Electrochemical Detection of Caffeic Acid on Diethyl 3,4-Dihydroxythiophene-2,5-Dicarboxylate-Modified Carbon Paste Electrode: Insights from Computational Analysis
Abstract
1. Introduction
2. Experimentation
2.1. Synthesize of Diethyl 3,4-Dihydroxythiophene-2,5-Dicarboxylate
2.2. Preparation of Bare and Ester-Modified CPE
2.3. Computational Methods DFT Studies
3. Results
3.1. Surface Morphology of BCPE and Ester-Modified CPE
3.2. Cyclic Voltammetric Behavior of [Fe(CN)6] on Bare and Ester-Modified CPE
3.3. Computational Studies
3.3.1. Global Electron Transfer Properties
3.3.2. Local Electron Transfer Properties
3.4. Electrochemical Behavior of Caffeic Acid on Bare and Ester-Modified CPE
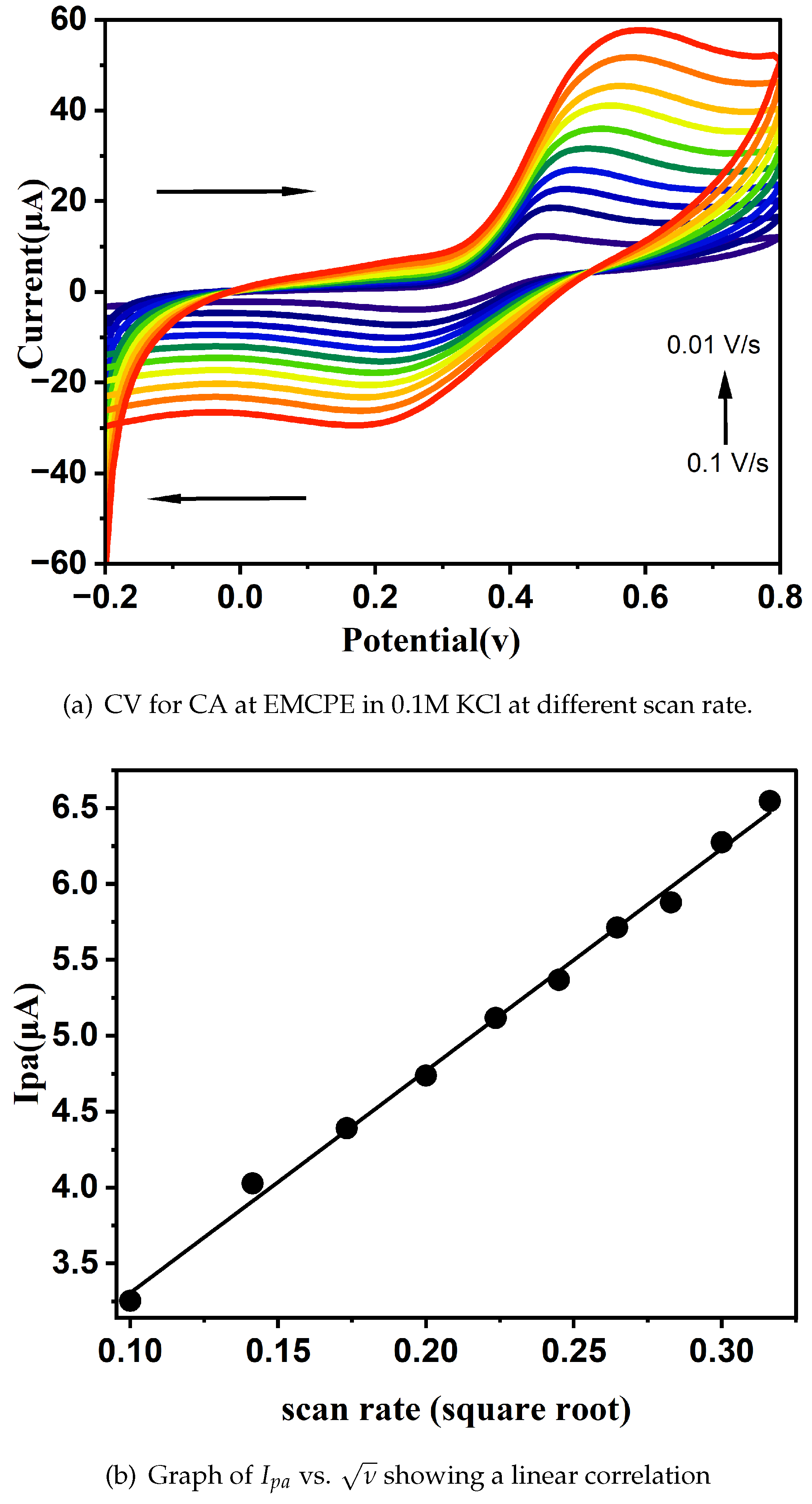
3.5. Influence of Scan Rate
4. Effect of Concentration for Caffeic Acid
5. Repeatability, Reproducibility, and Stability Assessment
6. Real Sample Analysis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Jabeen, N.; Ashraf, K.; Sultan, K.; Farhan, M.; Hussain, M.I.; Deng, G.; Alsudays, I.M.; Saleh, M.A.; Tariq, S.; et al. Exploring the role of caffeic acid in mitigating abiotic stresses in plants: A review. Plant Stress 2024, 12, 100487. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Voltamperometric sensors and biosensors based on carbon nanomaterials used for detecting caffeic acid—A review. Int. J. Mol. Sci. 2020, 21, 9275. [Google Scholar] [CrossRef]
- Bankova, V.; Trusheva, B.; Popova, M. Caffeic acid phenethyl ester (CAPE)—Natural sources, analytical procedures and synthetic approaches. C. R. L’Acad. Bulg. Des Sci. 2018, 71, 1157–1169. [Google Scholar]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Bankova, V.; Christoy, R.; Stoev, G.; Popov, S. Determination of phenolics from propolis by capillary gas chromatography. J. Chromatogr. A 1992, 607, 150–153. [Google Scholar] [CrossRef]
- Del Boccio, P.; Rotilio, D. Quantitative analysis of caffeic acid phenethyl ester in crude propolis by liquid chromatography-electrospray ionization mass spectrometry. J. Sep. Sci. 2004, 27, 619–623. [Google Scholar] [CrossRef]
- Yang, S.; Han, Y.; Wang, K.; Wang, Y.; Li, L.; Li, N.; Xu, X. Simultaneous determination of four phenolic acids in traditional Chinese medicine by capillary electrophoresis-chemiluminescence. RSC Adv. 2021, 11, 33996–34003. [Google Scholar] [CrossRef]
- Ramki, S.; Balasubramanian, P.; Chen, S.M.; Chen, T.W.; Tseng, T.W.; Lou, B.S. Voltammetric determination of caffeic acid using Co3O4 microballs modified screen printed carbon electrode. Int. J. Electrochem. Sci. 2018, 13, 1241–1249. [Google Scholar] [CrossRef]
- Filik, H.; Çetintaş, G.; Avan, A.A.; Aydar, S.; Koç, S.N.; Boz, İ. Square-wave stripping voltammetric determination of caffeic acid on electrochemically reduced graphene oxide–Nafion composite film. Talanta 2013, 116, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.E.; Chen, S.M.; Chen, Y.H.; Ali, M.A.; Al-Hemaid, F.M. An electrocatalytic oxidation and voltammetric method using a chemically reduced graphene oxide film for the determination of caffeic acid. J. Colloid Interface Sci. 2014, 423, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.S.; Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. Sensitive electrochemical detection of caffeic acid in wine based on fluorine-doped graphene oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [PubMed]
- Tonn, J.N.; Keithley, R.B. Waveform Optimization for the In Vitro Detection of Caffeic Acid by Fast-Scan Cyclic Voltammetry. ACS Meas. Sci. Au 2024, 4, 534–545. [Google Scholar] [CrossRef]
- Fukumoto, L.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit hydroxycinnamic acids inhibit human low-density lipoprotein oxidation in vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Jayaprakash, G.K. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2022, 789, 139295. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Casillas, N.; Flores-Moreno, R. Analytical Fukui and cyclic voltammetric studies on ferrocene modified carbon electrodes and effect of Triton X-100 by immobilization method. Electrochim. Acta 2017, 258, 1025–1034. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Chandrashekar, B.N.; Flores-Moreno, R. Theoretical and cyclic voltammetric studies on electrocatalysis of benzethonium chloride at carbon paste electrode for detection of dopamine in presence of ascorbic acid. J. Mol. Liq. 2017, 240, 395–401. [Google Scholar] [CrossRef]
- Lima, A.; Schottland, P.; Sadki, S.; Chevrot, C. Electropolymerization of 3, 4-ethylenedioxythiophene and 3, 4-ethylenedioxythiophene methanol in the presence of dodecylbenzenesulfonate. Synth. Met. 1998, 93, 33–41. [Google Scholar] [CrossRef]
- Geudtner, G.; Calaminici, P.; Carmona-Espíndola, J.; del Campo, J.M.; Domínguez-Soria, V.D.; Flores-Moreno, R.; Gamboa, G.U.; Goursot, A.; Köster, A.M.; Reveles, J.U.; et al. DeMon2k Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 548–555. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [PubMed]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Flores-Moreno, R.; Pineda-Urbina, K.; Gómez-Sandoval, Z. Sinapsis, Version XII–V; Sinapsis Developers: 2012. Available online: https://sourceforge.net/projects/sinapsis (accessed on 20 July 2020).
- Parr, R.G.; Yang, W. Density Functional Approach to the Frontier–Electron Theory of Chemical Reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Flores-Moreno, R.; Melin, J.; Ortiz, J.V.; Merino, G. Efficient Evaluation of Analytic Fukui Functions. J. Chem. Phys. 2008, 129, 224105. [Google Scholar] [CrossRef]
- Chermette, H. Chemical reactivity indexes in density functional theory. J. Comput. Chem. 1999, 20, 129–154. [Google Scholar] [CrossRef]
- Gázquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and electroaccepting powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Flores-Moreno, R. Symmetry Conservation in Fukui Functions. J. Chem. Theory Comput. 2010, 6, 48–54. [Google Scholar] [CrossRef]
- Kucukkolbasi, S.; Sayin, S.; Yilmaz, M. Fabrication and application of a new modified electrochemical sensor using newly synthesized calixarene-grafted MWCNTs for simultaneous determination of Cu (II) and Pb (II). Acta Chim. Slov. 2019, 66, 839–849. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.K.; Rajendrachari, S.; Sharma, S.; Flores-Moreno, R. Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications. J. Mol. Liq. 2021, 334, 116348. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Flores-Moreno, R. Regioselectivity in hexagonal boron nitride co-doped graphene. New J. Chem. 2018, 42, 18913–18918. [Google Scholar] [CrossRef]
- Revanappa, S.K.; Soni, I.; Siddalinganahalli, M.; Jayaprakash, G.K.; Flores-Moreno, R.; Bananakere Nanjegowda, C. A Fukui analysis of an arginine-modified carbon surface for the electrochemical sensing of dopamine. Materials 2022, 15, 6337. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.E. Strategies for assessing the limit of detection in voltammetric methods: Comparison and evaluation of approaches. Analyst 2024, 149, 4295–4309. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Electrochemical determination of chlorogenic acid in nutraceuticals using voltammetric sensors based on screen-printed carbon electrode modified with graphene and gold nanoparticles. Int. J. Mol. Sci. 2021, 22, 8897. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.P.; Bergamini, M.F.; Fogg, A.G.; Zanoni, M.V.B. Application of a glassy carbon electrode modified with poly (glutamic acid) in caffeic acid determination. Microchim. Acta 2005, 151, 127–134. [Google Scholar] [CrossRef]
- Bottari, D.; Pigani, L.; Zanardi, C.; Terzi, F.; Paţurcă, S.V.; Grigorescu, S.D.; Matei, C.; Lete, C.; Lupu, S. Electrochemical sensing of caffeic acid using gold nanoparticles embedded in poly (3, 4-ethylenedioxythiophene) layer by sinusoidal voltage procedure. Chemosensors 2019, 7, 65. [Google Scholar] [CrossRef]







| SI/No. | CA Spiked (M) | CA Sensed (M) | Deviation (M) | Recovery (%) |
|---|---|---|---|---|
| 1 | 150 | 144 | 6 | 96.0 |
| 2 | 200 | 196 | 4 | 98.0 |
| 3 | 250 | 246 | 4 | 98.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chethana Suresh, S.; Kudur Jayaprakash, G.; Shivashankar, S.M.; Shashanka, R.; Rikhari, B. Electrochemical Detection of Caffeic Acid on Diethyl 3,4-Dihydroxythiophene-2,5-Dicarboxylate-Modified Carbon Paste Electrode: Insights from Computational Analysis. Electrochem 2025, 6, 19. https://doi.org/10.3390/electrochem6020019
Chethana Suresh S, Kudur Jayaprakash G, Shivashankar SM, Shashanka R, Rikhari B. Electrochemical Detection of Caffeic Acid on Diethyl 3,4-Dihydroxythiophene-2,5-Dicarboxylate-Modified Carbon Paste Electrode: Insights from Computational Analysis. Electrochem. 2025; 6(2):19. https://doi.org/10.3390/electrochem6020019
Chicago/Turabian StyleChethana Suresh, Surya, Gururaj Kudur Jayaprakash, Sunitha Mughalihalli Shivashankar, Rajendrachari Shashanka, and Bhavana Rikhari. 2025. "Electrochemical Detection of Caffeic Acid on Diethyl 3,4-Dihydroxythiophene-2,5-Dicarboxylate-Modified Carbon Paste Electrode: Insights from Computational Analysis" Electrochem 6, no. 2: 19. https://doi.org/10.3390/electrochem6020019
APA StyleChethana Suresh, S., Kudur Jayaprakash, G., Shivashankar, S. M., Shashanka, R., & Rikhari, B. (2025). Electrochemical Detection of Caffeic Acid on Diethyl 3,4-Dihydroxythiophene-2,5-Dicarboxylate-Modified Carbon Paste Electrode: Insights from Computational Analysis. Electrochem, 6(2), 19. https://doi.org/10.3390/electrochem6020019









