Direct Electrochemical Reduction of Bicarbonate to Formate Using Tin Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Linear Sweep Voltammetry
2.3. Electroreduction Experiments
2.4. Product Analysis
3. Results and Discussion
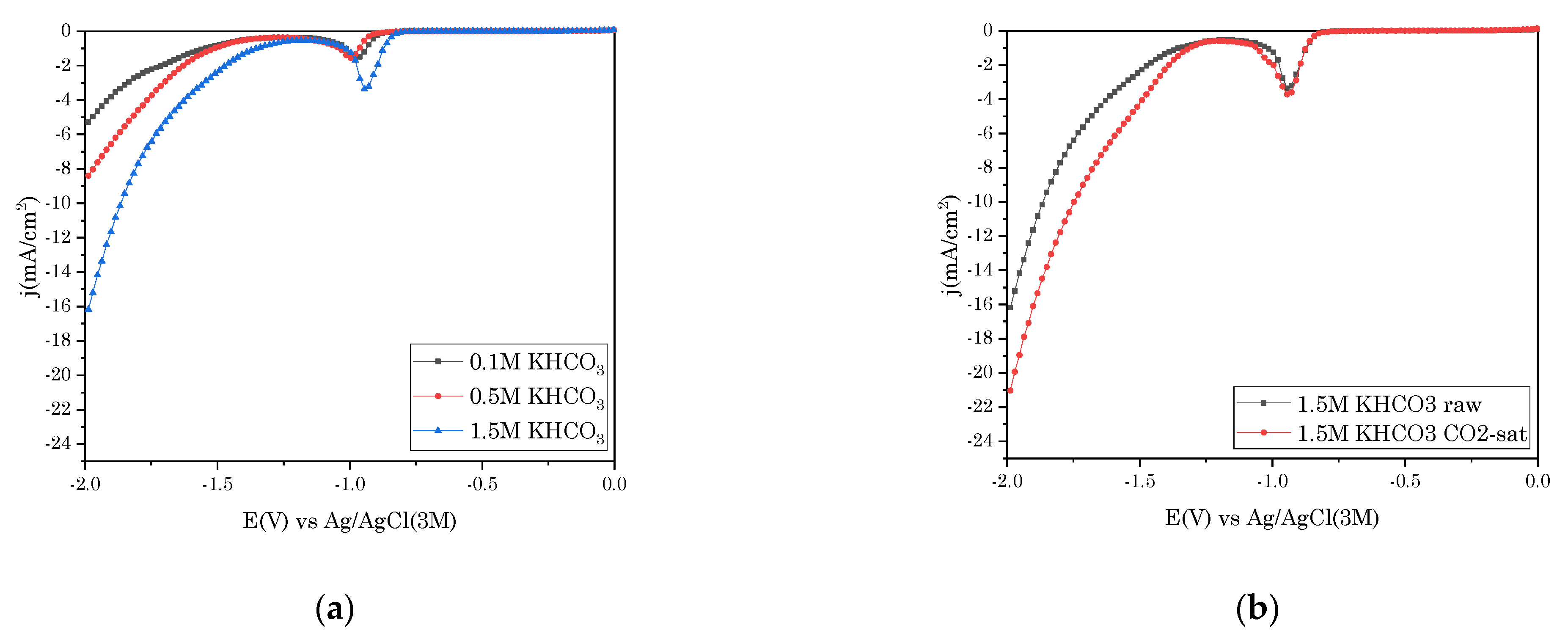
3.1. Linear Sweep Voltammetry (LSV)
3.2. Chronoamperometry
3.3. Analysis and Quantification of Products by 1H NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.R.; Myers, S.S. Impact of Anthropogenic CO2 Emissions on Global Human Nutrition. Nat. Clim. Chang. 2018, 8, 834–839. [Google Scholar] [CrossRef]
- Workman, M.; McGlashan, N.; Chalmers, H.; Shah, N. An Assessment of Options for CO2 Removal from the Atmosphere. Energy Procedia 2011, 4, 2877–2884. [Google Scholar] [CrossRef]
- Nogalska, A.; Zukowska, A.; Garcia-Valls, R. Atmospheric CO2 Capture for the Artificial Photosynthetic System. Sci. Total Environ. 2018, 621, 186–192. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Kheawhom, S.; Soltani, S.M.; Aroua, M.K. Electrochemical Reduction of Bicarbonate on Carbon Nanotube-Supported Silver Oxide: An Electrochemical Impedance Spectroscopy Study. J. Environ. Chem. Eng. 2018, 6, 1033–1043. [Google Scholar] [CrossRef]
- Spichiger-Ulmann, M.; Augustynski, J. Electrochemical Reduction of Bicarbonate Ions at a Bright Palladium Cathode. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1985, 81, 713–716. [Google Scholar] [CrossRef]
- Li, T.; Lees, E.W.; Goldman, M.; Salvatore, D.A.; Weekes, D.M.; Berlinguette, C.P. Electrolytic Conversion of Bicarbonate into CO in a Flow Cell. Joule 2019, 3, 1487–1497. [Google Scholar] [CrossRef]
- Min, X.; Kanan, M.W. Pd-Catalyzed Electrohydrogenation of Carbon Dioxide to Formate: High Mass Activity at Low Overpotential and Identification of the Deactivation Pathway. J. Am. Chem. Soc. 2015, 137, 4701–4708. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Fujii, K.; Nakano, Y.; Jin, F. Effect of CO2 Bubbling into Aqueous Solutions Used for Electrochemical Reduction of CO2 for Energy Conversion and Storage. J. Phys. Chem. C 2014, 119, 55–61. [Google Scholar] [CrossRef]
- Kortlever, R.; Tan, K.H.; Kwon, Y.; Koper, M.T.M. Electrochemical Carbon Dioxide and Bicarbonate Reduction on Copper in Weakly Alkaline Media. J. Solid State Electrochem. 2013, 17, 1843–1849. [Google Scholar] [CrossRef]
- Hori, Y. Electrolytic Reduction of Bicarbonate Ion at a Mercury Electrode. J. Electrochem. Soc. 1983, 130, 2387–2390. [Google Scholar] [CrossRef]
- Sreekanth, N.; Phani, K.L. Selective Reduction of CO2 to Formate through Bicarbonate Reduction on Metal Electrodes: New Insights Gained from SG/TC Mode of SECM. Chem. Commun. 2014, 50, 11143–11146. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.R.L.; Yeo, B.S. Recent Advances in Understanding Mechanisms for the Electrochemical Reduction of Carbon Dioxide. Curr. Opin. Electrochem. 2018, 8, 126–134. [Google Scholar] [CrossRef]
- Isa, P.; Louis, H.; Adesina, K.; Akpan, E. Review article Understanding the Mechanism of Electrochemical Reduction of CO2 Using Cu/Cu-Based Electrodes. Asian J. Nano Mater. 2018, 1, 183–224. [Google Scholar]
- Garza, A.J.; Bell, A.T.; Gordon, M.H. Mechanism of CO2 Reduction at Copper Surfaces: Pathways to C2 Products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef]
- Hussain, J.; Skúlason, E.; Jónsson, H. Computational Study of Electrochemical CO2 Reduction at Transition Metal Electrodes. Procedia Comput. Sci. 2015, 51, 1865–1871. [Google Scholar] [CrossRef]
- Aslam, N.; Masdar, M.; Kamarudin, S.; Daud, W.R.W. Overview on Direct Formic Acid Fuel Cells (DFAFCs) as an Energy Sources. APCBEE Procedia 2012, 3, 33–39. [Google Scholar] [CrossRef]
- Rees, N.V.; Compton, R.G. Sustainable Energy: A Review of Formic Acid Electrochemical Fuel Cells. J. Solid State Electrochem. 2011, 15, 2095–2100. [Google Scholar] [CrossRef]
- Nogalska, A.; Navarro, A.B.; Garcia-Valls, R. MEA Preparation for Direct Formate/Formic Acid Fuel Cell—Comparison of Palladium Black and Palladium Supported on Activated Carbon Performance on Power Generation in Passive Fuel Cell. Membranes 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, O.Z.; Orhan, M.F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Mohapatra, A.; Tripathy, S. A Critical Review of the use of Fuel Cells Towards Sustainable Management of Resources. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Mechanical, Materials and Renewable Energy, Sikkim, India, 8–10 December 2017; IOP Publishing: Bristol, UK, 2018; Volume 377, p. 012135. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Viva, F.A.; Olah, G.A. Electrochemical Reduction of CO2 over Sn-Nafion® Coated Electrode for a Fuel-Cell-like Device. J. Power Sources 2013, 223, 68–73. [Google Scholar] [CrossRef]
- Cui, C.; Wang, H.; Zhu, X.; Han, J.; Ge, Q. A DFT Study of CO2 Electrochemical Reduction on Pb(211) and Sn(112). Sci. China Chem. 2015, 58, 607–613. [Google Scholar] [CrossRef]
- Bumroongsakulsawat, P.; Kelsall, G. Effect of Solution pH on CO: Formate Formation Rates during Electrochemical Reduction of Aqueous CO2 at Sn Cathodes. Electrochim. Acta 2014, 141, 216–225. [Google Scholar] [CrossRef]
- Saravanan, K.; Basdogan, Y.; Dean, J.; Keith, J.A. Computational Investigation of CO2 Electroreduction on Tin Oxide and Predictions of Ti, V, Nb and Zr Dopants for Improved Catalysis. J. Mater. Chem. A 2017, 5, 11756–11763. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; Pupillo, R.C.; Keane, T.P.; DiMeglio, J.L.; Rosenthal, J. Efficient Conversion of CO2 to CO Using Tin and Other Inexpensive and Easily Prepared Post-Transition Metal Catalysts. J. Am. Chem. Soc. 2015, 137, 5021–5027. [Google Scholar] [CrossRef]
- Zhang, R.; Lv, W.; Lei, L. Role of the Oxide Layer on SN Electrode in Electrochemical Reduction of CO2 to Formate. Appl. Surf. Sci. 2015, 356, 24–29. [Google Scholar] [CrossRef]
- Sheng, W.; Kattel, S.; Yao, S.; Yan, B.; Liang, Z.; Hawxhurst, C.J.; Wu, Q.; Chen, J.G. Electrochemical Reduction of CO2 to Synthesis Gas with Controlled CO/H2 ratios. Energy Environ. Sci. 2017, 10, 1180–1185. [Google Scholar] [CrossRef]
- Rumayor, M.; Dominguez-Ramos, A.; Irabien, A. Formic Acid Manufacture: Carbon Dioxide Utilization Alternatives. Appl. Sci. 2018, 8, 914. [Google Scholar] [CrossRef]
- Noda, H.; Ikeda, S.; Oda, Y.; Imai, K.; Maeda, M.; Ito, K. Electrochemical Reduction of Carbon Dioxide at Various Metal Electrodes in Aqueous Potassium Hydrogen Carbonate Solution. Bull. Chem. Soc. Jpn. 1990, 63, 2459–2462. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kudo, A.; Azuma, M.; Sakata, T. Effect of Pressure on the Electrochemical Reduction of CO2 on Group VIII Metal Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1991, 308, 339–343. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective Electroreduction of Carbon Dioxide to Methanol on Copper Selenide Nanocatalysts. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]


| KHCO3 (aq) Concentration (M) | 0.1 M | 0.5 M | 1.5 M | 1.5 M |
|---|---|---|---|---|
| CO2 pre-saturation | No | No | No | Yes |
| Faradaic efficiency (%) | n.d. | 8 | 18 | 47 |
| Electrode | Reference Electrode | Electrolyte | CO2 Saturation | Faradaic Efficiency (%) towards HCOOK/HCOOH | Ref. |
|---|---|---|---|---|---|
| Ag (99.98%) electrode | −1.6 V vs. Ag/AgCl saturated with KCl | 0.1M KHCO3 aqueous solution | No | n | [30] |
| Au (99.95%) electrode | 6 | ||||
| Pd Metal | 1.8 V vs. Ag/AgCl | 0.1 M KHCO3 aqueous solution | No | 4.4 | [31] |
| Cu-Based catalysts | Ag/Ag+ with 0.01 M | 0.5 M KHCO3 aqueous solution | Yes | 3–15 | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonet Navarro, A.; Nogalska, A.; Garcia-Valls, R. Direct Electrochemical Reduction of Bicarbonate to Formate Using Tin Catalyst. Electrochem 2021, 2, 64-70. https://doi.org/10.3390/electrochem2010006
Bonet Navarro A, Nogalska A, Garcia-Valls R. Direct Electrochemical Reduction of Bicarbonate to Formate Using Tin Catalyst. Electrochem. 2021; 2(1):64-70. https://doi.org/10.3390/electrochem2010006
Chicago/Turabian StyleBonet Navarro, Andreu, Adrianna Nogalska, and Ricard Garcia-Valls. 2021. "Direct Electrochemical Reduction of Bicarbonate to Formate Using Tin Catalyst" Electrochem 2, no. 1: 64-70. https://doi.org/10.3390/electrochem2010006
APA StyleBonet Navarro, A., Nogalska, A., & Garcia-Valls, R. (2021). Direct Electrochemical Reduction of Bicarbonate to Formate Using Tin Catalyst. Electrochem, 2(1), 64-70. https://doi.org/10.3390/electrochem2010006





