Electrochemical Sensing of Amoxicillin Drug-Assisted Uropathogenic E. coli Bacteria Using Gold Nanostructures—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentations
2.3. Synthesis of Gold Nanowires
2.4. Electrochemical Experiments
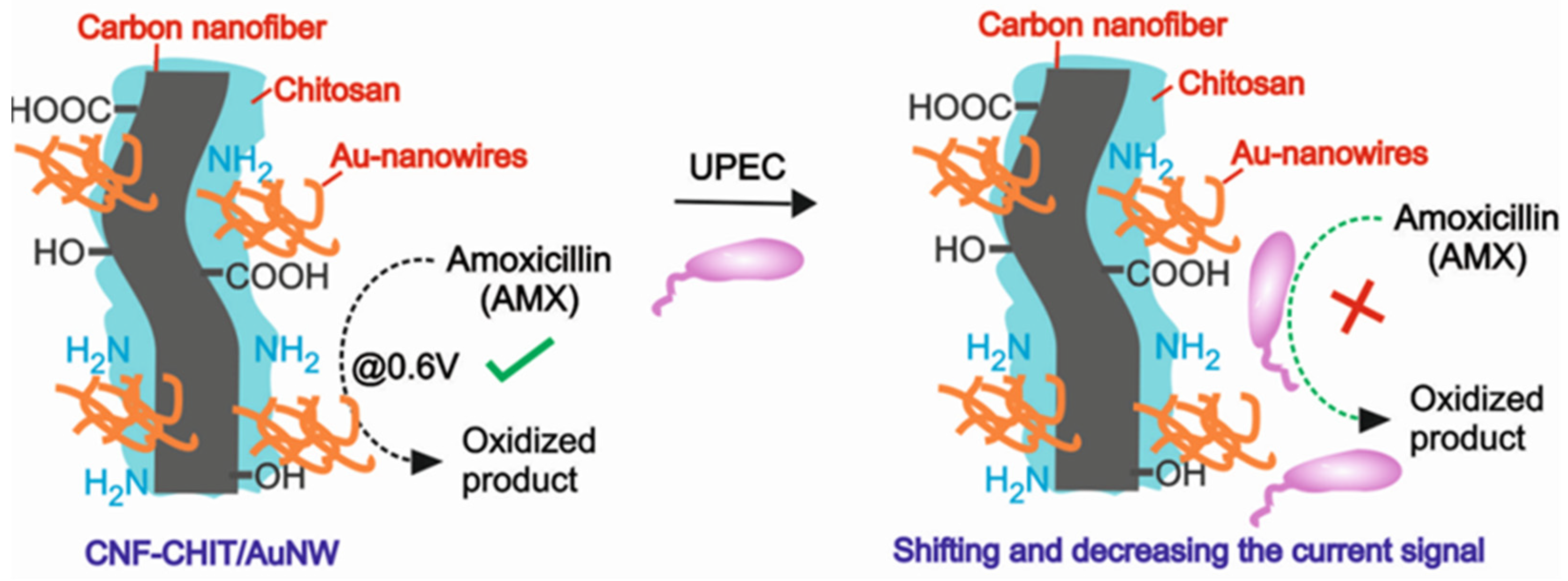
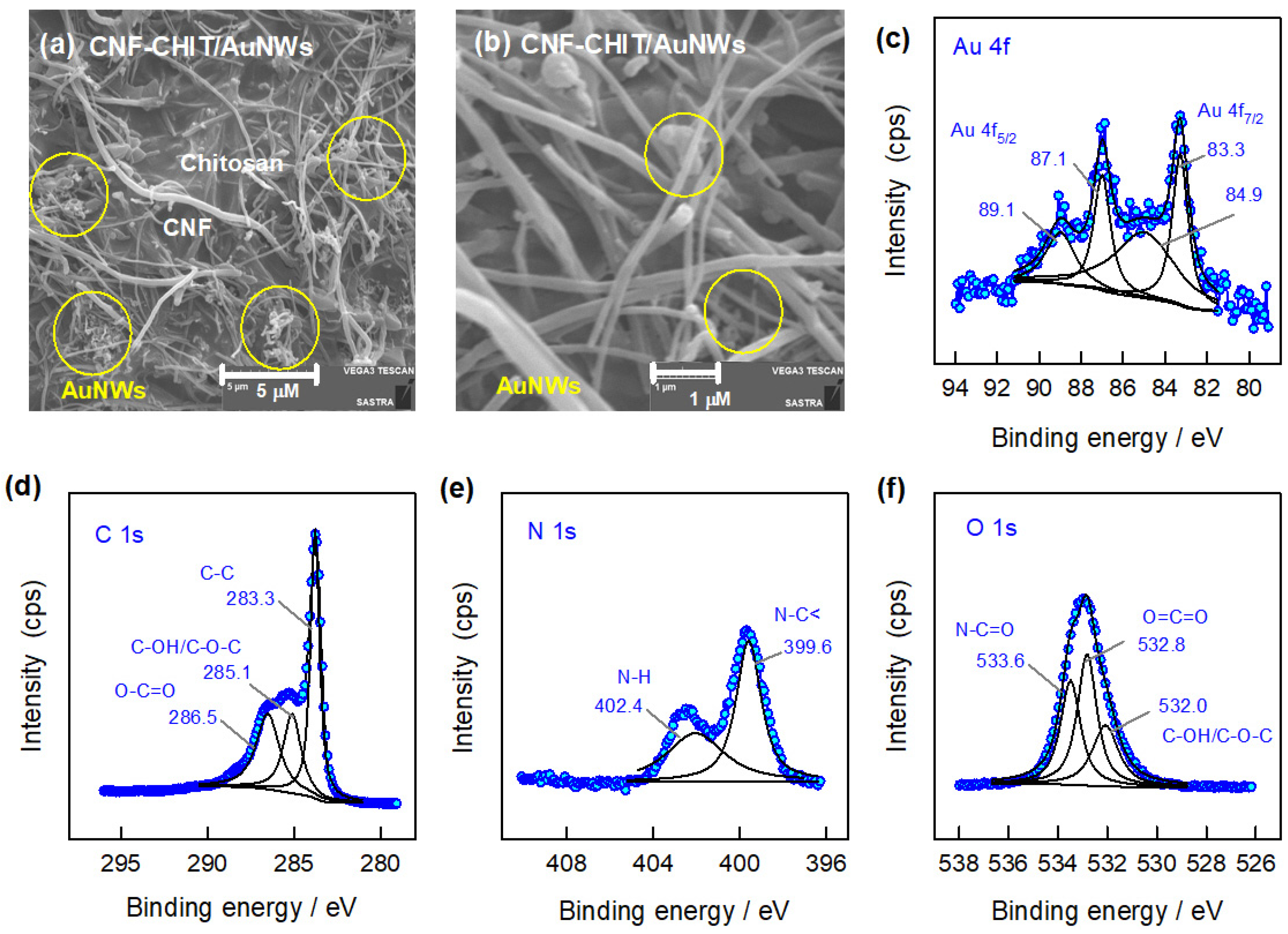
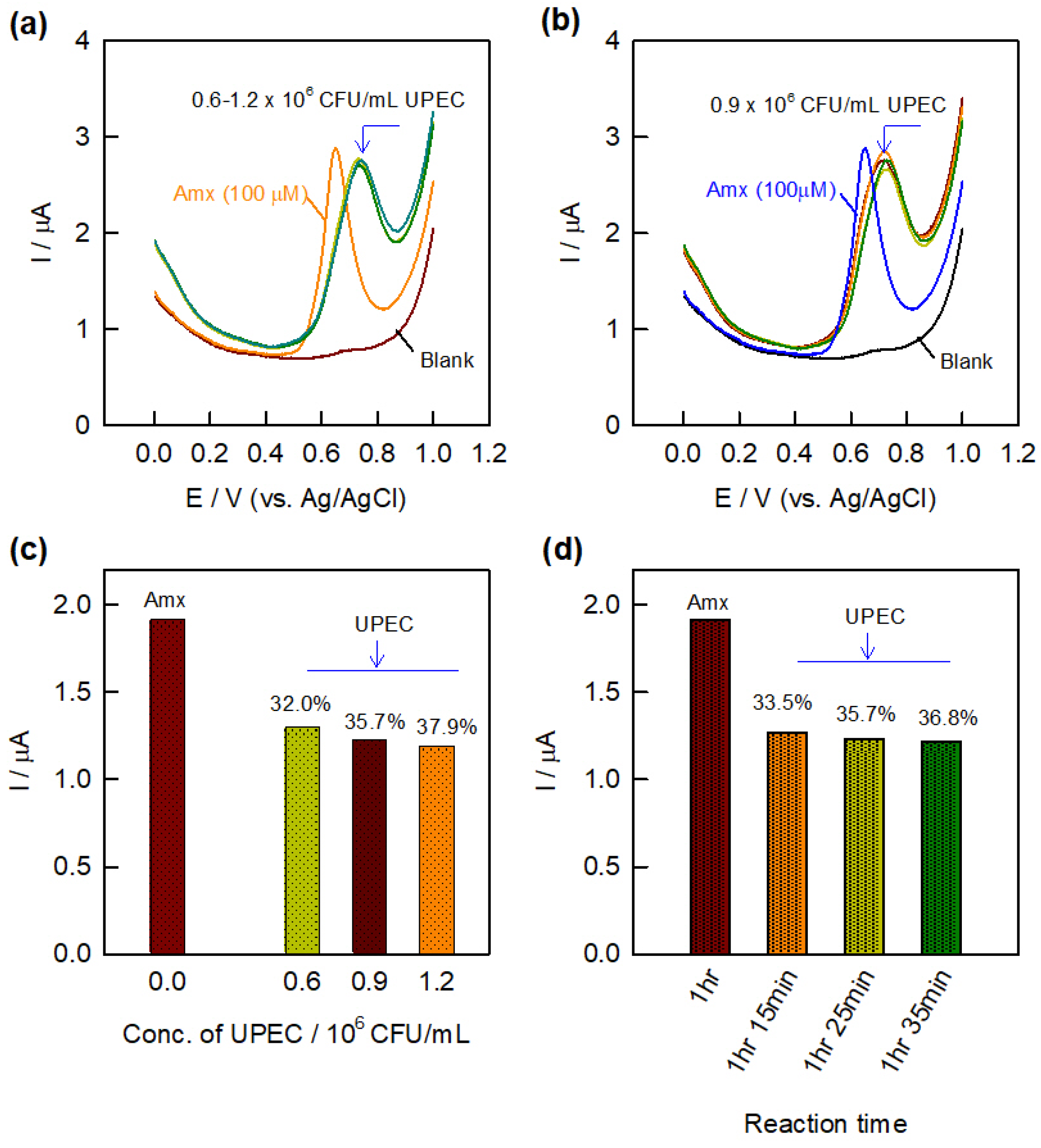
3. Results
3.1. Physicochemical Characterizations
3.2. Electrochemical Sensing of AMX on GCE/CNF-CHIT/AuNWs
3.3. Electrochemical Evaluation of Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- Karishetti, M.S.; Shaik, H.B. Clinicomicrobial assessment of urinary tract infections in a tertiary care hospital. Indian J. Health Sci. Biomed. Res. (KLEU) 2019, 12, 69. [Google Scholar]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef]
- Tasoglu, S. Toilet-based continuous health monitoring using urine. Nat. Rev. Urol. 2022, 19, 219–230. [Google Scholar] [CrossRef]
- Dospinescu, V.M.; Tiele, A.; Covington, J.A. Sniffing out urinary tract infection—Diagnosis based on volatile organic compounds and smell profile. Biosensors 2020, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Munir, T.; Rehman, S.; Hafiz, A.; Gilani, M.; Latif, M. Comparison of urine dipstick test with conventional urine culture in diagnosis of urinary tract infection. J. Coll. Physicians Surg. Pak. 2015, 25, 108–110. [Google Scholar] [PubMed]
- Gennifer, T.S.; Dwork, N.; Khan, S.A.; Millet, M.; Magar, K.; Javanmard, M.; Bowden, A.K.E. Robust dipstick urinalysis using a low-cost, micro-volume slipping manifold and mobile phone platform. Lab Chip 2016, 16, 2069–2078. [Google Scholar]
- Nellaiappan, S.; Mandali, P.K.; Prabakaran, A.; Krishnan, U.M. Electrochemical Immunosensors for Quantification of Procalcitonin: Progress and Prospects. Chemosensors 2021, 9, 182. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Shamagsumova, R.; Hianik, T. Advances in Electrochemical Aptasensors Based on Carbon Nanomaterials. Chemosensors 2020, 8, 96. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, S.; Yu, J.; Wang, H.; Liu, X.; Huang, J. Simultaneous voltammetric determination of E. coli and S. typhimurium based on target recycling amplification using self-assembled hairpin probes on a gold electrode. Microchim. Acta 2017, 184, 745–752. [Google Scholar] [CrossRef]
- Redondo-Marugan, J.; Petit-Dominguez, M.; Casero, E.; Vázquez, L.; García, T.; Parra-Alfambra, A.; Lorenzo, E. Sol–gel derived gold nanoparticles biosensing platform for Escherichia coli detection. Sens. Actuators B 2013, 182, 307–314. [Google Scholar] [CrossRef]
- Liao, J.C.; Mastali, M.; Gau, V.; Suchard, M.A.; Møller, A.K.; Bruckner, D.A.; Babbitt, J.T.; Li, Y.; Gornbein, J.; Landaw, E.M.; et al. Use of electrochemical DNA biosensors for rapid molecular identification of uropathogens in clinical urine specimens. J. Clin. Microbiol. 2006, 44, 561–570. [Google Scholar] [CrossRef]
- Lu, L.; Chee, G.; Yamada, K.; Jun, S. Electrochemical impedance spectroscopic technique with a functionalized microwire sensor for rapid detection of foodbornepathogens. Biosens. Bioelectron. 2013, 42, 492–495. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Erf, G.F. Interdigitated Array Microelectrode-Based Electrochemical Impedance Immunosensor for Detection of Escherichia coli O157: H7. Anal. Chem. 2004, 76, 1107–1113. [Google Scholar] [CrossRef]
- Wang, Y.; Alocilja, E.C. Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J. Biol. Eng. 2015, 9, 16. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, S.; Yu, J.; Wang, H.; Wang, Y.; Huang, J. Label-free and highly sensitive electrochemical detection of E. coli based on rolling circle amplifications coupled peroxidase-mimicking DNAzyme amplification. Biosens. Bioelectron. 2016, 75, 315–319. [Google Scholar] [CrossRef]
- Brosel-Oliu, S.; Ferreira, R.; Uria, N.; Abramova, N.; Gargallo, R.; Muñoz-Pascual, F.-X.; Bratov, A. Novel impedimetric aptasensor for label-free detection of Escherichia coli O157:H7. Sens. Actuators B 2018, 255, 2988–2995. [Google Scholar] [CrossRef]
- Burrs, S.; Bhargava, M.; Sidhu, R.; Kiernan-Lewis, J.; Gomes, C.; Claussen, J.; McLamore, E. A paper based graphene-nanocauliflower hybrid composite for point of care biosensing. Biosens. Bioelectron. 2016, 85, 479–487. [Google Scholar] [CrossRef]
- Kaur, H.; Shorie, M.; Sharma, M.; Ganguli, A.K.; Sabherwal, P. Bridged Rebar Graphene functionalized aptasensor for pathogenic E. coli O78:K80:H11 detection. Biosens. Bioelectron. 2017, 98, 486–493. [Google Scholar] [CrossRef]
- Li, Y.; Afrasiabi, R.; Fathi, F.; Wang, N.; Xiang, C.; Love, R.; She, Z.; Kraatz, H.-B. Impedance based detection of pathogenic E. coli O157: H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens. Bioelectron. 2014, 58, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, H.; Hao, H.; Gong, Q.; Nie, K. Detection of Escherichia coli with a label-free impedimetric biosensor based on lectin functionalized mixed self-assembled monolayer. Sens. Actuators B 2016, 229, 297–304. [Google Scholar] [CrossRef]
- Zhou, Y.; Marar, A.; Kner, P.; Ramasamy, R.P. Charge-directed immobilization of bacteriophage on nanostructured electrode for wholecell electrochemical biosensors. Anal. Chem. 2017, 89, 5734–5741. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Rehman, A.; Liu, H.; Zhang, J.; Zhu, S.; Zeng, X. Glycosylation of quinone-fused polythiophene for reagentless and labelfree detection of E. coli. Anal. Chem. 2015, 87, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.X.; Wang, R.; Wang, Y.; Su, X.; Ying, Y.; Wang, J.; Li, Y. Evaluation of different micro/nanobeads used as amplifiers in QCM immunosensor for more sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2011, 29, 23–28. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Zumpano, R.; Polli, F.; D’Agostino, C.; Antiochia, R.; Favero, G.; Mazzei, F. Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools. Electrochem 2021, 2, 10–28. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Schneider, R.; Lima, J.B.S.; Mercante, L.A.; Correa, D.S. Graphene Quantum Dots-Based Nanocomposites Applied in Electrochemical Sensors: A Recent Survey. Electrochem 2021, 2, 490–519. [Google Scholar] [CrossRef]
- Maurer, J.H.; González-García, L.; Reiser, B.; Kanelidis, I.; Kraus, T. Templated self-assembly of ultrathin gold nanowires by nanoimprinting for transparent flexible electronics. Nano Lett. 2016, 16, 2921–2925. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Kumar, A.S.; Nisha, S.; Pillai, K.C. In-situ preparation of Au(111) oriented nanoparticles trapped carbon nanofiber-chitosan modified electrode for enhanced bifunctional electrocatalysis and sensing of formaldehyde and hydrogen peroxide in neutral pH solution. Electrochim. Acta 2017, 249, 227–240. [Google Scholar] [CrossRef]
- Almeida, L.A.; Rodrigues, B.V.M.; Balogh, D.T.; Sanfelice, R.C.; Mercante, L.A.; Frade-Barros, A.F.; Pavinatto, A. Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing. Electrochem 2022, 3, 239–247. [Google Scholar] [CrossRef]
- Ito, Y.; Chang, T.-F.M.; Chien, Y.-A.; Chen, C.-Y.; Chakraborty, P.; Nakamoto, T.; Sone, M. Catalytic Activity of Atomic Gold-Decorated Polyaniline Support in Glucose Oxidation. Electrochem 2020, 1, 394–399. [Google Scholar] [CrossRef]
- Hrioua, A.; Loudiki, A.; Farahi, A.; Bakasse, M.; Lahrich, S.; Saqrane, S.; El Mhammedi, M.A. Recent advances in electrochemical sensors for amoxicillin detection in biological and environmental samples. Bioelectrochemistry 2021, 137, 107687. [Google Scholar] [CrossRef]
- Aihaiti, A.; Li, Z.; Qin, Y.; Meng, F.; Li, X.; Huangfu, Z.; Chen, K.; Zhang, M. Construction of electrochemical sensors for antibiotic detection based on carbon nanocomposites. Nanomaterials 2022, 12, 2789. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Q.A.; Luo, Z.; Ali, R.; Khan, M.I.; Li, F.; Qiu, B. advances in gold nanoparticles-based colorimetric aptasensors for the detection of antibiotics: An overview of the past decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Yusof, N.A.; Hajian, R.; Abdullah, J. Construction of an electrochemical sensor based on carbon nanotubes/gold nanoparticles for trace determination of amoxicillin in bovine milk. Sensors 2016, 16, 56. [Google Scholar] [CrossRef]
- Pollap, A.; Knihnicki, P.; Kus’trowski, P.; Kozak, J.; Cezpa, M.G.; Kotarba, A.; Kochana, J. Sensitive voltammetric amoxicillin sensor based on tio2 sol modified by cmk-3-type mesoporous carbon and gold ganoparticles. Electroanalysis 2018, 30, 2386–2396. [Google Scholar] [CrossRef]
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Mutair, A.A.; Alhumaid, S.; Alawi, Z.A.; et al. The molecular characterization of virulence determinants and antibiotic resistance patterns in human bacterial uropathogens. Antibiotics 2022, 11, 516. [Google Scholar] [CrossRef]
- Gupta, K.; Bhadelia, N. Management of urinary tract infections from multidrug-resistant organisms. Infect. Dis. Clin. 2014, 28, 49–59. [Google Scholar] [CrossRef]


| S. No | Pathogen | Electrode Modification | Methodology | Technique | Ref |
|---|---|---|---|---|---|
| 1 | E. coli O157:H7 | Au/Thiol-modified methylene blue/ferrocene/DNA | DNA-based sensing | DPV | [12] |
| 2 | E. coli | Au/AuNP/3-mercaptopropyl-trimethoxysilane/DNA | DNA-based sensing | DPV | [13] |
| 3 | E. coli UTI | Au/biotin-modified capture probe/horseradish peroxidase (HRP)-conjugated anti-fluorescein antibody/RNA | DNA-based sensing | Amp i-t | [14] |
| 4 | E. coli | Gold–Tungsten/Streptavidin/Biotinylated anti-b-galactosidase | Antibody-based sensing | EIS | [15] |
| 5 | E. coli O157:H7 | Indium–Tin oxide/EDC-NHS/ Anti-E. coli | Antibody-based sensing | EIS | [16] |
| 6 | E. coli O157:H7 | SPCE/Fe2O3-Polyaniline/Antibody/AuNP/Antibody | Antibody-based sensing | DPV | [17] |
| 7 | E. coli | Au/Antibody/BSA/E. coli/Aptamer–primer probe | Aptamer-mediated sensing | DPV | [18] |
| 8 | E. coli O157:H7 | 3D-interdigitated electrode/mercaptosilane/E. coli specific 5′disulfide-modified DNA aptamer | Aptamer-mediated sensing | EIS | [19] |
| 9 | E. coli O157:H7 | Graphene-nitrocellulose/Thiolated 64-mer RNAaptamer/Antigen | Aptamer-mediated sensing | EIS | [20] |
| 10 | E. coli O78:K80:H11 | SPE/Bridged Rebar Graphene/ Anti-E. coli aptamer | Aptamer-mediated sensing | EIS | [21] |
| 11 | E. coli O157:H7 | Au/LipoicacidNHS/Ferrocene/Antimicrobial peptides | Antimicrobial peptide-based sensing | EIS | [22] |
| 12 | E. coli | Au/11-mercapto-1-undecanoic acid/ dithiothreitol/Concanavalin A | Lectin-based sensing | EIS | [23] |
| 13 | E. coli K12 | GCE/Polyethylenimine-CNT/ Bacteriophage | Bacteriophage-based sensing | EIS | [24] |
| 14 | E. coli | Au/3-((2, 5-dimethoxyphenyl)ethynyl) thiophene/α-D-Mannopyranoside, 2-[2-(2-mercaptoethoxy) ethoxy]ethyl | Carbohydrate-based sensing | EIS | [25] |
| 15 | UroPathogenic E. coli (UPEC) | GCE/CNF-CHIT/AuNWs | Antibacterial drug interaction-based sensing | DPV | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sushmitha, J.; Nellaiappan, S. Electrochemical Sensing of Amoxicillin Drug-Assisted Uropathogenic E. coli Bacteria Using Gold Nanostructures—A Preliminary Study. Electrochem 2023, 4, 291-300. https://doi.org/10.3390/electrochem4020020
Sushmitha J, Nellaiappan S. Electrochemical Sensing of Amoxicillin Drug-Assisted Uropathogenic E. coli Bacteria Using Gold Nanostructures—A Preliminary Study. Electrochem. 2023; 4(2):291-300. https://doi.org/10.3390/electrochem4020020
Chicago/Turabian StyleSushmitha, Jayaprakash, and Subramanian Nellaiappan. 2023. "Electrochemical Sensing of Amoxicillin Drug-Assisted Uropathogenic E. coli Bacteria Using Gold Nanostructures—A Preliminary Study" Electrochem 4, no. 2: 291-300. https://doi.org/10.3390/electrochem4020020
APA StyleSushmitha, J., & Nellaiappan, S. (2023). Electrochemical Sensing of Amoxicillin Drug-Assisted Uropathogenic E. coli Bacteria Using Gold Nanostructures—A Preliminary Study. Electrochem, 4(2), 291-300. https://doi.org/10.3390/electrochem4020020







