Abstract
In situ corrosion inhibition in acid cleaning processes by using green inhibitors is at the forefront of corrosion chemistry. Plant extracts, especially alkaloids, are known to be good corrosion inhibitors against mild steel corrosion. In this research, alkaloids extracted from Acacia catechu have been used as green corrosion inhibitors for mild steel corrosion in a 1 M H2SO4 solution. Qualitative chemical tests and FTIR measurements have been performed to confirm the alkaloids in the extract. The inhibition efficiency of the extract has been studied by using weight-loss and potentiodynamic polarization methods. A weight-loss measurement has been adopted for the study of inhibitor’s concentration effect, with a variation employed to measure the inhibition efficiency for time and temperature. The weight-loss measurement revealed a maximum efficiency of 93.96% after 3 h at 28 °C for a 1000 ppm alkaloid solution. The 1000 ppm inhibitor is effective up to a temperature of 48 °C, with 84.39% efficiency. The electrochemical measurement results revealed that the alkaloids act as a mixed type of inhibitor. Inhibition efficiencies of 98.91% and 98.54% in the 1000 ppm inhibitor concentration solution for the as-immersed and immersed conditions, respectively, have been achieved. The adsorption isotherm has indicated the physical adsorption of alkaloids. Further, the spontaneous and endothermic adsorption processes have been indicated by the thermodynamic parameters. The results show that alkaloids extracted from the bark of Acacia catechu can be a promising green inhibitors for mild steel corrosion.
1. Introduction
The study of mild steel corrosion is important both theoretically and practically. Mild steel (MS) is a well-known ferrous alloy which is widely used as a construction material and a heat-exchange material by the defense industry and others owing to its great mechanical qualities and low purchase cost [1]. Corrosion occurs in mild steel, which is a distressing natural occurrence whose avoidance is extremely difficult and requires a significant financial expense to do so.
To reduce corrosion rates, methods such as surface modification, coatings, galvanization, passivation, use of inhibitors, etc. are at the forefront of current research [2]. These methods have their own inherent advantages and limitations. The selection and use of these methods depend on the nature of the aggressive environment.
Mineral acids are heavily used in processes such as pickling, cleaning, descaling, etc. in reaction tanks, vessels, and pipelines [3]. The use of acids removes the surface corrosion on the metal’s surface, and the residual acids may confer the corrosion [4,5].
Therefore, in recent years, attention has been focused on natural products such as greenery and eco-friendly inhibitors [5,6]. Natural products with high molecular weights and bulky hetero functional moieties such as alkaloids, flavonoids, amino acids, glucose, saponins, tannins, etc. are being used in corrosion inhibition processes [7,8]. Green corrosion inhibitors can either be scavengers or interface inhibitors. Scavengers lower the medium corrosivity by scavenging aggressive chemicals, whereas interface inhibitors prevent corrosion at the metal–environment contact by forming a coating. The availability of organic compounds with N, O, P, and S atoms that have a shielding effect and corrosion-inhibiting potential for material attack have been connected to the corrosion inhibition efficacy of organic green corrosion inhibitors (OGCIs) [9]. Their increasing order of corrosion inhibition efficiency has been stated to be oxygen < nitrogen < sulfur < phosphorus [10]. OGCIs exhibit their inhibiting action via physisorption or chemisorption onto the metal–solution interface by removing molecules of water on the surface for compact barrier film formations [7]. The occurrence of a coordinate covalent bond by the interaction between a lone pair and the π-electrons available in the molecules of OGCIs with the vacant metal d-orbitals has also been experienced [11]. Nevertheless, compound adsorption on a metal’s surface is enhanced by the p-d bond formation as a result of p-electron overlap to the 3d vacant orbital of the Fe atom [12] due to the availability of N, O, and S atoms and their organic structure double bonds [13]. The focus on natural products as inhibitors is due to their biodegradable, non-toxic, inexpensive, readily available, renewable, and eco-friendly properties [14]. Many varieties of plants have been reported to behave as effective green corrosion inhibitors in acidic environments. Examples of them are listed in Table 1.

Table 1.
Table showing a list of plants and the corrosion inhibition efficiency of their extracts.
Many inhibitors have been reported to possess good inhibition efficiency only below 45 °C. Nonetheless, plant abundance, extracts with higher inhibition efficiencies, and good inhibitions at higher temperatures remain to be considered. In the literature, the crude extract of Acacia catechu as a green inhibitor has been reported; however, the extraction of alkaloids from this plant and their use as corrosion inhibitors has not been performed. Therefore, in this study, the extraction of alkaloids from Acacia catechu bark and their inhibition efficacies has been carried out.
Acacia catechu, commonly known as the Cutch tree, or Khair in Nepali, is a 9–12 m tall tree. This tree is deciduous and has short-snared spines. It is a useful multi-purpose tree for producing fuel, wood, small timber, and fodder which are very popular among the local people in the Tarai region. Acacia catechu is commonly used in Ayurveda to cure a variety of ailments with its heartwood extract, whereas its bark is waste biomass [20]. Catechin, epicatechin, gallate, protocatechuic acid, tannins, and the alkaloids quercetin and kaempferol are some of the most important phyto-constituents found in this plant. Afzelechin gum and Porifera sterol glucosides have also been detected in small amounts [21]. The prime focus of this research is to analyze the efficiency of using alkaloids from its bark extract as green inhibitors on the surface of mild steel.
2. Materials and Methods
2.1. Chemicals and Instruments
Methanol (Thermo Fischer Scientific India Pvt., Ltd., molecular weight: 32.04, specific gravity: 0.792, and percentage purity: 99%), hexane (Thermo Fischer Scientific India Pvt. Ltd., percentage purity: 98.88% and specific gravity: 0.667), and sulphuric acid (specific gravity: 1.835 and 97% purity) were used as solvents. Deionized water (conductivity of 15 micro siemens/cm), ferric chloride, concentrated hydrochloric acid, Dragendorff’s reagent, concentrated nitric acid, Fehling solution, sodium hydroxide (Merc Life science, molecular weight.: 40, purity: 97%), and ethanol and silicon carbide sheets (100, 200, 400, 600, 800, 1000, and 1200 grit) were used. The chemicals were used as received, without further purification.
The instruments used consisted of: a grinder (AMEET, Delhi, India), a weighing balance (Phoenix, PH2204C, Sharjah, UAE), a funnel, beakers and a conical flask, a double-beam UV-Vis spectrophotometer (Labtronics, LT-2802, Haryana, India), an FTIR (PerkinElmer Spectrum IR; Version 10.6.2, Waltham, MA, USA), a rotatory evaporator (IKA RV 10, Karnataka India), a desiccator, a digital Vernier caliper, an Hokuto Denko potentiostat (HA151, Tokyo, Japan), and an optical microscope (Model: RXLT-4, Radical Scientific, Haryana, India).
2.2. Collection of Plants and Extraction of Alkaloids
Acacia catechu bark was collected in the month of July from the Letang (latitude: 26.75, longitude: 87.48, and altitude 850 m), Morang district of Nepal. The collected sample was shade-dried, and the dry bark was ground into fine powder. A total of 100 g of powdered sample was soaked in hexane for 24 h to remove unsaturated organic compounds and fats and then filtered. The residue was placed into a glass jar and approximately 500 mL of methanol was poured into it. The sample was kept for approximately 5 days to properly extract the phytochemicals, and then it was filtered until the filtrate was colorless. The filtrate was put into a large beaker, acidified with 5% tartaric acid, and filtered. Ammonia was added to deprotonate 1°, 2°, and 3° alkaloids and the pH was maintained at > 10. Alkaloids were extracted from this alkaline solution, employing chloroform. The alkaloids-containing chloroform layer was recovered in a beaker. It was first concentrated using a rotatory evaporator, and then it was dried in a water bath to obtain a solid residue that contained the Acacia catechu alkaloid. The dry alkaloid was kept in a moisture-free desiccator in the vessel.
2.3. Preparation of Mild Steel Sample
Mild steel coupons of the composition 0.15–0.30% C, 0.4% Si, 0.7–0.9% Mn, 0.04% S, and 0.04% P, with the remainder being Fe, with the dimensions 4 cm × 4 cm × 0.15 cm were obtained from Teku, Kathmandu. Each coupon was polished using a variety of silicon carbide sheets (# 100–1200 grits) and rinsed with hexane. Prior to each weight-loss and electrochemical measurement, the coupons were sonicated in ethanol for 30 min and then dried.
2.4. Preparation of Corrosive and Inhibitor Media
A corrosive environment was prepared by mixing 110.5 mL of concentrated sulphuric acid (specific gravity of 1.835 and 97% purity) with distilled water in a 2000 mL volumetric flask. Then, the solution was diluted by adding distilled water up to the mark. This solution was utilized as a corrosive medium for assessing the weight-loss and polarization measurements.
A total of 1 g of alkaloid was dissolved in 100 mL of the 1 M H2SO4 to make the inhibitor stock solution. The undissolved component of the mixture was filtered off, and the filtrate was transferred to a 1000 mL volumetric flask, where the 1 M H2SO4 was added up to the mark. As a consequence, a stock solution of 1000 ppm was formed. By serial dilution, further inhibitor solutions with concentrations of 200, 400, 600, and 800 ppm were prepared.
2.5. Tests for the Alkaloids
The extracted alkaloids were confirmed by chemical tests and a spectroscopic test. In the chemical method, Mayer’s, Dragendroff’s, and Wagner’s test methods were applied. The spectrophotometric characterization of the alkaloids was carried out using a Labtronics LT-2802 double-beam ultraviolet-visible (UV-Vis) spectrometer at the Department of Chemistry, Amrit Campus, Kathmandu, Nepal. The FTIR spectra of the alkaloid sample and the alkaloids adsorbed on the steel coupons were acquired at the Amrit Campus in Kathmandu using an FTIR spectrometer (PerkinElmer Spectrum IR; Version 10.6.2).
2.6. Weight-Loss Measurement
The impact of the immersion duration, the effect of the inhibitor concentration, and the influence of temperature on the corrosive media were investigated using the weight-loss measurement. The dimensions of the coupons were measured with a digital Vernier caliper and weighed with an electronic balance before each effect was studied. The weights of the coupons were recorded before they were dipped in 100 mL of caustic medium and inhibitor solutions of various concentrations. The coupons were removed from the solutions after 30 min, rinsed with distilled water, and dried. The weight of the dry coupons was measured again to determine the loss in weight. The corrosion rate was calculated using the difference in weight of the coupon before and after immersion. A similar procedure was carried out for a different immersion interval (1, 3, 6, and 24 h) in acid and with inhibitor solutions of various concentrations (200, 400, 600, 800, and 1000 ppm). The coupons were submerged in acid and in 200, 600, and 1000 ppm inhibitor solutions individually for 1 h at various temperatures (28, 38, 48, and 58 °C) to evaluate the temperature impact.
The corrosion inhibitor efficiency (IE percent) and surface coverage (θ) were computed from the weight-loss data using the following equations [18,22]:
where ΔWa and ΔWp represent the coupon’s weight-loss in the absence and presence of an inhibitor, respectively.
2.7. Electrochemical Measurements
The electrochemical measurements were carried out using an Hokuto Denko potentiostat (HA-151) at the APY Laboratory, Central Department of Chemistry, Tribhuvan University, Nepal. The measurements were completed with a three-electrode cell setup. The mild steel coupon was used as a working electrode, a graphite electrode was employed as a counter electrode, and a saturated calomel electrode (SCE) was used as a reference electrode in the three-electrode system. The open-circuit potential (OCP) was crucial for understanding how the inhibitor solution inhibited the cell. To achieve a constant OCP, each experiment was performed for 30 min before polarization. The sample was then polarized cathodically and anodically in the potential range of −0.8 to −0.2 (V) with a scan rate of 1 mV/s. The polarization measurements were performed on the steel samples immersed in various inhibitor doses, as well as on those immersed in acid solutions for under 3 h. The Tafel slope, corrosion potential, and corrosion current were computed using the polarization curves. Using the formula in Equation (3), the corrosion inhibition efficiency was computed [19].
where icorr and are the corrosion current densities in the absence and presence of an inhibitor, respectively.
2.8. Surface Morphological Study
A surface morphological study was used to take images of the steel coupons in the microscopic range. At the Central Department of Chemistry, Tribhuvan University, Nepal, using the different magnification scales of an optical microscope, images were taken for the steel coupons that were immersed for 1 h in acid and 1000 ppm inhibitor solution.
3. Results and Discussion
3.1. Characterization of the Alkaloids
A qualitative chemical test for the confirmation of the alkaloids extracted from Acacia catechu was performed. The test results of the alkaloids in different reagents are tabulated in Table 2.

Table 2.
Chemical tests of the Acacia catechu alkaloids.
The alkaloids were further characterized using the spectroscopic measurement method. It is used to determine whether an organic compound is unsaturated or contains a lone pair of electrons. In the spectra shown in Figure 1, the broad peak from 305 nm to 348 nm indicates the presence of lone pair of electrons and the unsaturation in the compound. The sharp peak is due to the n-π* transition of the alkaloid [23,24].
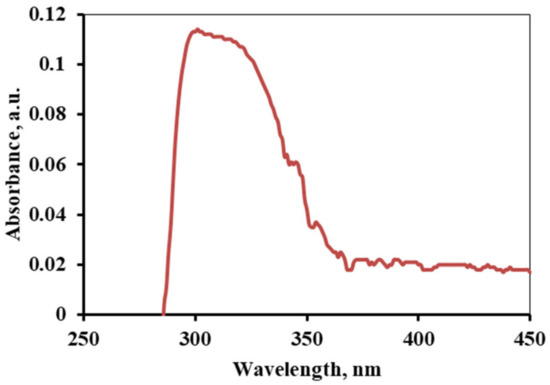
Figure 1.
UV-Vis spectrum of an alkaloid of Acacia catechu.
The FTIR spectrum reveals the chemical structure and confirms the presence of organic molecules, as well as various functional groups, in the sample. The stretching frequency in the FTIR spectrum can be used to identify the carbon–heteroatom bond.
The FTIR spectra of the alkaloids with typical functional groups are shown in Figure 2. The O-H stretching of alcohol, phenol, and carbohydrate and the N-H stretching of amine are responsible for the 3360 cm−1 to 3209 cm−1 bandwidth. A band at 2912 cm−1 depicts alkane C-H stretching, whereas a band at 1650 cm−1 symbolizes C=C stretching and C=N stretching, as well as imine or oxime, amide, or δ-lactum C=O stretching and amine N-H bending [20,24]. Aromatic C=C bending and the N-H bending of amine are denoted by a sharp band at 1570 cm−1. Similarly, the absorption band at 1435 cm−1 is due to carboxylic acid O-H bending and the strong peak at 1384 cm−1 is owed to alcohol, phenol and gem dimethyl, or aldehyde C-H bending. A band at 1284 cm−1 is attributed to aromatic amine C-N stretching, which is corroborated by a strong peak at 1037 cm−1. The absorption band at 1200 cm−1 is caused by the C-O stretching of aromatic ether and the 3° alcohol, ester and C-N stretching of amine, whereas the band at 1103 cm−1 is caused by the C-O stretching of 2° alcohols, ether, and the C-N stretching of amine. Because of the alkene’s C=C bending, there is another band at 987 cm−1 [18,19,23,25].

Figure 2.
FT-IR spectrum of a pure alkaloid and the alkaloids adsorbed on the MS surface.
3.2. Surface Morphological Evidence
The images of fresh MS, MS dipped in acid, and 1000 ppm inhibitor solutions were separately captured at a 10 μm scale using an optical microscope, and they are shown in Figure 3a–c. The images were captured by a high-definition optical microscope (Model: RXLT-4, Radical Scientific, India). The surface morphologies of each coupon, both undipped and dipped in acid and inhibitor solutions, were studied. As seen in the images, the undipped coupon is very smooth and clean, which, after dipping in an acid-only solution for 1 h, led to the formation of corrosion products that carbon leaves behind. However, for a similar immersion time in an inhibitor solution, a negligible amount of (i.e., almost none) corrosion products are observed. The production of corrosion products (pits and rusts) was detected in the MS surface on coupons dipped in acid, as shown in Figure 3b, but no corrosion products were visible in polished (undipped) MS coupons (Figure 3a). The large pits and areas of rust were seen due to the reaction between the acid molecules and the MS surface in the acid-dipped coupons, but in the case of coupons dipped in 1000 ppm inhibitor solution, there were generally small pits and little rust (Figure 3c). This means the inhibitor molecules blocked a large number of acid molecules from reacting with the MS surface [20]. This resulted in smooth surfaces, with remarkable patches on the MS surfaces. These very interesting greenish-brown patches over the MS surfaces of the coupons dipped in the 1000 ppm inhibitor solution (shown in Figure 3c) are due to the adsorption of the inhibitor molecules on the MS surfaces [18,19].

Figure 3.
Optical microscopic images of (a) polished MS, (b) MS immersed in acid, and (c) MS dipped in 1000 ppm inhibitor.
3.3. Weight-Loss Measurements
3.3.1. Effect of Immersion Time
Figure 4 shows the results of the weight-loss measurements for MS immersed in the 1 M H2SO4, both with and without an inhibitor. The findings show that the inclusion of an alkaloid inhibitor reduced the metal’s weight-loss in the acidic inhibitor medium as compared to that in the 1 M H2SO4 solution. The experiments were carried out using the 1 M H2SO4 in the presence and absence of an inhibitor, for different periods of time (namely, 0.5, 1, 3, 6, and 24 h) at 18 °C.

Figure 4.
Variation of weight-loss with immersion time for the corrosion of mild steel in a solution of 1 M H2SO4 and in solutions of different inhibitor concentrations at room temperature.
The experiments demonstrate that when the duration of the MS immersion is increased, the weight-loss of the metal is increased in both cases, but the ratio of the weight-loss was found to be less in the experiments using an inhibitor as compared to those using the 1 M H2SO4. As an example, the weight-loss of MS immersed in an acid-only solution for 24 h was 0.0272 g/cm2, whereas for MS immersed in a 1000 ppm inhibitor solution for the same time, the weight-loss was 0.0039 g/cm2. Here, the weight-loss related to the inhibitor medium was decreased by 0.0233 units due to presence of the inhibitor molecules. The surface adsorption of the inhibitors onto the MS surface could lead to this phenomenon. This adsorptive layer served as an acid barrier and decreased the extent of corrosion.
As compared to that of the inhibitor solutions, the weight-loss of MS in the acidic medium was quite substantial. Although there was weight-loss in the presence of the inhibitor solution, it was much lower than that in the absence of the inhibitor solution. This reduction in weight-loss of the immersed MS coupon is related to the inhibitor inhibition. The weight-loss rises in all cases as the immersion duration increases, but the associated inhibition efficiency improves, demonstrating the inhibitor molecules’ inhibitory actions.
The inhibition efficiency of the inhibitor molecules of different concentrations in the 1 M H2SO4 for the MS samples is shown in Figure 5. The inhibition efficiency increased with up to 3 h immersion time, and decreased with up to 6 h immersion time, and remained constant with up to 24 h. The inhibition efficiency of the 1000 ppm inhibitor solution was found to be the highest. The maximum inhibition efficiency was found to be 93.96% of 1000 ppm solution at a 3 h immersion time and 85.40% even for a 24 h immersion time, and similar trends were observed for all the concentrations of the inhibitors. The general hypothesis is that after formation of a protective layer of the inhibitor on the MS surface, the inhibition efficiency remains constant, even for long immersion times; however, the findings are do not accord with this. The orientation of the molecules on an adsorbed layer may lead to the formation of vacant sites that could allow the acid molecule to pass and interact with the MS.
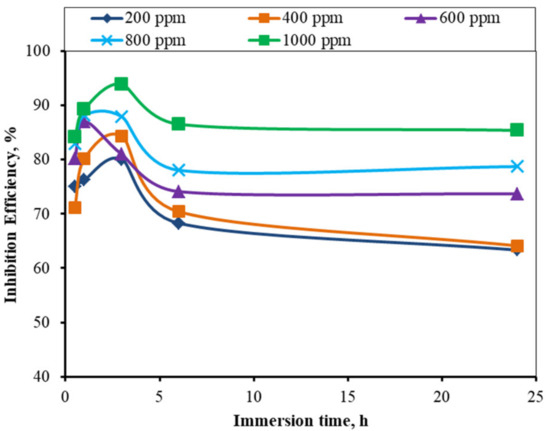
Figure 5.
Variations in the inhibition efficiency of the different inhibitor solutions in the 1 M H2SO4 solution versus time for the corrosion of mild steel (for various times).
3.3.2. Effect of Inhibitor Concentration
The weight-loss of the MS coupons is influenced by the nature of the MS surface, temperature, and solution concentration change. The MSs had significant weight-loss in the absence of an inhibitor. The loss in weight of MS was progressively diminished as the concentration of inhibitor increased, which may be due to the adsorption of the inhibitor molecules on the metal’s surface. After 24 h, the weight-loss was more significant than that after 1, 3, and 6 h, and this could be due to the desorption of the inhibitor molecule at longer time intervals [25]. The inhibition efficiency of the 200 ppm inhibitor in the 24 h immersed condition was small (63.34%), and it was higher for a lower immersion time (80.08%). However, it is lower than other concentrations of the inhibitor solution. The inhibition efficiency of the 1 h immersed sample was found to gradually increase up to a 600 ppm concentration, which remained constant for higher concentrations. For the 24 h immersed sample, the inhibition efficiency was nearly constant for both the 200 and 400 ppm solutions, and it increased drastically from the 600 ppm concentration, similar to the trend for the 3 and 6 h immersed samples (Figure 6). These peculiar findings reveal that lower concentrations of the inhibitor could be insufficient for prolonged immersion.
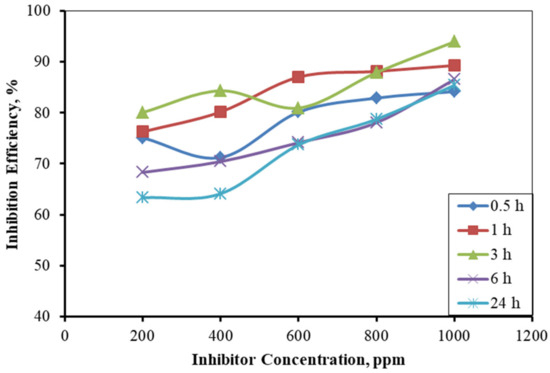
Figure 6.
Inhibition efficiencies at different concentrations of the inhibitor on mild steel in the 1 M H2SO4 solution for various times.
3.3.3. Effect of Working Temperature
The influence of temperature on the inhibition process was investigated using three different doses of inhibitor solution (200, 600, and 1000 ppm). The weight-loss in these three distinct inhibitor solutions at different temperatures was calculated using the acid solution as a reference. The MS coupons were submerged in these solutions for one hour at various temperatures (28, 38, 48, and 58 °C) for this measurement.
The MS weight-loss was greatest in the acid solution and lowest in the 1000 ppm inhibitor solution. The weight-loss of the MS immersed in acid linearly increased as the temperature increased, and the weight-loss of the MS immersed in the 200 ppm inhibitor solution followed nearly the same trend. However, the weight-loss of MS immersed in the 600 and 1000 ppm inhibitor solutions was controlled up to 48 °C. The weight-loss of the MS increased beyond this temperature.
As shown in Figure 7, the loss of weight was usually small at low temperatures and increased with increasing temperatures, which may be due to the fact that the inhibitor molecule becomes unstable at high temperatures, resulting in decreases in its effectiveness. The increase in temperature leads to an increase in the dynamic energy of the inhibitor molecules. This raises the rate of their collision with each other. This, in turn, impedes and slows the formation of the protective film of inhibitor on the metal’s surface. Thus, increases in temperature affect the strength of the adsorption molecules on the metal’s surface [26].
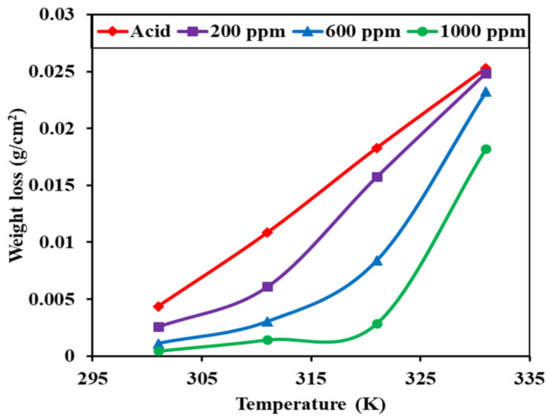
Figure 7.
Variations in the weight-loss of mild steel coupons in the 1 M H2SO4 solution and the 200, 600, and 1000 ppm inhibitor solutions at different temperatures.
Figure 8 depicts the temperature-dependent fluctuations in inhibitory efficiency. The figure clearly shows that the 200 ppm inhibitor solution is almost ineffective at all temperatures. The inhibitory efficiency of the 200 ppm inhibitor solution is essentially minimal at all temperatures, with the additional intriguing feature that there is no effect of temperature up to 48 °C for the 1000 ppm inhibitor solution. Up to 38 °C, there is satisfactory inhibition efficiency in the 600 ppm inhibitor solution. There is very low efficiency of the 1000 ppm solution at 58 °C, which is 28.02%. Up to 48 °C, the 1000 ppm inhibitor solution inhibited well. After 48 °C, the inhibition efficacy of the 1000 ppm decreased, further increasing the temperature by forming a hump shape. These findings reflect that these inhibitors could be ineffective at higher temperatures.
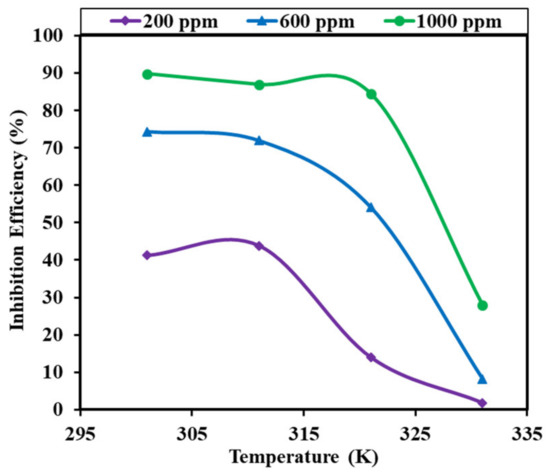
Figure 8.
Variations in the inhibition efficiency with temperature on mild steel in the presence of 200, 600, and 1000 ppm inhibitor in the 1 M H2SO4 solution.
3.4. Adsorption Isotherm
When MS is submerged a simple acid solution, the acid molecules attack the mild steel surface, and a violent reaction ensues that causes the deterioration of the metallic surface. In the presence of an acid-inhibitor solution mixture, inhibitor molecules are adsorbed on the MS surface, resulting in a reduction of the MS degradation. The adsorption isotherm should be known for a thorough comprehension of this adsorption process. Adsorption isotherms should be verified for a better understanding of the adsorption isotherm and the type of adsorption between the MS surface and the inhibitor molecule.
The adsorption isotherm provides fundamental information about the interaction between the inhibitor and the MS surface. Water molecules are initially adsorbed on an MS surface in an aqueous solution, after which inhibitor molecules replace some of the adsorbed water molecules. As a result, the adsorption of inhibitor molecules in aqueous mediums is a quasi-substitution process. The Langmuir adsorption isotherm equation can be applied to determine whether the adsorption process is a monolayer or a multilayer one, as follows:
If the slope of the curve obtained by plotting versus Cinh in the above equation is unity, then it indicates the monolayer adsorption. As in the equation, if one uses the molar concentration of the inhibitor, then the value of the adsorption constant Kads can be calculated from the intercept of a straight line.
As in Figure 9, the value of the regression coefficient (R2) and the slope are nearly equal to unity, which means that the adsorption of the inhibitor molecules obeys the Langmuir adsorption isotherm. This suggests that adsorption can be mono- or multilayer, and that the entire monolayer had formed before the creation of the multilayer [27,28]. Similarly, the Freuldlich adsorption was verified by plotting ln θ versus ln C (from Equation (5)), as seen in Figure 10. The slope of the straight line equal to 0.1053 was obtained, and it lies in between 0 < 1/n < 1, indicating that the alkaloids can be adsorbed on the MS surface very easily. The value of the interaction parameter ‘a’ was calculated from Figure 11 by plotting ln C versus θ (Equation (6)), and it was found to be negative 5.74. This indicates that there is are strong interactions among the alkaloids [29].
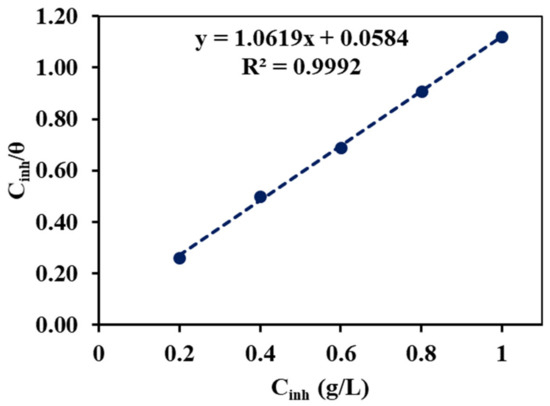
Figure 9.
Langmuir adsorption isotherm plot for the MS in the 1 M H2SO4 with different concentrations of the inhibitor.
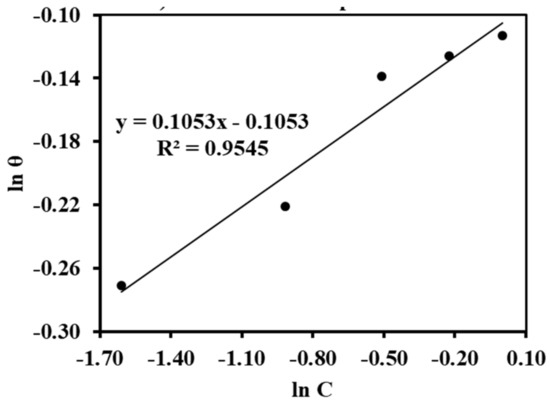
Figure 10.
Freuldlich adsorption plot.
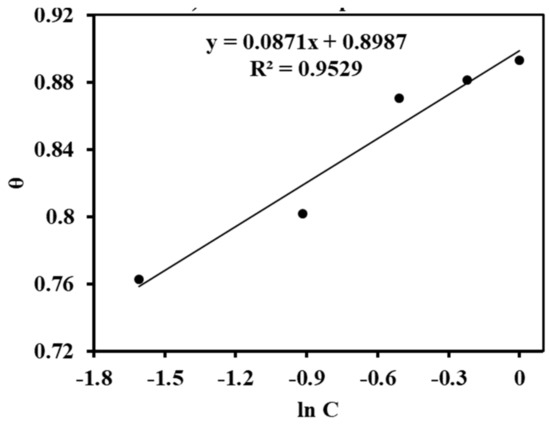
Figure 11.
Temkin adsorption plot.
3.5. Corrosion Kinetics
The activation energy of a process in an electrochemical cell in the presence and absence of an inhibitor may be described by rearranging the Arrhenius equation. The activation energy of the process is proportional to the corrosion rate (CR), as follows:
where A is the Arrhenius pre-exponential factor and T is the absolute temperature. Equation (7) shows that the activation energy of the reaction is equal to the slope of the Arrhenius plot, i.e., a plot obtained between the logarithms of the corrosion rate with along the axes.
Here, the activation energy for the reaction between the acid and the mild steel is 48.22 kJ/mol. When the inhibitor is added, the energy of activation is increased due to the blockage of acid molecules from the MS surface by the inhibitor molecules. The energy of activation is 64.38, 83.60, and 97.36 kJ/mol for 200, 600, and 1000 ppm inhibitor solutions, respectively (Figure 12).
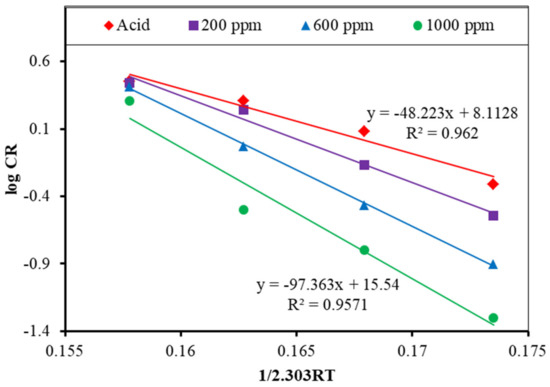
Figure 12.
Arrhenius plot for the MS in the 1 M H2SO4, both with and without an inhibitor.
This rise in activation energy indicates that when inhibitor molecules are added, the reaction rate between the acid and the MS is suppressed, leading to a drop in the corrosion rate. These computed values fall somewhere between physical (less than or equal to 20 kJ/mol) and chemical (more than 80 kJ/mol) adsorption. The adsorption of alkaloids on an MS surface in a 1 M H2SO4 solution, therefore, comprises a mixed kind of adsorption, with chemisorption dominance [22,30].
3.6. Thermodynamics of Corrosion
The enthalpy and entropy of the system were calculated by using the transition state equation, where the symbols have their usual meanings, as follows:
The enthalpy of activation () is obtained as the slope of a straight line obtained by plotting the graph of vs and the entropy of activation ( from its intercept [22,30,31].
From the above and Figure 13, the enthalpy of activation was found to be 45.60 kJ/mol in the absence of an inhibitor, and it increased to 61.76, 80.97, and 94.74 kJ/mol for 200, 600, and 1000 ppm, respectively. The positive value of the for inhibitor adsorption on the MS surface suggests that the adsorption is an endothermic process. The progressive increase in enthalpy value implies a decrease in the corrosion rate regulated by activation kinetic factors. The values of Ea and also show that alkaloid adsorption occurs through both physical and chemical interactions [22,28].
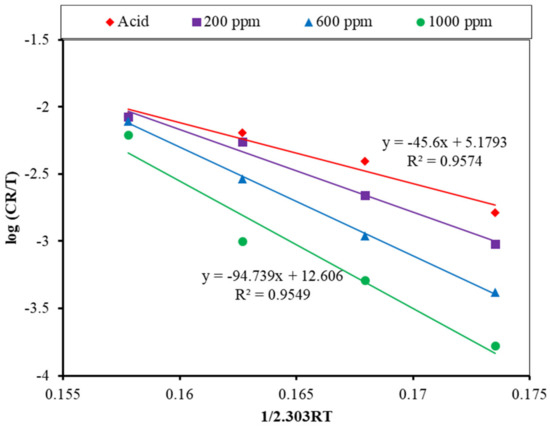
Figure 13.
Transition state plot for the MS in the 1 M H2SO4, both with and without an inhibitor.
The degree of randomness of the molecules onto the MS surface is calculated from the intercept of the transition state plot. The calculated values of the entropies are −98.42, −49.86, 6.42, and 43.80 JK−1 mol−1 for the acid and the 200, 600, and 1000 ppm solutions, respectively. The addition of three different doses of inhibitors boosted the system’s activation energy. An increase in activation energy decreases the reaction rate, which suppresses the corrosion rate. With the addition of an inhibitor concentration, the rise in Ea demonstrates the high adsorption of inhibitor molecules on the metal surface, with total or almost complete coverage, such that the acid molecules have little or no possibility of reacting with the metal. The calculated values of Ea, , and for acid, both without and with an inhibitor, are tabulated in Table 3.

Table 3.
Activation parameters of the MS dissolution in the 1 M H2SO4, both without and with an inhibitor.
3.7. Electrochemical Results
By observing the variations in the corrosion potential over time, the fluctuation of the mild steel’s open circuit potential (OCP) in the 1 M H2SO4 was investigated. The OCP modifications of the MS were exposed to acid solutions and inhibitors at various concentrations (200, 400, 600, 800, and 1000 ppm) for 30 min at 18 °C.
Figure 14 shows that, initially, the potential shifted to be more positive, but the shift in the value of the OCP was less than 85 mV, indicating that the plant extract acts as a mixed corrosion inhibitor [18,32]. The shifting of potential from the OCP to a more positive value indicates the formation of a protective layer by the inhibitor molecules in acid solutions on the MS surface (i.e., passivation), which limits the interaction of the aggressive ions with the MS surface [22,33]. The creation of a protective layer on the MS surface by the inhibitor molecules in acid solutions, as indicated by the positive shifting of the potential from the OCP, inhibits the interaction of hostile ions with the MS surface.
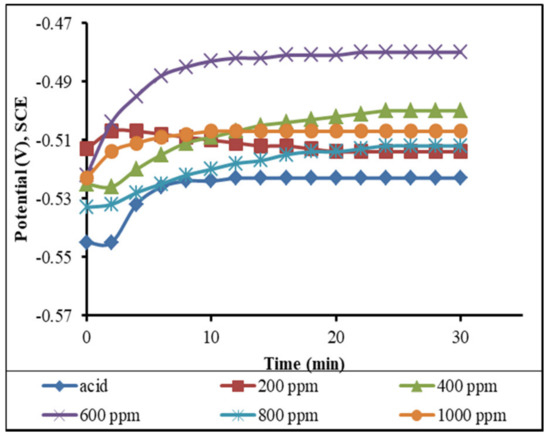
Figure 14.
Variation of the OCP with the time of immersion of the mild steel in the different concentrations of inhibitors in the 1 M H2SO4 solution (measured at the time of immersion).
However, in Figure 15, there is a slight increase in the OCP towards a negative potential because there is no inhibitor molecule. In the case of the solution having different concentrations of inhibitors, there is a slight increase in the OCP towards a positive potential firstly, and it remains constant after a certain amount of time.
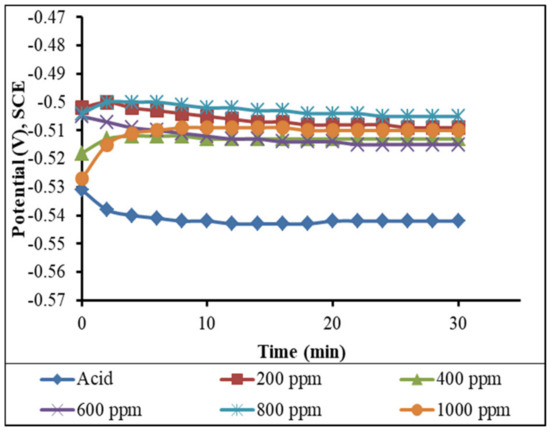
Figure 15.
Variations of the OCP with the time of immersion of the mild steel in the different concentrations of inhibitors in the 1 M H2SO4 solution (measured after 3 h of immersion in the solutions).
The polarization of the mild steel samples in the acid and various inhibitor concentrations (200, 400, 600, 800, and 1000 ppm) was accomplished by applying 350 mV in both anodic and cathodic directions using a potentiostat. The Evans diagram and Tafel extrapolation (Figure 16) show that the reduction in current density with the addition of an inhibitor signifies that the MS surface was covered with inhibitor molecules, resulting in a tiny active site for the process.
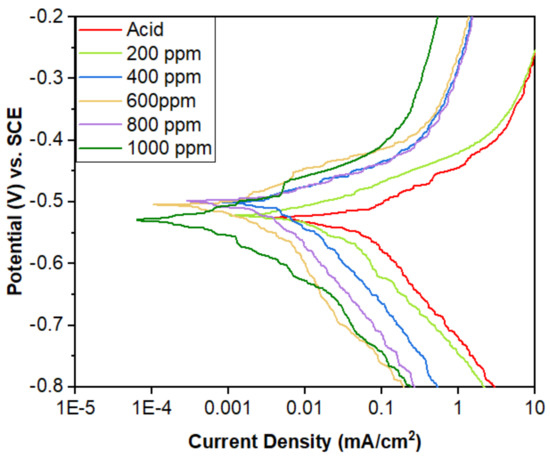
Figure 16.
Potentiodynamic polarization curves for the mild steel in the 1 M H2SO4 containing different concentrations of inhibitors in as-immersed conditions.
This polarization measurement of the as-immersed MS was carried out at a laboratory temperature of 18 °C, and the obtained results are tabulated in Table 4. The resulting decrease in the corrosion current in the presence of an inhibitor indicates the blockage of the reacting sites by the inhibitor molecules.

Table 4.
Anodic and cathodic slopes and inhibition efficiencies for the as-immersed samples.
Similar measurements were carried out after immersion of the MS coupons in different solutions for 3 h (Figure 17). Starting from the acid solution and progressing to the inhibitor 1000 ppm solution, the current density was found to decrease due to the resistance of the inhibitor solution toward the corrosion reaction. Hence, as in the as-immersed conditions, the current density decrease was due to coverage of the MS surface by the inhibitor molecules, resulting in a small active site.
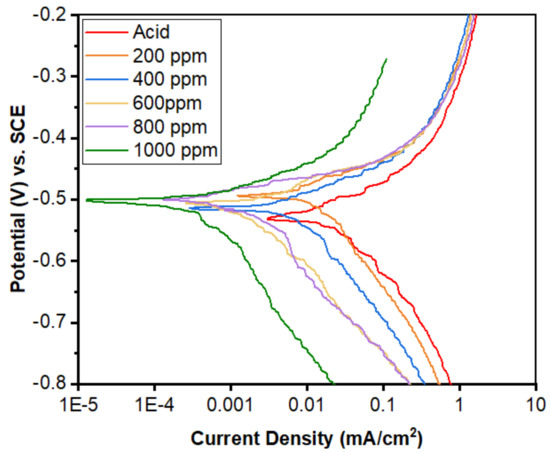
Figure 17.
Potentiodynamic polarization curves for the mild steel in the 1 M H2SO4 containing different concentrations of the inhibitor in immersed conditions.
The reaction in this small area produced a small current density. This also indicates that the alkaloids used in the experiment are good inhibitors. The inhibition efficiency, cathodic and anodic slopes, corrosion potential, and corrosion current densities of the alkaloid are tabulated in Table 5. In the acid-only solution, the current density was 0.0205 mA/cm2 and the corrosion potential was −0.527 V. The addition of varied concentrations of inhibitors induced drops in the current density. At 1000 ppm inhibitor solution, the lowest current density was 0.0003 mA/cm2 and the corrosion potential was −0.507 V.

Table 5.
The anodic slopes, cathodic slopes, and inhibition efficiencies for the 3-h-immersed samples.
The inhibition efficacy of the inhibitor at the different concentrations was assessed using the polarization technique for both the immersed and as-immersed samples. The efficiency figures are shown in the tables above (Table 4 and Table 5). The inhibitor’s inhibition efficacy is better in the as-immersed condition than that in the 3-h-immersion condition. Figure 18 depicts the variance in the inhibitor’s effectiveness with varied concentrations for the mild steel in both the immersed and as-immersed circumstances. The maximum efficiency was found to be 98.91% and 98.54% in as- immersed and 3-h-immersed MS samples, respectively, at a 1000 ppm concentration.
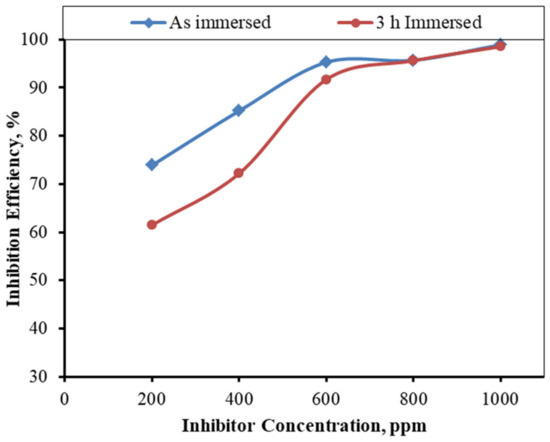
Figure 18.
Inhibition efficiencies of the inhibitors obtained from the polarization of both the immersed and as-immersed MS samples in the different concentrations of the inhibitor.
4. Discussion
The inhibitor concentration has a significant impact on the inhibitor’s capacity in corrosion inhibition. The inhibitory efficacy of the inhibitor solution at all concentrations is greater than 50% throughout any immersion period (Figure 6). This demonstrates that there is enough inhibitor present to completely cover the MS surface, leading to substantially higher inhibition. An increase in the surface coverage of the MS by the inhibitor molecule is indicated by the increasing order in which inhibition occurs with concentration. For maximal inhibition, a concentration of 1000 ppm is the ideal inhibitor solution. The inhibitory effectiveness was found to be highest at a 1000 ppm concentration because there are enough inhibitor molecules to be adsorbed on the MS surface, resulting in the lowest weight-loss. This leads to the maximum inhibition efficiency.
The inhibitory action of the inhibitor is also affected by immersion time. In the study of immersion time, the weight-loss of the MS was found to be almost constant for all concentrations of the inhibitor solutions from 6 h onwards (Figure 5). This may be due to the formation of a passive layer of the inhibitor which completely blocks the reaction between the acid molecules and the MS surface, resulting in the stoppage of further weight-loss, even in increasing immersion times. These findings are further supported by the morphological evidence.
The working temperature greatly influences the effectiveness of the inhibitor. It was found that the MS weight-loss in inhibitor solutions increased with increasing temperatures (Figure 7). This increase in weight-loss may be attributed to two factors: desorption of the inhibitor molecules from the MS surface and structural distortion of the inhibitor molecules [27]. As weight reduction progresses, the inhibitor’s inhibitory effectiveness became decreased.
The effect of higher temperatures is a sped-up a chemical reaction and reduced oxygen solubility, which allow the cathodic reaction to occur. In addition, the viscosity of water became decreased with the increase in temperature, which leads to increased diffusion. This will allow the increased transport of reactants (dissolved oxygen or other electrons acceptors) and products (Fe2+ species) on the metal surface and increased weight-loss, which, in turn, decreases the inhibition efficiency [26]. From the results obtained from temperature effect study, it can be concluded that the structural deformation of the molecules may occur more than that of the desorption of the molecules from the MS surface. This deformation leads to a decrease in the inhibition efficiency of the inhibitors for the MS in an acidic medium. The results show that there is less thermal stability of the inhibitors at high temperatures.
The activation value of greater than 80 kJ/mol indicates the chemical adsorption, while less than 20 kJ/mol, indicates physical adsorption [34,35]. The energy of activation for these alkaloids was found to be 64.38 kJ/mol in 200 ppm, 83.60 kJ/mol in 600 ppm, and 97.36 kJ/mol in 1000 ppm inhibitor solutions (Table 3). These data reveal that there is chemical-dominated physical adsorption between the MS and the alkaloid molecules. The positive value of transition enthalpy (Table 3) for alkaloids adsorption indicates that the adsorption process is endothermic [1]. Seemingly, the increase in transition enthalpy upon the addition of an inhibitor confirms the decrease in corrosion rate, which is controlled by kinetic activation [36]. The value of activation energy is greater than that of the transition enthalpy (). This implies the participation of a gaseous reaction, i.e., cathodic hydrogen evolution process, which leads to a reduction in total reaction volume. The activation energy and enthalpy values support the relation Ea- = RT (Table 3), indicating the adsorption of the alkaloids on the MS surface by both physical and chemical processes [35,37]. Very interestingly, the increase in the transition state entropy of the system is due to the quasi-substitution process, as well as to increased density of the proton roaming in the solution. The corrosion inhibition is due to the adsorption of the alkaloids on the MS surface by the replacement of the water molecules, as follows:
where Alk(sol) and Alk(ads) represent the solvated and adsorbed alkaloid molecules, respectively. Similarly, H2O(ads) represents the adsorbed water molecules on the MS surface and x represents the size ratio, i.e., the number of water molecules that are replaced by one alkaloid molecule [38].
Alk(sol) + xH2O(ads) ⇌ Alk(ads) + H2O(sol)
The OCP of the MS in the corrosion cell containing aggressive media in both the presence and absence of inhibitor was measured. It was found that the OCP value shifted to a more positive direction in the presence of an inhibitor solution compared to that of the acid-only solution. This positive shifting of the potential reveals that the MS surface is positively charged, which interacts with the negatively charged sulphate ions in the medium and becomes negative. Then, the negatively charged surface interacts with the protonated alkaloids electrostatically. Consequently, the deportation of alkaloids occurs, liberating H2 gas [22]. Afterwards, there may exist a donor–acceptor relation between the vacant d-orbital and the highest occupied molecular orbital of the alkaloids, forming a coordinate bond. Here, lone pair of electrons, as well as the π-electrons of the alkaloids, act as donors, and the vacant d-orbitals of the Fe act as acceptors [35,37]. The wavy nature of the polarization curve may be due to the increase in randomness in the solution in the evolution of the H2. This randomness could influence the conductance of the electrolytic solution.
For a short while, the degree of randomness increases. This is supported by the transition entropy of the system (Table 3). Based on the enthalpy, entropy, activation energy, and OCP of the system, the following may be the possible adsorption mechanism:
where Alk(sol) and H-Alk represent the solvated and protonated alkaloids, respectively, Fe(+) and Fe-SO42−(Ads) are the positively charged MS and the sulphate ions adsorbed on the MS, respectively, and Fe-Alk(Ads) is the alkaloid adsorbed MS.
Fe + Acid + Alk(sol) ⇌ Fe(+) + H-Alk + SO42−(Free)
Fe(+) + SO42−(Free) ⇌ Fe-SO42−(Ads)
Fe- SO42−(Ads) + H-Alk ⇌ Fe-Alk(Ads) + SO42−(Free) + H
H + H ⇌ H2
The polarization measurement reveals that there is a decrease in the current density in the presence of an inhibitor for both the immersed and as-immersed conditions (Figure 16 and Figure 17). The inhibition efficiency of the as-immersed sample is nearly equal to the efficiency obtained in the polarization of the immersed samples (Table 4 and Table 5). This finding reflects that the alkaloids work from the very beginning. The corrosion potential values are slightly increasing towards a positive direction as the concentration of the inhibitor increases. This implies that the MS surface becomes more positive as the inhibitor concentration increases, which favors the adsorption of alkaloids on the MS surface [35]. The cathodic slope of the polarization curve is found to be parallel, i.e., no change in the trend of slope, which indicates that the hydrogen evolution is activation-controlled and the reaction mechanism has not been changed upon the addition of alkaloids to the acid solution [39,40]. Both the anodic and cathodic slopes in the polarization measurements have not been changed upon the addition of alkaloids, which indicates that the inhibition is due to the adsorption of alkaloids on the MS surface, entirely blocking the sites [41,42].
5. Conclusions
Alkaloids were successfully extracted from Acacia catechu bark and used as green inhibitors against mild steel corrosion in a 1 M H2SO4 solution. From the methanol extract, the alkaloids were effectively extracted utilizing the liquid–liquid extraction technique and characterized using phytochemical and spectroscopic methods during the process. The positive results for the Mayer’s and Dragendroff’s tests, as well as the characteristic functional group peaks in the FTIR spectra, confirm the alkaloids. The effect of immersion time, inhibitor concentration, and working temperature were evaluated using the weight-loss method, which concluded that these alkaloids could work perfectly for up to 24 h of immersion with maximum inhibition efficacy. The alkaloids protect the MS through a chemical-dominated physical adsorption process in temperatures of up to 48 °C. Polarization measurements revealed that the 1000 ppm inhibitor could protect the MS from an aggressive environment of up to 98.5% for up to 3 h of immersion. From these findings, it can be concluded that the Acacia catechu alkaloids could be commercialized as an effective green corrosion inhibitor.
Author Contributions
Conceptualization, H.B.O. and D.P.B.; methodology, H.B.O.; software, H.B.O.; validation, H.B.O. and D.P.B.; formal analysis, R.K.; investigation, R.K. and A.K.B.; resources, R.K.; data curation, A.K.B.; writing—original draft preparation, R.K., A.K.B., N.K., and O.T.; writing—review and editing, H.B.O., T.M., and D.P.B.; visualization, A.K.B.; supervision, H.B.O.; project administration, D.P.B. and H.B.O.; funding acquisition, H.B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University Grants Commission of Bhaktapur, Nepal, under grant number MRS-78-79-S&T-32.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the Department of Chemistry, Amrit Campus, Tribhuvan Univesity, Kathmandu, Nepal for providing the use of chemical accessories and laboratory facilities, as well as the University Grants Commission, Bhaktapur, Nepal for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot Juice as Green Corrosion Inhibitor of Mild Steel in Phosphoric Acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef]
- Pritzl, M.D.; Tabatabai, H.; Ghorbanpoor, A. Laboratory Evaluation of Select Methods of Corrosion Prevention in Reinforced Concrete Bridges. Int. J. Concr. Struct. Mater. 2014, 8, 201–212. [Google Scholar] [CrossRef]
- Tan, B.; Lan, W.; Zhang, S.; Deng, H.; Qiang, Y.; Fu, A.; Ran, Y.; Xiong, J.; Marzouki, R.; Li, W. Passiflora Edulia Sims Leaves Extract as Renewable and Degradable Inhibitor for Copper in Sulfuric Acid Solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128892. [Google Scholar] [CrossRef]
- Ikeuba, A.; Okafor, P.; Ekpe, U.; Ebenso, E.E. Alkaloid and Non-Alkaloid Ethanolic Extracts from Seeds of Garcinia Kola as Green Corrosion Inhibitors of Mild Steel in H2SO4 Solution. Int. J. Electrochem. Sci. 2013, 8, 7455–7467. [Google Scholar]
- Gupta, D.K.; Kafle, K.A.; Das, A.K.; Neupane, S.; Ghimire, A.; Yadav, B.D.; Chaudhari, Y.; Karki, N.; Yadav, A.P. Study of Jatropha Curcas Extract as a Corrosion Inhibitor in Acidic Medium on Mild Steel by Weight Loss and Potentiodynamic Methods. J. Nepal Chem. Soc. 2020, 41, 87–93. [Google Scholar] [CrossRef]
- Shrestha, P.R.; Oli, H.B.; Thapa, B.; Chaudhary, Y.; Gupta, D.K.; Das, A.K.; Nakarmi, K.B.; Singh, S.; Karki, N.; Yadav, A.P. Bark Extract of Lantana Camara in 1M HCl as Green Corrosion Inhibitor for Mild Steel. Eng. J. 2019, 23, 205–211. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of Corrosion Inhibitors for Steels in Acidic Media for the Oil and Gas Industry: A Review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Rahim, M.A.A.; Hassan, H.B.; Khalil, M.W. Naturally Occurring Organic Substances as Corrosion Inhibitors for Mild Steel in Acid Medium: Concentration and Temperature Effects. Mater. Werkst. 1997, 28, 198–204. [Google Scholar] [CrossRef]
- Yıldırım, A.; Çetin, M. Synthesis and Evaluation of New Long Alkyl Side Chain Acetamide, Isoxazolidine and Isoxazoline Derivatives as Corrosion Inhibitors. Corros. Sci. 2008, 50, 155–165. [Google Scholar] [CrossRef]
- Patni, N.; Agarwal, S.; Shah, P. Greener Approach towards Corrosion Inhibition. Chin. J. Eng. 2013, 2013, 784186. [Google Scholar] [CrossRef]
- Abdallah, M. Rhodanine Azosulpha Drugs as Corrosion Inhibitors for Corrosion of 304 Stainless Steel in Hydrochloric Acid Solution. Corros. Sci. 2002, 44, 717–728. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M. Adsorption and Inhibitive Properties of Some New Mannich Bases of Isatin Derivatives on Corrosion of Mild Steel in Acidic Media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Ju, H.; Kai, Z.-P.; Li, Y. Aminic Nitrogen-Bearing Polydentate Schiff Base Compounds as Corrosion Inhibitors for Iron in Acidic Media: A Quantum Chemical Calculation. Corros. Sci. 2008, 50, 865–871. [Google Scholar] [CrossRef]
- Sivakumar, P.R.; Srikanth, A.P. Anticorrosive Activity of Schreabera Swietenioids Leaves as Green Inhibitor for Mild Steel in Acidic Solution. Asian J. Chem. 2017, 29, 274–278. [Google Scholar] [CrossRef]
- Kamal, C.; Sethuraman, M.G. Caulerpin—A Bis-Indole Alkaloid As a Green Inhibitor for the Corrosion of Mild Steel in 1 M HCl Solution from the Marine Alga Caulerpa Racemosa. Ind. Eng. Chem. Res. 2012, 51, 10399–10407. [Google Scholar] [CrossRef]
- Raja, P.B.; Qureshi, A.K.; Abdul Rahim, A.; Osman, H.; Awang, K. Neolamarckia Cadamba Alkaloids as Eco-Friendly Corrosion Inhibitors for Mild Steel in 1M HCl Media. Corros. Sci. 2013, 69, 292–301. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Experimental and Theoretical Evaluation of Acacia Catechu Extract as a Natural, Economical and Effective Corrosion Inhibitor for Mild Steel in an Acidic Environment. J. Bio-Tribo-Corros. 2020, 6, 76. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis Retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- Parajuli, D.; Sharma, S.; Oli, H.B.; Bohara, D.S.; Bhattarai, D.P.; Tiwari, A.P.; Yadav, A.P. Comparative Study of Corrosion Inhibition Efficacy of Alkaloid Extract of Artemesia Vulgaris and Solanum Tuberosum in Mild Steel Samples in 1 M Sulphuric Acid. Electrochem 2022, 3, 416–433. [Google Scholar] [CrossRef]
- Sunil, M.A.; Sunitha, V.S.; Radhakrishnan, E.K.; Jyothis, M. Immunomodulatory Activities of Acacia Catechu, a Traditional Thirst Quencher of South India. J. Ayurveda Integr. Med. 2019, 10, 185–191. [Google Scholar] [CrossRef]
- Hashmat, M.A.; Hussain, R. A Review on Acacia Catechu Willd. Interdiscip. J. Contemp. Res. Bus. 2013, 5, 593–600. [Google Scholar]
- Karki, N.; Neupane, S.; Gupta, D.K.; Das, A.K.; Singh, S.; Koju, G.M.; Chaudhary, Y.; Yadav, A.P. Berberine Isolated from Mahonia Nepalensis as an Eco-Friendly and Thermally Stable Corrosion Inhibitor for Mild Steel in Acid Medium. Arab. J. Chem. 2021, 14, 103423. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Oli, H.B.; Sharma, N.; Ekaraj, K.; Subedee, A.; Timilsina, R. Green Synthesis of Copper Nanoparticles Using Zingiber Officinale Extract and Characterization. J. Nepal Chem. Soc. 2018, 39, 10–17. [Google Scholar] [CrossRef]
- Karki, N.; Neupane, S.; Chaudhary, Y.; Gupta, D.K.; Yadav, A.P. Berberis Aristata: A Highly Efficient and Thermally Stable Green Corrosion Inhibitor for Mild Steel in Acidic Medium. Anal. Bioanal. Electrochem. 2020, 12, 970–988. [Google Scholar]
- Khaleel, H.; Ateeq, A.A.; Ali, A.A. The Effect of Temperature and Inhibitor on Corrosion of Carbon Steel in Acid Solution under Static Study. Int. J. Appl. Eng. Res. 2018, 13, 3638–3647. [Google Scholar]
- Andoor, P.A.; Okeoma, K.B.; Mbamara, U.S. Adsorption and Thermodynamic Studies of the Corrosion Inhibition Effect of Rosmarinus Officinalis L. Leaves on Aluminium Alloy in 0.25 M HCl and Effect of an External Magnetic Field. Int. J. Phys. Sci. 2021, 16, 79–95. [Google Scholar]
- Ituen, E.; Akaranta, O.; James, A. Evaluation of Performance of Corrosion Inhibitors Using Adsorption Isotherm Models: An Overview. Chem. Sci. Int. J. 2017, 18, 1–34. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Cao, X.; Fu, A.; Guo, L.; Marzouki, R.; Li, W. Insight into the Anti-Corrosion Performance of Two Food Flavors as Eco-Friendly and Ultra-High Performance Inhibitors for Copper in Sulfuric Acid Medium. J. Colloid Interface Sci. 2022, 609, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Karki, N.; Choudhary, Y.; Yadav, A.P. Thermodynamic, Adsorption and Corrosion Inhibition Studies of Mild Steel by Artemisia Vulgaris Extract from Methanol as Green Corrosion Inhibitor in Acid Medium. J. Nepal Chem. Soc. 2018, 39, 76–85. [Google Scholar] [CrossRef]
- Oli, H.B.; Parajuli, D.L.; Sharma, S.; Chapagain, A.; Yadav, A.P. Adsorption Isotherm and Activation Energy of Inhibition of Alkaloids on Mild Steel Surface in Acidic Medium. Amrit Res. J. 2021, 2, 59–67. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr.; Nathan, C. Corrosion Inhibitors; National Association of Corrosion Engineers: Houston, TX, USA, 1973. [Google Scholar]
- Ijuo, G.A.; Chahul, H.F.; Eneji, I.S. Kinetic and Thermodynamic Studies of Corrosion Inhibition of Mild Steel Using Bridelia Ferruginea Extract in Acidic Environment. J. Adv. Electrochem. 2016, 2, 107–112. [Google Scholar]
- Li, Y.; Zhao, P.; Liang, Q.; Hou, B. Berberine as a Natural Source Inhibitor for Mild Steel in 1 M H2SO4. Appl. Surf. Sci. 2005, 252, 1245–1253. [Google Scholar] [CrossRef]
- Erami, R.S.; Amirnasr, M.; Meghdadi, S.; Talebian, M.; Farrokhpour, H.; Raeissi, K. Carboxamide Derivatives as New Corrosion Inhibitors for Mild Steel Protection in Hydrochloric Acid Solution. Corros. Sci. 2019, 151, 190–197. [Google Scholar] [CrossRef]
- Bhat, J.I.; Alva, V.D. Meclizine Hydrochloride as a Potential Non-Toxic Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Medium. Arch. Appl. Sci. Res. 2011, 3, 343–356. [Google Scholar]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo Leaf Extract as an Eco-Friendly Corrosion Inhibitor of X70 Steel in HCl Solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Dhakal, K.; Bohara, D.S.; Bist, B.B.; Oli, H.B.; Bhattarai, D.P.; Singh, S.; Karki, N.; Yadav, A.P. Alkaloids Extract of Alnus Nepalensis Bark as a Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. J. Nepal Chem. Soc. 2022, 43, 76–92. [Google Scholar] [CrossRef]
- Chkirate, K.; Azgaou, K.; Elmsellem, H.; El Ibrahimi, B.; Sebbar, N.K.; Benmessaoud, M.; El Hajjaji, S.; Essassi, E.M. Corrosion Inhibition Potential of 2-[(5-Methylpyrazol-3-Yl) Methyl] Benzimidazole against Carbon Steel Corrosion in 1 M HCl Solution: Combining Experimental and Theoretical Studies. J. Mol. Liq. 2021, 321, 114750. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; He, J.; Li, W.; Qiang, Y.; Wang, Q.; Xu, C.; Chen, S. Insight into Anti-Corrosion Mechanism of Tetrazole Derivatives for X80 Steel in 0.5 M H2SO4 Medium: Combined Experimental and Theoretical Researches. J. Mol. Liq. 2021, 321, 114464. [Google Scholar] [CrossRef]
- Fekkar, G.; Yousfi, F.; Elmsellem, H.; Aiboudi, M.; Ramdani, M.; Abdel-Rahman, I.; Hammouti, B.; Bouyazza, L. Eco-Friendly Chamaerops Humilis L. Fruit Extract Corrosion Inhibitor for Mild Steel in 1 M HCl. Int. J. Corros. Scale Inhib. 2020, 9, 446–459. [Google Scholar]
- Hafez, B.; Mokhtari, M.; Elmsellem, H.; Steli, H. Environmentally Friendly Inhibitor of the Corrosion of Mild Steel: Commercial Oil of Eucalyptus. Int. J. Corros. Scale Inhib. 2019, 8, 573–585. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).