Protein-Bound Nano-Injectable Suspension: Unveiling the Promises and Challenges
Abstract
1. Introduction
2. Fundamentals of Protein-Bound Nano-Injectable Suspensions
2.1. Types of Carrier Proteins
2.2. Mechanisms of Drug Binding
2.2.1. Covalent Interactions
2.2.2. Non-Covalent Interactions
2.2.3. Hydrophobic/Hydrophilic Balance
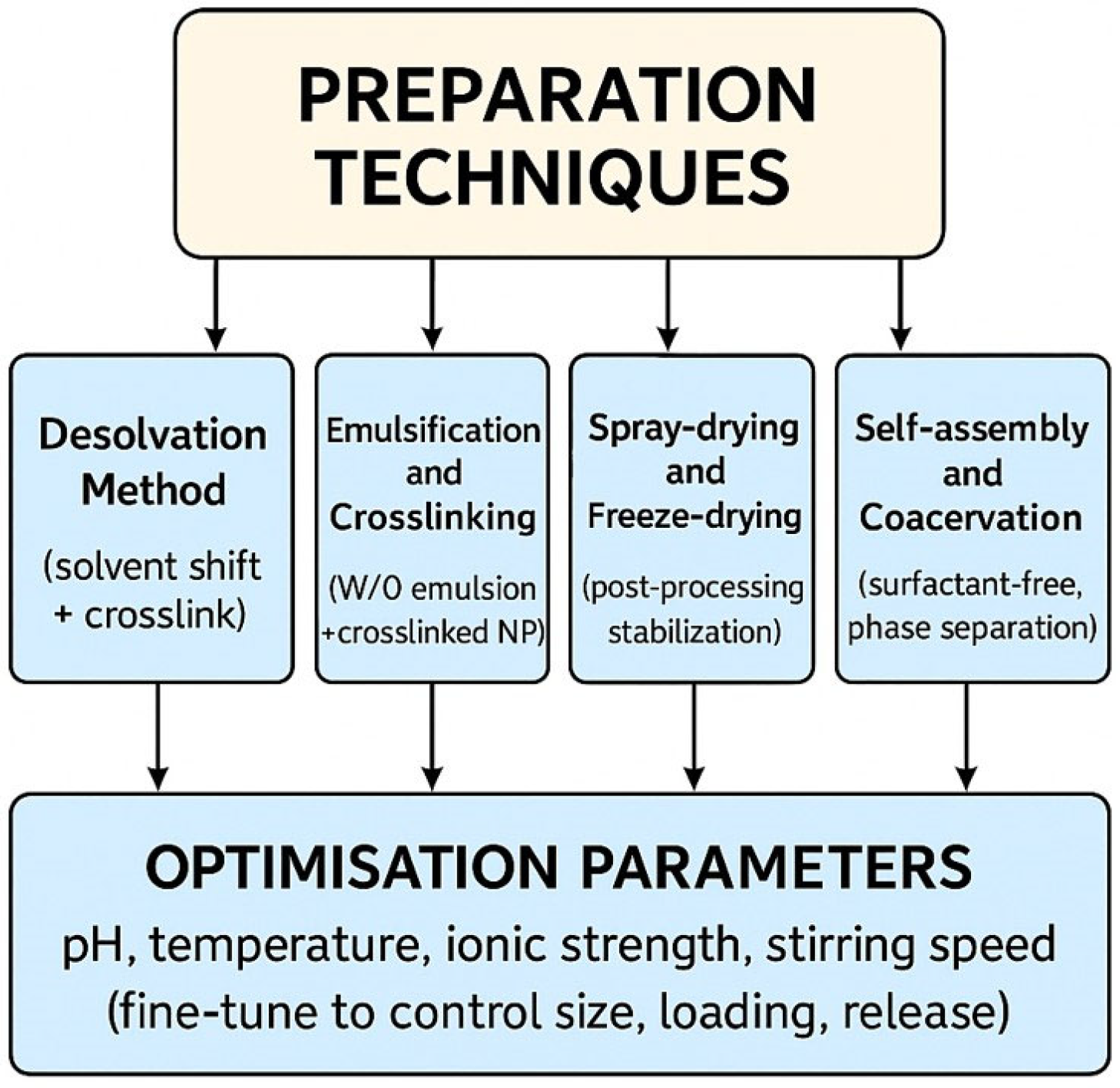
3. Preparation Techniques
3.1. Desolvation Method
3.2. Emulsification and Crosslinking
3.3. Spray-Drying and Freeze-Drying
3.4. Self-Assembly and Coacervation
3.5. Optimization Parameters
4. Physicochemical and Biological Characterization
4.1. Particle Size and Zeta Potential
4.2. Surface Morphology
4.3. Drug Loading and Entrapment Efficiency
4.4. Stability Studies
4.5. In Vitro Release Profiles
4.6. Protein Structure Integrity
5. Advantages and Therapeutic Promises
6. Challenges and Limitations
7. Clinical and Preclinical Applications
7.1. Oncology
7.2. Anti-Inflammatory and Autoimmune Disorders
7.3. Antimicrobial and Antiviral Delivery
7.4. Neurological Disorders
7.5. Case Studies and Ongoing Clinical Trials
| Application Area | Target Diseases/Conditions | Key Benefits | Examples |
|---|---|---|---|
| Oncology | Breast cancer, pancreatic cancer, non-small cell lung cancer | Enhanced tumor targeting, reduced toxicity, improved efficacy | Nab-paclitaxel (Abraxane®), Albumin-bound gemcitabine [4,5,12] |
| Anti-inflammatory and Autoimmune Disorders | Rheumatoid arthritis, IBD, Psoriasis | Controlled release, targeted delivery, reduced systemic side effects | Albumin-based anti-inflammatory agents [88,100] |
| Antimicrobial and Antiviral Delivery | Bacterial infections, viral infections (e.g., COVID-19) | Improved bioavailability, site-specific delivery, reduced resistance | Protein-bound amphotericin B [92], remdesivir-loaded nanoparticles [101] |
| Neurological Disorders | Alzheimer’s, Parkinson’s, glioblastoma | BBB penetration, sustained CNS (central nervous system) delivery, reduced peripheral toxicity | Albumin nanoparticles with BBB-targeting ligands [94,96] |
| Case Studies and Clinical Trials | Various cancers, autoimmune, CNS disorders | Validates efficacy and safety, supports regulatory approval and broader clinical application | Abraxane® trials, Albumin/transferrin nanoparticle trials [102] |
8. Regulatory Landscape and Safety Concerns
8.1. Guidelines from FDA, EMA and ICH [105,106]
8.2. Preclinical Safety Requirements
8.3. Risk Assessment: Toxicokinetic and Immunotoxicity
8.4. Pharmacovigilance Post-Approval
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADCs | Antibody–drug conjugates |
| ADME | Absorption, distribution, metabolism, and excretion |
| Al | Artificial intelligence |
| BBB | Blood–brain barrier |
| CD | Circular dichroism |
| CNS | Central nervous system |
| DLS | Dynamic Light Scattering |
| DNA | Deoxyribonucleic acid |
| DoE | Design of Experiments |
| EMA | European Medicines Agency |
| EPR | Enhanced permeability and retention |
| FDA | Food and Drug Administration |
| FTIR | Fourier transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| HSA | Human serum albumin |
| IBD | Inflammatory bowel disease |
| ICH | International Council for Harmonization |
| IND | Investigational new drug |
| ML | Machine learning |
| MRI | Magnetic resonance imaging |
| NOAEL | No Observed Adverse Effect Level |
| NSCLC | Non-small cell lung cancer |
| PDI | Polydispersity index |
| PEG | Polyethylene glycol |
| pI | Isoelectric point |
| PSURs | Periodic safety update reports |
| RMPs | Risk Management Plans |
| RSM | Response surface methodology |
| RWE | Real-world evidence |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
References
- Zhang, Q.; Zhang, J.; Song, J.; Liu, Y.; Ren, X.; Zhao, Y. Protein-Based Nanomedicine for Therapeutic Benefits of Cancer. ACS Nano 2021, 15, 8001–8038. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of Polymeric Nanoparticles for Biomedical Delivery Applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; Tambuwala, M.M. Protein-Based Drug Delivery Nanomedicine Platforms: Recent Developments. Pharm. Nanotechnol. 2022, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Nanoparticle Albumin-Bound Paclitaxel (Abraxane®). In Albumin in Medicine: Pathological and Clinical Applications; Springer: Singapore, 2016; pp. 101–119. [Google Scholar]
- U.S. Food and Drug Administration. Highlights of Prescribing Information for Paclitaxel Protein-Bound Particles for Injectable Suspension (Albumin-Bound); U.S. Food and Drug Administration (FDA): Silver Spring, MD, USA, 2022. [Google Scholar]
- European Medicines Agency (EMEA). Reflection Paper on Nanotechnology-Based Medicinal Products for Human Use; European Medicines Agency (EMEA): Amsterdam, The Netherlands, 2006. [Google Scholar]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold Nanoparticles as Novel Agents for Cancer Therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef]
- Mishra, V.; Kesharwani, P.; Jain, N.K. Biomedical Applications and Toxicological Aspects of Functionalized Carbon Nanotubes. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 293–330. [Google Scholar] [CrossRef] [PubMed]
- Rabinow, B.E. Nanosuspensions in Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, J.; Liu, Y.; Yu, M.; Zhao, L.; Zhu, X.; Bhasin, S.; Li, Q.; Ha, E.; Shi, J.; et al. Ultra-PH-Responsive and Tumor-Penetrating Nanoplatform for Targeted SiRNA Delivery with Robust Anti-Cancer Efficacy. Angew. Chem.—Int. Ed. 2016, 55, 7091–7094. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced Materials and Processing for Drug Delivery: The Past and the Future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef]
- Yardley, D.A. Nab-Paclitaxel Mechanisms of Action and Delivery. J. Control. Release 2013, 170, 365–372. [Google Scholar] [CrossRef]
- Cho, H.; Jeon, S.I.; Ahn, C.H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef]
- Mustafai, A.; Zubair, M.; Hussain, A.; Ullah, A. Recent Progress in Proteins-Based Micelles as Drug Delivery Carriers. Polymers 2023, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.B.; Borse, D.M.; More, M.P.; Patil, D.A. Gossypol-Embedded Casein Nanoparticles for Potential Targeting of Ovarian Cancer: Formulation, Characterization, and Anticancer Activity. J. Pharm. Innov. 2023, 18, 563–574. [Google Scholar] [CrossRef]
- Spanu, C.; Camorani, S.; Tortorella, S.; Agnello, L.; Maturi, M.; Comes Franchini, M.; Cerchia, L.; Locatelli, E. Synthesis and Functionalization of Casein Nanoparticles with Aptamers for Triple-Negative Breast Cancer Targeting. New J. Chem. 2022, 46, 21995–21999. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M. The Role of Surface Charge in the Desolvation Process of Gelatin: Implications in Nanoparticle Synthesis and Modulation of Drug Release. Int. J. Nanomed. 2017, 12, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Sleep, D. Albumin and Its Application in Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Benival, D. Peptide Functionalised Nanocarriers for Bone Specific Delivery of PTH (1-34) in Osteoporosis. Curr. Nanomed. 2021, 11, 142–148. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Fei, S.; He, H.; Zhang, Y.; Yin, T.; Tang, X. Formulation and Pharmacokinetics of HSA-Core and PLGA-Shell Nanoparticles for Delivering Gemcitabine. AAPS PharmSciTech 2018, 19, 812–819. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Helmy, M.W.; Samy, W.M.; Elgindy, N.A. Novel Ionically Crosslinked Casein Nanoparticles for Flutamide Delivery: Formulation, Characterization, and in Vivo Pharmacokinetics. Int. J. Nanomed. 2013, 8, 1721–1732. [Google Scholar] [CrossRef]
- Xu, J.; Gattacceca, F.; Amiji, M. Biodistribution and Pharmacokinetics of EGFR-Targeted Thiolated Gelatin Nanoparticles Following Systemic Administration in Pancreatic Tumor-Bearing Mice. Mol. Pharm. 2013, 10, 2031–2044. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ain, Q.U.; Sunny, A.; Raza, F.; Mohsin, M.; Khan, S.; Weigmann, B.; Ali, H. QbD-Based Fabrication of Transferrin-Anchored Nanocarriers for Targeted Drug Delivery to Macrophages and Colon Cells for Mucosal Inflammation Healing. Biomater. Sci. 2023, 11, 1373–1397. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Chopra, V.; Alvarado, A.G.; Gómez Siono, J.A.; Madou, M.J.; Martinez-Chapa, S.O.; Kulkarni, M.M. Doxorubicin Conjugated γ-Globulin Functionalised Gold Nanoparticles: A PH-Responsive Bioinspired Nanoconjugate Approach for Advanced Chemotherapeutics. Pharmaceutics 2024, 16, 208. [Google Scholar] [CrossRef]
- Schaefer, D.; Cheng, X. Recent Advances in Covalent Drug Discovery. Pharmaceuticals 2023, 16, 663. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Singh, S.K.; Li, N.; Toler, M.R.; King, K.R.; Nema, S. Immunogenicity of Protein Aggregates—Concerns and Realities. Int. J. Pharm. 2012, 431, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide–Drug Conjugates as Effective Prodrug Strategies for Targeted Delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 112–126. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-Directed Antibody-Drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol. Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Liu, B.; Dudu, O.E.; Zhang, S.; Meng, L.; Wang, Y.; Wei, W.; Cheng, J.; Yan, T. Modification of Structural and Functional Characteristics of Casein Treated with Quercetin via Two Interaction Modes: Covalent and Non-Covalent Interactions. Food Hydrocoll. 2023, 137, 108394. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Qu, Q.; Li, B.; Zhang, L.; Gu, R.; Zuo, J.; Wei, W.; Ma, C.; Liu, L.; et al. Engineering Non-Covalently Assembled Protein Nanoparticles for Long-Acting Gouty Arthritis Therapy. J. Mater. Chem. B 2021, 9, 9923–9931. [Google Scholar] [CrossRef]
- Abdel-Bar, H.M.; Tulbah, A.S.; Darwish, H.W.; Salama, R.; Naguib, I.A.; Yassin, H.A.; Abo El-Enin, H.A. Quetiapine Albumin Nanoparticles as an Efficacious Platform for Brain Deposition and Potentially Improved Antipsychotic Activity. Pharmaceutics 2023, 15, 1785. [Google Scholar] [CrossRef]
- Qing, R.; Hao, S.; Smorodina, E.; Jin, D.; Zalevsky, A.; Zhang, S. Protein Design: From the Aspect of Water Solubility and Stability. Chem. Rev. 2022, 122, 14085–14179. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Li, Y.; Wang, W.; Shi, J.; Fu, F.; Huang, Y.; Pan, X.; Wu, C. Impact of Particle Size and PH on Protein Corona Formation of Solid Lipid Nanoparticles: A Proof-of-Concept Study. Acta Pharm. Sin. B 2021, 11, 1030–1046. [Google Scholar] [CrossRef]
- Guo, X.; Wu, X.; Sun, Z.; Li, D.; Jia, H.; Zhang, K.; Zhao, Y.; Zheng, H. Preparation, Characterization, and Binding Mechanism of PH-Driven Gliadin/Soy Protein Isolate Nanoparticles. Food Res. Int. 2025, 208, 116289. [Google Scholar] [CrossRef]
- Brancolini, G.; Rotello, V.M.; Corni, S. Role of Ionic Strength in the Formation of Stable Supramolecular Nanoparticle–Protein Conjugates for Biosensing. Int. J. Mol. Sci. 2022, 23, 2368. [Google Scholar] [CrossRef]
- Chen, B.; He, X.Y.; Yi, X.Q.; Zhuo, R.X.; Cheng, S.X. Dual-Peptide-Functionalized Albumin-Based Nanoparticles with PH-Dependent Self-Assembly Behavior for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 15148–15153. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, H.; Zhang, H. Protein Nanospheres and Nanofibers Prepared by Ice-Templating for the Controlled Release of Hydrophobic Drugs. ACS Appl. Nano Mater. 2024, 7, 21692–21704. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted Drug Delivery to Tumors: Myths, Reality and Possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Rana, D.; Kumar, H.; Kommineni, N.; Benival, D. Anabolic Peptide-Enriched Stealth Nanoliposomes for Effective Anti-Osteoporotic Therapy. Pharmaceutics 2022, 14, 2417. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Fang, D.; Pi, M.; Pan, Z.; Song, N.; He, X.; Li, J.; Luo, F.; Tan, H.; Li, Z. Stable, Bioresponsive, and Macrophage-Evading Polyurethane Micelles Containing an Anionic Tripeptide Chain Extender. ACS Omega 2019, 4, 16551–16563. [Google Scholar] [CrossRef]
- Petros, R.A.; Desimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lin, H.; Dai, J.; Wen, X.; Yu, X.; Xu, C.; Ruan, G. Protein-Nanoparticle Co-Assembly Supraparticles for Drug Delivery: Ultrahigh Drug Loading and Colloidal Stability, and Instant and Complete Lysosomal Drug Release. Int. J. Pharm. 2024, 658, 124231. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation Process and Surface Characterisation of Protein Nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, Y.P.; Dewi, M.K.; Gatera, V.A.; Barliana, M.I.; Joni, I.M.; Chaerunisaa, A.Y. Factors Affecting the Synthesis of Bovine Serum Albumin Nanoparticles Using the Desolvation Method. Nanotechnol. Sci. Appl. 2024, 17, 21–40. [Google Scholar] [CrossRef]
- Sadeghi, R.; Kalbasi, A.; Moosavi-Movahedi, A.A.; Karimi, M.; Kokini, J. Preparation of BSA Nanoparticles by Desolvation Method as a Delivery System for Nutraceuticals. In Proceedings of the 2013 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2013, Washington, DC, USA, 12–16 May 2013; Volume 3. [Google Scholar]
- Salehiabar, M.; Nosrati, H.; Javani, E.; Aliakbarzadeh, F.; Kheiri Manjili, H.; Davaran, S.; Danafar, H. Production of Biological Nanoparticles from Bovine Serum Albumin as Controlled Release Carrier for Curcumin Delivery. Int. J. Biol. Macromol. 2018, 115, 83–89. [Google Scholar] [CrossRef]
- Lin, J.; Meng, H.; Yu, S.; Wang, Z.; Ai, C.; Zhang, T.; Guo, X. Genipin-Crosslinked Sugar Beet Pectin-Bovine Serum Albumin Nanoparticles as Novel Pickering Stabilizer. Food Hydrocoll. 2021, 112, 106306. [Google Scholar] [CrossRef]
- Long, X.; Ren, J.; Zhang, C.; Ji, F.; Jia, L. Facile and Controllable Fabrication of Protein-Only Nanoparticles through Photo-Induced Crosslinking of Albumin and Their Application as Dox Carriers. Nanomaterials 2019, 9, 797. [Google Scholar] [CrossRef]
- Zimmermann, C.M.; Baldassi, D.; Chan, K.; Adams, N.B.P.; Neumann, A.; Porras-Gonzalez, D.L.; Wei, X.; Kneidinger, N.; Stoleriu, M.G.; Burgstaller, G.; et al. Spray Drying SiRNA-Lipid Nanoparticles for Dry Powder Pulmonary Delivery. J. Control. Release 2022, 351, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, L.; Choudhary, P.; Moses, J.A.; Anandharamakrishnan, C. Emulsion Electrospraying and Spray Drying of Whey Protein Nano and Microparticles with Curcumin. Food Hydrocoll. Health 2023, 3, 100122. [Google Scholar] [CrossRef]
- Khramtsov, P.; Burdina, O.; Lazarev, S.; Novokshonova, A.; Bochkova, M.; Timganova, V.; Kiselkov, D.; Minin, A.; Zamorina, S.; Rayev, M. Modified Desolvation Method Enables Simple One-Step Synthesis of Gelatin Nanoparticles from Different Gelatin Types with Any Bloom Values. Pharmaceutics 2021, 13, 1537. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Melo, V.A.; Buzgo, M.; Bremer, E.; Simaite, A. Antibody Desolvation with Sodium Chloride and Acetonitrile Generates Bioactive Protein Nanoparticles. PLoS ONE 2024, 19, e0300416. [Google Scholar] [CrossRef] [PubMed]
- Luebbert, C.C.E.; Mansa, R.; Rahman, R.; Jakubek, Z.J.; Frahm, G.E.; Zou, S.; Johnston, M.J.W. Influence of Bound Dodecanoic Acid on the Reconstitution of Albumin Nanoparticles from a Lyophilized State. Sci. Rep. 2021, 11, 4768. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, M.; Li, W.; Zhao, L.; Wang, Z.; Yan, X.; Norde, W.; Li, Y. Pickering Emulsions Stabilized by Whey Protein Nanoparticles Prepared by Thermal Cross-Linking. Colloids Surf. B Biointerfaces 2015, 127, 96–104. [Google Scholar] [CrossRef]
- Olshefsky, A.; Richardson, C.; Pun, S.H.; King, N.P. Engineering Self-Assembling Protein Nanoparticles for Therapeutic Delivery. Bioconjug. Chem. 2022, 33, 2018–2034. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein Nanoparticles for Pickering Emulsions: A Comprehensive Review on Their Shapes, Preparation Methods, and Modification Methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Hedayati, R.; Jahanshahi, M.; Attar, H. Fabrication and Characterization of Albumin-Acacia Nanoparticles Based on Complex Coacervation as Potent Nanocarrier. J. Chem. Technol. Biotechnol. 2012, 87, 1401–1408. [Google Scholar] [CrossRef]
- Vogelaar, T.D.; Agger, A.E.; Reseland, J.E.; Linke, D.; Jenssen, H.; Lund, R. Crafting Stable Antibiotic Nanoparticles via Complex Coacervation of Colistin with Block Copolymers. Biomacromolecules 2024, 25, 4267–4280. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Mauser, A.; Ko, Y.; Lahann, J. Protein Nanoparticles: Uniting the Power of Proteins with Engineering Design Approaches. Adv. Sci. 2022, 9, 2104012. [Google Scholar] [CrossRef]
- Desai, N.; Nayi, S.; Khunt, D.; Kapoor, D.U.; Salave, S.; Prajapati, B.; Vora, C.; Malviya, R.; Maheshwari, R.; Patel, R. Zein: Potential Biopolymer in Inflammatory Bowel Diseases. J. Biomed. Mater. Res. A 2025, 113, e37785. [Google Scholar] [CrossRef]
- Doan, C.D.; Ghosh, S. Formation and Stability of Pea Proteins Nanoparticles Using Ethanol-Induced Desolvation. Nanomaterials 2019, 9, 949. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kalashnikova, T.; Bochkova, M.; Kropaneva, M.; Timganova, V.; Zamorina, S.; Rayev, M. Measuring the Concentration of Protein Nanoparticles Synthesized by Desolvation Method: Comparison of Bradford Assay, BCA Assay, Hydrolysis/UV Spectroscopy and Gravimetric Analysis. Int. J. Pharm. 2021, 599, 120422. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Li, Y.; Hu, H.; Zhong, J.; Zhong, X.; Wang, F.; Pan, H.; Bi, Y.; Kong, F. Characterization, Stability and Activity of Apigenin-Gelatin Nanoparticles Prepared by PH-Driven Method. Colloids Surf. A Physicochem. Eng. Asp. 2025, 718, 136962. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Dawoud, M.H.S.; Abdel-Daim, A.; Nour, M.S.; Sweed, N.M. A Quality by Design Paradigm for Albumin-Based Nanoparticles: Formulation Optimization and Enhancement of the Antitumor Activity. J. Pharm. Innov. 2023, 18, 1395–1414. [Google Scholar] [CrossRef]
- Nadkarni, A.; Rana, D.; Desai, N.; Benival, D.; Joshi, V.; Salave, S.; Khunt, D. Advanced Characterization and Sample Preparation Strategies for Nanoformulations. J. Nanotheranostics 2024, 5, 104–127. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- FDA; CDER; YEATON; AYSE. Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry; U.S. Food and Drug Administration: Rockville, MD, USA, 2022. [Google Scholar]
- Wu, S.; Xia, J.; Wei, Z.; Sun, W.; Zhang, X.; Xiang, N. Preparation, Characterization, and Foaming Properties of Soy Protein Nanoparticles by the Cross-Linking Reaction Induced by Microbial Transglutaminase. Food Hydrocoll. 2023, 140, 108627. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Factors Affecting Drug Encapsulation and Stability of Lipid-Polymer Hybrid Nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, S.H.; Decker, C.G.; Wong, D.Y.; Loo, J.A.; Maynard, H.D. A Heparin-Mimicking Polymer Conjugate Stabilizes Basic Fibroblast Growth Factor. Nat. Chem. 2013, 5, 221–227. [Google Scholar] [CrossRef]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri, S. Shape and Size-Dependent Immune Response to Antigen-Carrying Nanoparticles. J. Control. Release 2015, 220, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.C.; Chou, D.K.; Murphy, B.M.; Payne, R.W.; Katayama, D.S. Stability of Protein Pharmaceuticals: An Update. Pharm Res 2010, 27, 544–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nema, S.; Teagarden, D. Protein Aggregation-Pathways and Influencing Factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef]
- Rasmussen, K.; Rauscher, H.; Mech, A.; Riego Sintes, J.; Gilliland, D.; González, M.; Kearns, P.; Moss, K.; Visser, M.; Groenewold, M.; et al. Physico-Chemical Properties of Manufactured Nanomaterials—Characterisation and Relevant Methods. An Outlook Based on the OECD Testing Programme. Regul. Toxicol. Pharmacol. 2018, 92, 8–28. [Google Scholar] [CrossRef]
- Bosetti, R. Cost-Effectiveness of Nanomedicine: The Path to a Future Successful and Dominant Market? Nanomedicine 2015, 10, 1851–1853. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of Repeated Failures in Cancer Therapy for Solid Tumors: Poor Tumor-selective Drug Delivery, Low Therapeutic Efficacy and Unsustainable Costs. Clin. Transl. Med. 2018, 7, e11. [Google Scholar] [CrossRef]
- Park, K. Questions on the Role of the EPR Effect in Tumor Targeting. J. Control. Release 2013, 172, 391. [Google Scholar] [CrossRef]
- Hollis, C.P.; Weiss, H.L.; Leggas, M.; Evers, B.M.; Gemeinhart, R.A.; Li, T. Biodistribution and Bioimaging Studies of Hybrid Paclitaxel Nanocrystals: Lessons Learned of the EPR Effect and Image-Guided Drug Delivery. J. Control. Release 2013, 172, 391. [Google Scholar] [CrossRef]
- Murphy, G.; Brayden, D.J.; Cheung, D.L.; Liew, A.; Fitzgerald, M.; Pandit, A. Albumin-Based Delivery Systems: Recent Advances, Challenges, and Opportunities. J. Control. Release 2025, 380, 375–395. [Google Scholar] [CrossRef]
- Bristol Myers Squibb Abraxane® Side Effects & Expectations for Metastatic Breast Cancer, Labelling Information. Available online: https://www.abraxane.com/mbc/expectations-and-side-effects-of-abraxane (accessed on 27 May 2025).
- Danışman-Kalındemirtaş, F.; Kariper, İ.A.; Erdemir, G.; Sert, E.; Erdem-Kuruca, S. Evaluation of Anticancer Effects of Carboplatin–Gelatin Nanoparticles in Different Sizes Synthesized with Newly Self-Assembly Method by Exposure to IR Light. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Khatun, S.; Pebam, M.; Sankaranarayanan, S.A.; Pogu, S.V.; Bantal, V.S.; Rengan, A.K. Glutathione—IR 797 Coupled Casein Nano-Trojan for Augmenting the Therapeutic Efficacy of Camptothecin in Highly Invasive Triple Negative Breast Cancer. Biomater. Adv. 2024, 159, 213802. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.S.; Chan Kwon, I.; Tae, G. Targeted Delivery of Anti-Inflammatory Cytokine by Nanocarrier Reduces Atherosclerosis in Apo E−/- Mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.M. Biodegradable Nanoparticles for Targeted Drug Delivery in Treatment of Inflammatory Bowel Disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar] [CrossRef]
- Tang, H.; Dong, L.; Xia, X.; Chen, X.; Ren, M.; Shu, G.; Fu, H.; Lin, J.; Zhao, L.; Zhang, L.; et al. Preparation, Optimization, and Anti-Pulmonary Infection Activity of Casein-Based Chrysin Nanoparticles. Int. J. Nanomed. 2024, 19, 5511–5522. [Google Scholar] [CrossRef]
- Grela, E.; Stączek, S.; Nowak, M.; Pawlikowska-Pawlega, B.; Zdybicka-Barabas, A.; Janik, S.; Cytryńska, M.; Grudzinski, W.; Gruszecki, W.I.; Luchowski, R. Enhanced Antifungal Activity of Amphotericin B Bound to Albumin: A “Trojan Horse” Effect of the Protein. J. Phys. Chem. B 2023, 127, 3632–3640. [Google Scholar] [CrossRef]
- Park, H.H.; Kim, H.; Lee, H.S.; Seo, E.U.; Kim, J.E.; Lee, J.H.; Mun, Y.H.; Yoo, S.Y.; An, J.; Yun, M.Y.; et al. PEGylated Nanoparticle Albumin-Bound Steroidal Ginsenoside Derivatives Ameliorate SARS-CoV-2-Mediated Hyper-Inflammatory Responses. Biomaterials 2021, 273, 120827. [Google Scholar] [CrossRef]
- Tong, T.; Deng, S.; Zhang, X.; Fang, L.; Liang, J.; Xiao, S. Inhibitory Effect and Mechanism of Gelatin Stabilized Ferrous Sulfide Nanoparticles on Porcine Reproductive and Respiratory Syndrome Virus. J. Nanobiotechnol. 2022, 20, 70. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood–Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Oh, J.S.; Lee, Y.; Lee, E.C.; Yang, M.; Kwon, N.; Ha, T.W.; Hong, D.Y.; Song, Y.; Kim, H.K.; et al. Albumin-Binding Photosensitizer Capable of Targeting Glioma via the SPARC Pathway. Biomater. Res. 2023, 27, 23. [Google Scholar] [CrossRef]
- Zensi, A.; Begley, D.; Pontikis, C.; Legros, C.; Mihoreanu, L.; Wagner, S.; Büchel, C.; von Briesen, H.; Kreuter, J. Albumin Nanoparticles Targeted with Apo E Enter the CNS by Transcytosis and Are Delivered to Neurones. J. Control. Release 2009, 137, 78–86. [Google Scholar] [CrossRef]
- Annu; Sartaj, A.; Qamar, Z.; Aldosari, E.; Baboota, S.; Ali, J. Intranasal Delivery of Transferrin Conjugated Chitosan Nanoparticles to the Brain: A Comparative Analysis of Conjugated and Un-Conjugated Nanoparticles in Vivo. J. Drug Deliv. Sci. Technol. 2025, 104, 106550. [Google Scholar] [CrossRef]
- Yoneshima, Y.; Morita, S.; Ando, M.; Nakamura, A.; Iwasawa, S.; Yoshioka, H.; Goto, Y.; Takeshita, M.; Harada, T.; Hirano, K.; et al. Phase 3 Trial Comparing Nanoparticle Albumin-Bound Paclitaxel With Docetaxel for Previously Treated Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A. Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease. Toxics 2021, 9, 212. [Google Scholar] [CrossRef]
- Nogueira-Librelotto, D.R.; Codevilla, C.F.; Farooqi, A.; Rolim, C.M.B. Transferrin-Conjugated Nanocarriers as Active-Targeted Drug Delivery Platforms for Cancer Therapy. Curr. Pharm. Des. 2016, 23, 454–466. [Google Scholar] [CrossRef]
- Jeon, W.J.; Lee, H.K.; Na, Y.G.; Jung, M.; Han, S.C.; Hwang, J.H.; Jung, E.; Hwang, D.; Shin, J.S.; Cho, C.W. Antiviral Lipid Nanocarrier Loaded with Remdesivir Effective Against SARS-CoV-2 in Vitro Model. Int. J. Nanomed. 2023, 18, 1561–1575. [Google Scholar] [CrossRef]
- Gianni, L.; Mansutti, M.; Anton, A.; Calvo, L.; Bisagni, G.; Bermejo, B.; Semiglazov, V.; Thill, M.; Chacon, J.I.; Chan, A.; et al. Comparing Neoadjuvant Nab-Paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women with ERBB2/HER2-Negative Breast Cancer-the Evaluating Treatment with Neoadjuvant Abraxane (ETNA) Trial a Randomized Phase 3 Clinical Trial. JAMA Oncol. 2018, 4, 302–308. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory Pathways and Guidelines for Nanotechnology-Enabled Health Products: A Comparative Review of EU and US Frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Tahir, D.; Syarifuddin, S.; Noor, E.E.M.; Heryanto, H.; Mohamed, M.A. Advancements in Protein-Based Bionanocomposites for Targeted and Controlled Drug Delivery Systems: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2025, 106, 106698. [Google Scholar] [CrossRef]
- US Food and Drug Administration, Department of Health and Human Services. The Biologics License Application (BLA) Process Explained; 2024. Available online: https://www.thefdagroup.com/blog/2014/07/test-the-biologics-license-application-bla-process/ (accessed on 19 April 2025).
- US Food and Drug Administration, Department of Health and Human Services.21 CFR PART 600—Biological Products: General. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-F/part-600 (accessed on 19 April 2025).
- International Conference on Harmonization ICH. Q8 (R2): Pharmaceutical Development Q8; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2009. [Google Scholar]
- International Conference on Harmonization ICH. Q9: Quality Risk Management; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2005. [Google Scholar]
- International Conference on Harmonization ICH. Q10: Pharmaceutical Quality System; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2008. [Google Scholar]
- International Conference on Harmonization ICH. M3(R2): Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2009. [Google Scholar]
- Dorato, M.A.; Engelhardt, J.A. The No-Observed-Adverse-Effect-Level in Drug Safety Evaluations: Use, Issues, and Definition(s). Regul. Toxicol. Pharmacol. 2005, 42, 265–274. [Google Scholar] [CrossRef]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory Overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef]
- Food and Drug Administration. USA MedWatch: The FDA Safety Information and Adverse Event Reporting Program. Available online: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program (accessed on 21 May 2025).
- European Medicines Agency. EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance (accessed on 21 May 2025).
- Arora, S.; Rajwade, J.M.; Paknikar, K.M. Nanotoxicology and in Vitro Studies: The Need of the Hour. Toxicol. Appl. Pharmacol. 2012, 258, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, Stimuli-Sensitive Nanoparticulate Systems for Drug Delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin: Review of Animal and Human Studies. Clin. Pharmacokinet 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Wang, A.Z.; Farokhzad, O.C. Current Progress of Aptamer-Based Molecular Imaging. J. Nucl. Med. 2014, 55, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Gervits, N.E.; Gippius, A.A.; Tkachev, A.V.; Demikhov, E.I.; Starchikov, S.S.; Lyubutin, I.S.; Vasiliev, A.L.; Chekhonin, V.P.; Abakumov, M.A.; Semkina, A.S.; et al. Magnetic Properties of Biofunctionalized Iron Oxide Nanoparticles as Magnetic Resonance Imaging Contrast Agents. Beilstein J. Nanotechnol. 2019, 10, 1964–1972. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Nasif, K.F.A.; Xie, Y.; Deng, B.; Niu, S.; Pouriyeh, S.; Dai, Z.; Chen, J.; Xie, C.Y. AI-Driven Deep Learning Techniques in Protein Structure Prediction. Int. J. Mol. Sci. 2024, 25, 8426. [Google Scholar] [CrossRef]
- Kopac, T. Leveraging Artificial Intelligence and Machine Learning for Characterizing Protein Corona, Nanobiological Interactions, and Advancing Drug Discovery. Bioengineering 2025, 12, 312. [Google Scholar] [CrossRef]

| Protein | Source | Key Properties | Applications | Example | Pharmacokinetics/Pharmacodynamics Performance | |
|---|---|---|---|---|---|---|
| HSA | Human plasma | Biocompatible, non-immunogenic, multiple binding sites, long circulation half-life | Cancer therapy, targeted delivery | Abraxane® (paclitaxel) | ↑ AUC, ↑ Cmax, ↑ t1/2 vs. free gemcitabine; sustained in vitro release via diffusion and erosion [21]. | Extended therapeutic window; improved efficacy due to sustained drug levels; reduced dosing frequency |
| Casein | Milk | Amphiphilic, forms micelles, biodegradable | Oral/injectable delivery, nutraceuticals | Flutamide NPs | ↑ Half-life from 0.88 h to 14.64 h, ↓ clearance, sustained release up to 4 days [22]. | Extended circulation and retention, improved therapeutic window for poorly soluble anticancer drugs |
| Gelatin | Collagen (animal origin) | Thermoresponsive, surface modifiable, biodegradable | Injectable delivery, tissue targeting | EGFR-targeted gelatin nanoparticles | EGFR-targeted gelatin nanoparticles showed higher blood AUC (19.56% ID/mL·h) and tumor AUC (322% ID/g·h) vs. unmodified particles (10.71 and 138, respectively); PEG-modified NPs had intermediate values [23]. | EGFR-targeted gelatin NPs achieved 2× greater tumor accumulation and sustained tumor retention, confirming the success of active tumor targeting and enhancing therapeutic potential in pancreatic cancer models. |
| Transferrin | Blood plasma glycoprotein | Tumor targeting via transferrin receptors, receptor-mediated uptake | Cancer, gene delivery | Transferrin-conjugated NPs | The Tofa-P/tfr NCs demonstrated sustained drug release at colon-relevant pH 7.4, enhancing site-specific retention [24]. | In vivo, Tofa-P/tfr NCs significantly reduced pro-inflammatory cytokines and STAT-1/TFR-1 expression, restored histopathology and vascular integrity, and normalized hematological and microbial markers in DSS-induced ulcerative colitis. |
| Globulins | Blood serum | Immune recognition, drug binding, receptor specificity | Immunotherapy, vaccine delivery | Doxorubicin-γ-globulin-AuNPs | γG-AuNPs improved biostability in harsh serum, pH-sensitive release at acidic pH (5.5) enhanced drug delivery in tumor microenvironment [25]. | Dox-γG-AuNPs showed 10-fold higher cytotoxic potency via p53-mediated ROS apoptosis pathway; enhanced uptake and targeted cell death in cancer cells through pH-triggered release. |
| Parameter | Description | Techniques/Methods |
|---|---|---|
| Particle Size and Zeta Potential | Key indicators of nanoparticle behavior (biodistribution, stability). | DLS: Measures size and PDI. Zeta Potential: Assesses stability. |
| Surface Morphology | Analyzes shape and texture of nanoparticles for injectable formulations. | SEM, TEM: High-resolution imaging of surface and internal structure. |
| Drug Loading and Entrapment Efficiency | Measures drug amount and encapsulation efficiency in nanoparticles. | UV-Vis spectrophotometry, HPLC (high-performance liquid chromatography): Quantifies drug concentration and entrapment. |
| Stability Studies | Assesses physical and chemical stability under various conditions. | Monitoring of aggregation, degradation, and sedimentation over time. |
| In Vitro Release Profiles | Examines drug release kinetics under simulated physiological conditions. | Dialysis or Sample-and-Separate: Measures release rate and pattern. |
| Protein Structure Integrity | Ensures protein maintains its natural conformation to prevent denaturation. | SDS-PAGE, FTIR, CD spectroscopy: Analyzes protein purity, structure, and conformation. |
| Challenge/Limitations | Description |
|---|---|
| Protein Denaturation and Aggregation | Protein instability can lead to loss of functionality and aggregation, affecting efficacy and safety. |
| Immunogenicity and Hypersensitivity | Potential immune responses or allergic reactions due to the presence of foreign proteins. |
| Drug–Protein Binding Variability | Variability in how drugs bind to proteins can impact drug release, bioavailability, and therapeutic outcomes. |
| Scale-up and Reproducibility Issues | Difficulties in scaling up production while maintaining consistency and quality of nanoparticles. |
| Regulatory and Quality Control Hurdles | Challenges in meeting regulatory standards and maintaining rigorous quality control during formulation and production. |
| Cost-effectiveness and Economic Constraints | High production costs may limit the economic feasibility and accessibility of the final product. |
| Section | Key Focus | Details/Highlights |
|---|---|---|
| Guidelines from FDA, EMA, ICH | Regulatory framework and guidance |
|
| Preclinical Safety Requirements | Safety evaluation before human trials |
|
| Risk Assessment | Toxicokinetic and immunotoxicity evaluation |
|
| Pharmacovigilance Post-approval | Ongoing safety monitoring after marketing |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahire, E.D.; Savaliya, N.; Makwana, K.V.; Salave, S.; Banth, M.K.; Bhavsar, B.; Khunt, D.; Prajapati, B.G. Protein-Bound Nano-Injectable Suspension: Unveiling the Promises and Challenges. Appl. Nano 2025, 6, 9. https://doi.org/10.3390/applnano6020009
Ahire ED, Savaliya N, Makwana KV, Salave S, Banth MK, Bhavsar B, Khunt D, Prajapati BG. Protein-Bound Nano-Injectable Suspension: Unveiling the Promises and Challenges. Applied Nano. 2025; 6(2):9. https://doi.org/10.3390/applnano6020009
Chicago/Turabian StyleAhire, Eknath D., Namrata Savaliya, Kalarav V. Makwana, Sagar Salave, Mandeep Kaur Banth, Bhavesh Bhavsar, Dignesh Khunt, and Bhupendra G. Prajapati. 2025. "Protein-Bound Nano-Injectable Suspension: Unveiling the Promises and Challenges" Applied Nano 6, no. 2: 9. https://doi.org/10.3390/applnano6020009
APA StyleAhire, E. D., Savaliya, N., Makwana, K. V., Salave, S., Banth, M. K., Bhavsar, B., Khunt, D., & Prajapati, B. G. (2025). Protein-Bound Nano-Injectable Suspension: Unveiling the Promises and Challenges. Applied Nano, 6(2), 9. https://doi.org/10.3390/applnano6020009










