A Review of Electrospun Carbon Nanofiber-Based Negative Electrode Materials for Supercapacitors
Abstract
:1. Introduction
2. Negative Electrode Materials
3. Carbon-Based Negative Electrode Materials
4. Electrospun-Based Fibers as Negative Electrode Materials for Supercapacitors
5. Challenges, Opportunities, and Future Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Agency, I.E. World Energy Outlook 2018. 2018. Available online: https://www.oecd-ilibrary.org/energy/world-energy-outlook-2018_weo-2018-en (accessed on 1 November 2020).
- Jin, H.; Li, J.; Yuan, Y.; Wang, J.; Lu, J.; Wang, S. Recent Progress in Biomass-Derived Electrode Materials for High Volumetric Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1801007. [Google Scholar] [CrossRef]
- Capellán-Pérez, I.; Arto, I.; Polanco-Martínez, J.M.; González-Eguino, M.; Neumann, M.B. Likelihood of climate change pathways under uncertainty on fossil fuel resource availability. Energy Environ. Sci. 2016, 9, 2482–2496. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Dilshad, S.; Khalid, R.; Kalair, A.R.; Abas, N. Review of energy storage and transportation of energy. Energy Storage 2019, 1, e49. [Google Scholar] [CrossRef]
- Palacín, M.R.; de Guibert, A. Why do batteries fail? Science 2016, 351, 1253292. [Google Scholar] [CrossRef] [Green Version]
- El Kharbachi, A.; Zavorotynska, O.; Latroche, M.; Cuevas, F.; Yartys, V.; Fichtner, M. Exploits, advances and challenges benefiting beyond Li-ion battery technologies. J. Alloys Compd. 2020, 817, 153261. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, C.-H.; Lu, W.; Dai, L. Recent Advances in Fiber-Shaped Supercapacitors and Lithium-Ion Batteries. Adv. Mater. 2020, 32, 1902779. [Google Scholar] [CrossRef]
- Gou, Q.; Zhao, S.; Wang, J.; Li, M.; Xue, J. Recent Advances on Boosting the Cell Voltage of Aqueous Supercapacitors. Nano Micro Lett. 2020, 12, 98. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel–cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Liang, H.; Jia, H.; Lin, T.; Wang, Z.; Li, C.; Chen, S.; Qi, J.; Cao, J.; Fei, W.; Feng, J. Oxygen-vacancy-rich nickel-cobalt layered double hydroxide electrode for high-performance supercapacitors. J. Colloid Interface Sci. 2019, 554, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, C.; Hu, C.; Wang, M.; Li, S.; Huang, H.; Bustillo, K.; Han, X.; Zhao, C.; Guo, W.; et al. Surface-Confined Fabrication of Ultrathin Nickel Cobalt-Layered Double Hydroxide Nanosheets for High-Performance Supercapacitors. Adv. Funct. Mater. 2018, 28, 1803272. [Google Scholar] [CrossRef]
- Wei, Z.; Yuan, J.; Tang, S.; Wu, D.; Wu, L. Porous nanorods of nickel–cobalt double hydroxide prepared by electrochemical co-deposition for high-performance supercapacitors. J. Colloid Interface Sci. 2019, 542, 15–22. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Zhang, X.; Zhang, Z. New Ti3C2 aerogel as promising negative electrode materials for asymmetric supercapacitors. J. Power Sources 2017, 364, 234–241. [Google Scholar] [CrossRef]
- Grote, F.; Zhao, H.; Lei, Y. Self-supported carbon coated TiN nanotube arrays: Innovative carbon coating leads to an improved cycling ability for supercapacitor applications. J. Mater. Chem. A 2015, 3, 3465–3470. [Google Scholar] [CrossRef]
- Han, X.; Zhang, D.; Qin, Y.; Kong, X.; Zhang, F.; Lei, X. Construction of Ta-Cu7S4 negative electrode for high-performance all-solid-state asymmetric supercapacitor. Chem. Eng. J. 2021, 403, 126471. [Google Scholar] [CrossRef]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/vertically-aligned carbon nanotubes as negative electrode for asymmetric supercapacitor in neutral aqueous electrolyte. J. Colloid Interface Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Zheng, K.; Zeng, Y.; Liu, S.; Zeng, C.; Tong, Y.; Zheng, Z.; Zhu, T.; Lu, X. Valence and surface modulated vanadium oxide nanowires as new high-energy and durable negative electrode for flexible asymmetric supercapacitors. Energy Storage Mater. 2019, 22, 410–417. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.-B.; Liu, M.-C.; Dai, Y.-H.; Yan, K.; Hu, B.; Luo, Y.-C.; Kang, L. Design and preparation of MoO2/MoS2 as negative electrode materials for supercapacitors. Mater. Des. 2016, 112, 88–96. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Nguyen, T.; Silva, T.M.; Carmezim, M.J.; Montemor, M.F. Electrodeposited MoOx films as negative electrode materials for redox supercapacitors. Electrochim. Acta 2017, 225, 19–28. [Google Scholar] [CrossRef]
- Long, C.; Wei, T.; Yan, J.; Jiang, L.; Fan, Z. Supercapacitors based on graphene-supported iron nanosheets as negative electrode materials. ACS Nano 2013, 7, 11325–11332. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, Z.; Kumamoto, A.; Unah, W.I.; Yan, S.; Ikuhara, Y.H.; Xiang, X.; Zu, X.; Zhou, W. PEDOT coated iron phosphide nanorod arrays as high-performance supercapacitor negative electrodes. Chem. Commun. 2018, 54, 794–797. [Google Scholar] [CrossRef]
- Liang, B.; Zheng, Z.; Retana, M.; Lu, K.; Wood, T.; Ai, Y.; Zu, X.; Zhou, W. Synthesis of FeP nanotube arrays as negative electrode for solid-state asymmetric supercapacitor. Nanotechnology 2019, 30, 295401. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Chhetri, K.; Kim, H.; Ji, S.; Chae, S.-H.; Kim, T.; Kim, H.Y. Self-assembled polypyrrole hierarchical porous networks as the cathode and porous three dimensional carbonaceous networks as the anode materials for asymmetric supercapacitor. J. Energy Storage 2021, 33, 102080. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Lai, C.-C.; Ho, C.-L.; Lo, C.-T. Preparation of interconnected carbon nanofibers as electrodes for supercapacitors. Electrochim. Acta 2014, 127, 369–376. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Zhao, Y.-F.; Xiao, Q.-Q.; Zhang, Y.-X.; Jing, L.; Yan, Y.-M.; Sun, K.-N. Controllable Growth of CNTs on Graphene as High-Performance Electrode Material for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 8497–8504. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, S.; Hong, Z.; Wu, N. Electrospun activated carbon nanofibers for supercapacitor electrodes. RSC Adv. 2014, 4, 43619–43623. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Chae, S.-H.; Ojha, G.P.; Dahal, B.; Mukhiya, T.; Lee, M.; Chhetri, K.; Kim, T.; Kim, H.-Y. Three-dimensional porous carbonaceous network with in-situ entrapped metallic cobalt for supercapacitor application. J. Colloid Interface Sci. 2019, 553, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; He, Y.; Song, Y.; Yang, T.; Li, X.; Liu, Z. Flexible Cross-Linked Electrospun Carbon Nanofiber Mats Derived from Pitch as Dual-Functional Materials for Supercapacitors. Energy Fuels 2020, 34, 14975–14985. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Q.; Xue, H.; Pang, H.; Xu, Q. A highly alkaline-stable metal oxide@ metal–organic framework composite for high-performance electrochemical energy storage. Natl. Sci. Rev. 2020, 7, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Siraj, N.; Macchi, S.; Berry, B.; Viswanathan, T. Metal-Free Carbon-Based Supercapacitors—A Comprehensive Review. Electrochem 2020, 1, 410–438. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Denmark, I.; Macchi, S.; Watanabe, F.; Viswanathan, T.; Siraj, N. Effect of KOH on the Energy Storage Performance of Molasses-Based Phosphorus and Nitrogen Co-Doped Carbon. Electrochem 2021, 2, 29–40. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Zhang, X.; Shi, H.; Zeng, W.; Zhang, H.; Liu, Q.; Li, C.; Liu, Q.; Duan, H. Porous ultrathin carbon nanobubbles formed carbon nanofiber webs for high-performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 14801–14810. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid supercapacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar] [CrossRef]
- Song, B.; Wu, F.; Zhu, Y.; Hou, Z.; Moon, K.-s.; Wong, C.-P. Effect of polymer binders on graphene-based free-standing electrodes for supercapacitors. Electrochim. Acta 2018, 267, 213–221. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, P.; Liu, H.; Yan, L.; Song, H.; Liu, D.; Zhao, X. Metal-organic framework derived porous flakes of cobalt chalcogenides (CoX, X = O, S, Se and Te) rooted in carbon fibers as flexible electrode materials for pseudocapacitive energy storage. Electrochim. Acta 2021, 369, 137681. [Google Scholar] [CrossRef]
- Dahal, B.; Mukhiya, T.; Ojha, G.P.; Muthurasu, A.; Chae, S.-H.; Kim, T.; Kang, D.; Kim, H.Y. In-built fabrication of MOF assimilated B/N co-doped 3D porous carbon nanofiber network as a binder-free electrode for supercapacitors. Electrochim. Acta 2019, 301, 209–219. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Singh, T.I.; Kim, N.H.; Lau, K.-t.; Lee, J.H. Effects of the composition of reduced graphene oxide/carbon nanofiber nanocomposite on charge storage behaviors. Compos. Part B Eng. 2019, 178, 107500. [Google Scholar] [CrossRef]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.-H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Shen, Z.; Cao, D. ZIF-derived porous carbon: A promising supercapacitor electrode material. J. Mater. Chem. A 2014, 2, 12873–12880. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, G.; Hu, H.; Yang, J.; Qian, B.; Ling, Z.; Qiu, J. Ultrasound-assisted preparation of electrospun carbon nanofiber/graphene composite electrode for supercapacitors. J. Power Sources 2013, 243, 350–353. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, X.; Liang, Q.; Bai, Y.; Huang, Z.-H.; Kang, F. Nitrogen-doped hollow activated carbon nanofibers as high performance supercapacitor electrodes. J. Electroanal. Chem. 2015, 739, 84–88. [Google Scholar] [CrossRef]
- Chen, L.-F.; Zhang, X.-D.; Liang, H.-W.; Kong, M.; Guan, Q.-F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wu, X. Transition metal oxides anchored on graphene/carbon nanotubes conductive network as both the negative and positive electrodes for asymmetric supercapacitor. J. Alloys Compd. 2020, 842, 155838. [Google Scholar] [CrossRef]
- Ishita, I.; Singhal, R. Porous multi-channel carbon nanofiber electrodes using discarded polystyrene foam as sacrificial material for high-performance supercapacitors. J. Appl. Electrochem. 2020, 50, 809–820. [Google Scholar] [CrossRef]
- Sheng, J.; Ma, C.; Ma, Y.; Zhang, H.; Wang, R.; Xie, Z.; Shi, J. Synthesis of microporous carbon nanofibers with high specific surface using tetraethyl orthosilicate template for supercapacitors. Int. J. Hydrogen Energy 2016, 41, 9383–9393. [Google Scholar] [CrossRef]
- Choudhury, A.; Kim, J.-H.; Sinha Mahapatra, S.; Yang, K.-S.; Yang, D.-J. Nitrogen-Enriched Porous Carbon Nanofiber Mat as Efficient Flexible Electrode Material for Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 2109–2118. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Shi, J.; Song, Y.; Liu, L. High-performance supercapacitor electrodes based on porous flexible carbon nanofiber paper treated by surface chemical etching. Chem. Eng. J. 2014, 249, 216–225. [Google Scholar] [CrossRef]
- Zhu, T.; Zhou, J.; Li, Z.; Li, S.; Si, W.; Zhuo, S. Hierarchical porous and N-doped carbon nanotubes derived from polyaniline for electrode materials in supercapacitors. J. Mater. Chem. A 2014, 2, 12545–12551. [Google Scholar] [CrossRef]
- Kshetri, T.; Thanh, T.D.; Singh, S.B.; Kim, N.H.; Lee, J.H. Hierarchical material of carbon nanotubes grown on carbon nanofibers for high performance electrochemical capacitor. Chem. Eng. J. 2018, 345, 39–47. [Google Scholar] [CrossRef]
- Kim, T.; Tiwari, A.P.; Chhetri, K.; Ojha, G.P.; Kim, H.; Chae, S.-H.; Dahal, B.; Lee, B.M.; Mukhiya, T.; Kim, H.Y. Phytic acid controlled in situ synthesis of amorphous cobalt phosphate/carbon composite as anode materials with a high mass loading for symmetrical supercapacitor: Amorphization of the electrode to boost the energy density. Nanoscale Adv. 2020, 2, 4918–4929. [Google Scholar] [CrossRef]
- Mukhiya, T.; Dahal, B.; Ojha, G.P.; Kang, D.; Kim, T.; Chae, S.-H.; Muthurasu, A.; Kim, H.Y. Engineering nanohaired 3D cobalt hydroxide wheels in electrospun carbon nanofibers for high-performance supercapacitors. Chem. Eng. J. 2019, 361, 1225–1234. [Google Scholar] [CrossRef]
- Mukhiya, T.; Tiwari, A.P.; Chhetri, K.; Kim, T.; Dahal, B.; Muthurasu, A.; Kim, H.Y. A metal–organic framework derived cobalt oxide/nitrogen-doped carbon nanotube nanotentacles on electrospun carbon nanofiber for electrochemical energy storage. Chem. Eng. J. 2021, 420, 129679. [Google Scholar] [CrossRef]
- Yu, M.; Huang, Y.; Li, C.; Zeng, Y.; Wang, W.; Li, Y.; Fang, P.; Lu, X.; Tong, Y. Building three-dimensional graphene frameworks for energy storage and catalysis. Adv. Funct. Mater. 2015, 25, 324–330. [Google Scholar] [CrossRef]
- Dahal, B.; Chhetri, K.; Muthurasu, A.; Mukhiya, T.; Tiwari, A.P.; Gautam, J.; Lee, J.Y.; Chung, D.C.; Kim, H.Y. Biaxial Stretchability in High-Performance, All-Solid-State Supercapacitor with a Double-Layer Anode and a Faradic Cathode Based on Graphitic-2200 Knitted Carbon Fiber. Adv. Energy Mater. 2021, 11, 2002961. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Yue, Z.; Dunya, H.; Ashuri, M.; Kucuk, K.; Aryal, S.; Antonov, S.; Alabbad, B.; Segre, C.U.; Mandal, B.K. Synthesis of a very high specific surface area active carbon and its electrical double-layer capacitor properties in organic electrolytes. ChemEngineering 2020, 4, 43. [Google Scholar] [CrossRef]
- He, X.; Mao, X.; Zhang, C.; Yang, W.; Zhou, Y.; Yang, Y.; Xu, J. Flexible binder-free hierarchical copper sulfide/carbon cloth hybrid supercapacitor electrodes and the application as negative electrodes in asymmetric supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 2145–2152. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, S.; Zhang, J.; Ning, F.; Ma, C.; Qiu, Z. Facile fabrication of oxygen vacancy-rich α-Fe2O3 microspheres on carbon cloth as negative electrode for supercapacitors. Electrochim. Acta 2020, 338, 135820. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yu, Z.-Y.; Ma, X.; Li, Z.-Y.; Yu, S.-H. In situ hydrothermal growth of ferric oxides on carbon cloth for low-cost and scalable high-energy-density supercapacitors. Nano Energy 2014, 9, 345–354. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, J.-N.; Wang, H.-T.; Kang, X.-H.; Bian, S.-W. Boosting the electrochemical performance of carbon cloth negative electrodes by constructing hierarchically porous nitrogen-doped carbon nanofiber layers for all-solid-state asymmetric supercapacitors. Mater. Chem. Front. 2019, 3, 25–31. [Google Scholar] [CrossRef]
- Patiño, J.; López-Salas, N.; Gutiérrez, M.C.; Carriazo, D.; Ferrer, M.L.; del Monte, F. Phosphorus-doped carbon–carbon nanotube hierarchical monoliths as true three-dimensional electrodes in supercapacitor cells. J. Mater. Chem. A 2016, 4, 1251–1263. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon Nanofibers Prepared via Electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Lee, S.; Tiwari, A.P.; Maharjan, B.; Poudel, S.B.; Park, C.H.; Kim, C.S. Integrated design and fabrication strategies for biomechanically and biologically functional PLA/β-TCP nanofiber reinforced GelMA scaffold for tissue engineering applications. Int. J. Biol. Macromol. 2020, 164, 976–985. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Bhattarai, D.P.; Maharjan, B.; Ko, S.W.; Kim, H.Y.; Park, C.H.; Kim, C.S. Polydopamine-based Implantable Multifunctional Nanocarpet for Highly Efficient Photothermal-chemo Therapy. Sci. Rep. 2019, 9, 2943. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Tiwari, A.P.; Maharjan, B.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Sacrificial template-based synthetic approach of polypyrrole hollow fibers for photothermal therapy. J. Colloid Interface Sci. 2019, 534, 447–458. [Google Scholar] [CrossRef]
- Boys, C.V. On the Production, Properties, and some suggested Uses of the Finest Threads. Proc. Phys. Soc. Lond. 1887, 9, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.I. Disintegration of water drops in an electric field. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar] [CrossRef]
- Taylor, G.I.; Van Dyke, M.D. The force exerted by an electric field on a long cylindrical conductor. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1966, 291, 145–158. [Google Scholar] [CrossRef]
- Taylor, G.I.; Van Dyke, M.D. Electrically driven jets. Proc. R. Soc. Lond. A Math. Phys. Sci. 1969, 313, 453–475. [Google Scholar] [CrossRef]
- Dai, H.; Gong, J.; Kim, H.; Lee, D. A novel method for preparing ultra-fine alumina-borate oxide fibres via an electrospinning technique. Nanotechnology 2002, 13, 674–677. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Barrero, A.; Márquez, M.; Spretz, R.; Velarde-Ortiz, R.; Larsen, G. Electrically Forced Coaxial Nanojets for One-Step Hollow Nanofiber Design. J. Am. Chem. Soc. 2004, 126, 5376–5377. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core–Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Joshi, M.K.; Kim, J.I.; Unnithan, A.R.; Lee, J.; Park, C.H.; Kim, C.S. Bimodal fibrous structures for tissue engineering: Fabrication, characterization and in vitro biocompatibility. J. Colloid Interface Sci. 2016, 476, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.P.; Kim, M.H.; Park, H.; Park, W.H.; Kim, B.S.; Kim, C.S. Coaxially fabricated polylactic acid electrospun nanofibrous scaffold for sequential release of tauroursodeoxycholic acid and bone morphogenic protein2 to stimulate angiogenesis and bone regeneration. Chem. Eng. J. 2020, 389, 123470. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Mukhiya, T.; Ojha, G.P.; Dahal, B.; Kim, T.; Chhetri, K.; Lee, M.; Chae, S.-H.; Muthurasu, A.; Tiwari, A.P.; Kim, H.Y. Designed Assembly of Porous Cobalt Oxide/Carbon Nanotentacles on Electrospun Hollow Carbon Nanofibers Network for Supercapacitor. ACS Appl. Energy Mater. 2020, 3, 3435–3444. [Google Scholar] [CrossRef]
- Kaerkitcha, N.; Chuangchote, S.; Sagawa, T. Control of physical properties of carbon nanofibers obtained from coaxial electrospinning of PMMA and PAN with adjustable inner/outer nozzle-ends. Nanoscale Res. Lett. 2016, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- El-Deen, A.G.; Barakat, N.A.M.; Khalil, K.A.; Kim, H.Y. Hollow carbon nanofibers as an effective electrode for brackish water desalination using the capacitive deionization process. New J. Chem. 2014, 38, 198–205. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, T.; Khan, A.U.; Liu, G. Block copolymer–based porous carbon fibers. Sci. Adv. 2019, 5, eaau6852. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Electrospun metal–organic framework derived hierarchical carbon nanofibers with high performance for supercapacitors. Chem. Commun. 2017, 53, 1751–1754. [Google Scholar] [CrossRef]
- Li, W.-H.; Ding, K.; Tian, H.-R.; Yao, M.-S.; Nath, B.; Deng, W.-H.; Wang, Y.; Xu, G. Conductive Metal–Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Kong Yoong Lee, J.; Tian, L.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Electrospun carbon nanofibers and their hybrid composites as advanced materials for energy conversion and storage. Nano Energy 2016, 22, 361–395. [Google Scholar] [CrossRef]
- Lai, C.-C.; Lo, C.-T. Preparation of Nanostructural Carbon Nanofibers and Their Electrochemical Performance for Supercapacitors. Electrochim. Acta 2015, 183, 85–93. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Ting, J.-M. Direct Growth of MoS2 Nanowalls on Carbon Nanofibers for Use in Supercapacitor. Sci. Rep. 2017, 7, 5999. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, I.W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Ding, Y.; Niu, J.; Xia, Z.; Roy, A.; Chen, H.; Qu, J.; Wang, Z.L.; Dai, L. Rationally designed graphene-nanotube 3D architectures with a seamless nodal junction for efficient energy conversion and storage. Sci. Adv. 2015, 1, e1400198. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Jeong, Y.I.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Endo, M.; Lee, J.-W. Synthesis and Characterization of Porous Carbon Nanofibers with Hollow Cores Through the Thermal Treatment of Electrospun Copolymeric Nanofiber Webs. Small 2007, 3, 91–95. [Google Scholar] [CrossRef]
- Mukhiya, T.; Dahal, B.; Ojha, G.P.; Chhetri, K.; Lee, M.; Kim, T.; Chae, S.-H.; Tiwari, A.P.; Muthurasu, A.; Kim, H.Y. Silver nanoparticles entrapped cobalt oxide nanohairs/electrospun carbon nanofibers nanocomposite in apt architecture for high performance supercapacitors. Compos. Part B Eng. 2019, 178, 107482. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Dong, K.; Zhang, Y.; Wang, X.; Aboalhassan, A.A.; Yu, J.; Ding, B. Multifunctional flexible membranes from sponge-like porous carbon nanofibers with high conductivity. Nat. Commun. 2019, 10, 5584. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhou, J.; Wang, S.; Zhang, W.; Wang, Z.; Lv, F.; Wang, K.; Sun, Q.; Guo, S. Freestanding film made by necklace-like N-doped hollow carbon with hierarchical pores for high-performance potassium-ion storage. Energy Environ. Sci. 2019, 12, 1605–1612. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.-C.; Huang, K.-J.; Wu, X. Metal–organic framework derived hollow materials for electrochemical energy storage. J. Mater. Chem. A 2018, 6, 6754–6771. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Yang, W.; Shen, W.; Xue, H.; Pang, H. MOF-Derived Metal Oxide Composites for Advanced Electrochemical Energy Storage. Small 2018, 14, 1704435. [Google Scholar] [CrossRef] [PubMed]
- Arbulu, R.C.; Jiang, Y.-B.; Peterson, E.J.; Qin, Y. Metal–Organic Framework (MOF) Nanorods, Nanotubes, and Nanowires. Angew. Chem. Int. Ed. 2018, 57, 5813–5817. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-C.; Liu, P.-F.; Liu, R.; Liu, M.; Tao, K.; Zhu, S.-R.; Wu, M.-K.; Yi, F.-Y.; Han, L. MOF-derived hierarchical double-shelled NiO/ZnO hollow spheres for high-performance supercapacitors. Dalton Trans. 2016, 45, 13311–13316. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, Y.; Cui, J.; Yu, D.; Zhang, X.; Shu, X.; Zhang, J.; Zhang, Y.; Vajtai, R.; Ajayan, P.; et al. MOF-74 derived porous hybrid metal oxide hollow nanowires for high-performance electrochemical energy storage. J. Mater. Chem. A 2018, 6, 8396–8404. [Google Scholar] [CrossRef]
- Na, K.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Metal Nanocrystals Embedded in Single Nanocrystals of MOFs Give Unusual Selectivity as Heterogeneous Catalysts. Nano Lett. 2014, 14, 5979–5983. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal–Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Muthurasu, A.; Chae, S.-H.; Kim, T.; Mukhiya, T.; Kim, H.Y. Template-Assisted Fabrication of ZnO/Co3O4 One-Dimensional Metal–Organic Framework Array Decorated with Amorphous Iron Oxide/Hydroxide Nanoparticles as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. Energy Fuels 2020, 34, 7716–7725. [Google Scholar] [CrossRef]
- Tan, Y.; Meng, L.; Wang, Y.; Dong, W.; Kong, L.; Kang, L.; Ran, F. Negative electrode materials of molybdenum nitride/N-doped carbon nano-fiber via electrospinning method for high-performance supercapacitors. Electrochim. Acta 2018, 277, 41–49. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Shen, K.; Liu, Y.; Zhao, X.; Wu, Y.; Wang, Y.; Ran, F. Ultra-small vanadium nitride quantum dots embedded in porous carbon as high performance electrode materials for capacitive energy storage. J. Power Sources 2016, 333, 61–71. [Google Scholar] [CrossRef]
- Kim, C.; Yang, K. Electrochemical properties of carbon nanofiber web as an electrode for supercapacitor prepared by electrospinning. Appl. Phys. Lett. 2003, 83, 1216–1218. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Jia, D. Coal/PAN interconnected carbon nanofibers with excellent energy storage performance and electrical conductivity. Electrochim. Acta 2016, 194, 239–245. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Zhu, C.; van Aken, P.A.; Maier, J. Tin Nanoparticles Encapsulated in Porous Multichannel Carbon Microtubes: Preparation by Single-Nozzle Electrospinning and Application as Anode Material for High-Performance Li-Based Batteries. J. Am. Chem. Soc. 2009, 131, 15984–15985. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, X.; Wang, Y. Co3O4 carbon nanofiber mats as negative electrodes for sodium-ion batteries. Mater. Lett. 2016, 170, 21–24. [Google Scholar] [CrossRef]
- Yao, S.; Guo, R.; Xie, F.; Wu, Z.; Gao, K.; Zhang, C.; Shen, X.; Li, T.; Qin, S. Electrospun three-dimensional cobalt decorated nitrogen doped carbon nanofibers network as freestanding electrode for lithium/sulfur batteries. Electrochim. Acta 2020, 337, 135765. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Sun, X.; Chang, Z. 3D hierarchical carbon nanofibers/TiO2@MoS2 core-shell heterostructures by electrospinning, hydrothermal and in-situ growth for flexible electrode materials. Mater. Des. 2020, 189, 108503. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Park, M.; Kang, W.-S.; Rhee, K.Y.; Park, S.-J. Preparation and characterization of carbon black/pitch-based carbon fiber paper composites for gas diffusion layers. Compos. Part B Eng. 2019, 159, 362–368. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Niu, H.Q.; Qi, S.L.; Tian, G.F.; Wang, X.D.; Wu, D.Z. Structure evolutions involved in the carbonization of polyimide fibers with different chemical constitution. Mater. Today Commun. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Wang, N.; Haque, M.; Naboka, O.; Flygare, M.; Svensson, K.; Gatenholm, P.; Liu, J.; Enoksson, P. Cellulose-derived carbon nanofibers/graphene composite electrodes for powerful compact supercapacitors. RSC Adv. 2017, 7, 45968–45977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xia, G.; Guo, Z.; Sun, D.; Li, X.; Yu, X. In situ fabrication of three-dimensional nitrogen and boron co-doped porous carbon nanofibers for high performance lithium-ion batteries. J. Power Sources 2016, 324, 294–301. [Google Scholar] [CrossRef]
- Wei, J.; Geng, S.; Pitkänen, O.; Järvinen, T.; Kordas, K.; Oksman, K. Green Carbon Nanofiber Networks for Advanced Energy Storage. ACS Appl. Energy Mater. 2020, 3, 3530–3540. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Zhang, Y.; Rhee, K.Y.; Park, S.-J. Synthesis of PAN/PVDF nanofiber composites-based carbon adsorbents for CO2 capture. Compos. Part B Eng. 2019, 156, 95–99. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Luo, J.; Chen, L. Highly nitrogen-doped graphitic carbon fibers from sustainable plant protein for supercapacitor. Ind. Crops Prod. 2018, 121, 226–235. [Google Scholar] [CrossRef]
- Lu, J.; Wan, H.; Ju, T.; Ying, Z.; Zhang, W.; Li, B.; Zhang, Y. Super flexible electrospun carbon/nickel nanofibrous film electrode for supercapacitors. J. Alloys Compd. 2019, 774, 593–600. [Google Scholar] [CrossRef]
- Tian, D.; Lu, X.; Nie, G.; Gao, M.; Wang, C. Direct growth of Ni–Mn–O nanosheets on flexible electrospun carbon nanofibers for high performance supercapacitor applications. Inorg. Chem. Front. 2018, 5, 635–642. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, Q.; Cai, T.; Wang, Y.; Wang, D.; Kong, D.; Ren, H.; Zhou, J.; Xing, W. Graphitized electrospun carbon fibers with superior cyclability as a free-standing anode of potassium-ion batteries. J. Power Sources 2020, 474, 228479. [Google Scholar] [CrossRef]
- Kim, S.E.; Tiwari, A.P. Three dimensional polycaprolactone/cellulose scaffold containing calcium-based particles: A new platform for bone regeneration. Carbohydr. Polym. 2020, 250, 116880. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, G.; Zhang, H.; Wang, L.; Shi, H.; Wei, D.; Duan, H. MOF-derived N-doped carbon bubbles on carbon tube arrays for flexible high-rate supercapacitors. Energy Storage Mater. 2018, 10, 75–84. [Google Scholar] [CrossRef]
- Cao, X.-M.; Sun, Z.-J.; Zhao, S.-Y.; Wang, B.; Han, Z.-B. MOF-derived sponge-like hierarchical porous carbon for flexible all-solid-state supercapacitors. Mater. Chem. Front. 2018, 2, 1692–1699. [Google Scholar] [CrossRef]
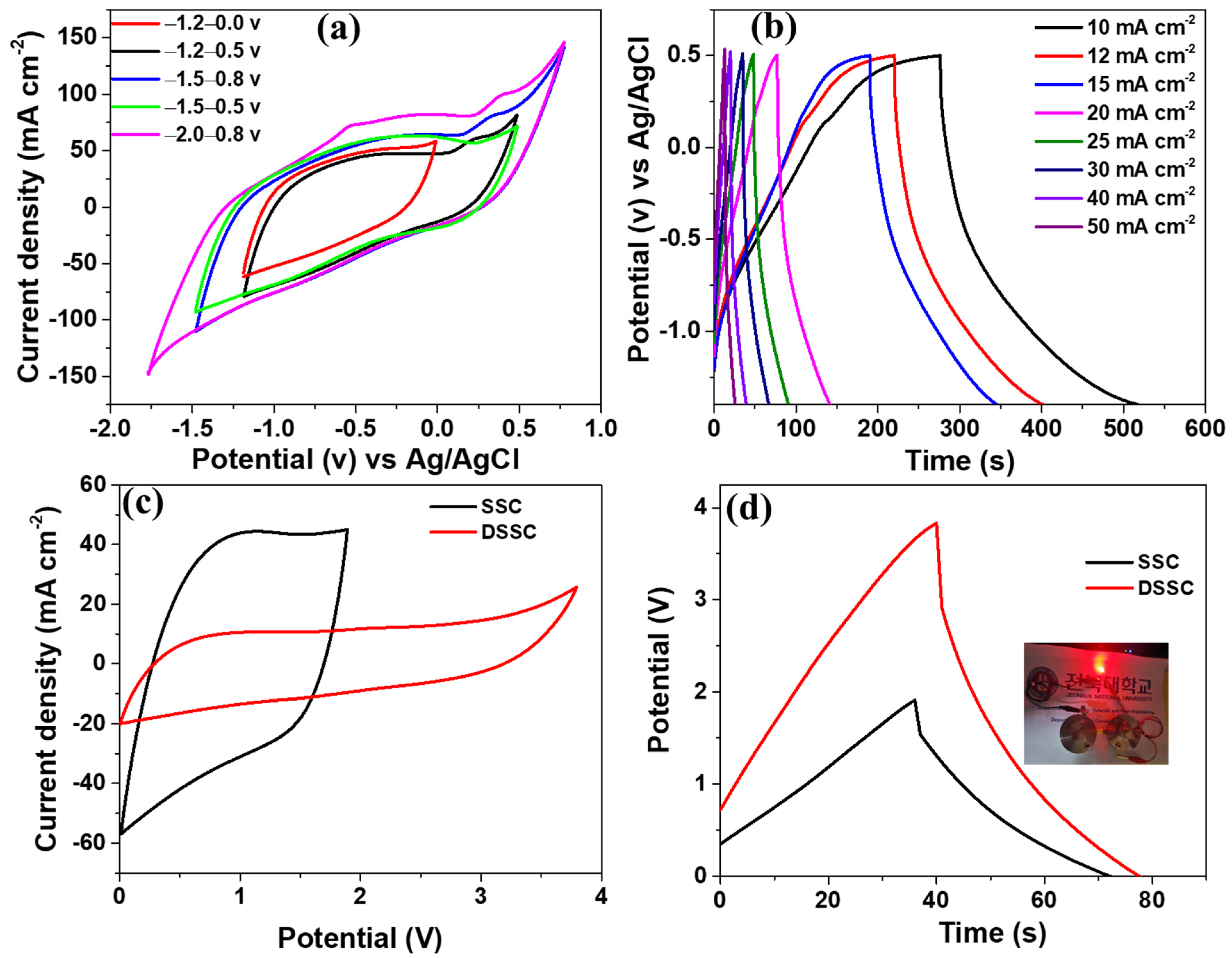
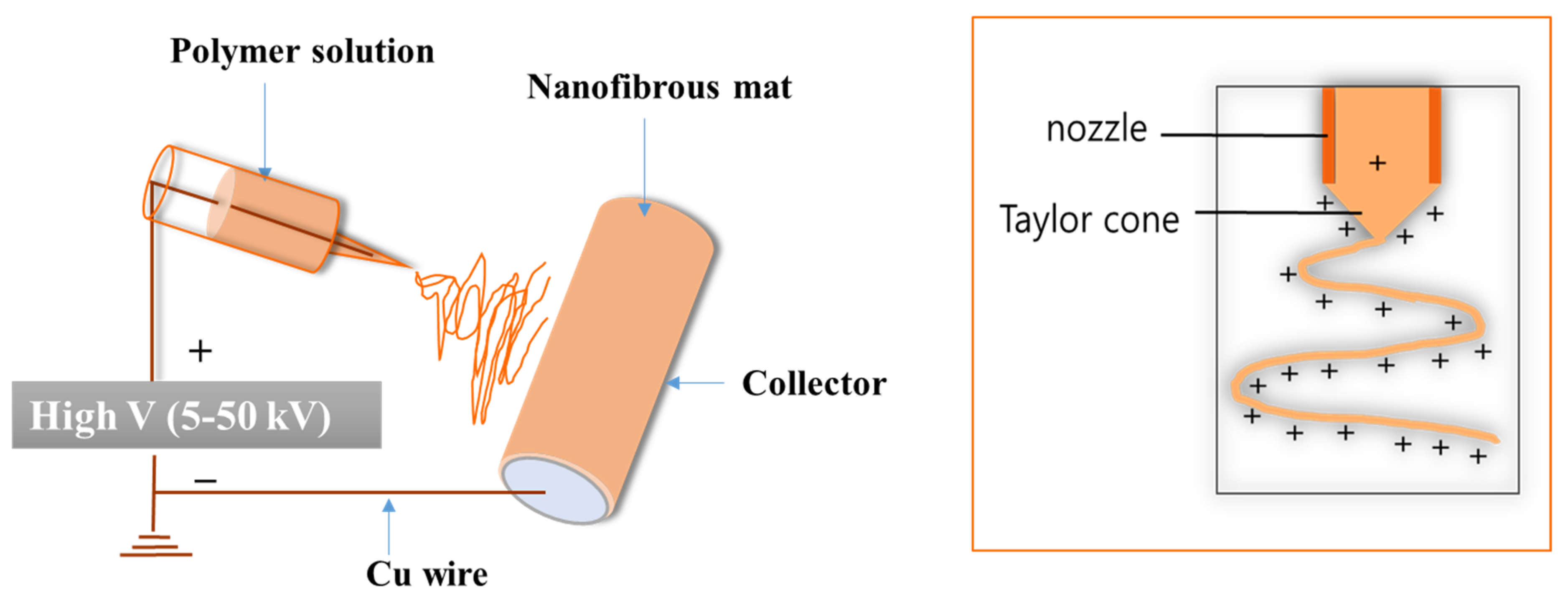
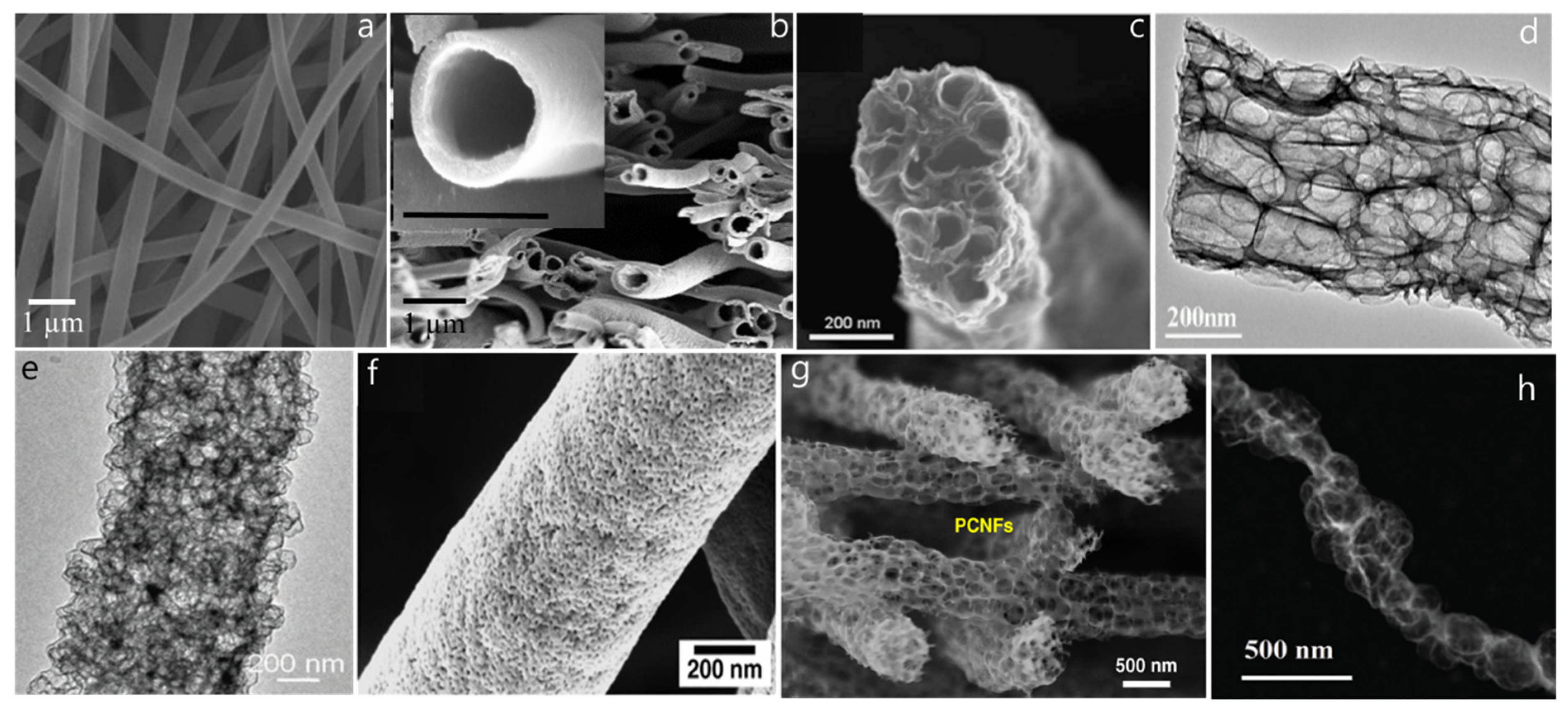
| S.N. | Electrode Materials | Electrolyte | Capacitance | Current density | Reference |
|---|---|---|---|---|---|
| 1. | Iron phosphide (FeP) nanotubes | 1 M LiCl | 149.11 F g−1 | 1 mA cm−2 | [27] |
| 2. | Activated CNF | 6 M KOH | 156.0 F g−1 | 0.5 A g−1 | [31] |
| 3. | Carbon-coated tin nitride (TiN) | 1 M KOH | 167.0 F g−1 | 1 A g−1 | [19] |
| 4. | 80:20 PAN, Poly(acrylonitrile-co-butadiene (PAN/PAN-co-PB) derived CNF | 2 M KOH | 172.0 F g−1 | 1 A g−1 | [29] |
| 5. | CNF/graphene | 6 M KOH | 183.0 F g−1 | 1 A g−1 | [47] |
| 6 | ZIF-8 derived nanoporous carbon (ZIF-8/NPC) | 3 M (KOH) | 190.0 F g−1 | 1 A g−1 | [45,48] |
| 7. | Nitrogen-doped hollow activated carbon nanofibers (HACNFs) | 6 M KOH | 197.0 F g−1 | 0.2 A g−1 | [49] |
| 8. | Nitrogen doped NCFs@polypyrrole (NCNF-900@PPy) | 6 M KOH | 202.0 F g−1 | 1 A g−1 | [50] |
| 9. | Three-dimensional porous CNFs (P@3D-CNF) | 2 M KOH | 205.5 F g−1 | 1 A g−1 | [28] |
| 10. | ZIF-7/glucose composite-derived carbon-L-950 | 6 M KOH | 228.0 F g−1 | 0.1 A g−1 | [46] |
| 11. | Graphene/carbon nanotube/iron oxide (G/CNT/Fe2O3-150) | 1 M Li2SO4 | 258.0 F g−1 | 1 A g−1 | [51] |
| 12. | CNF-40 (Polystyrene foam/PAN, PF:PAN = 40:60) | 1 M H2SO4 | 271.6 F g−1 | 0.5 A g−1 | [52] |
| 13. | Vanadium pentoxide V2O5/vertically aligned CNTs composites (V2O5/VACNT) | 1 M sodium sulphate (Na2SO4) | 284.0 F g−1 | 2 A g−1 | [21] |
| 14 | Three dimensional boron-doped CNF (3D-BN-CNF-F900) | 2 M KOH | 295.0F g−1 | 0.5 A g−1 | [42] |
| 15. | Porous CNF-3 | 6 M KOH | 314.0 F g−1 | 0.5 A g−1 | [53] |
| 16. | Reduced graphene oxide-CNF (rGO-CNF, 1:1) | 6 M KOH | 316.5F g−1 | 0.25 A g−1 | [43] |
| 17. | PAN: poly(m-aminophenol (PmAP)-NCNF | 6 M KOH | 347.5 F g−1 | 0.5 mA cm-2 | [54] |
| 18. | Porous CNFs | 6 M KOH | 362.0 F g−1 | 0.2 A g−1 | [55] |
| 19. | hierarchical porous carbon nanotube (HPCT) | 6 M KOH | 386.2 F g−1 | 0.1 A g−1 | [56] |
| 20. | CNT@Graphene | 6 M KOH | 401.0 F g−1 | 1 A g−1 | [30] |
| 21. | Molybdenum oxide/sulphide (MoO2/MoS2) | 1 M Na2SO4 | 433.3 F g−1 | 5 mV s−1 | [23] |
| 22. | CNT@CNF | 6 M KOH | 464.2 F g−1 | 0.5 A g−1 | [57] |
| 23. | Electrodeposited molybdenum oxide (MoOx) film | 1 M H2SO4 | 507.0 F g−1 | 1 A g−1 | [24] |
| 24. | Carbon nanotube@graphene-CNFs (CNT@Gr-CNF-5) | 6 M KOH | 521.5 F g−1 | 0.25 A g−1 | [39] |
| 25. | Amorphous cobalt phosphate/porous carbon on CC (a-PC@CoPi-CC8) | 2 M KOH | 606.1 F g−1(2.15 F cm−2) | 1 A g−1(4 mA cm−2) | [58] |
| 26. | Tantalum in copper sulphide (Ta-Cu7S4) | 1 M KOH | 675.0 F g−1 | 1 A g−1 | [20] |
| 27. | 3D Titanium Carbide (Ti3C2) aerogel | 1 M KOH | 1012.0 mF cm−2 | 2 mV s−1 | [18] |
| 28. | Vanadium oxides (VOx) | 5 M lithium chloride (LiCl) | 1.57 F cm−21652.3 F g−1 | 2 mA cm−2 | [22] |
| S.N. | Polymer/Solvent | Fiber Diameter/Surface Area | Application | References |
|---|---|---|---|---|
| 1 | Polyacrylonitrile /dimethylformaide (PAN/DMF) with a metal precursor | 200–500 nm/N/A | Energy storage | [59,88,117] |
| 2 | Coal, PAN/DMF | N/A | Energy storage | [118] |
| 3 | [PAN + PMMA + tin octoate]/DMF | N/A | Lithium-based batteries | [119] |
| 4 | PVP, cobalt nitrate [Co(NO3)] in water/ethanol | 150 nm/N/A | Sodium ion batteries | [120] |
| 5 | ZIF-67, PAN/DMF | 200 nm/338.37 m2 g−1 | Li–S batteries | [121] |
| 6 | PAN, cobalt salt/DMF | Energy storage | [32] | |
| 7 | PAN, terephthalic acid/DMF | 400–600 nm/N/A | Electrochemical test | [122] |
| 8 | Pitch/DMF | Micrometer/N/A | Gas diffusion | [123] |
| 9 | Polyimide/dimethylacetamide (PI/DMAc) | Micrometer/N/A | n/a | [124] |
| 10 | Cellulose/acetone-dimethylacetamide | 50–500 nm/N/A | Energy storage | [125] |
| 11 | PVP, ammonia borane/methanol | 150 nm/145 m2 g−1 | Lithium-ion batteries | [126] |
| 12 | Lignin, polyvinyl alcohol/distilled water (PVA/DW) | 100 ± 23 nm/1670 m2 g−1 | Energy storage | [127] |
| 14 | PAN, PVDF/DMF | 200–300 nm/29 m2 g−1 | CO2 adsorbents | [128] |
| 15 | Plant protein/acetic acid | 413–900 nm/N/A | Energy storage | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Chhetri, K.; Lee, M.; Dahal, B.; Lohani, P.C.; Kim, H.-Y. A Review of Electrospun Carbon Nanofiber-Based Negative Electrode Materials for Supercapacitors. Electrochem 2021, 2, 236-250. https://doi.org/10.3390/electrochem2020017
Tiwari AP, Mukhiya T, Muthurasu A, Chhetri K, Lee M, Dahal B, Lohani PC, Kim H-Y. A Review of Electrospun Carbon Nanofiber-Based Negative Electrode Materials for Supercapacitors. Electrochem. 2021; 2(2):236-250. https://doi.org/10.3390/electrochem2020017
Chicago/Turabian StyleTiwari, Arjun Prasad, Tanka Mukhiya, Alagan Muthurasu, Kisan Chhetri, Minju Lee, Bipeen Dahal, Prakash Chandra Lohani, and Hak-Yong Kim. 2021. "A Review of Electrospun Carbon Nanofiber-Based Negative Electrode Materials for Supercapacitors" Electrochem 2, no. 2: 236-250. https://doi.org/10.3390/electrochem2020017







