Abstract
Electrochemotherapy (ECT) has evolved significantly during the last decade, expanding treatment indications from superficial skin lesions to advanced-stage, deep-seated tumors in hard-to-reach areas. Electrodes have also shown steady technological improvement throughout the years. Besides standard and VEG (variable geometry electrode) electrodes, the introduction of laparoscopic electrodes has brought on a new era in ECT treatment, making the minimally invasive approach a reality. The exact role of ECT in the oncological dashboard is yet to be determined; however, increased tumor response, pain relief, and a low number of adverse events may yield the way for more widespread application of the technique with possible further inclusion of ECT in international oncological guidelines. The aim of this review is to give an overview on the current status of ECT in deep-seated tumor treatment and shed light on its emerging role in local anticancer therapy.
1. Introduction
Electroporation is the phenomenon that occurs when a cell is exposed to an electric field, which causes transient cell membrane permeabilization. Electrochemotherapy (ECT) combines the oncological effect of cytotoxic agents—most frequently bleomycin (BLM)—and electroporation, resulting in a successful local anticancer therapy used for a variety of malignancies with mixed histologies. Bleomycin-based ECT in particular has proven to be effective in the treatment of skin tumors, such as metastatic malignant melanoma, or basal cell, squamous cell carcinoma and Kaposi sarcoma and is used with increasing frequency for the treatment of numerous deep-seated tumors (e.g.,pancreatic carcinoma, primary and secondary liver tumors, colon tumors, breast cancer, gynecological tumors, soft tissue sarcomas, and bone metastases) as well [1,2,3,4].
Bleomycin may be administered intravenously based on body surface index for larger tumors or multiple tumors or intratumorally for smaller and solitary lesions. During ECT, the concentration of bleomycin may reach up to 10.000 folds inside the target cells, resulting in increased damage to tumorous tissue. ECT stands as an easily reproducible procedure with reduced burden to the patient [5]. Furthermore, unlike other ablative modalities such as radiofrequency ablation, major vascular structures, organs (e.g., the duodenum, bile ducts, etc.), or nerves (e.g., fascial nerves) often involved by tumors do not suffer notable heat-damage during ECT, making it an applicable choice even in cases of extensive tumor involvement.
During the last decade, ECT has been extendedly applied in the treatment of advanced-stage, deep-seated, bulky soft tissue tumors and malignancies involving intraabdominal viscera [6,7]. Standard electrodes have been successfully used for the treatment of more superficial tumors and those not exceeding 3 cm in depth. However, with the introduction of variable electrode geometry (VEG) electrodes, ECT became feasible for the treatment of larger neoplasms, even in hard-to-reach areas, applying electrodes directly (standard) or percutaneously through the skin (VEG) with CT (computed tomography) guidance [8,9].
The aim of this review was to shed light on the oncological values of bleomycin-based ECT and potentially define its place in oncological patient care.
2. Benefits of Electrochemotherapy
The direct effects of ECT on blood flow include but are not limited to the vascular lock effect, meaning that cytotoxic agents, such as BLM, after being injected intravenously or directly inside the tumor, due to consecutive vasoconstriction, become trapped inside the vessels, initiating a damaging effect in the vasculature through the disruption of blood flow [10,11]. Besides locally banishing tumor cells, a distant, abscopal effect (i.e., away from the target) has also been observed, meaning that, e.g., it has an effect not only on the primary liver tumor but also on simultaneous distant metastases during the same treatment session [12,13]. In recent years, it has been also noted that ECT in combination with other systemic therapies, such as immunotherapy, potentiates the oncological effect, resulting in improved tumor response rates [14]. Moreover, by adding gene electro-transfer to the recipe and transferring IL-12 into tumor cells, former “cold tumors” (lesions unresponsive to IT), can be transformed into “hot tumors”, now responding to immunotherapy, e.g., pembrolizumab, hence significantly improving the objective response rate (up to 41%, according to RECIST—Response Evaluation Criteria In Solid Tumors) [15].
Deep-seated tumors are frequently located in hard-to-reach areas, in the vicinity of important structures, such as great vessels (common iliac artery- retroperitoneal sarcomas) or nerves (femoral nerve retroperitoneal sarcomas and fascial nerve parotid tumors) and even the bile ducts (Klatskin tumors, located at the intersection of hepatic ducts). Since preserving such pivotal structures is essential, ablation techniques such as radio frequency ablation, microwave ablation, cryoablation, high-intensity focused ultrasound, or LASER are unavailable for treating lesions infiltrating such structures, since these modalities result in a heat sink effect. ECT on the other hand has been proven to be capable of sparing vital structures, by excluding the heat sink effect or mechanical damage, even in direct contact with vital structures and organs [16,17,18]. During a phase I/II study, Izzo et al. performed bleomycin-based ECT on 25 patients with locally advanced pancreatic cancer and found that at 1 month, 76% had a partial response (PR) and 20% had stable disease (SD), while at 6 months, 44% had PR and 12% had SD, with overall survival reaching 11.5 months [19]. This study only included inoperable cases and although in these particular cases ECT posed as a palliative modality, with improved overall survival, it confirmed the safety and feasibility of ECT, which are obvious benefits of the technique [19]. Furthermore, Djokic et al. treated 17 liver metastases with both standard and VEG electrodes and at the 1-month follow-up found complete response (CR) in 88%, with the results still valid 20.5 months after ECT [20].
The effects of ECT strongly depend on the extension of the lesions. It has been previously confirmed that small lesions (2–3 cm) have a significantly better response to ECT than larger ones (<3 cm) [21]. Djokic et al. [20] also noted that 11/17 liver metastases were indeed smaller than 3 cm, with significantly higher CR rates for the latter, than those larger than 3 cm [20]. Edhemovic et al. [22] treated 27 liver metastases with VEG electrodes and found an 86% CR rate 33 days after ECT [22]. In our own prospective study conducted on VEG ECT treatment for advanced-stage, bulky soft tissue sarcomas (STS), PR was found in 71.42%, however with a median tumor volume and diameter of 131.13 cm3 (35.6–2456.22) and 5.9 cm (3.7–22.5), respectively [1]. Hence, the results concerning STSs also confirm the above-mentioned observation, with the smaller tumors responding better to ECT.
3. ECT for Deep-Seated Tumors
The first experimental application and outcome evaluations with ECT were carried out in the early 1990s by Mir et al. [23], with the then uncertain role of bleomycin slowly clarifying and gradually coming into the limelight [23,24,25]. The first breakthrough was the realization that although having a low ability to penetrate the cell membrane, in the presence of an electric field, BLM can reach the cytosol and have a several thousandfold intracellular effect compared to chemotherapy on its own without an electric field [26]. Since the introduction of the ESOPE (European Standard Operating Procedures for Electrochemotherapy), the foundations for standardized treatment have been laid, leading to easily reproducible and interpretable results [27,28]. The evaluation of treatment is carried out according to the RECIST guidelines, mostly based on imaging results (CT—computed tomography, MRI—magnetic resonance imaging, or PET-CT—positron emission tomography–computed tomography) [29], serving as a precise follow-up tool for the treated tumors (Figure 1) [1].
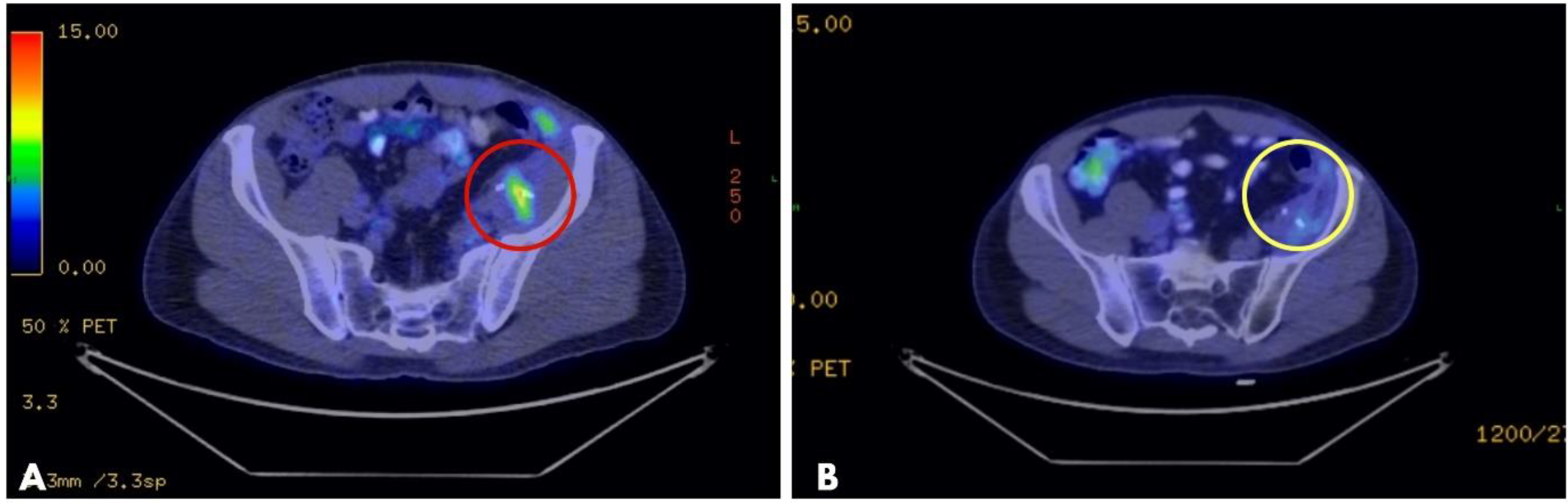
Figure 1.
PET-CT of a myofibroblastic sarcoma of the retroperitoneum: (A): high metabolic activity tumor recurrence after surgical resection (red circle); (B): partial tumor response after CT-guided VEG ECT treatment, without a metabolically active tumor (2-month follow-up). Metal clips, placed during the initial surgery with the intention to mark the tumor’s location, are visible on both images (the two white lines inside the circles), guiding the placement of long needle electrodes during ECT. PET-CT: positron emission tomography–computed tomography; VEG: variable electrode geometry; ECT: electrochemotherapy.
3.1. Previous Oncological Treatment
In the majority of cases, patients scheduled for ECT have already exhausted numerous oncological treatment options, such as surgery (often multiple), chemotherapy, radiotherapy, immunotherapy, chemoradiation, and even previous ECT treatments. In our own study conducted on VEG ECT treatment for STSs, we found that the median elapsed time from the first diagnosis to ECT was 19 (8–144) months [1], a relatively long time, somewhat pinpointing the current role of ECT among oncological regimens. According to an INSPECT study on cutaneous metastases of breast cancers treated by ECT, 87% received previous chemotherapy and 81% received previous radiotherapy [30]. Furthermore, Edhemovic et al. also note that 48.7% of patients received chemotherapy + targeted therapy for colorectal liver metastases prior to ECT [22]. According to the above data, it seems to be clear that ECT still stands as a last resort in the oncological armamentarium for deep-seated tumors.
Despite the reportedly high complete response rate (62–91%) and positive patient-reported outcomes, major guidelines such as those of the ESMO (European Society of Medical Oncology) and the NCCN (National Comprehensive Cancer Network) still do not include ECT as a potential treatment option for deep-seated tumors even in reserved cases [31].
3.2. Electrode Placement Planning
During VEG ECT of deep-seated lesions, planning of electrode placement is essential on one hand for accurate assessment and complete covering of target tissue and also to keep healthy tissue damage to a minimum. In order to successfully treat extended, bulky tumors, the diameter/volume of the tumor and the tumor depth and distance from the surface of the skin should all be accurately measured through -CT/MRI/PET-CT prior to the intervention. Pre-treatment imaging is not only important for tumor staging (defining the extent of the primary lesion and excluding/confirming lymph nodes and distant metastases) but also for follow-up and comparison of the target lesion(s). Patient follow-up is carried out according to RECIST, with the first image-based control after 2 months. The time interval between image-based treatment planning should be minimized as tumor volume can change with time, jeopardizing accurate electrode placement and tumor targeting. During our own practice, we used Pulsar software version 1.0 (IGEA, S.p.A, Carpi (MO), Italy) for electrode placement planning, supplemented by intraoperative ultrasound (US), which enables confirmation of precise tissue depth and adequate coverage of tumor volume, maintaining safety margins (10 mm). Recently, patient-specific planning for electroporation-based treatments has also shown major improvements. Three-dimensional modelling of tumors inside solid organs in relation to neighboring vessels and vital structures has made treatment outcomes and electric field distribution more predictable compared to simply uploading medical images (e.g., CT, or MRI) [32,33]. Moreover, web-based planning software has been developed, facilitating more precise treatment planning through uploading of DICOM images; hence, clinicians, otherwise laymen, regarding biomedical engineering are easily able to assess electric field calculation and visualization [34]. Standard electrodes do not require placement planning; hence, these treatments are more rapid and straightforward than VEG treatments. Placement of VEG electrodes can be indeed time-consuming; however, accurate positioning of electrodes is paramount for successful target covering. Soft tissue VEG electrodes can vary from 12 to 24 cm in length with an active tip of 3–4 mm, depending on the lesion size and depth. Electrodes have to be placed parallel with one another, with the inter-electrode distance not extending 30 mm. Hence, in some cases, VEG electrode placement can be quite difficult, resulting in an overall prolonged procedure.
Each organ and tissue requires different treatment planning, taking tissue heterogeneity (meaning that electric field distribution and effect can also differ within the same tissue) into account. Cindric et al. [14] reported on electric field distribution changes observed in liver tissue and found that major hepatic structures indeed affect EFD; hence, this phenomenon should be carefully considered in order to reach a sufficient electric threshold in the target tissue [14,35]. It is important to keep in mind that bleomycin-based ECT can be carried out in a 20–40 min time window, 8 min after the intravenous administration of bleomycin; thus, VEG electrode placement—which in the case of bulky lesions takes a substantial amount of time—should be completed before administering the cytotoxic drug. In the case of bulky tumors with large treatment areas, multiple VEG electrodes are used, which can be repositioned during the same session in order to treat the complete extent of the lesion. Both vertical and horizontal repositioning can be carried out during treatment, meaning that the complete tumor volume can be sufficiently covered (Figure 2). Considering fragile structures such as major liver vessels or the bile ducts, an important question rises as to whether the train of electric pulses and eventually ECT have any kind of damaging effect on them. Zmuc et al. [17] conducted an animal model study, which confirmed no clinically significant damage or severe inflammatory response to major liver structures after ECT [17]. In their phase II study, Edhemovic et al. [36] reported no serious adverse events during the ECT treatment of 39 patients with inoperable colorectal liver metastases of which 44% were located centrally next to liver vessels and the bile ducts [36].
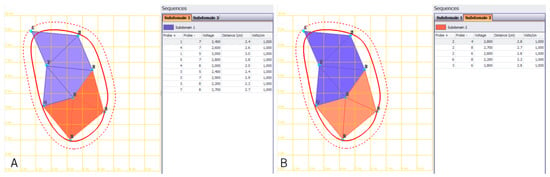
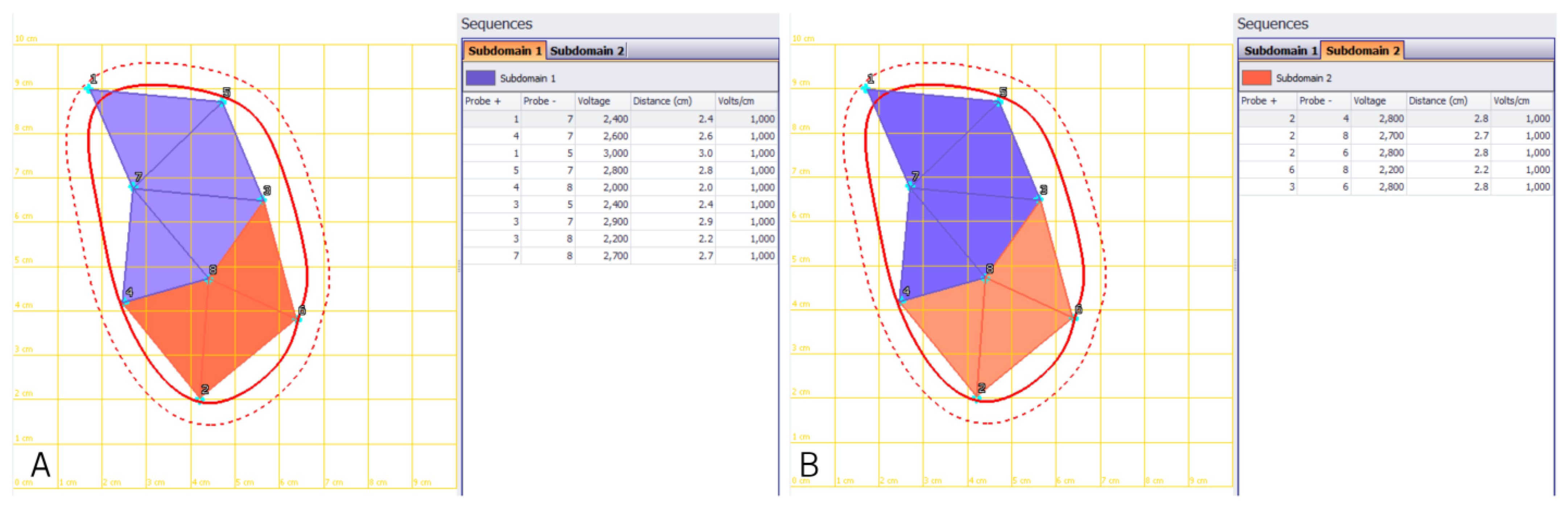
Figure 2.
Treatment planning of an extended retroperitoneal sarcoma with long needle VEG electrodes. Planning of electrode re-positioning in order to cover the complete tumor extent (red line) including safety margins (dotted line). (A): Treatment of subdomain 1—area in orange and (B): subdomain 2—area in purple. VEG: variable electrode geometry.
3.3. Electrodes
During the last decade, the improvement of electrodes has granted treatment for not only extended, bulky lesions (with VEG electrodes) but also the endoscopic and laparoscopic treatment of intraabdominal tumors (the introduction of IGEA Stinger®), further upgrading reversible electroporation and electrochemotherapy by covering each aspect of tumor access [37,38]. Standard electrodes have enabled the treatment of both superficial and deep-seated lesions with linear, hexagonal constellation and even finger electrodes for hard-to-reach areas, especially body cavities (e.g., vulva carcinomas).
The introduction of a new pulse generator called the Cliniporator Vitae (IGEA SpA, Carpi, Italy) and long needle VEG electrodes has paved the way for the treatment of malignancies in specific deep locations (e.g., lesions invading great vascular structures such as the inferior vena cava in the case of liver tumors) and prompted the introduction of percutaneous US/CT-guided techniques with less burden to the patient, further reducing in-patient stays [8].
Deep-seated malignancies often include advanced-stage, already metastatic neoplasms, previously treated with numerous different modalities. In our previous study conducted on bleomycin-based VEG ECT treatment of STSs, the average time from the initial diagnosis to ECT treatment was 19 months [1]. This time delay is mainly attributed to the fact that the use of ECT has increased during the last decade. With a wide spectrum of treatable tumors and objective response rates as high as 85% [31], the exact role of ECT is yet to be defined in the oncological ball game [8].
As regards ECT being safe, with mild side effects, and the fact that it can be used in combination with different treatment modalities (surgery, chemotherapy, radiation therapy, and immunotherapy), we believe that earlier integration into treatment strategies would be more beneficial in the management of advanced tumors than reserving it only as an “ultimum refugium” (with palliative intent). In addition, in case of recurrence or the development of novum metastases, ECT is easily repeatable without precluding additional treatment modalities on the same patient.
3.4. Adverse Events
As regards serious adverse events, they are only sporadically mentioned in the literature [1]. Common side effects include involuntary muscle contractions during the application of electric pulses. These are transient contractions, with only mild discomfort to the patient. During ECT of visceral tumors localized in the upper quadrant of the abdomen, the train of electric pulses must be synchronized to the heart’s absolute refractory period in order to avoid interference with cardiac electrical activity [39,40].
The minor side effects of deep-seated ECT are similar to the well-published side effects of ECT used on superficial tumors: skin ulceration, hyperpigmentation, maculopapular rash, skin suppuration and odor, flu-like symptoms, and headaches.
The necrosis of tumorous tissue, if near the surface of the skin, becomes visible with a firm, black, ulcerated area at the treatment site. However, in the case of deep-seated or visceral lesions treated with the percutaneous approach, skin marks can only be seen according to electrode placement (Figure 3C).
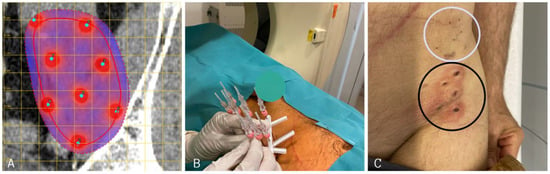
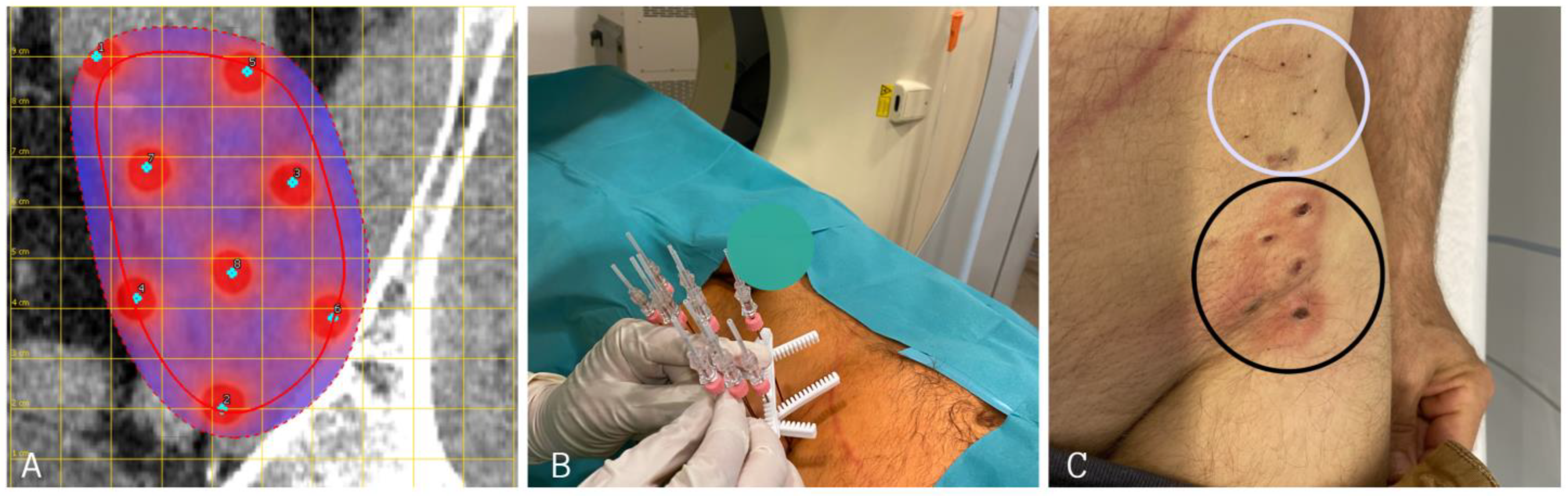
Figure 3.
(A): Planning of long needle electrode placement in the case of a left-sided retroperitoneal sarcoma near the left iliac crest. (Red line indicates tumor extent, dotted red line indicates safety margin from tumor boarder). (B): Positioning of VEG electrodes before starting ECT treatment. (C): Skin marks of long needle VEG electrodes after ECT. In order to cover the complete tumor extent, two sets of electrodes were applied (cranial set—white circle; caudal set—black circle). VEG: variable electrode geometry; ECT: electrochemotherapy.
In the case of the deep-seated treatment of liver tumors, mainly mild side effects are reported, such as post-ECT fever, transient ascites formation, or mild pain elevation [16,22,41]. After ECT treatment of patients with locally advanced pancreatic cancer, Izzo et al. found delayed gastric emptying in 30.8% and 25% and pleural effusion in 30.8% and 16.7% with standard and VEG electrodes, respectively [19]. Our own study on STSs showed 4/7 cases of local ulceration at the treatment site, all of which could be managed conservatively. However, nerve damage and transient plegia of the quadriceps femoris muscle were also observed in one case of left-sided retroperitoneal myofibroblastic sarcoma treated with CT-guided VEG ECT, which gradually improved with medication and physiotherapy [1].
An additional observation after ECT is the reduced level of pain experienced by patients. In our practice, a VAS (visual analogue scale) was used, which is a simple, bedside score, that easily indicates patient pain levels. Ranieri et al. analyzed VAS results after percutaneous VEG ECT in 20 patients with a variety of primary tumors (lung, breast, colorectal, kidney, melanoma, etc.) and found a mean pre-ECT VAS of 7.5, shifting to 3 one month after ECT [42]. Simioni et al. reported on 30 patients treated mostly for STSs and melanoma (43%) with bleomycin-based ECT, without any serious adverse events and notably improved pain levels and tumor response (CR: 63%, PR: 27%, and SD: 10%) [43]. Electrochemotherapy in the treatment of bone metastases also showed promising results, especially in terms of pain reduction after treatment [44].
4. Summary and Future Perspectives
With low toxicity and fast recovery, ECT serves as a promising local anticancer therapy that can successfully supplement other systemic treatment modalities, such as chemo- or immunotherapy. The question, however, still arises as whether ECT will be able to possess a more autonomous role in the near future and whether it will be upgraded as a first line treatment option. Although phase I/II studies have been conducted on assessing the efficacy of ECT for deep-seated tumors, a proper number of randomized studies are still needed.
Clinical application of ECT has been expanding in recent years with improved objective response rates and a low number of adverse events, making it indeed a feasible supplementary treatment option in combination with other treatment modalities. With positive results and the emerging popularity of the technique, we hope that this promising modality will no longer serve as an “ultimum refugium” treatment and can be built into oncological practice.
Author Contributions
Conceptualization: A.O. and E.K.; methodology: A.O., E.K., G.V. and M.V.; software: A.O., R.P. and A.N.; validation: E.K. and G.L.; formal analysis: J.O., G.V. and A.N.; investigation: A.O., E.K. and G.V.; resources: A.O., M.V. and G.V.; data curation: M.V. and R.P.; writing—original draft preparation: A.O. and E.K.; writing—review and editing: A.O. and E.K.; visualization: G.L. and J.O.; supervision: A.O. and E.K.; project administration: A.O. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Regional Institutional Review Board of Human Investigations at the University of Szeged. Approval number: ECT-REPRO-002. Approval date: 10 October 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors state that they have no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Institutional Review Board Statement. This change does not affect the scientific content of the article.
Abbreviations
BLM: bleomycin; CR: complete response; CT: computed tomography; ECT: electrochemotherapy; ESMO: European Society of Medical Oncology; ESOPE: European Standard Operating Procedures on Electrochemotherapy; MRI: magnetic resonance imaging; NCCN: National Comprehensive Cancer Network; PET: positron emission tomography; PR: partial response; RECIST: Response Evaluation Criteria in Solid Tumors; STS: soft tissue sarcoma; US: ultrasound; VAS: visual analogue scale; VEG: variable electrode geometry.
References
- Ottlakan, A.; Lazar, G.; Hideghety, K.; Koszo, R.L.; Deak, B.; Nagy, A.; Besenyi, Z.; Bottyan, K.; Vass, G.Z.; Olah, J.; et al. Clinical considerations of bleomycin based electrochemotherapy with variable electrode geometry electrodes for inoperable, deep-seated soft tissue sarcomas. Bioelectrochemistry 2022, 148, 108220. [Google Scholar] [CrossRef]
- Mali, B.; Jarm, T.; Snoj, M.; Sersa, G.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2013, 39, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Edhemovic, I.; Soden, D.; Perrone, A.M.; Scarpa, M.; Campanacci, L.; Cemazar, M.; Valpione, S.; Miklavčič, D.; Mocellin, S.; et al. Electrochemotherapy-Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur. J. Surg. Oncol. 2019, 45, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, E.; Curatolo, P.; Iacovino, C.; Careri, R.; Calvieri, S.; Giustini, S. Electrochemotherapy in Gorlin-Goltz syndrome. Ital. J. Dermatol. Venereol. 2021, 156 (Suppl. 1), 95–97. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; D’Alessio, V.; Simonetti, I.; Grassi, F.; Silvestro, L.; Palaia, R.; Belli, A.; Patrone, R.; Piccirillo, M.; et al. Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review. Diagnostics 2023, 13, 209. [Google Scholar] [CrossRef]
- Spiliotis, A.E.; Holländer, S.; Rudzitis-Auth, J.; Wagenpfeil, G.; Eisele, R.; Nika, S.; Mallis Kyriakides, O.; Laschke, M.W.; Menger, M.D.; Glanemann, M.; et al. Evaluation of Electrochemotherapy with Bleomycin in the Treatment of Colorectal Hepatic Metastases in a Rat Model. Cancers 2023, 15, 1598. [Google Scholar] [CrossRef] [PubMed]
- Groselj, A.; Kos, B.; Cemazar, M.; Urbancic, J.; Kragelj, G.; Bosnjak, M.; Veberic, B.; Strojan, P.; Miklavcic, D.; Sersa, G. Coupling treatment planning with navigation system: A new technological approach in treatment of head and neck tumors by electrochemotherapy. Biomed. Eng. Online 2015, 14 (Suppl. 3), S2. [Google Scholar] [CrossRef]
- Fuhrmann, I.; Probst, U.; Wiggermann, P.; Beyer, L. Navigation Systems for Treatment Planning and Execution of Percutaneous Irreversible Electroporation. Technol. Cancer Res. Treat. 2018, 17, 1533033818791792. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Jarm, T.; Kotnik, T.; Coer, A.; Podkrajsek, M.; Sentjurc, M.; Miklavcic, D.; Kadivec, M.; Kranjc, S.; Secerov, A.; et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br. J. Cancer 2008, 98, 388–398. [Google Scholar] [CrossRef]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of bleeding metastases. Expert Rev. Anticancer Ther. 2010, 10, 729–746. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Justesen, T.F.; Orhan, A.; Raskov, H.; Nolsoe, C.; Gögenur, I. Electroporation and Immunotherapy-Unleashing the Abscopal Effect. Cancers 2022, 14, 2876. [Google Scholar] [CrossRef]
- Djokic, M.; Dezman, R.; Cemazar, M.; Stabuc, M.; Petric, M.; Smid, L.M.; Jansa, R.; Plesnik, B.; Bosnjak, M.; Tratar, U.L.; et al. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: Technological advancement. Radiol. Oncol. 2020, 54, 347–352. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Z.; Hu, J.J.; Liu, C. Advances of Electroporation-Related Therapies and the Synergy with Immunotherapy in Cancer Treatment. Vaccines 2022, 10, 1942. [Google Scholar] [CrossRef] [PubMed]
- Spallek, H.; Bischoff, P.; Zhou, W.; de Terlizzi, F.; Jakob, F.; Kovàcs, A. Percutaneous electrochemotherapy in primary and secondary liver malignancies-local tumor control and impact on overall survival. Radiol. Oncol. 2022, 56, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zmuc, J.; Gasljevic, G.; Sersa, G.; Edhemovic, I.; Boc, N.; Seliskar, A.; Plavec, T.; Brloznik, M.; Milevoj, N.; Brecelj, E.; et al. Large Liver Blood Vessels and Bile Ducts Are Not Damaged by Electrochemotherapy with Bleomycin in Pigs. Sci. Rep. 2019, 9, 3649. [Google Scholar] [CrossRef] [PubMed]
- Kos, B.; Voigt, P.; Miklavcic, D.; Moche, M. Careful treatment planning enables safe ablation of liver tumors adjacent to major blood vessels by percutaneous irreversible electroporation (IRE). Radiol. Oncol. 2015, 49, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Tafuto, S.; et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021, 10, 1305. [Google Scholar] [CrossRef]
- Djokic, M.; Cemazar, M.; Popovic, P.; Kos, B.; Dezman, R.; Bosnjak, M.; Zakelj, M.N.; Miklavcic, D.; Potrc, S.; Stabuc, B.; et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur. J. Surg. Oncol. 2018, 44, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Groselj, A.; Campana, L.G.; Kunte, C.; Schepler, H.; Gehl, J.; Muir, T.; Clover, J.A.P.; Quaglino, P.; Kis, E.; et al. Electrochemotherapy for the treatment of cutaneous squamous cell carcinoma: The INSPECT experience (2008-2020). Front Oncol. 2022, 12, 951662. [Google Scholar] [CrossRef] [PubMed]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Marolt Music, M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Grcar Kuzmanov, B.; et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef]
- Mir, L.M.; Glass, L.F.; Sersa, G.; Teissié, J.; Domenge, C.; Miklavcic, D.; Jaroszeski, M.J.; Orlowski, S.; Reintgen, D.S.; Rudolf, Z.; et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br. J. Cancer 1998, 77, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Belehradek Paoletti, C., Jr. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer 1991, 27, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, Z.; Stabuc, B.; Cemazar, M.; Miklavcic, D.; Vodovnik, L.; Sersa, G. Electrochemotherapy with bleomycin. The first clinical experience in malignant melanoma patients. Radiol. Oncol. 1995, 29, 229–235. [Google Scholar]
- Orlowski, S.; Belehradek JJr Paoletti, C.; Mir, L.M. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. EJC Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Matthiessen, L.W.; Keshtgar, M.; Curatolo, P.; Kunte, C.; Grischke, E.M.; Odili, J.; Muir, T.; Mowatt, D.; Clover, J.P.; Liew, S.H.; et al. Electrochemotherapy for Breast Cancer-Results From the INSPECT Database. Clin. Breast Cancer 2018, 18, e909–e917. [Google Scholar] [CrossRef]
- Clover, A.J.P.; de Terlizzi, F.; Bertino, G.; Curatolo, P.; Odili, J.; Campana, L.G.; Kunte, C.; Muir, T.; Brizio, M.; Sersa, G.; et al. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur. J. Cancer 2020, 138, 30–40. [Google Scholar] [CrossRef]
- Perera-Bel, E.; Aycock, K.N.; Salameh, Z.S.; Gomez-Barea, M.; Davalos, R.V.; Ivorra, A.; Ballester, M.A.G. PIRET-A Platform for Treatment Planning in Electroporation-Based Therapies. IEEE Trans. Biomed. Eng. 2022, 1–9, Epub ahead of print. [Google Scholar] [CrossRef]
- Zupanic, A.; Kos, B.; Miklavcic, D. Treatment planning of electroporation-based medical interventions: Electrochemotherapy, gene electrotransfer and irreversible electroporation. Phys. Med. Biol. 2012, 57, 5425–5440. [Google Scholar] [CrossRef] [PubMed]
- Marčan, M.; Pavliha, D.; Kos, B.; Forjanič, T.; Miklavčič, D. Web-based tool for visualization of electric field distribution in deep-seated body structures and planning of electroporation-based treatments. Biomed. Eng. Online 2015, 14 (Suppl. 3), S4. [Google Scholar] [CrossRef] [PubMed]
- Marčan, M.; Kos, B.; Miklavčič, D. Effect of blood vessel segmentation on the outcome of electroporation-based treatments of liver tumors. PLoS ONE 2015, 10, e0125591. [Google Scholar] [CrossRef]
- Edhemovic, I.; Brecelj, E.; Cemazar, M.; Boc, N.; Trotovsek, B.; Djokic, M.; Dezman, R.; Ivanecz, A.; Potrc, S.; Bosnjak, M.; et al. Intraoperative electrochemotherapy of colorectal liver metastases: A prospective phase II study. Eur. J. Surg. Oncol. 2020, 46, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Trotovsek, B.; Hadzialjevic, B.; Cemazar, M.; Sersa, G.; Djokic, M. Laparoscopic electrochemotherapy for the treatment of hepatocellular carcinoma: Technological advancement. Front. Oncol. 2022, 12, 996269. [Google Scholar] [CrossRef]
- Schipilliti, F.M.; Onorato, M.; Arrivi, G.; Panebianco, M.; Lerinò, D.; Milano, A.; Roberto, M.; Capalbo, C.; Mazzuca, F. Electrochemotherapy for solid tumors: Literature review and presentation of a novel endoscopic approach. Radiol. Oncol. 2022, 56, 285–291. [Google Scholar] [CrossRef]
- Mali, B.; Gorjup, V.; Edhemovic, I.; Brecelj, E.; Cemazar, M.; Sersa, G.; Strazisar, B.; Miklavcic, D.; Jarm, T. Electrochemotherapy of colorectal liver metastases-an observational study of its effects on the electrocardiogram. Biomed. Eng. Online 2015, 14 (Suppl. 3), S5. [Google Scholar] [CrossRef]
- Deodhar, A.; Dickfeld, T.; Single, G.W.; Hamilton, W.C., Jr.; Thornton, R.H.; Sofocleous, C.T.; Maybody, M.; Gónen, M.; Rubinsky, B.; Solomon, S.B. Irreversible electroporation near the heart: Ventricular arrhythmias can be prevented with ECG synchronization. AJR Am. J. Roentgenol. 2011, 196, W330–W335. [Google Scholar] [CrossRef]
- Djokic, M.; Cemazar, M.; Bosnjak, M.; Dezman, R.; Badovinac, D.; Miklavcic, D.; Kos, B.; Stabuc, M.; Stabuc, B.; Jansa, R.; et al. A Prospective Phase II Study Evaluating Intraoperative Electrochemotherapy of Hepatocellular Carcinoma. Cancers 2020, 12, 3778. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Laface, C.; Fazio, V.; De Ceglia, D.; Macina, F.; Gisone, V.; Porcelli, M.; Vinciarelli, G.; Carella, C.; Molinari, P.; et al. Local treatment with deep percutaneous electrochemotherapy of different tumor lesions: Pain relief and objective response results from an observational study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7764–7775. [Google Scholar] [CrossRef] [PubMed]
- Simioni, A.; Valpione, S.; Granziera, E.; Rossi, C.R.; Cavallin, F.; Spina, R.; Sieni, E.; Aliberti, C.; Stramare, R.; Campana, L.G. Ablation of soft tissue tumours by long needle variable electrode-geometry electrochemotherapy: Final report from a single-arm, single-centre phase-2 study. Sci. Rep. 2020, 10, 2291. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, L.; Bianchi, G.; Cevolani, L.; Errani, C.; Ciani, G.; Facchini, G.; Spinnato, P.; Tognù, A.; Massari, L.; Cornelis, F.H.; et al. Operating procedures for electrochemotherapy in bone metastases: Results from a multicenter prospective study on 102 patients. Eur. J. Surg. Oncol. 2021, 47, 2609–2617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).