Investigation of Ionic Conductivity of Electrolytes for Anode-Free Lithium-Ion Batteries by Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrolytes
2.2. Assembly of Samples of the 2032 Format (Cells)
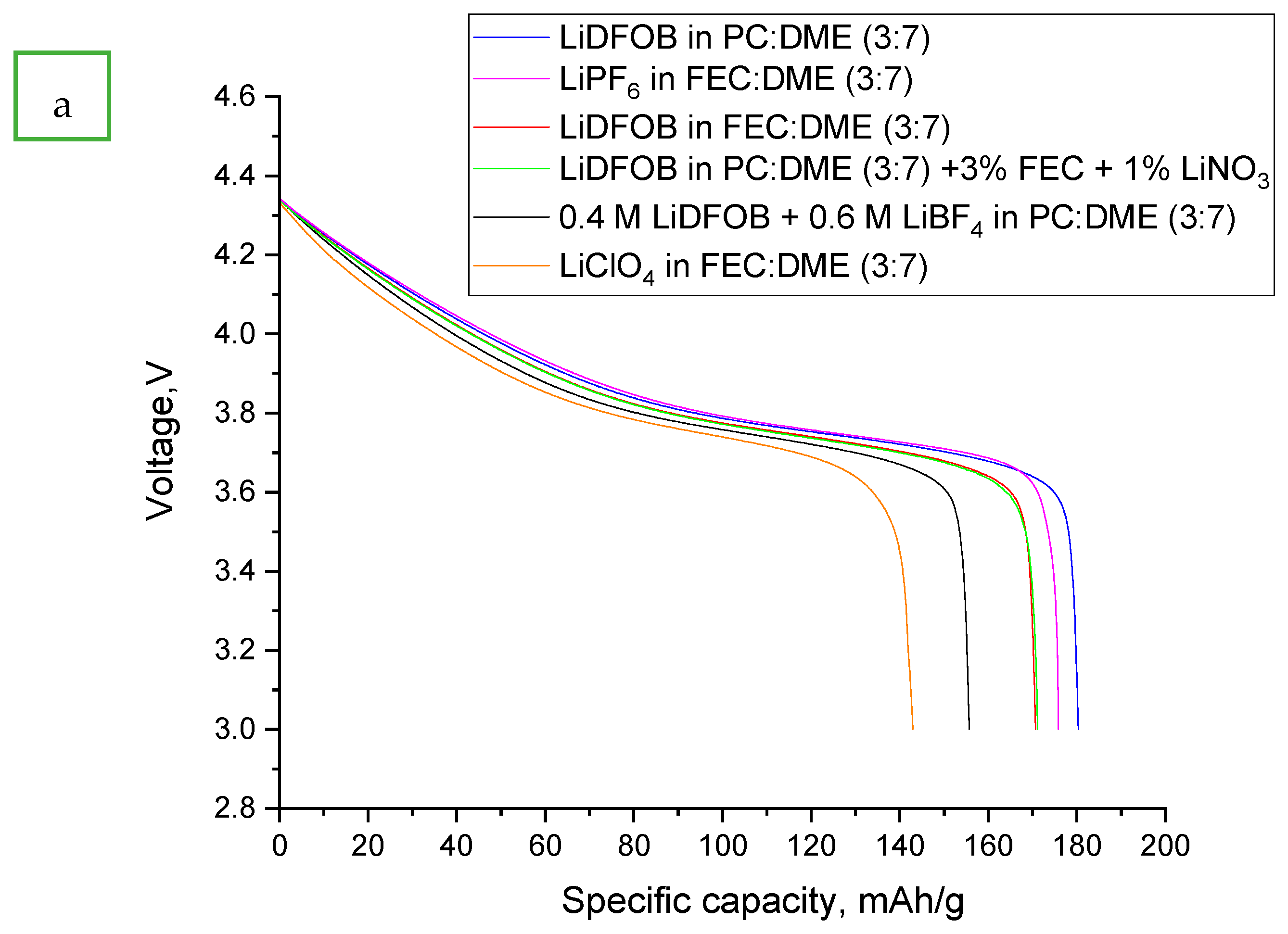
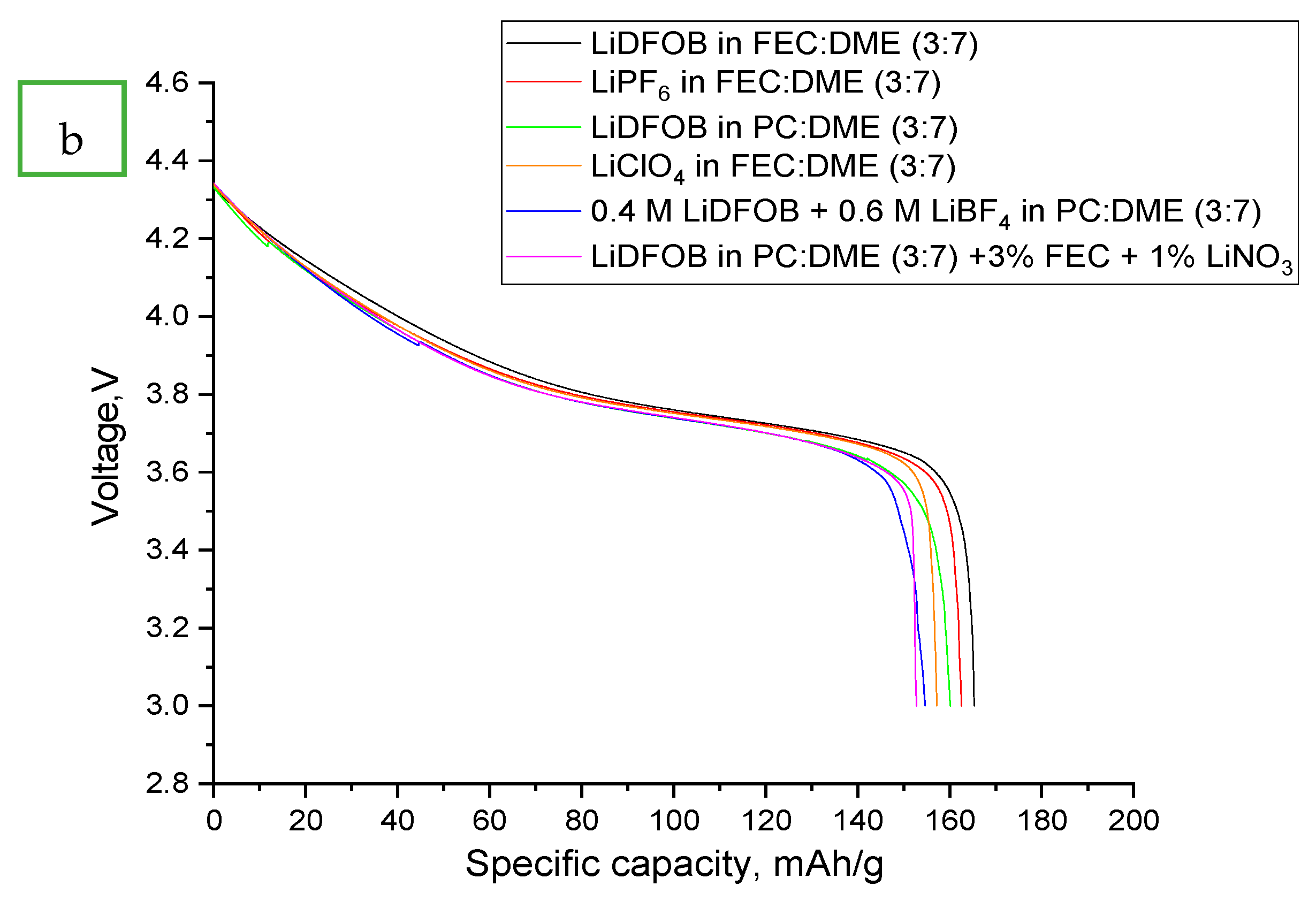
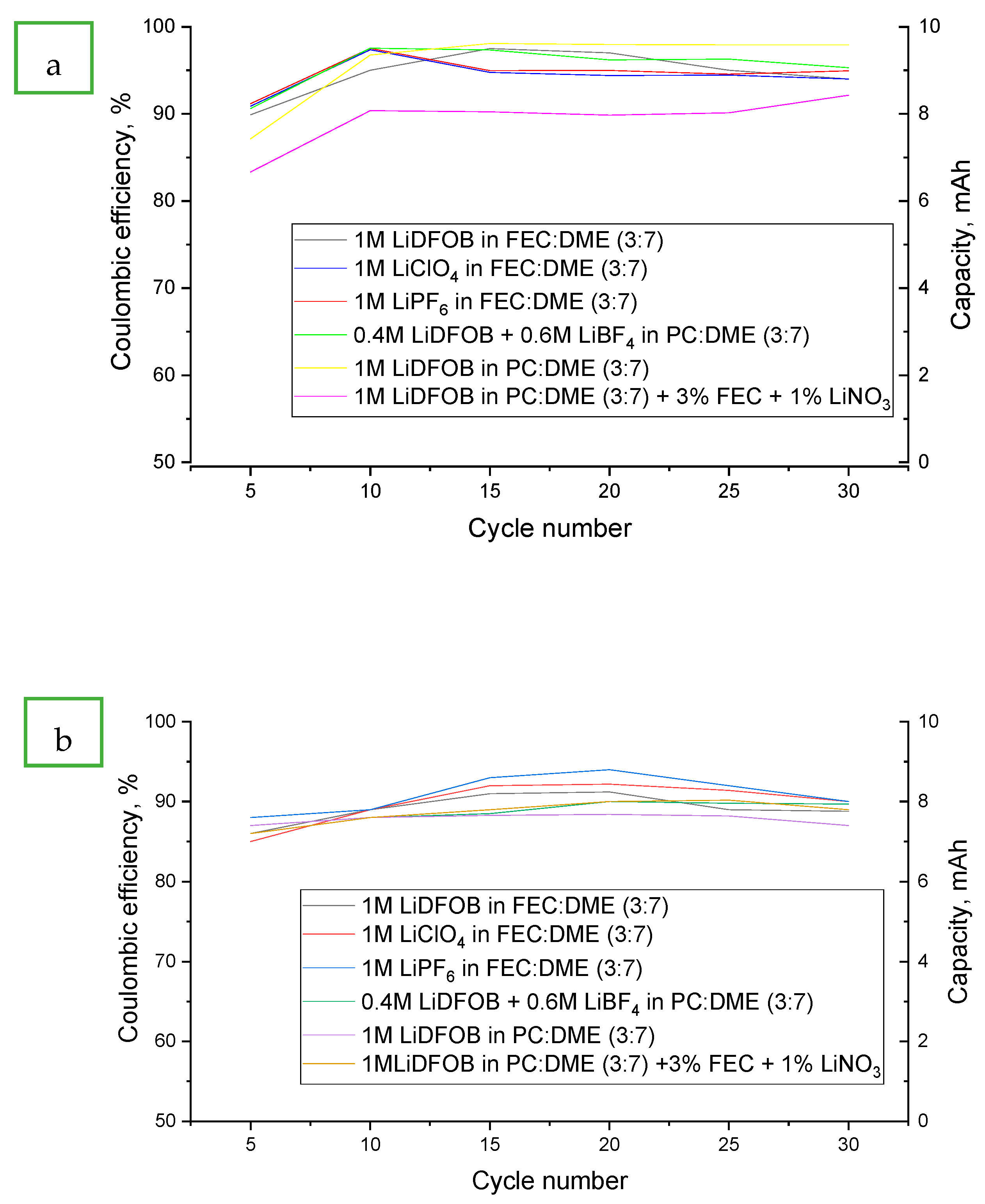
- LiDFOB in FEC:DME (3:7)
- LiClO4 in FEC:DME (3:7)
- LiPF6 in FEC:DME (3:7)
- 0.4 M LiDFOB + 0.6 M LiBF4 in PC:DME (3:7)
- LiDFOB in PC:DME (3:7)
- LiDFOB in PC:DME (3:7) + 3% FEC + 1% LiNO3
- LiDFOB in PC:DME:EA (3:7:3) + 5% FEC + 3% LiNO3
- Polyvinylidene fluoride (Solef 5130) was dissolved in N-methyl-2-pyrrolidone under mechanical stirring at 60 °C for 2 h;
- Carbon nanotubes were dispersed in N-methyl-2-pyrrolidone using an ultrasonic homogenizer Bandelin Sonopuls HD 3100 (Bandelin electronic GmbH & Co. KG, Berlin, Germany) in 5 min intervals for 15 min;
- The polymer solution and nanotube dispersion were combined and sonicated for an additional 5–10 min;
- Carbon black and NMC111 active material were added under overhead stirring without heating for 40 min;
- The mixture was homogenized to obtain a uniform paste;
- Final mechanical stirring was carried out for at least 12 h at 1500 rpm, followed by 20 min of vacuum mixing to remove residual gases.
3. Results
- σ is the specific ionic conductivity;
- l is the spacer distance (70 µm, based on two 35 µm cardboard rings);
- R is the resistance value obtained from Nyquist plots;
- S is the cross-sectional area (0.79 cm2).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Qiu, L.; Cheng, H.-M. Towards the practical use of flexible lithium ion batteries. Energy Storage Mater. 2019, 23, 434–438. [Google Scholar] [CrossRef]
- Xue, W.; Qin, T.; Li, Q.; Zan, M.; Yu, X.; Li, H. Exploiting the synergistic effects of multiple components with a uniform design method for developing low-temperature electrolytes. Energy Storage Mater. 2022, 50, 598–605. [Google Scholar] [CrossRef]
- Xu, G.; Shangguan, X.; Dong, S.; Zhou, X.; Cui, G. Formulation of Blended-Lithium-Salt Electrolytes for Lithium Batteries. Angew. Chem. Int. Ed. 2020, 59, 3400–3415. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Cheng, H.; Sun, Q.; Zhang, J.; Ming, J. Low-Temperature Electrolyte Design for Lithium-Ion Batteries: Prospect and Challenges. Chem. A Eur. J. 2021, 27, 15842–15865. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, L.; Li, Z.; Dou, H.; Zhang, X. Design strategies and research progress for Water-in-Salt electrolytes. Energy Storage Mater. 2022, 44, 10–28. [Google Scholar] [CrossRef]
- Tashenov, A.K.; Omarova, N.M.; Belgibayeva, D.S.; Itkis, D.M.; Krivchenko, B.A. The influence of nanostructured carbon additives to the functional electrode characteristics of lithium-ion batteries. Bull. Karaganda Univ. 2018, 1, 32–35. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, W.; et al. Post-mortem analysis of aged lithium-ion batteries: Disassembly methodology and physico-chemical analysis techniques. J. Electrochem. Soc. 2016, 163, 2149–2164. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, S.; Wang, C. Recent Advances in Anode-Free Lithium-Metal Batteries: Challenges and Opportunities. Adv. Energy Mater. 2025, 15, 2301125. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, D.; Yang, J.; Cui, G. Strategies for High Energy Density and Long Life Anode-Free Lithium-Metal Batteries. Energy Storage Mater. 2024, 64, 856–874. [Google Scholar]
- Han, L.; Xu, Z.; Sun, M.; Zhang, Y. High-Performance Anode-Free Li-Metal Batteries Enabled by Novel SEI Engineering. Joule 2025, 9, 150–167. [Google Scholar] [CrossRef]
- Kim, S.; Cho, K.; Lee, J.; Lee, S. Interface Engineering for Stable Anode-Free Lithium Metal Batteries. Nat. Energy 2025, 10, 420–431. [Google Scholar]
- Zhao, Y.; Chen, X.; Lin, D.; Zhang, J. Minimizing Dead Lithium Formation in Anode-Free Lithium Metal Batteries. Adv. Funct. Mater. 2024, 34, 2308795. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z.; Wang, H.; Yang, W. Limitations of Thick Lithium Foils in High-Energy Lithium Metal Batteries. Electrochim. Acta 2024, 489, 142124. [Google Scholar]
- Park, J.; Choi, S.; Kim, H.; Yoon, W. Industrial Feasibility of Anode-Free Lithium Batteries Without Lithium Foil. Energy Environ. Sci. 2025, 18, 690–707. [Google Scholar]
- Abrha, L.H.; Zegeye, T.A.; Hagos, T.T.; Sutiono, H.; Hagos, T.M.; Berhe, G.B.; Huang, C.-J.; Jiang, S.-K.; Su, W.-N.; Yang, Y.-W.; et al. Li7La2.75Ca0.25Zr1.75Nb0.25O12@LiClO4 composite film derived solid electrolyte interphase for anode-free lithium metal battery. Electrochim. Acta 2019, 325, 134825. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.-H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef]
- Wood, K.; Kazyak, E.; Chadwick, A.F.; Chen, K.-H.; Zhang, J.-G.; Thornton, K.; Dasgupta, N.P. Dendrites and pits: Untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Cent. Sci. 2016, 2, 790–801. [Google Scholar] [CrossRef]
- Rodriguez, R.; Edison, R.A.; Stephens, R.M.; Sun, H.; Heller, A.; Mullins, C.B. Separator-free and concentrated LiNO3 electrolyte cells enable uniform lithium electrodeposition. J. Mater. Chem. A 2020, 8, 3999–4006. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Cao, X.; Engelhard, M.H.; Liu, W.; Burton, S.D.; Lee, H.; Niu, C.; Matthews, B.E.; Zhu, Z.; et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 2019, 3, 1662–1676. [Google Scholar] [CrossRef]
- Louli, A.J.; Genovese, M.; Weber, R.; Hames, S.G.; Logan, R.; Dahn, J.R. Exploring the impact of mechanical pressure on the performance of anode-free lithium metal cells. J. Electrochem. Soc. 2019, 166, A1291–A1299. [Google Scholar] [CrossRef]
- Niu, C.; Lee, H.; Chen, S.; Li, Q.; Du, J.; Xu, W.; Zhang, J.G.; Whittingham, M.S.; Xiao, J.; Liu, J. Highnergy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 2019, 4, 551–559. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009–2013. [Google Scholar] [CrossRef]
- Jehnichen, P.; Wedlich, K.; Korte, C. Degradation of high-voltage cathodes for advanced lithium-ion batteries—differential capacity study on differently balanced cells. Sci. Technol. Adv. Mater. 2019, 20, 1–9. [Google Scholar] [CrossRef]
- Li, Q.; Lu, D.; Zheng, J.; Jiao, S.; Luo, L.; Wang, C.M.; Xu, K.; Zhang, J.G.; Xu, W. Li+-Desolvation Dictating Lithium-Ion Battery’s Low-Temperature Performances. ACS Appl. Mater. Interfaces 2017, 9, 42761–42768. [Google Scholar] [CrossRef]
- Koo, B.; Lee, H.; Hwang, S.; Lee, J.; Han, Y.-K.; Ahn, K.H.; Lee, C.; Lee, H. Role of Solvent Isomerism in Mixed Carbonate Electrolytes for Li-Ion Batteries. J. Phys. Chem. C 2023, 127, 18271–18278. [Google Scholar] [CrossRef]
- Ding, M.S.; Xu, K.; Jow, T.R. Liquid-Solid Phase Diagrams of Binary Carbonates for Lithium Batteries. J. Electrochem. Soc. 2000, 147, 1688. [Google Scholar] [CrossRef]
- Plichta, E.J.; Behl, W.K. A low-temperature electrolyte for lithium and lithium-ion batteries. J. Power Sources 2000, 88, 192–196. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Ding, M.S.; Xu, K.; Zhang, S.S.; Amine, K.; Henriksen, G.L.; Jow, T.R. Change of Conductivity with Salt Content, Solvent Composition, and Temperature for Electrolytes of LiPF6 in Ethylene Carbonate-Ethyl Methyl Carbonate. J. Electrochem. Soc. 2001, 148, A1196. [Google Scholar] [CrossRef]
- Villaluenga, I.; Pesko, D.M.; Timachova, K.; Feng, Z.; Newman, J.; Srinivasan, V.; Balsara, N.P. Negative Stefan-Maxwell Diffusion Coefficients and Complete Electrochemical Transport Characterization of Homopolymer and Block Copolymer Electrolytes. J. Electrochem. Soc. 2018, 165, A2766–A2773. [Google Scholar] [CrossRef]
- Landesfeind, J.; Gasteiger, H.A.; Soc, J.E.; Landesfeind, J. Temperature and Concentration Dependence of the Ionic Transport Properties of Lithium-Ion Battery Electrolytes. J. Electrochem. Soc. 2019, 166, A3079. [Google Scholar] [CrossRef]
- Mestre-Aizpurua, F.; Hamelet, S.; Masquelier, C.; Palacín, M.R. High temperature electrochemical performance of nanosized LiFePO4. J. Power Sources 2010, 195, 6897–6901. [Google Scholar] [CrossRef]
- Reale, P.; Fernicola, A.; Scrosati, B. Compatibility of the Py24TFSI–LiTFSI ionic liquid solution with Li4Ti5O12 and LiFePO4 lithium ion battery electrodes. J. Power Sources 2009, 194, 182–189. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Chen, L.; Lu, J.; Wang, Y.; He, P.; Huang, S.; Liu, Y.; Wu, Y.; Cao, G.; Wang, L.; He, X.; et al. Double-salt electrolyte for Li-ion batteries operated at elevated temperatures. Energy Storage Mater. 2022, 49, 493–501. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Y.S.; Xu, K. Perspective—Fluorinating Interphases. J. Electrochem. Soc. 2019, 166, A5184–A5186. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Jia, H.; Yang, J.; Monroe, C.W.; Nuli, Y. Towards a Safe Lithium–Sulfur Battery with a Flame-Inhibiting Electrolyte and a Sulfur-Based Composite Cathode. Angew. Chem. Int. Ed. 2014, 53, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tao, L.; Hahn, H.; Schultz, C.; Gallus, D.R.; Cao, X.; Nowak, S.; Röser, S.; Li, J.; Cekic-Laskovic, I.; et al. Lifetime limit of tris(trimethylsilyl) phosphite as electrolyte additive for high voltage lithium ion batteries. RSC Adv. 2016, 6, 38342–38349. [Google Scholar] [CrossRef]
- Han, Y.K.; Yoo, J.; Yim, T. Why is tris(trimethylsilyl) phosphite effective as an additive for high-voltage lithium-ion batteries? J. Mater. Chem. A 2015, 3, 10900–10909. [Google Scholar] [CrossRef]
- Liu, B.; Li, Q.; Engelhard, M.H.; He, Y.; Zhang, X.; Mei, D.; Wang, C.; Zhang, J.G.; Xu, W. Constructing Robust Electrode/Electrolyte Interphases to Enable Wide Temperature Applications of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 21496–21505. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huang, S.; Cui, Z.; Du, X.; Wang, X.; Lu, D.; Shangguan, X.; Ma, J.; Han, P.; Zhou, X.; et al. Functional additives assisted ester-carbonate electrolyte enables wide temperature operation of a high-voltage (5 V-Class) Li-ion battery. Power Sources 2019, 416, 29–36. [Google Scholar] [CrossRef]
- Liao, L.; Fang, T.; Zhou, X.; Gao, Y.; Cheng, X.; Zhang, L.; Yin, G. Enhancement of low-temperature performance of LiFePO4 electrode by butyl sultone as electrolyte additive. Solid State Ion. 2014, 254, 27–31. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdrakhmanova, A.; Sabitova, A.; Mussabayeva, B.; Bayakhmetova, B.; Sharipkhan, Z.; Yermoldina, E. Investigation of Ionic Conductivity of Electrolytes for Anode-Free Lithium-Ion Batteries by Impedance Spectroscopy. Electrochem 2025, 6, 20. https://doi.org/10.3390/electrochem6020020
Abdrakhmanova A, Sabitova A, Mussabayeva B, Bayakhmetova B, Sharipkhan Z, Yermoldina E. Investigation of Ionic Conductivity of Electrolytes for Anode-Free Lithium-Ion Batteries by Impedance Spectroscopy. Electrochem. 2025; 6(2):20. https://doi.org/10.3390/electrochem6020020
Chicago/Turabian StyleAbdrakhmanova, Azhar, Alfira Sabitova, Binur Mussabayeva, Bulbul Bayakhmetova, Zhanna Sharipkhan, and Elmira Yermoldina. 2025. "Investigation of Ionic Conductivity of Electrolytes for Anode-Free Lithium-Ion Batteries by Impedance Spectroscopy" Electrochem 6, no. 2: 20. https://doi.org/10.3390/electrochem6020020
APA StyleAbdrakhmanova, A., Sabitova, A., Mussabayeva, B., Bayakhmetova, B., Sharipkhan, Z., & Yermoldina, E. (2025). Investigation of Ionic Conductivity of Electrolytes for Anode-Free Lithium-Ion Batteries by Impedance Spectroscopy. Electrochem, 6(2), 20. https://doi.org/10.3390/electrochem6020020





