RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors
Abstract
1. Introduction
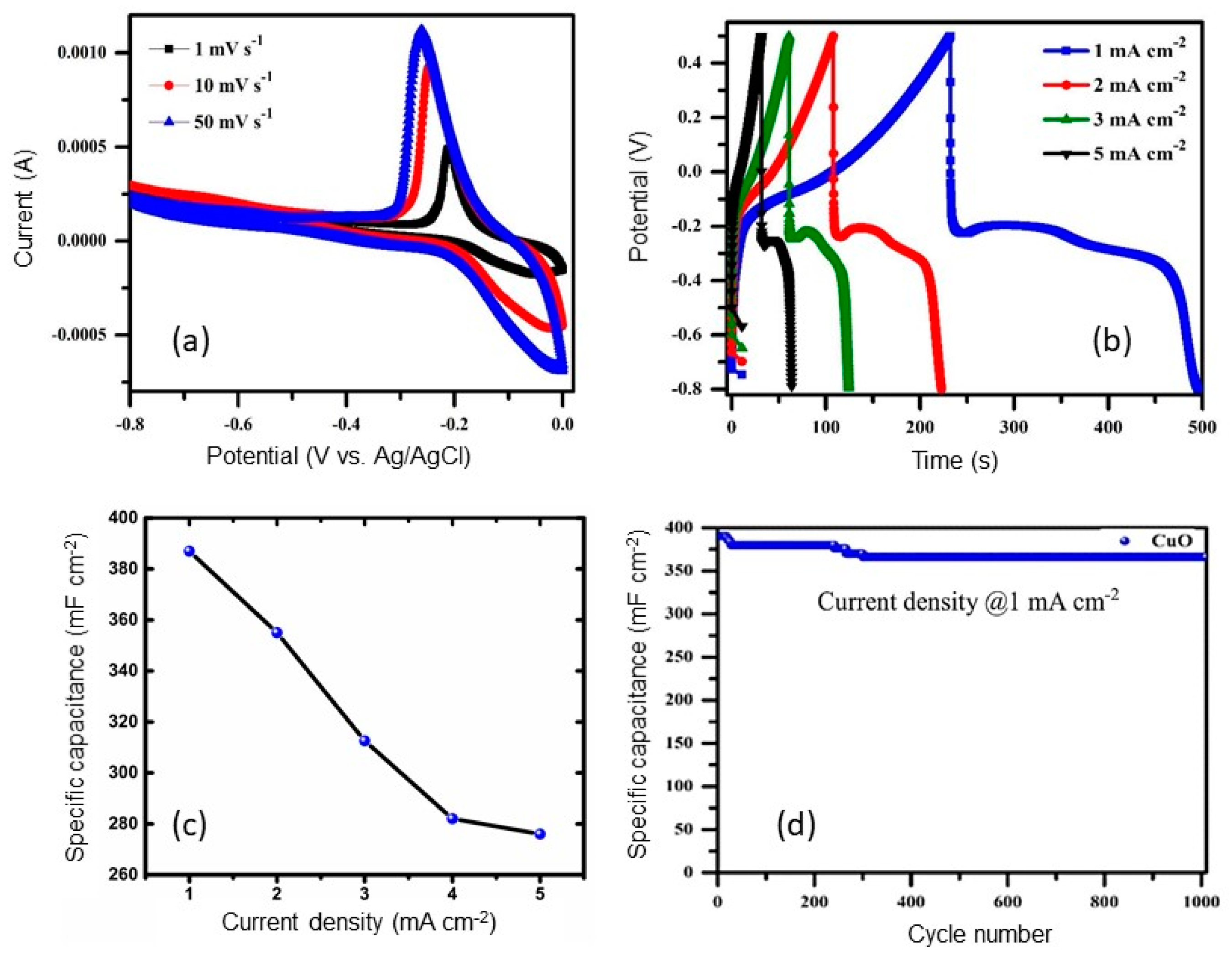
- (a)
- Stable crystallographic structure with good integrity between the crystallites to avoid running-off of the active material during the electrochemical intercalation and de-intercalation process for long cycle stability.
- (b)
- The microstructure of the electrode with a high specific surface area and high mesoporosity provides more intercalation sites for enhancement of the specific capacitance.
- (c)
- Reasonable conductivity of the electrode yields low charge transfer resistance and, in turn, improves the transportation of electrons and ions.
- (d)
- Good adhesion among the layers avoids the running-off of the material.
2. Materials and Methods
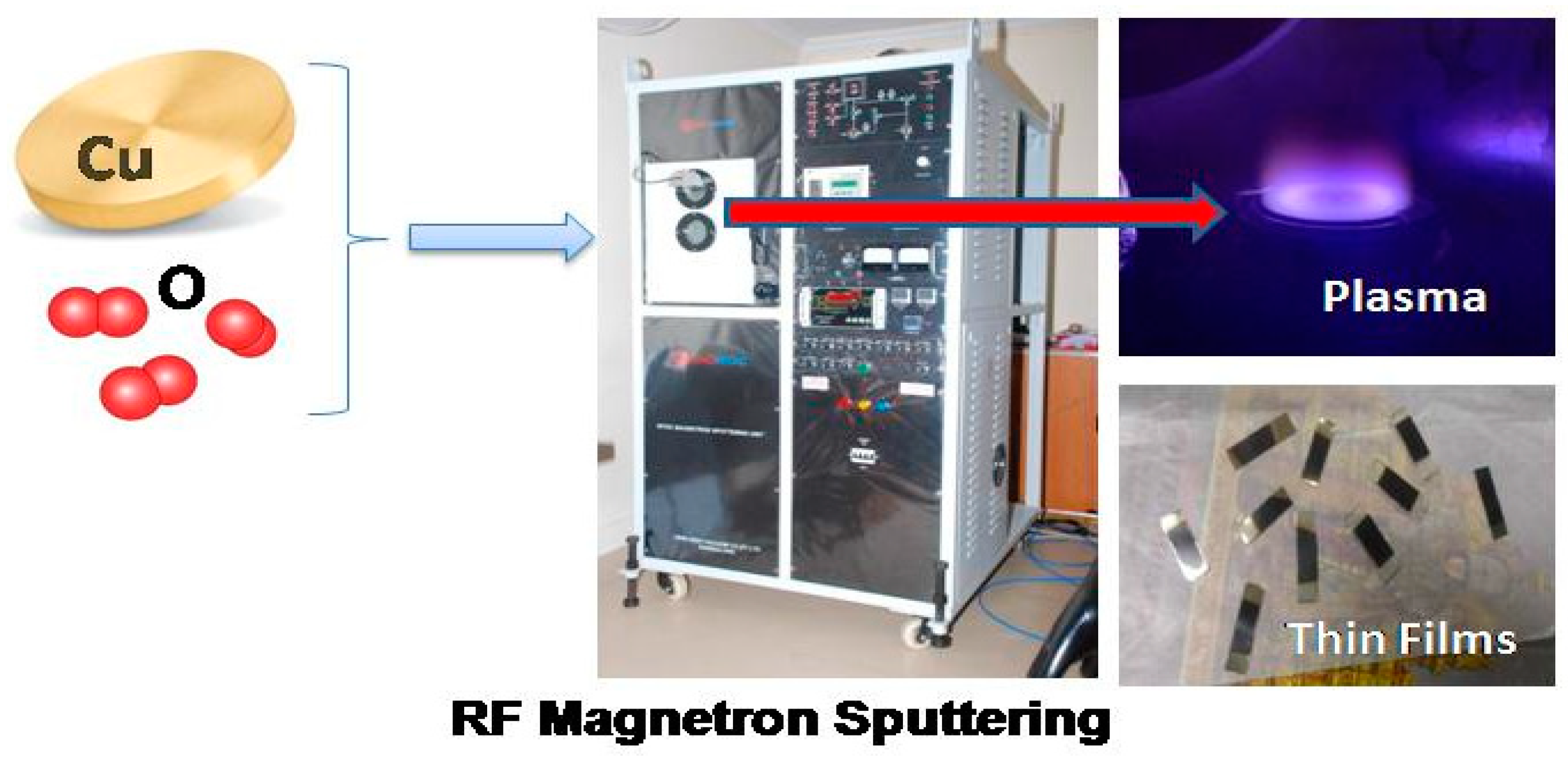
2.1. CuO Film Deposition
2.2. Characterization
3. Results
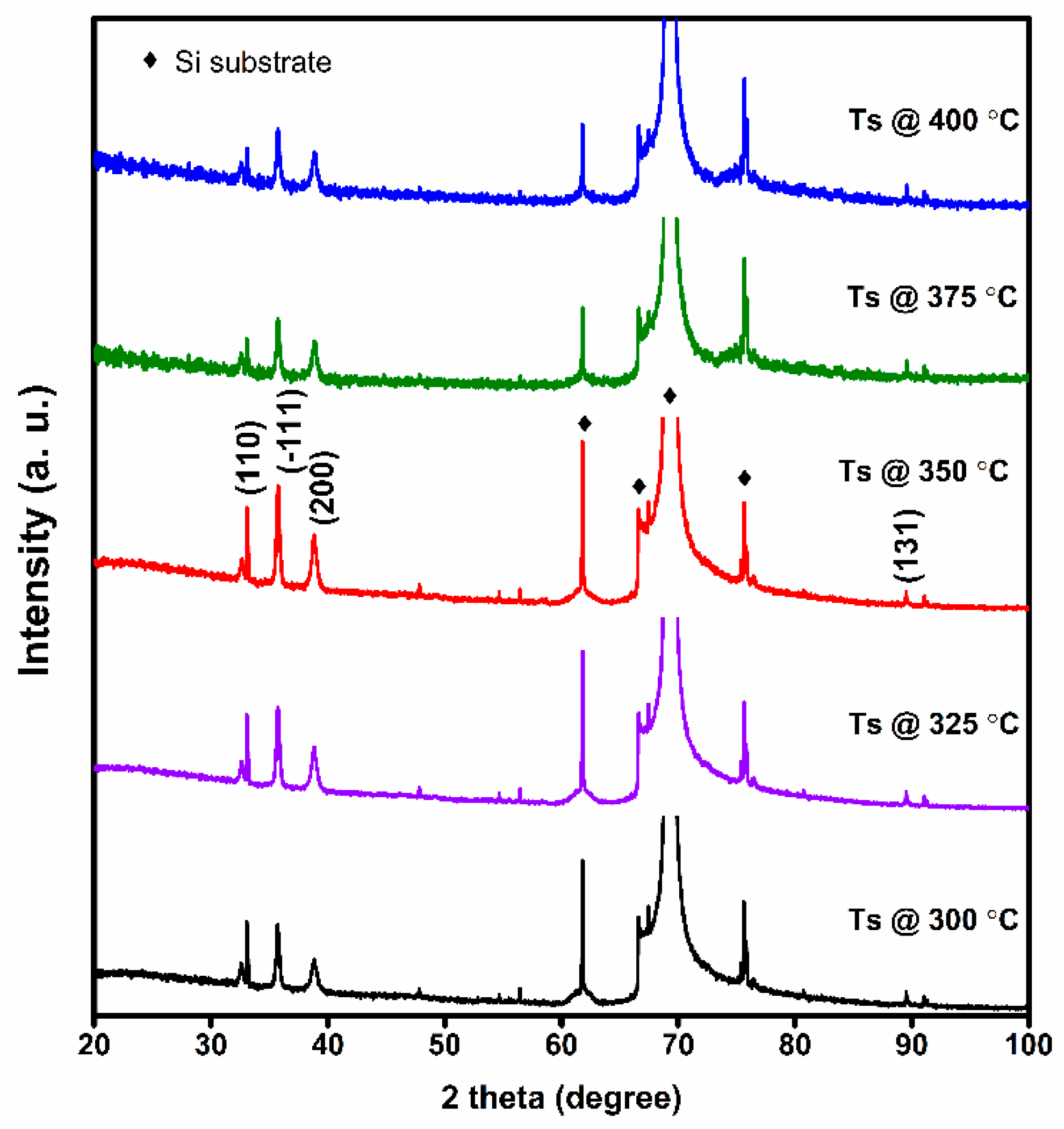
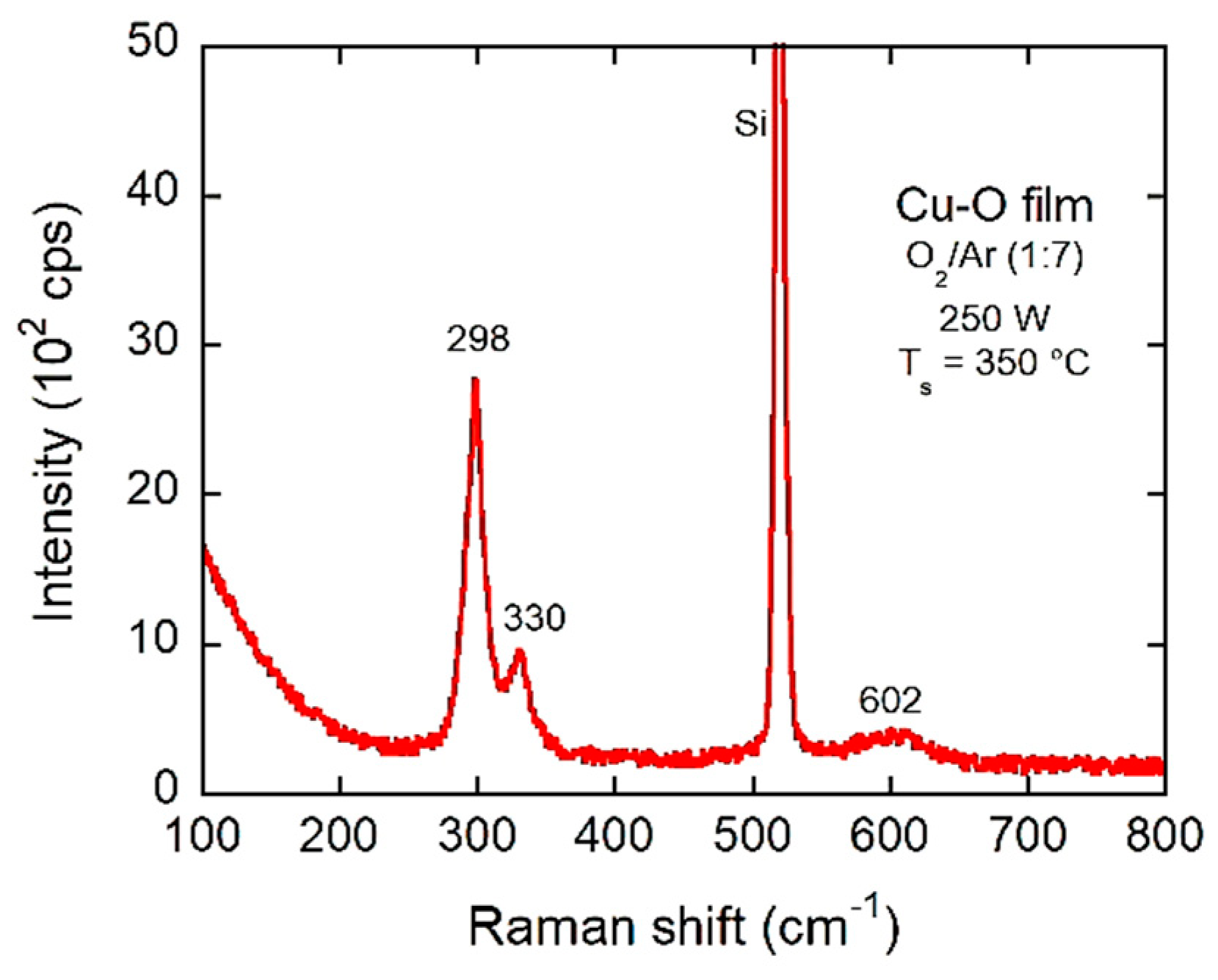
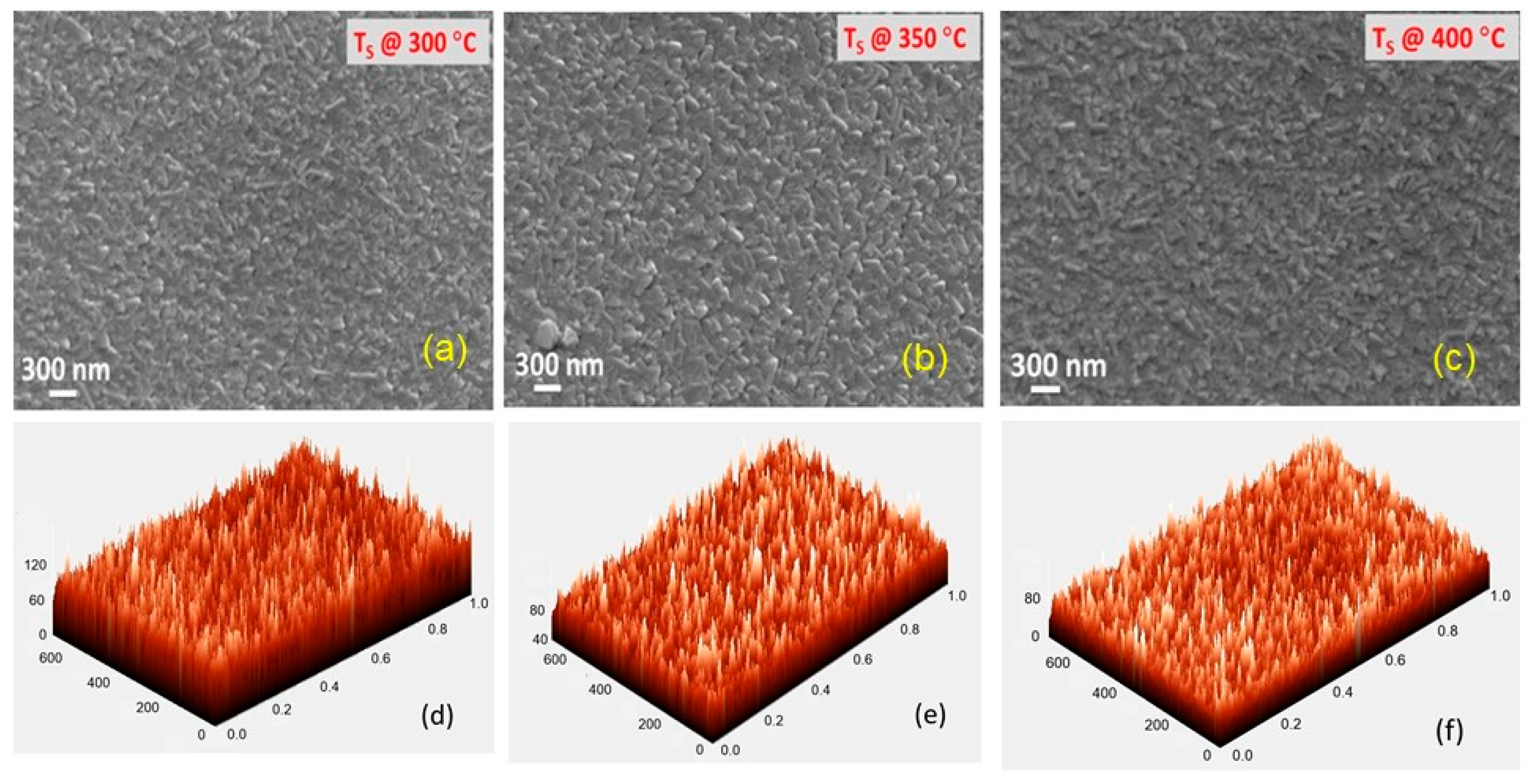
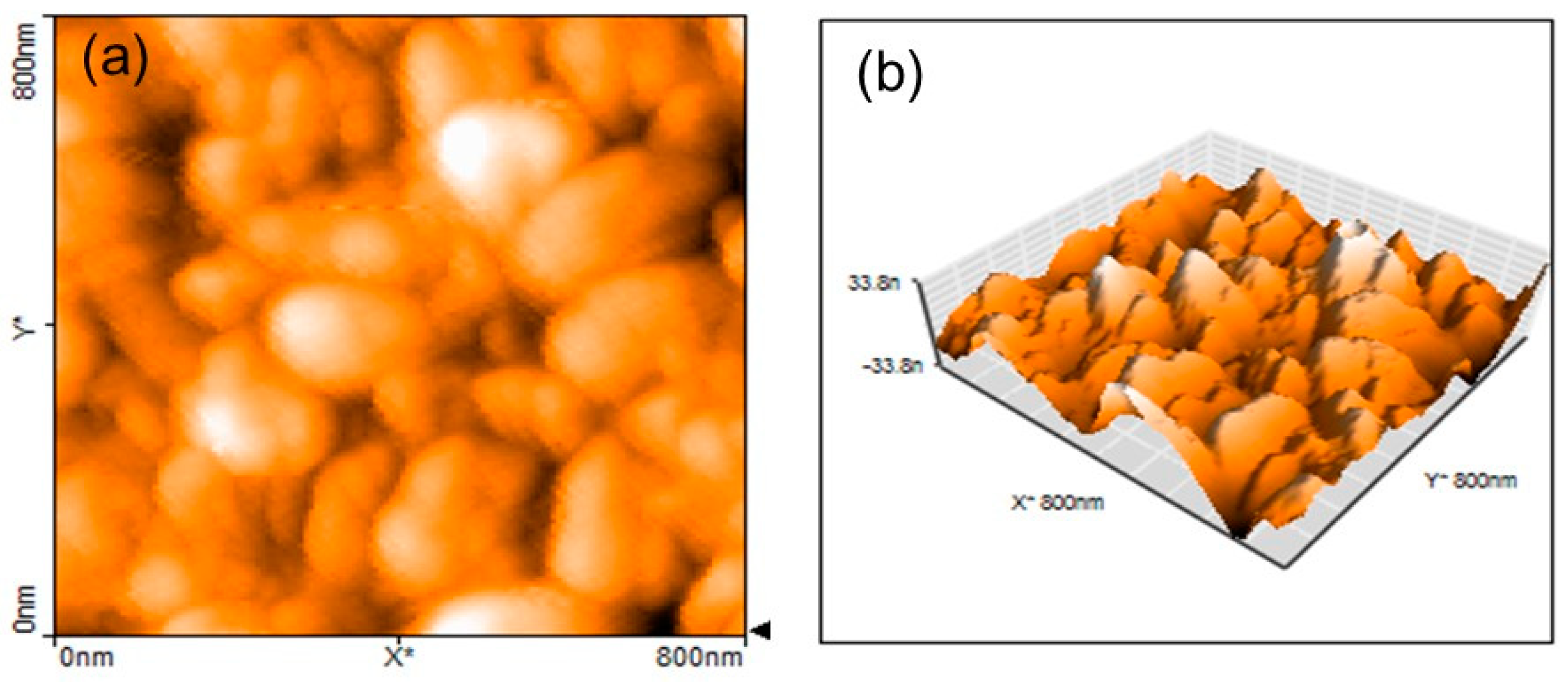
3.1. Structure and Morphology
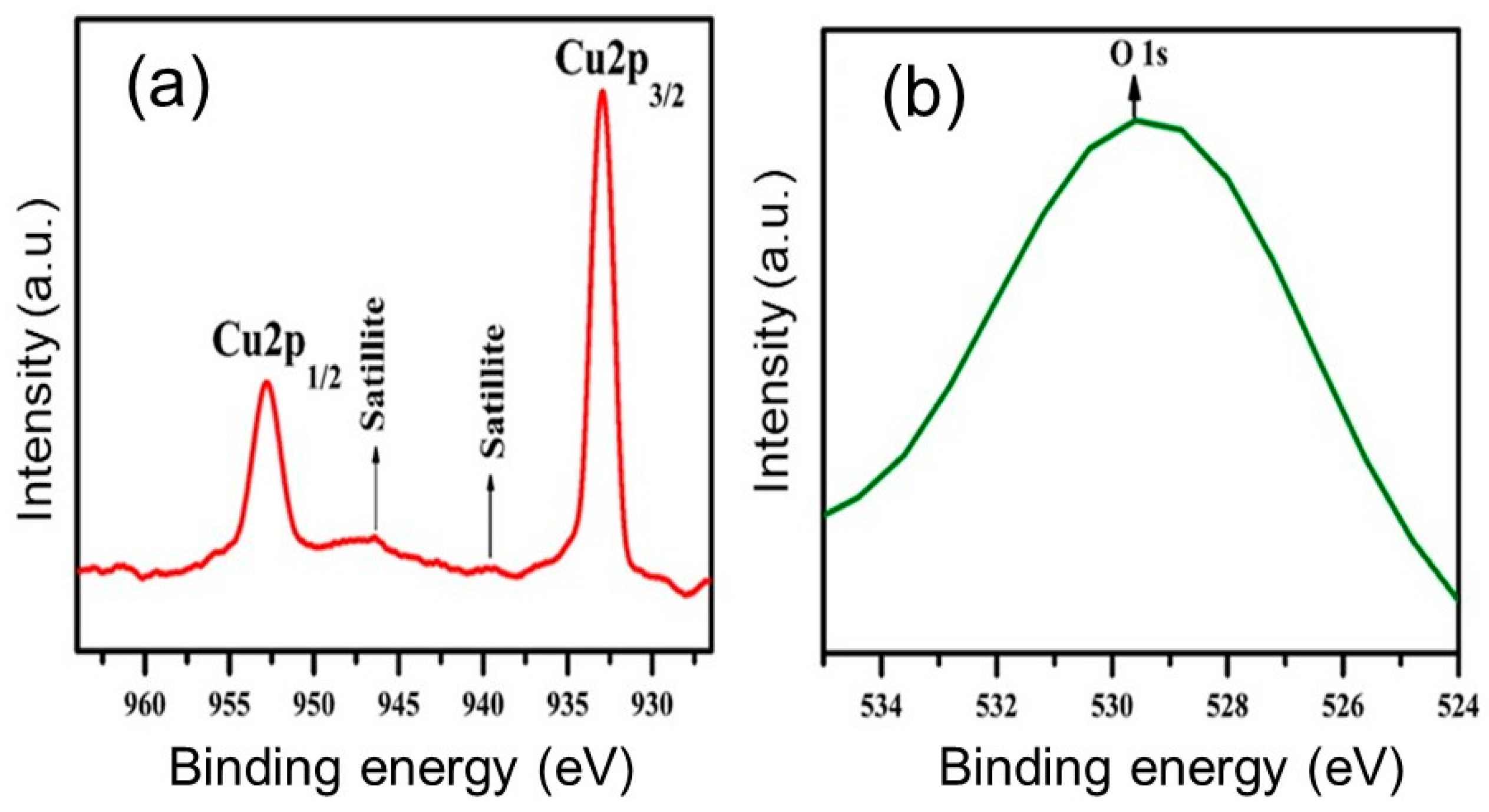
3.2. XPS Studies
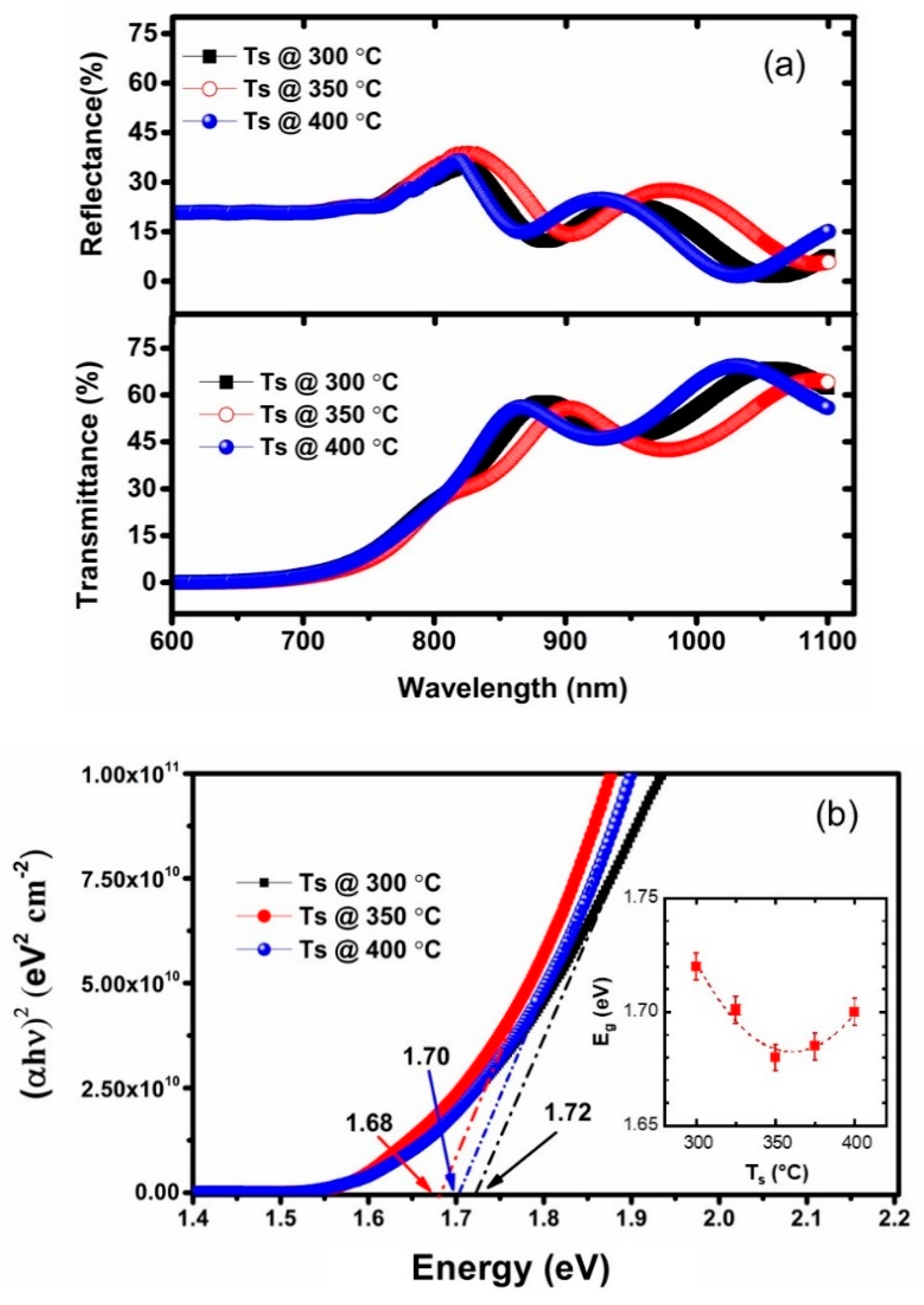
3.3. Optical Bandgap
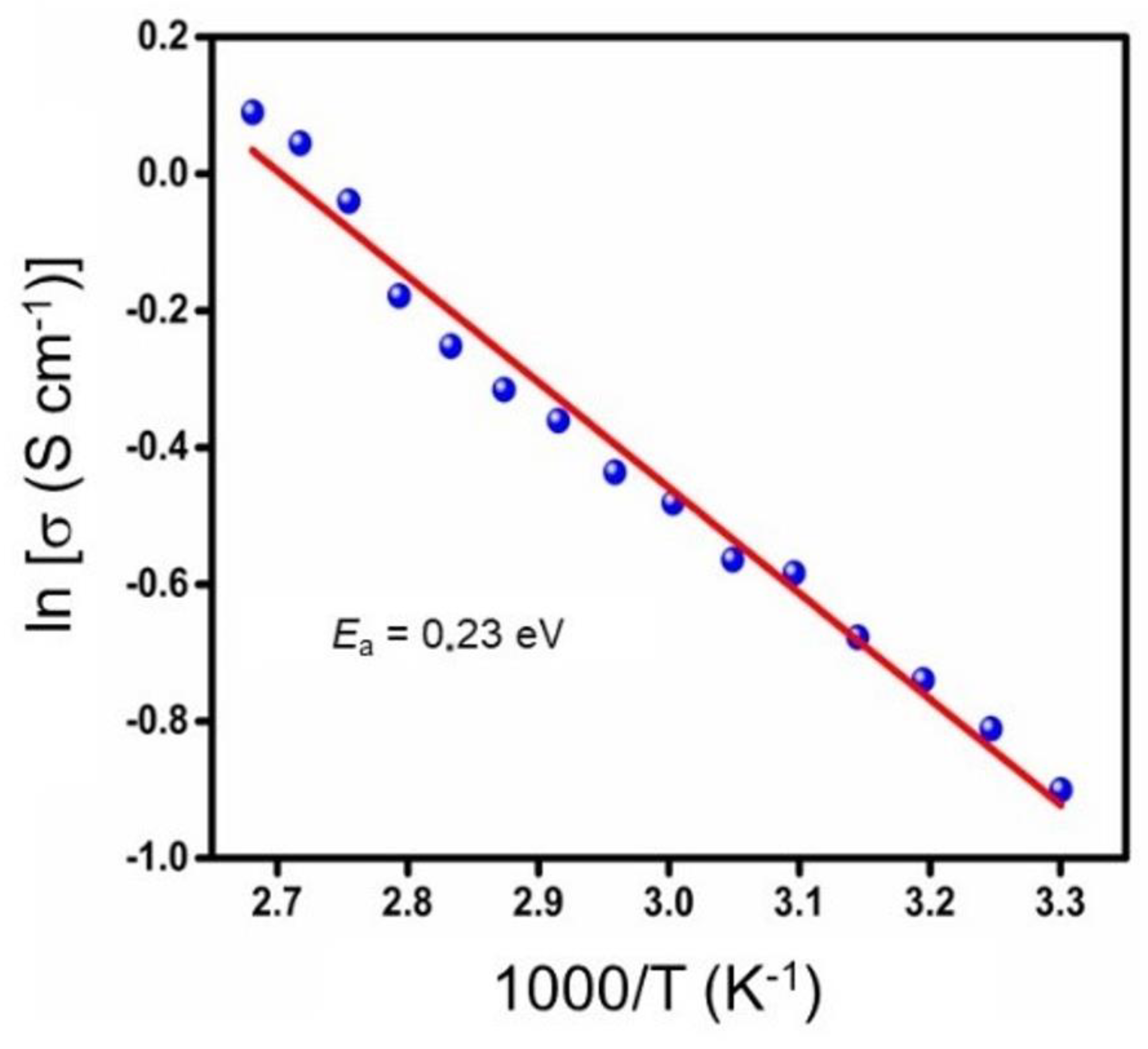
3.4. Electrical Properties
3.5. Electrochemical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lethien, C.; Bideau, J.L.; Brousse, T. Challenges and prospects of 3D micro–supercapacitors for powering the internet of things. Energy Environ. Sci. 2019, 12, 96–115. [Google Scholar] [CrossRef]
- Eustache, E.; Douard, C.; Demortiere, A.; De Andrade, V.; Brachet, M.; Le Bideau, J.; Brousse, T.; Lethien, C. High areal energy 3D-interdigitated micro-supercapacitors in aqueous and ionic liquid electrolytes. Adv. Mater. Technol. 2017, 2, 1700126. [Google Scholar] [CrossRef]
- Ferris, A.; Garbarino, S.; Guay, D.; Pech, D. 3D RuO2 micro supercapacitors with remarkable areal energy. Adv. Mater. 2015, 27, 6625–6629. [Google Scholar] [CrossRef]
- Kotz, R.; Carlen, M. Principles and Applications of Electrochemical Capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Materials science, electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Li, L.; Wang, X.-L.; Gu, C.-D.; Tu, J.-P. Metal oxide/hydroxide based materials for supercapacitors. RSC Adv. 2014, 4, 41910–41921. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.S. Metal oxide thin film based supercapacitors. Current Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Bahloul, A.; Nessark, B.; Briot, E.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Polypyrrole-covered MnO2 as electrode material for hydrid supercapacitor. J. Power Sources 2013, 240, 267–272. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. CuO cauliflowers for supercapacitor application: Novel potentiodynamic deposition. Mater. Res. Bull. 2013, 48, 923–928. [Google Scholar] [CrossRef]
- Shinde, S.K.; Yadav, H.M.; Ghogake, G.S.; Kadam, A.A.; Kumbhar, V.S.; Yang, J.; Hwang, K.; Jagadale, A.D.; Kumar, S.; Kim, D.Y. Using chemical bath deposition to create nanosheet-like CuO electrodes for supercapacitor applications. Colloids Surf. B Biointerfaces 2019, 181, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Huang, M.; Li, F.; Wen, Z.Q. Controlled synthesis of hierarchical CuO nanostructures for electrochemical capacitor electrodes. Int. J. Electrochem. Sci. 2013, 8, 8645–8661. [Google Scholar]
- Grondahl, L.O. The copper-cuprous-oxide rectifier and photoelectric cell. Rev. Mod. Phys. 1933, 5, 141–160. [Google Scholar] [CrossRef]
- Constable, F.H. Electrical conductivity of copper oxide films showing interference colours. Nature 1935, 136, 517. [Google Scholar] [CrossRef]
- Son, D.I.; You, C.H.; Kim, T.W. Structural, optical, and electronic properties of colloidal CuO nanoparticles formed by using a colloid-thermal synthesis process. Appl. Surf. Sci. 2009, 255, 8794–8797. [Google Scholar] [CrossRef]
- Papadimitropoulos, G.; Vourdas, N.; Vamvakas, V.E.; Davazoglou, D. Optical and structural properties of copper oxide thin films grown by oxidation of metal layers. Thin Solid Films 2006, 515, 2428–2432. [Google Scholar] [CrossRef]
- Sohn, J.; Song, S.-H.; Nam, D.-W.; Cho, I.-T.; Cho, E.-S.; Lee, J.-H.; Kwon, H.-I. Effects of vacuum annealing on the optical and electrical properties of p-type copper-oxide thin-film transistors. Semicond. Sci. Technol. 2013, 28, 015005. [Google Scholar] [CrossRef]
- Hoa, N.D.; An-Sca, S.Y.; Dung, N.Q.; Quy, N.V.; Kim, D. Synthesis of p-type semiconducting cupric oxide thin films and their application to hydrogen detection. Sens. Actuator B 2010, 146, 239–244. [Google Scholar] [CrossRef]
- Dahrul, M.; Alatas, H.; Izaman, X. Preparation and optical properties study of CuO thin film as applied solar cell on LAPAN-IPB satellite. Proc. Environ. Sci. 2016, 33, 661–667. [Google Scholar] [CrossRef]
- Figueiredo, V.; Elangovan, E.; Gonçalves, G.; Barquinha, P.; Pereira, L.; Franco, N.; Alves, E.; Martins, R.; Fortunato, E. Effect of post-annealing on the properties of copper oxide thin films obtained from the oxidation of evaporated metallic copper. Appl. Surf. Sci. 2008, 254, 3949–3954. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Switzer, J.A. Galvanostatic electrodeposition and microstructure of copper(I) oxide film. Mater. Res. Innov. 1998, 2, 22–27. [Google Scholar] [CrossRef]
- Sagu, J.S.; Nirmal-Peiris, T.A.; Wijayantha, U. Rapid and simple potentiostatic deposition of copper(II) oxide thin films. Electrochem. Commun. 2014, 42, 68–71. [Google Scholar] [CrossRef]
- Faiz, H.; Siraj, K.; Khan, M.F.; Irshad, M.; Majeed, S.; Rafique, M.S.; Naseem, S. Microstructural and optical properties of dysprosium doped copper oxide thin films fabricated by pulsed laser deposition technique. J. Mater. Sci. Mater. Electron. 2016, 27, 8197–8205. [Google Scholar] [CrossRef]
- Santra, K.; Sarkar, C.K.; Mukherjee, M.K.; Ghosh, B. Copper-oxide thin-films grown by plasma evaporation method. Thin Solid Films 1992, 213, 226–229. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Ganapathi, S.K.; Kaur, M.; Datta, N.; Muthe, K.P.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators B 2010, 151, 90–96. [Google Scholar] [CrossRef]
- Lu, H.C.; Chu, C.L.; Lai, C.Y.; Wang, Y.H. Property variations of direct-current reactive magnetron sputtered copper oxide thin films deposited at different oxygen partial pressures. Thin Solid Films 2009, 517, 4408–4412. [Google Scholar] [CrossRef]
- Sravanthi, V.; Srikanth, T.; Reddy, A.S.; Reddy, P.S.; Reddy, C.S. Electron beam evaporated copper oxide thin films. IOSR J. Eng. 2018, 8, 82–87. [Google Scholar]
- Muthe, K.P.; Vyas, J.C.; Narang, S.N.; Aswal, D.K.; Gupta, S.K.; Bhattacharya, D.; Pinto, R.; Kothiyal, G.P.; Sabharwal, S.C. A study of the CuO phase formation during thin film deposition by molecular beam epitaxy. Thin Solid Films 1998, 324, 37–43. [Google Scholar] [CrossRef]
- Shabu, R.; Moses-Ezhil-Raj, A.; Sanjeeviraja, C.; Ravidhas, C. Assessment of CuO thin films for its suitablity as window absorbing layer in solar cell fabrications. Mater. Res. Bull. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Morales, J.; Sanchez, L.; Martin, F.; Ramos-Barrado, J.R.; Sanchez, M. Nanostructured CuO thin film electrodes prepared by spray pyrolysis: A simple method for enhancing the electrochemical performance of CuO in lithium cells. Electrochim. Acta 2004, 49, 4589–4597. [Google Scholar] [CrossRef]
- Baturay, S.; Tombak, A.; Kaya, D.; Ocak, Y.S.; Tokus, M.; Aydemir, M.; Kilicoglu, T. Modification of electrical and optical properties of CuO thin films by Ni doping. J. Sol-Gel Sci. Technol. 2016, 78, 422–429. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, M.D.; Lohar, G.M.; Jadhav, S.T.; Fulari, V.J. Supercapacitive properties of CuO thin films using modified SILAR method. Ionics 2017, 23, 1259–1266. [Google Scholar] [CrossRef]
- Nair, M.T.S.; Guerrero, L.; Arenas, O.L.; Nair, P.K. Chemically deposited copper oxide thin films: Structural, optical and electrical characteristics. Appl. Surf. Sci. 1999, 150, 143–151. [Google Scholar] [CrossRef]
- Nicolau, Y.F. Solution deposition of thin solid compound films by a successive ionic-layer adsorption and reaction process. Appl. Surf. Sci. 1985, 22–23, 1061–1074. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Hussain, O.M. Sputtered LiCoO2 cathode materials for all-solid-state thin-film lithium microbatteries. Materials (Basel) 2019, 12, 2687. [Google Scholar] [CrossRef]
- Pierson, J.F.; Thobor-Keck, A.; Billard, A. Cuprite, paramelaconite and tenorite films deposited by reactive magnetron sputtering. Appl. Surf. Sci. 2003, 210, 359–367. [Google Scholar] [CrossRef]
- Cho, S. Optical and electrical properties of CuO thin films deposited at several growth temperatures by reactive RF magnetron sputtering. Met. Mater. Int. 2013, 19, 1327–1331. [Google Scholar] [CrossRef]
- Khalaf, M.K.; Said, S.N.; Abbas, A.J. Characterization of the copper oxide thin films deposited by DC sputtering technique. Eng. Tech. J. 2014, 32, 770–776. [Google Scholar]
- Sanal, K.C.; Vikas, L.S.; Jayaraj, M.K. Room temperature deposited transparent p-channel CuO thin film transistors. Appl. Surf. Sci. 2014, 297, 153–157. [Google Scholar] [CrossRef]
- Ooi, P.K.; Ching, C.G.; Ahmad, M.A.; Ng, S.S.; Abdullah, M.J.; Abu Hassan, H.; Hassan, Z. Characterizations of cupric oxide thin films on glass and silicon substrates by radio frequency magnetron sputtering. Sains Malays. 2014, 43, 617–621. [Google Scholar]
- Pecquenard, B.; Le Cras, F.; Poinot, D.; Sicardy, O.; Manaud, J.P. Thorough characterization of sputtered CuO thin films used as conversion material electrodes for lithium batteries. ACS Appl. Mater. Interfaces 2014, 6, 3413–3420. [Google Scholar] [CrossRef]
- Purusottam-Reddy, B.; Sivajee-Ganesh, K.; Jayanth-Babu, K.; Hussain, O.M.; Julien, C.M. Microstructure and supercapacitive properties of rf-sputtered copper oxide thin films: Influence of O2/Ar ratio. Ionics 2015, 21, 2319–2328. [Google Scholar] [CrossRef]
- Shelke, A.R.; Lokhande, A.C.; Pujari, R.B.; Lokhande, C.D. Effect of N-complexing agents on the supercapacitive properties of reflux deposited CuO thin films. Int. J. Eng. Res. Technol. 2017, 10, 561–567. [Google Scholar]
- Sayson, L.V.A.; Lopez, J.M.; Estacio, E.S.; Salvador, A.A.; Somintac, A.S. Nanostructured CuO thin film deposited on stainless steel using spray pyrolysis as supercapacitor electrode. Mater. Res. Express 2020, 6, 125551. [Google Scholar] [CrossRef]
- Kambale, S.V.; Jadhav, A.L.; Kore, R.M.; Thakur, A.V.; Lokhande, B.J. Cyclic voltammetric study of CuO thin film electrodes prepared by automatic spray pyrolysis. Macromolecular Symp. 2019, 387, 1800213. [Google Scholar] [CrossRef]
- Shinde, A.V.; Chodankar, N.R.; Lokhande, V.C.; Lokhande, A.C.; Ji, T.; Kim, J.H.; Lokhande, C.D. Highly energetic flexible all-solid-state asymmetric supercapacitor with Fe2O3 and CuO thin films. RSC Adv. 2016, 6, 58839–58843. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Holze, R.; Jadhav, H.S.; Lokhande, C.D.; Park, C.-J. Surfactant-assisted morphological tuning of hierarchical CuO thin films for electrochemical supercapacitors. Dalton Trans. 2013, 42, 6459–6467. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Holze, R.; Lokhande, C.D. Enhancement in supercapacitive properties of CuO thin films due to the surfactant mediated morphological modulation. J. Electroanal. Chem. 2014, 712, 40–46. [Google Scholar] [CrossRef]
- Khatavkar, S.N.; Sartale, S.D. Superior supercapacitive performance of grass-like CuO thin films deposited by liquid phase deposition. New, J. Chem. 2020, 44, 6778–6790. [Google Scholar] [CrossRef]
- Endut, Z.; Hamdi, M.; Basirun, W.J. Pseudocapacitive performance of vertical coper oxide nanoflakes. Thin Solid Films 2013, 528, 213–216. [Google Scholar] [CrossRef]
- Purusottam-Reddy, B.; Sivajee-Ganesh, K.; Hussain, O.M. Growth, microstructure and supercapacitive performance of copper oxide thin films prepared by RF magnetron sputtering. Applied Phys. A 2016, 122, 128. [Google Scholar] [CrossRef]
- Ali, M.; Gobinner, C.R.; Kekuda, D. Role of oxygen flow rate on the structural and optical properties of copper oxide thin films grown by reactive magnetron sputtering. Indian J. Phys. 2016, 90, 219–224. [Google Scholar] [CrossRef]
- Dulmaa, A.; Vrielinck, H.; Khelifi, S.; Depla, D. Sputter deposition of copper oxide films. Appl. Surf. Sci. 2019, 492, 711–717. [Google Scholar] [CrossRef]
- Du, Y.; Gao, X.; Zhang, X.; Meng, X. Characterization of the microstructure and the optical and electrical properties of the direct-current magnetron sputtered CuO films at different substrate temperatures. Phys. B Cond. Matter 2018, 546, 28–32. [Google Scholar] [CrossRef]
- Muhammad, K.S.; Shazia, B.; Qazi, S.A.; Nazish, Y.; Sohail, A.J.; Mahreen, A.; Khaliq, M.; Ayesha, K. Effect of substrate temperature on the growth of copper oxide thin films deposited by pulsed laser deposition technique. Surf. Rev. Lett. 2018, 25, 1850053. [Google Scholar]
- Xu, L.; Zhang, G.; Pei, S.; Wang, J. Investigation of optical bandgap variation and photoluminescence behavior in nanocrystalline CuO thin films. Optik 2018, 158, 382–390. [Google Scholar] [CrossRef]
- Calos, N.J.; Forrester, J.S.; Schaffer, G.B. A crystallographic contribution to the mechanism mechanically induced solid state reaction. J. Solid State Chem. 1996, 122, 272–280. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Sander, T.; Reindl, C.T.; Giar, M.; Eifert, B.; Heinemann, M.; Heiliger, C.; Klar, P.J. Correlation of intrinsic point defects and the Raman modes of cuprous oxide. Phys. Rev. B Condens. Matter 2014, 90, 045203. [Google Scholar] [CrossRef]
- Debbichi, L.; de Lucas, M.C.M.; Pierson, J.F.; Krüger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Hodson, M.E.; Lee, M.R.; Parsons, I. Origins of the surface roughness of unweathered alkali feldspar grains. Geochim. Cosmochim. Acta 1997, 61, 3885–3896. [Google Scholar] [CrossRef]
- Ghodselhi, T.; Vesaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Li, Z.; Tong, K.; Shi, R.; Shen, Y.; Zhang, Y.; Yao, Z.; Fan, J.; Thwaites, M.; Shao, G. Reactive plasma deposition of high quality single phase CuO thin films suitable for metal oxide solar cells. J. Alloys Compd. 2017, 695, 3116–3123. [Google Scholar] [CrossRef]
- Sakai, Y.; Ninomiya, S.; Hiraoka, K. XPS depth analysis of CuO by electrospray droplet impact. Surf. Interface Anal. 2012, 43, 1605–1609. [Google Scholar] [CrossRef]
- Rusu, D.I.; Rusu, G.G.; Luca, D. Structural characteristics and optical properties of thermally oxidized zinc films. Acta Phys. Polonica A 2011, 119, 850–856. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Solids; Abeles, F., Ed.; North-Holland: Amsterdam, The Netherlands, 1972. [Google Scholar]
- Moosavifard, S.E.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Dedigning 3D highly ordered nanoporous CuO electrodes for high-performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, J.A.; Min, B.K.; Kim, J.H.; Kang, S.H.; Kim, H.; Ahn, K.S. Facile hydrothermal synthesis and electrochemical supercapacitor performance of hierarchical coral-like ZnCo2O4 nanowires. J. Electroanal. Chem. 2017, 785, 48–57. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.-M. Energy storage: Pseudocapacitance in prospect. Chem. Sci. 2019, 10, 5656–5666. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, P.; Zhou, Y.; Zhu, Y. High-performance symmetric supercapacitors based on carbon nanotubes/graphite nanofiber nanocomposites. Sci. Rep. 2018, 8, 9005. [Google Scholar] [CrossRef]
- Parretta, A.; Jayaraj, M.K.; Di Noceras, A.; Loreti, S.; Quercia, L.; Agati, A. Electrical and optical properties of copper oxide films prepared by reactive RF magnetron sputtering. Phys. Status Sol. 1996, 155, 399–404. [Google Scholar] [CrossRef]
- Asl, H.Z.; Rozati, S.M. Spray deposited nanostructured CuO thin films: Influence of substrate temperature and annealing process. Mater. Res. 2018, 21, e20170754. [Google Scholar]
- Ghosh, S.; Avasthi, D.K.; Shah, P.; Ganesan, V.; Gupta, A.; Sarangi, D.; Bhattacharya, R.; Assmann, W. Deposition of thin films of different oxides of copper by RF sputtering and their characterization. Vacuum 2000, 57, 377–385. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Khedr, M.H.; Ansari, M.S.; Hasan, P.M.Z.; Abdel-Wahab, M.S.; Farghali, A.A. RF sputtered CuO thin films: Structural, optical and photo-catalytic behavior. Phys. E 2016, 81, 83–90. [Google Scholar] [CrossRef]
- Chan, K.-Y.; Teo, B.-S. Atomic force microscopy (AFM) and X-ray diffraction (XRD) investigations of copper thin films prepared by dc magnetron sputtering technique. Microelectron. J. 2006, 37, 1064–1071. [Google Scholar] [CrossRef]
- Dhanasekaran, V.; Mahalingam, T.; Ganesan, V. SEM and AFM studies of dip-coated CuO nanofilms. Microsc. Res. Techn. 2013, 76, 58–65. [Google Scholar] [CrossRef]
- Hammoodi, R.A.; Abbas, A.K.; Elttayef, A.K. Structural and optical properties of CuO thin films prepared via RF magnetron sputtering. Int. J. Appl. Innov. Eng. Manag. 2014, 3, 1–7. [Google Scholar]
- Yousif, A.K.; Bader, B.A.; Mahdi, R.O. Morphological and optical properties of CuO/sapphire thin films prepared by pulsed laser deposition. Eng. Techn. J. 2014, 32, 892–898. [Google Scholar]
- Raebiger, H.; Lany, S.; Zunger, A. Origins of the p-type nature and cation deficiency in Cu2O and related materials. Phys. Rev. B 2007, 76, 045209. [Google Scholar] [CrossRef]
- Prabu, R.D.; Valanarasu, S.; Ganesh, V.; Shkir, M.; Al Faify, S.; Kathalingam, A.; Srikumar, S.R.; Chandramohan, R. An effect of temperature on structural, optical, photoluminescence and electrical properties of copper oxide thin films deposited by nebulizer spray pyrolysis technique. Mater. Sci. Semicond. Proc. 2018, 74, 129–135. [Google Scholar] [CrossRef]
- Baturay, S.; Tombak, A.; Batibay, D.; Ocak, Y.S. n-type conductivity of CuO thin films by metal doping. Appl. Surf. Sci. 2019, 477, 91–95. [Google Scholar] [CrossRef]
- Drobny, V.F.; Pulfrey, D.L. Properties of reactively-sputtered copper oxide films. Thin Solid Films 1979, 61, 89–98. [Google Scholar] [CrossRef]
- Asl, H.Z.; Rozati, S.M. Spray deposition of n-type cobalt-doped CuO thin films: Influence of cobalt doping on structural, morphological, electrical and optical properties. J. Electron. Mater. 2020, 49, 1534–1540. [Google Scholar] [CrossRef]
- Singh, I.; Bedi, R.K. Studies and correlation among the structural, electrical and gas response properties of aerosol spray deposited self assembled nanocrystalline CuO. Appl. Surf. Sci. 2011, 257, 7592–7599. [Google Scholar] [CrossRef]
- Abdel-Rafea, M.; Roushdy, N. Determination of the optical band gap for amorphous and nanocrystalline copper oxide thin films prepared by SILAR technique. J. Phys. D: Appl. Phys. 2008, 42, 015413. [Google Scholar] [CrossRef]
- Johan, M.R.; Suan, M.S.M.; Hawari, N.L.; Ching, H.A. Annealing effects on the properties of copper oxide thin films prepared by chemical deposition. Int. J. Electrochem. Sci. 2011, 6, 6094–6104. [Google Scholar]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Jandale, D.; Pawar, S.; Chougule, M.; Godse, P.; Patil, S.; Raut, B.; Sen, S.; Patil, V. Nanocrystalline CuO thin films for H2S monitoring: Microstructural and optoelectronic characterization. J. Sens. Technol. 2011, 1, 36–46. [Google Scholar]
- Dolai, S.; Dey, R.; Das, S.; Hussain, S.; Bhar, R.; Pal, A.K. Cupric oxide (CuO) thin films prepared by reactive d.c. magnetron sputtering technique for photovoltaic application. J. Alloys Compd. 2017, 724, 456–464. [Google Scholar] [CrossRef]
- Waikar, M.R.; Shaikh, A.A.; Sonkawade, R.G. The supercapacitive performance of woollen-like structure of CuO thin films prepared by the chemical method. Vacuum 2019, 161, 168–175. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Kim, Y.S.; Chandrasekaran, S.; Rajagopalan, B.; Kim, E.J.; Chung, J.S. Comparative supercapacitance performance of CuO nanostructures for energy storage device applications. RSC Adv. 2015, 5, 20545–20553. [Google Scholar] [CrossRef]
- Xu, L.; Sithambaram, S.; Zhang, Y.; Chen, C.-H.; Jin, L.; Joesten, R.; Suib, S.L. Novel urchin-like CuO synthesized by a facile reflux method with efficient olefin epoxidation catalytic performance. Chem. Mater. 2009, 21, 1253–1259. [Google Scholar] [CrossRef]
- Bhise, S.C.; Awale, D.V.; Vadiyar, M.M.; Patil, S.K.; Kokare, B.N.; Kolekar, S.S. Facile synthesis of CuO nanosheets as electrode for supercapacitor with long cyclic stability in novel methyl imidazole-based ionic liquid electrolyte. J. Solid State Electrochem. 2017, 21, 2585–2591. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Chen, Y.-C.; Lin, Y.-G. Characteristics and electrochemical performances of lotus-like CuO/Cu(OH)2 hybrid material electrodes. J. Electroanal. Chem. 2012, 673, 43–47. [Google Scholar] [CrossRef]
- Li, Y.; Ye, K.; Cheng, K.; Cao, D.; Pan, Y.; Kong, S.; Zhang, X.; Wang, G. Anchoring CuO nanoparticles on nitrogen-doped reduced graphene oxide nanosheets as electrode material for supercapacitors. J. Electroanal. Chem. 2014, 727, 154–162. [Google Scholar] [CrossRef]
- Patake, V.D.; Lokhande, C.D.; Joo, O.S. Electrodeposited ruthenium oxide thin films for supercapacitor: Effect of surface treatments. Appl. Surf. Sci. 2009, 255, 4192–4196. [Google Scholar] [CrossRef]
- Tobari, S.; Cherry, M.; Duijnstee, E.A.; Le Corre, V.M.; Qiu, L.; Hummelen, J.C.; Palasantzas, G.; Koster, L.J.A. Rough electrode creates excess capacitance in thin-film capacitors. ACS Appl. Mater. Interfaces 2017, 9, 27290–27297. [Google Scholar]

| Number of Steps | Successive Processes | Ref. |
|---|---|---|
| 3 | Solution + SILAR (90 cycles) + annealing | [31] |
| 4 | Solution + refluxing + washing + drying | [43] |
| 3 | Solution + spray pyrolysis + annealing | [44] |
| 3 | Ultrasonication + spray pyrolysis + drying | [45] |
| 3 | Solution + chemical bath (pH control) + drying | [46] |
| 3 | Solution + precipitation process + annealing | [47] |
| 3 | Solution + chemical bath + annealing | [48] |
| 5 | Solution + filtration + washing + drying + annealing | [49] |
| 4 | Acid treatment + surface oxidation + washing + drying | [50] |
| Ts (°C) | Lattice Parameters | Grain Size (nm) | Strain <e> ×10−3 | Dislocation Density 1017 cm−2 | |||
|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | β (°) | ||||
| 300 | 4.664(2) | 3.466(1) | 5.111(5) | 98.70(1) | 18 | 4.12 | 3.1 |
| 325 | 4.669(9) | 3.467(5) | 5.114(3) | 98.65(2) | 20 | 3.88 | 2.5 |
| 350 | 4.674(2) | 3.471(8) | 5.117(0) | 98.61(0) | 24 | 3.32 | 1.7 |
| 375 | 4.677(7) | 3.472(2) | 5.121(3) | 98.64(5) | 22 | 3.64 | 2.0 |
| 400 | 4.689(3) | 3.478(1) | 5.127(1) | 98.66(3) | 22 | 3.97 | 2.1 |
| Growth Conditions | Conductivity at 25 °C (S cm−1) | Activation Energy (eV) | Ref. |
|---|---|---|---|
| sputtering; Ts = 25 °C; p(O2) = 2.5 Pa; glass slide | 2.0 × 10−3 | 0.14 | [82] |
| sputtering; Ts = 25 °C; p(O2) = 0.08 Pa; glass slide | 5.5 × 10−3 | 0.22 | [71] |
| spray deposition; Ts = 350 °C; glass slide | 4.0 × 10−6 | 0.97 | [29] |
| spray pyrolysis; Ts = 320 °C; glass slide | 2.1 × 10−3 | 0.012 | [80] |
| sputtering; Ts = 25 °C; F(O2) = 5 sccm; silicon | 3.9× 10−3 (a) | 0.25 | [41] |
| sputtering; Ts = 25 °C; F(O2) = 5 sccm; silicon | 9.3× 10−3 (a) | 0.11 | [41] |
| spray pyrolysis; Ts = 475 °C; glass slide | 3.4 × 10−4 | - | [83] |
| spray pyrolysis; Ts = 400 °C; glass slide | 1.8 × 10−3 | 0.31 | [84] |
| sputtering; Ts = 350 °C; p(O2) = 2 Pa; Si wafer | 4.0 × 10−1 | 0.23 | this work |
| Preparation Method | Specific Capacitance (F g−1) | Electrolyte | Ref. |
|---|---|---|---|
| Liquid–solid reaction | 278 | NaOH | [94] |
| Reflux process | 225 | KOH | [95] |
| Chemical deposition | 79 | Na2SO4 | [90] |
| Surface oxidation of Cu | 190 | KOH | [50] |
| Reactive RF sputtering | 375 | PBS | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahendra, G.; Malathi, R.; Kedhareswara, S.P.; Lakshmi-Narayana, A.; Dhananjaya, M.; Guruprakash, N.; Hussain, O.M.; Mauger, A.; Julien, C.M. RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Appl. Nano 2021, 2, 46-66. https://doi.org/10.3390/applnano2010005
Mahendra G, Malathi R, Kedhareswara SP, Lakshmi-Narayana A, Dhananjaya M, Guruprakash N, Hussain OM, Mauger A, Julien CM. RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Applied Nano. 2021; 2(1):46-66. https://doi.org/10.3390/applnano2010005
Chicago/Turabian StyleMahendra, Goddati, Reddappagari Malathi, Sairam P. Kedhareswara, Ambadi Lakshmi-Narayana, Merum Dhananjaya, Nunna Guruprakash, Obili M. Hussain, Alain Mauger, and Christian M. Julien. 2021. "RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors" Applied Nano 2, no. 1: 46-66. https://doi.org/10.3390/applnano2010005
APA StyleMahendra, G., Malathi, R., Kedhareswara, S. P., Lakshmi-Narayana, A., Dhananjaya, M., Guruprakash, N., Hussain, O. M., Mauger, A., & Julien, C. M. (2021). RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Applied Nano, 2(1), 46-66. https://doi.org/10.3390/applnano2010005









