium-labelled sestamibi-single photon emission CT scan) can localize the lesion before surgical excision. At times, the parathyroid adenoma/tumour may be ectopic [20,38]. Radiographical findings of osteopenia, metaphyseal widening and irregularity, subperiosteal resorption, and fractures should be included. Renal sonograms may also reveal nephrocalcinosis [20]. Genetic testing is indicated if hyperparathyroidism syndrome is suspected.
2.4. Parathyroid Hormone- Hypoparathyroidism
Primary hypoparathyroidism is a group of disorders caused by deficient parathyroid hormone (PTH) secretion leading to hypocalcemia and hyperphosphatemia (Table 3) [43,50].

Table 3.
Hypoparathyroidism genetic mutations.
- A.
- Isolated Hypoparathyroidism
- Autosomal dominant hypocalcemia type 1 results from a gain of function mutations in CaSR increasing the sensitivity and response to serum concentrations of calcium leading to inappropriately low PTH production [37,50]. It may be associated with Bartter syndrome type 5 caused by poor renal tubular reabsorption of sodium chloride [50].
- Autosomal dominant hypocalcemia type 2 results from GNA11 (guanine nucleotide-binding protein alpha-11) mutations that usually initiates intracellular signal transduction from CaSR and calcium binding [37,50,51].
- B.
- Familial Isolated Hypoparathyroidism
- Mutation of PTH on 11p15.3 is either autosomal dominant or autosomal recessive and leads to hypocalcemia-mediated increased neuromuscular irritability: paresthesias, tetany, and seizures [37,50].
- Mutations in GCM2 (glial cells missing 2) on 6p24.2 encodes a transcription factor essential for parathyroid gland differentiation [52].
- C.
- Diagnosis
In hypoparathyroidism, serum calcium concentrations are low and phosphate levels high. The PTH levels are low or undetectable [54]. Vitamin D1,25(OH)2 levels are low, but alkaline phosphatase activity is unaffected [54]. Despite increased fractional excretion of calcium, there is suppressed intestinal calcium absorption and bone resorption. Decreased renal filtered load of calcium, reduced 24-h urinary calcium excretion and low nephrogenous cyclic AMP excretion is observed; renal tubular reabsorption of phosphate is elevated [54]. The clinical symptoms of hypoparathyroidism result from hypocalcemia and comprise various degrees of neuromuscular excitability.
Symptoms include circumoral numbness, paresthesias of the distal extremities, muscle cramping, carpopedal spasm, tetany, positive Chvostek, or Trousseau signs. It can also present as laryngospasm, bronchospasm, or seizures [54]. Less specific, subtle signs are fatigue and irritability. In contrast, severe acute hypocalcemia could lead to a prolonged QTc interval and be complicated by life-threatening arrhythmias (torsades de pointes) [54]. Some with chronic hypoparathyroidism may have calcification of the basal ganglia or more widespread intracranial calcification, detected by skull X-ray or CT scan [50,54]. Others exhibit rare extrapyramidal neurological symptoms (more often with intracranial calcification), subcapsular cataracts, band keratopathy, abnormal dentition, alopecia, coarse brittle hair.
2.5. Parathyroid Hormone-Pseudohypoparathyroidism
Pseudohypoparathyroidism (PHP) is a heterogeneous group of disorders characterized by PTH end-organ resistance. Though the pathophysiology is different from hypoparathyroidism, this group presents with similar features of hypocalcemia and hyperphosphatemia (Table 4) [22].

Table 4.
Different subtypes of Pseudohypoparathyroidism.
- Pseudohypoparathyroidism 1A (PHP1A), the most common subtype of PHP, is caused by inactivating maternal GNAS mutations. The gene GNAS encodes the alpha subunit of the stimulatory guanine nucleotide-binding protein Gsα. The maternal allele is expressed exclusively in the renal tubules while in other tissues, both maternal and paternal alleles are expressed. Other hormones such as TSH, gonadotropins, and growth hormone-releasing hormone act via G protein-coupled receptors too [2,22]. Albright Hereditary Osteodystrophy (AHO) clinical phenotype describes specific features such as short stature, developmental delay, round faces, depressed nasal bridge, brachydactyly, and dental abnormalities associated with PHP1A [22,53].
- Pseudopseudohypoparathyroidism is a disorder of the mutated paternal allele of GNAS, not maternal as in PHP1A [50,53]. These patients only have AHO but without the biochemical changes of PHP1A such as PTH resistance since the maternal allele is still expressed in the renal tubules [50,53].
- Pseudohypoparathyroidism 1B (PHP1B) is caused by isolated renal resistance to PTH, resulting from methylation defects at differentially methylated regions (DMRs) of the GNAS maternal allele, causing decreased expression of Gsα [22,53]. These patients are usually difficult to diagnose as they lack the AHO findings, present with a normal phenotype [55], and typically have no other endocrine abnormalities [22,53]. As only the maternal allele is expressed in the kidney, renal PTH resistance manifests without the skeletal affects due to the intact paternal allele. Sporadic cases of PHP1B are most common, however, autosomal dominant transmission has also been reported [22,53]. Biochemically PHP1A and PHP1B are similar, leading to hyperphosphatemia, hypocalcemia, and elevated PTH (Table 4).
- Pseudohypoparathyroidism 1C (PHP1C) is caused by impaired Gsα-receptor interaction due to different GNAS mutation in exon 13 [55,56]. Clinical and biochemical presentation of abnormalities is similar to PHPIa [55,56].
- Pseudohypoparathyroidism 2 (PHP2) is caused by mutations in PRKAR1A (protein kinase, cAMP-dependent, regulatory, type 1, alpha), which encodes the catalytic subunit of adenylate cyclase [57,58,59]. Patients do not have features of AHO [57,58].
2.6. FGF23
Hypophosphatemic rickets are disorders that result in renal phosphate loss due to impaired metabolic pathways leading to the increased serum concentration of FGF23.
- Activating FGF23 mutations lead to increased urinary phosphate loss via sodium-dependent phosphate transporter in the proximal renal tubule. Specific genetic mutations and electrolyte abnormalities are shown in Table 5 [1,60].
 Table 5. Hypophosphatemic rickets genetic mutations.
Table 5. Hypophosphatemic rickets genetic mutations. - Loss-of-function mutation of PHEX (phosphate-regulating endopeptidase homolog) on chromosome Xp22.2-p22.1 is associated with increased expression of FGF23 and leads to X-linked dominant hypophosphatemic rickets [1,60,61]. Normally PHEX is expressed in mature osteoblasts and odontoblasts and plays a role in the down-regulation of FGF23 expression [62,63].
Hypophosphatemia in phosphopenic rickets results in impaired apoptosis of terminally differentiated chondrocytes and the growth plate’s mineralization failure [64]. Chronic hypophosphatemia causes impaired mineralization of osteoid and leads to rickets in children and osteomalacia in older adolescents [64]. Hypophosphatemia prevents apoptosis in the hypertrophic cells in the growth plate with consequent accumulation of the hypertrophic cells in the growth plate and formation of the rachitic bone [64].
It is the most common inherited refractory rickets in children [62,64]. Two disorders may be acquired, comprised of tumour-induced osteomalacia (TIO) and drug-induced Fanconi syndrome.
The TMP/GFR ratio provides the best estimate of renal phosphate loss. In the absence of secondary hyperparathyroidism, low TMP/GFR usually indicates renal phosphate loss as the primary defect. However, concomitant vitamin D deficiency must be treated prior to diagnosing hypophosphatemic rickets [61,62,63,64].
2.7. Hypophosphatasia
- A.
- Hypophosphatasia (HPP) is a rare inherited disorder of bone and mineral metabolism caused by loss of function mutations in the ALPL gene encoding the tissue nonspecific alkaline phosphatase (TNSALP) [65,66] (Table 6). Osteoblast synthesis of TNSALP is decreased due to monoallelic or biallelic inactivating mutations in ALPL [66,67].
 Table 6. Genetic Mutations leading to Hypophosphatasia.
Table 6. Genetic Mutations leading to Hypophosphatasia.
The TNSALP is expressed in the extracellular bone matrix and serum circulation at the outer surface of osteoblasts and chondrocytes [68]. More than 340 monoallelic or biallelic mutations of the ALPL gene have been described, explaining a broad range of clinical presentations [68,69]. Hypophosphatasia might have an autosomal dominant or autosomal recessive mode of inheritance [66,68].
Normally, the TNSALP enzyme is responsible for degrading extracellular inorganic pyrophosphate, a strong inhibitor of mineralization, into phosphate. A low concentration of extracellular inorganic pyrophosphate initiates the process of extracellular matrix mineralization [66,68]. In contrast, the high serum concentration of inorganic phosphate due to enzyme inactivity suppresses the formation of hydroxyapatite crystals and bone mineralization. Insufficient bone mineralization contributes to characteristic futures of bone changes in rickets and osteomalacia. Therefore, TNSALP is critical in promoting osteoblastic mineralization [68].
Other substrates of TNSALP are pyridoxal-5 phosphate, an active form of Vitamin B6, that facilitates the delivery of pyridoxal 5-phosphate into cells. Therefore, absence or decreased activity of enzyme results in high serum concentration of pyridoxal-5 phosphate could explain the epileptic seizures observed in babies with HPP [68].
- B.
- Clinical Symptoms
- The severe infantile form of hypophosphatasia is due to biallelic inactivating mutation presenting before six months and is fatal. Initial signs are failure to thrive, impaired feeding, motor delays and weakness, limb deformities and rachitic deformations of the thorax leading to respiratory failure. Vitamin B6-dependent seizures could be present as well [68].
- Mild clinical forms of HPP are due to monoallelic mutations leading to milder forms of the HPP presenting with bone pain, leg bowing, joint enlargement, and fractures. A specific and constant feature of HPP is the premature, painless, and atraumatic loss of the primary teeth with intact roots. Nephrocalcinosis due to elevated hypercalciuria could develop in older children and adolescents [66,68,69].
In HPP, the primary defect is absent or low TNSALP, therefore, PTH and FGF23 levels are normal [69]. In severe forms, calcium and phosphorus could not be integrated into minerals to form a bone, causing hypercalcemia, hyperphosphatemia, suppressed low PTH levels, and 1,25(OH)2 vitamin D levels. Vitamin D25(OH) may be high due to lack of conversion to vitamin D1,25(OH)2. Hypercalciuria causes nephrocalcinosis [65,67,68,69] PEA and PLP levels are elevated. The vitamin PLP is a kind of vitamin B6 that accumulates because it is the physiological extracellular substrate of ALP.
In the infantile form of HPP, one can present with demineralized calvarium and peripheral skeleton, rickets, spurs of cartilage, and bone extending from the sides of the knee and elbow joints. Neurologically, increased intracranial pressure is possible due to premature craniosynostosis [65,68,69]. Older children present with global bone demineralization. The metaphyses are irregularly enlarged, similar to rickets. Focal defects at the central zone of the metaphyses of the long bones, ‘tongues of radiolucencies,’ are typical of the disease [65,68].
3. Treatment
Treatment of genetic disorders of calcium metabolism varies based on the specific pathology, but the basis of treatment remains consistent [37]. Initial treatment depends on the acuity and severity of the clinical condition. An immediate goal of treatment is to stabilize serum calcium level within the normal range to avoid seizures and fatal arrhythmias. After stabilization, the goal is to optimize the levels of calcium, phosphorus, magnesium, and keep adequate hydration. The next step will be to ensure optimal bone mineralization, and to prevent long term complications and side effects of treatment such as nephrolithiasis. In addition, it is important to identify the cause and conduct genetic testing if clinically indicated [37].
3.1. Vitamin D Deficiency and Nutritional Rickets
For vitamin D deficiency and nutritional rickets, moderate to severe symptoms are recommended to be treated with vitamin D 50,000 IU weekly for 8–12 weeks in either formulation: ergocalciferol (D2) or cholecalciferol (D3) [18]. Once serum and urine levels of calcium, alkaline phosphatase, PTH, and vitamin D have normalized, a daily maintenance dose of 1000–2000 units can be given [70].
3.2. Vitamin D-Dependent Rickets
For vitamin D-dependent rickets, where the disease is resistant to typical doses of vitamin D, high doses of oral calcitriol (1–6 μg/kg/day, divided 2 doses), and calcium (1–3 g/day elementary calcium) are the recommended treatment [64]. The additional calcium is used to enhance the remineralization of bone [71]. This is particularly important in patients with elevated PTH levels to avoid worsening hypocalcemia when beginning vitamin D therapy to prevent hungry bone syndrome [71]. After roughly four weeks of therapy, serum calcium, phosphorus, and alkaline phosphatase concentrations and urine calcium to creatinine ratio should begin to normalize [67,71]. Serum calcium concentrations should be normalizing, though urine calcium to creatinine ratio may still be low [67,71].
To monitor progress and prevent toxicity, regular follow-up of chemistries should be checked until dosage has been lowered to a normal daily supplement level, generally occurring around three months after the start of therapy. Imaging should also be taken to assess for proper healing of bones or fractures [71].
3.3. Hyperparathyroidism
Hyperactive parathyroid gland(s) are surgically removed [72,73].
3.4. Hypoparathyroidism
In cases of hypoparathyroidism, treatment is typically managed with calcium (25–50 mg/kg elemental calcium per day) and calcitriol (initial dose of 0.25 mcg daily, increased as needed) with possible magnesium treatment as needed [73,74,75]. Urinary and serum calcium and phosphate should be measured weekly until serum calcium has stabilized, after which three to six-month follow-ups are sufficient. Hypercalciuria is the earliest sign of toxicity in these patients and should be monitored carefully to avoid complications such as nephrolithiasis, nephrocalcinosis, and renal failure [73,74,75].
3.5. Hypophosphatemic Rickets
Calcitriol and oral phosphate supplements are typically given. For calcitriol, twice-daily doses for a total of 20–40 ng/kg/day should be given [5,76]. Four to five doses per day for phosphate with a starting dose of 40 mg/kg/day should be administered. Phosphorus supplements can be increased as needed until a maximum of 2000 mg/day [76]. Children should be followed up on every three months to monitor height, serum calcium, phosphorus, alkaline phosphatase, and creatinine, as well as urine calcium to creatinine ratio [76]. Patients should also annually undergo renal ultrasonography to evaluate for nephrocalcinosis. Bone imaging should also be obtained to monitor bone age and rachitic healing [76]. In newer studies, burosumab, a recombinant human immunoglobulin monoclonal antibody, has been tested in clinical trials. Burosumab binds to the FGF23 receptor for inhibition and increases phosphate and calcitriol levels with only one dose. Dosing in children begins at 0.8 mg/kg given through subcutaneous injection every 2 weeks and increased as needed until a maximum dose of 2 mg/kg. Long-term effects are still being studied [76].
3.6. Hypophosphatasia
Recently the treatment asfotase alfa was approved for hypophosphatasia. It is a human recombinant enzyme replacement therapy for low alkaline phosphatase. Recommended dosing is roughly 6 mg/kg/week injected subcutaneously divided 3 or 6 times a week [77,78]. In most cases, dosing and regimen vary significantly by personal preference, severity, and resource availability. Supplementation also requires frequent monitoring of serum and urine calcium and phosphorus levels to prevent additional complications. Periodic renal ultrasounds and ophthalmic examinations should also be conducted to evaluate any ectopic calcifications associated with asfotase alfa.
It is important to note that symptomatic relief is achieved much quicker than biochemical or radiologic progress, so maintenance is important. Even after normal values and imaging are achieved, patients are still at risk of fracture due to the loss of cortical bone. The goal of treatment is to alleviate symptoms and strengthen bone long-term and improve mineralization [71,77,78,79].
3.7. Modifiable Factors
In our review, we described the most common and well-known genetic disorders of calcium and phosphorus metabolism. Co-existing conditions can be found in the same patient. It could present with a complex clinical picture and biochemical profile reflecting compensatory mechanisms. For example, case reports of a patient with pseudohypoparathyroidism and concomitant nutritional vitamin D deficiency, masquerading as rickets showed that treatment with vitamin D unmasked the main disorder [80,81]. Vitamin D–dependent rickets (VDDR) and vitamin D co-existing could be suspected if the patient does not respond to vitamin D treatment [82,83]. While nutritional rickets could be prevented with conventional doses of Vitamin D supplement [84], VDDR requires significantly higher doses. Another factor such as oral sodium phosphate supplementation can affect calcium phosphorus homeostasis, especially in patients with nephrolithiasis. Patients with nephrolithiasis had higher PTH levels and expressed a more prominent hypocalciuria after treatment with sodium phosphate [85].
4. Conclusions
We have summarized the genetic mutations and biochemical profiles of the most common homeostasis disorders involving calcium and phosphorus. Recent advances in molecular genetic testing have the potential to shed a light on the understanding of precise mechanisms and treatment options of disorders of calcium and phosphorus homeostasis. While management of some entities is still challenging, the treatment includes new modalities. Further research is crucial to elucidate the precise mechanisms, diagnostics, and management of genetic disorders of calcium-phosphorus homeostasis.
Author Contributions
Conceptualization, A.M. and V.L.C.; Writing, A.M., S.M., S.P., V.L.C.; Editing: A.M., L.F., V.L.C., Supervision, V.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakker, R.V.; Richard Bringhurst, F.; Jüppner, H. Chapter 61—Regulation of Calcium Homeostasis and Genetic Disorders that Affect Calcium Metabolism. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., de Kretser, D.M., Giudice, L.C., Grossman, A.B., Melmed, S., Potts, J.T., Jr., Weir, G.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2016; pp. 1063–1089.e10. [Google Scholar] [CrossRef]
- Thakker, R.V. The Parathyroid Glands, Hypercalcemia, and Hypocalcemia. In Goldman-Cecil Medicine, 26th ed.; Schafer, L.G.A.I., Ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 1611–1623.e2. [Google Scholar]
- Root, A.W.; Levine, M.A. 20—Disorders of Mineral Metabolism II. Abnormalities of Mineral Homeostasis in the Newborn, Infant, Child, and Adolescent. In Sperling Pediatric Endocrinology, 5th ed.; Sperling, M.A., Ed.; Elsevier: Philadelphia, PA, USA, 2021; pp. 705–813. [Google Scholar]
- Root, A.W. Genetic disorders of calcium, phosphorus, and bone homeostasis. Transl. Sci. Rare Dis. 2018, 3, 1–36. [Google Scholar] [CrossRef]
- Kliegman, R.M.; St. Geme, J.W.; Blum, N.J.; Shah, S.S.; Tasker, R.C.; Wilson, K.M.; Greenbaum, L.A. Electrolyte and Acid-Base Disorders. In Nelson Textbook of Pediatrics; Elsevier: Philadelphia, PA, USA, 2020; pp. 389–425. [Google Scholar]
- Moutsatsou, P.; Kassi, E.; Papavassiliou, A.G. Glucocorticoid receptor signaling in bone cells. Trends Mol. Med. 2012, 18, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.; Högler, W. Rickets and Osteomalacia. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; pp. 339–354. [Google Scholar]
- Waller, D.G.; Sampson, A.P. Calcium metabolism and metabolic bone disease. In Medical Pharmacology and Therapeutics; Elsevier: Philadelphia, PA, USA, 2017; pp. 481–489. [Google Scholar]
- Clinical disorders of bone and mineral metabolism 5th international symposium. Calcif. Tissue Int. 1993, 52, S1–S46. [CrossRef] [PubMed]
- Watson, R.R. Handbook of Vitamin D in Human Health; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013. [Google Scholar]
- Sharma, A.; Thakker, R.V.; Jüppner, H. Physiology of the Developing Kidney: Disorders and Therapy of Calcium and Phosphorous Homeostasis. In Pediatric Nephrology; Avner, E., Harmon, W., Niaudet, P., Yoshikawa, N., Emma, F., Goldstein, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 291–339. [Google Scholar]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Konrad, M. Chapter 74—Infantile Hypercalcemia and CYP24A1 Mutations. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Oxford, UK, 2018; pp. 317–330. [Google Scholar]
- Mirea, A.-M.; Pop, R.M.; Căinap, S.S.; Trifa, A.P. Presymptomatic diagnosis of CYP24A1-related infantile idiopathic hypercalcemia: A case report. Eur. J. Med. Genet. 2020, 63, 104100. [Google Scholar] [CrossRef]
- Bikle, D.D.; Adams, J.S.; Christakos, S. Vitamin D: Production, Metabolism, Action, and Clinical Requirements. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 230–240. [Google Scholar]
- Masi, L.; Agnusdei, D.; Bilezikian, J.; Chappard, D.; Chapurlat, R.; Cianferotti, L.; Devolgelaer, J.-P.; El Maghraoui, A.; Ferrari, S.; Javaid, M.K.; et al. Taxonomy of rare genetic metabolic bone disorders. Osteoporos. Int. 2015, 26, 2529–2558. [Google Scholar] [CrossRef]
- Gardella, T.J.; Nissenson, R.A.; Jüppner, H. Parathyroid Hormone. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 205–211. [Google Scholar]
- Underland, L.; Markowitz, M.; Gensure, R. Calcium and Phosphate Hormones: Vitamin D, Parathyroid Hormone, and Fibroblast Growth Factor 23. Pediatr. Rev. 2020, 41, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tfelt-Hansen, J.; Brown, E.M. The calcium-sensing receptor in normal physiology and pathophysiology: A review. Crit. Rev. Clin. Lab. Sci. 2005, 42, 35–70. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M. Disorders involving calcium, phosphorus, and magnesium. Prim. Care Clin. Off. Pract. 2008, 35, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Koves, I.H.; Ness, K.D.; Nip, A.S.-Y.; Salehi, P. 95—Disorders of Calcium and Phosphorus Metabolism. In Avery’s Diseases of the Newborn, 10th ed.; Gleason, C.A., Juul, S.E., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 1333–1350.e4. [Google Scholar]
- Goel, N.J.; Meyers, L.L.; Frangos, M. Pseudohypoparathyroidism type 1B in a patient conceived by in vitro fertilization: Another imprinting disorder reported with assisted reproductive technology. J. Assist. Reprod. Genet. 2018, 35, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fukumoto, S. Fibroblast Growth Factor 23 (FGF23) and Disorders of Phosphate Metabolism. Int. J. Pediatr. Endocrinol. 2009, 2009, 496514. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.P. Disorders of Calcium Metabolism. In Fluid and Electrolytes in Pediatrics; Feld, L.G., Kaskel, F.J., Eds.; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Schlingmann, K.P.; Kaufmann, M.; Weber, S.; Irwin, A.; Goos, C.; John, U.; Misselwitz, J.; Klaus, G.; Kuwertz-Bröking, E.; Fehrenbach, H.; et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011, 365, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Ruminska, J.; Kaufmann, M.; Dursun, I.; Patti, M.; Kranz, B.; Pronicka, E.; Ciara, E.; Akcay, T.; Bulus, D.; et al. Autosomal-Recessive Mutations in SLC34A1 Encoding Sodium-Phosphate Cotransporter 2A Cause Idiopathic Infantile Hypercalcemia. J. Am. Soc. Nephrol. 2016, 27, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Hureaux, M.; Chantot-Bastaraud, S.; Cassinari, K.; Casado, E.M.; Cuny, A.; Frébourg, T.; Vargas-Poussou, R.; Bréhin, A.-C. When a maternal heterozygous mutation of the CYP24A1 gene leads to infantile hypercalcemia through a maternal uniparental disomy of chromosome 20. Mol. Cytogenet. 2021, 14, 23. [Google Scholar] [CrossRef]
- Lodefalk, M.; Frykholm, C.; Esbjörner, E.; Ljunggren, Ö. Hypercalcaemia in a Patient with 2p13.2-p16.1 Duplication. Horm. Res. Paediatr. 2016, 85, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Cassar, W.; Konrad, M. Juvenile onset IIH and CYP24A1 mutations. Bone Rep. 2018, 9, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Jobst-Schwan, T.; Pannes, A.; Schlingmann, K.P.; Beck, B.B.; Wiesener, M.S.; Eckardt, K.-U. Discordant Clinical Course of Vitamin-D-Hydroxylase (CYP24A1) Associated Hypercalcemia in Two Adult Brothers with Nephrocalcinosis. Kidney Blood Press. Res. 2015, 40, 443–451. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Bhansali, A.; Aggarwal, A.; Parthan, G.; Gogate, Y. Rickets–Osteomalacia. In Clinical Rounds in Endocrinology; Springer: New Delhi, India, 2016; pp. 131–170. [Google Scholar]
- Ahmad, N.; Sobaihi, M.M.; Al-Jabri, M.; Al-Esaei, N.A.; Alzaydi, A. Acute respiratory failure and generalized hypotonia secondary to vitamin D dependent rickets type 1A. Int. J. Pediatr. Adolesc. Med. 2018, 5, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, A.; Castells, S.; Perez-Colon, S. Genetic Disorders of Vitamin D Metabolism: Case Series and Literature Review. Clin. Pediatr. 2016, 55, 404–414. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Genetic Testing Registry; U.S. National Library of Medicine: Bethesda, MD, USA, 2022. [Google Scholar]
- Gattineni, J.; Baum, M. Genetic disorders of phosphate regulation. Pediatr. Nephrol. 2012, 27, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Root, A.W. Genetic disorders of calcium and phosphorus metabolism. Crit. Rev. Clin. Lab. Sci. 2000, 37, 217–260. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bandeira, L.; Khan, A.; Cusano, N.E. Hyperparathyroidism. Lancet 2018, 391, 168–178. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Cusano, N.E.; Khan, A.A.; Liu, J.M.; Marcocci, C.; Bandeira, F. Primary hyperparathyroidism. Nat. Rev. Dis. Primers 2016, 2, 16033. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.H. Approach to the Child with Hypercalcaemia. In Calcium and Bone Disorders in Children and Adolescents; Karger: Basel, Switzerland, 2015; Volume 28, pp. 101–118. [Google Scholar] [CrossRef]
- Silva, B.C.; Cusano, N.E.; Bilezikian, J.P. Primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.; Wysolmerski, J. Chapter 66—Disorders of Calcium Metabolism. In Seldin and Giebisch’s The Kidney, 5th ed.; Alpern, R.J., Moe, O.W., Caplan, M., Eds.; Academic Press: Oxford, UK, 2013; pp. 2273–2309. [Google Scholar]
- Robinson, C. Genetics of Mineral Disorders. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: Oxford, UK, 2020; pp. 92–107. [Google Scholar]
- Diao, J.; DeBono, A.; Josephs, T.M.; Bourke, J.E.; Capuano, B.; Gregory, K.J.; Leach, K. Therapeutic Opportunities of Targeting Allosteric Binding Sites on the Calcium-Sensing Receptor. ACS Pharmacol. Transl. Sci. 2021, 4, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.V.; Nyholt, D.; Busfield, F.; Epstein, M.; Burgess, J.; Stranks, S.; Hill, P.; Perry-Keene, D.; Learoyd, D.; Robinson, B.; et al. Familial isolated hyperparathyroidism is linked to a 1.7 Mb region on chromosome 2p13.3–14. J. Med. Genet. 2006, 43, e12. [Google Scholar] [CrossRef][Green Version]
- Shibata, Y.; Yamazaki, M.; Takei, M.; Uchino, S.; Sakurai, A.; Komatsu, M. Early-onset, severe, and recurrent primary hyperparathyroidism associated with a novel CDC73 mutation. Endocr. J. 2015, 62, 627–632. [Google Scholar] [CrossRef]
- Simonds, W.F.; James-Newton, L.A.; Agarwal, S.K.; Yang, B.; Skarulis, M.C.; Hendy, G.N.; Marx, S.J. Familial Isolated Hyperparathyroidism: Clinical and Genetic Characteristics of 36 Kindreds. Medicine 2002, 81, 1–26. [Google Scholar] [CrossRef]
- Battista, C.; Guarnieri, V.; Carnevale, V.; Baorda, F.; Pileri, M.; Garrubba, M.; Salcuni, A.S.; Chiodini, I.; Minisola, S.; Romagnoli, E.; et al. Vitamin D status in primary hyperparathyroidism: Effect of genetic background. Endocrine 2017, 55, 266–272. [Google Scholar] [CrossRef]
- Kabadi, U.M. Low 25-Hydroxyvitamin D in Primary Hyperparathyroidism: Enhanced Conversion into 1,25-Hydroxyvitamin D May Not Be “True” Deficiency. JBMR Plus 2020, 4, e10415. [Google Scholar] [CrossRef]
- Hendy, G.N.; Cole, D.E.C.; Bastepe, M. Hypoparathyroidism and Pseudohypoparathyroidism. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Roszko, K.L.; Bi, R.D.; Mannstadt, M. Autosomal Dominant Hypocalcemia (Hypoparathyroidism) Types 1 and 2. Front. Physiol. 2016, 7, 458. [Google Scholar] [CrossRef]
- Thomée, C.; Schubert, S.W.; Parma, J.; Lê, P.Q.; Hashemolhosseini, S.; Wegner, M.; Abramowicz, M. GCMB Mutation in Familial Isolated Hypoparathyroidism with Residual Secretion of Parathyroid Hormone. J. Clin. Endocrinol. Metab. 2005, 90, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Levine, M.A. Genetic Disorders of Parathyroid Development and Function. Endocrinol. Metab. Clin. N. Am. 2018, 47, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Hendy, G.N.; Bastepe, M.; Cole, D.E.C. Chapter 85—Parathyroid Disorders. In Emery and Rimoin’s Principles and Practice of Medical Genetics; Rimoin, D., Pyeritz, R., Korf, B., Eds.; Academic Press: Oxford, UK, 2013; pp. 1–34. [Google Scholar]
- Brix, B.; Werner, R.; Staedt, P.; Struve, D.; Hiort, O.; Thiele, S. Different Pattern of Epigenetic Changes of the GNAS Gene Locus in Patients with Pseudohypoparathyroidism Type Ic Confirm the Heterogeneity of Underlying Pathomechanisms in This Subgroup of Pseudohypoparathyroidism and the Demand for a New Classification of GNAS-Related Disorders. J. Clin. Endocrinol. Metab. 2014, 99, E1564–E1570. [Google Scholar] [PubMed]
- Thiele, S.; de Sanctis, L.; Werner, R.; Grötzinger, J.; Aydin, C.; Jüppner, H.; Bastepe, M.; Hiort, O. Functional characterization of GNAS mutations found in patients with pseudohypoparathyroidism type Ic defines a new subgroup of pseudohypoparathyroidism affecting selectively Gsα-receptor interaction. Hum. Mutat. 2011, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.J.; Villarreal, H.; Klahr, S.; Slatopolsky, E. Pseudohypoparathyroidism Type II: Restoration of Normal Renal Responsiveness to Parathyroid Hormone by Calcium Administration. J. Clin. Endocrinol. Metab. 1974, 39, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Linglart, A.; Menguy, C.; Couvineau, A.; Auzan, C.; Gunes, Y.; Cancel, M.; Motte, E.; Pinto, G.; Chanson, P.; Bougnères, P.; et al. Recurrent PRKAR1A mutation in acrodysostosis with hormone resistance. N. Engl. J. Med. 2011, 364, 2218–2226. [Google Scholar] [CrossRef]
- Drezner, M.; Neelon, F.A.; Lebovitz, H.E. Pseudohypoparathyroidism type II: A possible defect in the reception of the cyclic AMP signal. N. Engl. J. Med. 1973, 289, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Al Juraibah, F.; Al Amiri, E.; Al Dubayee, M.; Al Jubeh, J.; Al Kandari, H.; Al Sagheir, A.; Al Shaikh, A.; Beshyah, S.A.; Deeb, A.; Habeb, A.; et al. Diagnosis and management of X-linked hypophosphatemia in children and adolescent in the Gulf Cooperation Council countries. Arch. Osteoporos. 2021, 16, 52. [Google Scholar] [CrossRef]
- Raimann, A.; Mehany, S.N.; Feil, P.; Weber, M.; Pietschmann, P.; Boni-Mikats, A.; Klepochova, R.; Krššák, M.; Häusler, G.; Schneider, J.; et al. Decreased Compressional Sound Velocity Is an Indicator for Compromised Bone Stiffness in X-Linked Hypophosphatemic Rickets (XLH). Front. Endocrinol. 2020, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Şıklar, Z.; Turan, S.; Bereket, A.; Baş, F.; Güran, T.; Akberzade, A.; Abacı, A.; Demir, K.; Böber, E.; Özbek, M.N.; et al. Nationwide Turkish Cohort Study of Hypophosphatemic Rickets. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 150–159. [Google Scholar] [CrossRef]
- Acar, S.; Demir, K.; Shi, Y. Genetic Causes of Rickets. J. Clin. Res. Pediatr. Endocrinol. 2017, 9 (Suppl. 2), 88–105. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, V.S.; Sarathi, V.; Lila, A.R.; Bandgar, T.; Menon, P.; Shah, N.S. Hypophosphatemic rickets. Indian J. Endocrinol. Metab. 2012, 16, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Josse, R.; Kannu, P.; Villeneuve, J.; Paul, T.; Van Uum, S.; Greenberg, C.R. Hypophosphatasia: Canadian update on diagnosis and management. Osteoporos Int. 2019, 30, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Girschick, H.; Mornet, E.; Schneider, D.; Jakob, F.; Mentrup, B. Unexpected high intrafamilial phenotypic variability observed in hypophosphatasia. Eur. J. Hum. Genet. 2014, 22, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Hypophosphatasia: An overview For 2017. Bone 2017, 102, 15–25. [Google Scholar] [CrossRef]
- Linglart, A.; Biosse-Duplan, M. Hypophosphatasia. Curr. Osteoporos Rep. 2016, 14, 95–105. [Google Scholar] [CrossRef]
- Mornet, E. Hypophosphatasia. Metabolism 2018, 82, 142–155. [Google Scholar] [CrossRef]
- Bhan, A.; Rao, A.; Bhadada, S.; Rao, S. Rickets and Osteomalacia. In Williams Textbook of Endocrinology; Elsevier: Philadelphia, PA, USA, 2020; pp. 1298–1317.e6. [Google Scholar]
- Carpenter, T. Etiology and Treatment of Calcipenic Rickets in Children. 6 February 2020. Available online: https://www.medilib.ir/uptodate/show/5804 (accessed on 25 January 2022).
- Bollerslev, J.; Schalin-Jäntti, C.; Rejnmark, L.; Siggelkow, H.; Morreau, H.; Thakker, R.; Sitges-Serra, A.; Cetani, F.; Marcocci, C.; Guistina, A.; et al. Management of Endocrine Disease: Unmet therapeutic, educational and scientific needs in parathyroid disorders. Eur. J. Endocrinol. 2019, 181, P1–P19. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, R.D.; Rakel, D.; Conn, H.F.; Marcocci, C. Hyperparathyroidism and Hypoparathyroidism. In Conn’s Current Therapy; Elsevier: Philadelphia, PA, USA, 2021; pp. 330–337. [Google Scholar]
- Goltzman, D. Hypoparathyroidism; UpToDate. 2021. Available online: https://www.uptodate.com/contents/hypoparathyroidism (accessed on 25 January 2022).
- Rubin, M.R.; Levine, M.A. Chapter 75. Hypoparathyroidism and Pseudohypoparathyroidism. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 354–361. [Google Scholar]
- Scheinman, S.J.; Carpenter, T. Hereditary Hypophosphatemic Rickets and Tumor-Induced Osteomalacia; UpToDate, 2021. Available online: https://www.uptodate.com/contents/hereditary-hypophosphatemic-rickets-and-tumor-induced-osteomalacia (accessed on 25 January 2022).
- Seefried, L.; Kishnani, P.S.; Moseley, S.; Denker, A.E.; Watsky, E.; Whyte, M.P.; Dahir, K.M. Pharmacodynamics of asfotase alfa in adults with pediatric-onset hypophosphatasia. Bone 2021, 142, 115664. [Google Scholar] [CrossRef] [PubMed]
- Seefried, L.; Rak, D.; Petryk, A.; Genest, F. Bone turnover and mineral metabolism in adult patients with hypophosphatasia treated with asfotase alfa. Osteoporos. Int. 2021, 32, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Stokes, V.J.; Nielsen, M.F.; Hannan, F.M.; Thakker, R.V. Hypercalcemic Disorders in Children. J. Bone Miner. Res. 2017, 32, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Vlachopapadopoulou, E.-A.; Anagnostou, E.; Dikaiakou, E.; Hanna, P.; Tsolia, M.; Michalacos, S.; Linglart, A.; Karavanaki, K. Pseudohypoparathyroidism type 1B (PHP1B), a rare disorder encountered in adolescence. J. Pediatr. Endocrinol. Metab. 2020, 33, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Ramalho e Silva, J.D.; da Rocha, G.F.M.A.; Oliveira, M.J.M. An intricate case of sporadic pseudohypoparathyroidism type 1B with a review of literature. Arch. Endocrinol. Metab. 2021, 65, 112–116. [Google Scholar] [PubMed]
- Bouchard, G.; Laberge, C.; Scriver, C.R. Hereditary tyrosinemia and vitamin-dependent rickets in Saguenay. A genetic and demographic approach. Union Med. Can. 1985, 114, 633–636. [Google Scholar] [PubMed]
- El Kholy, M.; Elsedfy, H.; Cancio, M.F.; Hamza, R.T.; Amr, N.H.; Ahmed, A.; Toaima, N.N.; Audí, L. Nutritional rickets: Vitamin D, calcium, and the genetic make-up. Pediatr. Res. 2017, 81, 356–363. [Google Scholar] [CrossRef]
- Zittermann, A.; Pilz, S.; Berthold, H.K. Serum 25-hydroxyvitamin D response to vitamin D supplementation in infants: A systematic review and meta-analysis of clinical intervention trials. Eur. J. Nutr. 2020, 59, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Irzyniec, T.; Boryń, M.; Kasztalska, J.; Nowak-Kapusta, Z.; Maciejewska-Paszek, I.; Grochowska-Niedworok, E. The effect of an oral sodium phosphate load on parathyroid hormone and fibroblast growth factor 23 secretion in normo- and hypercalciuric stone-forming patients. Clin. Nutr. 2020, 39, 3804–3812. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).