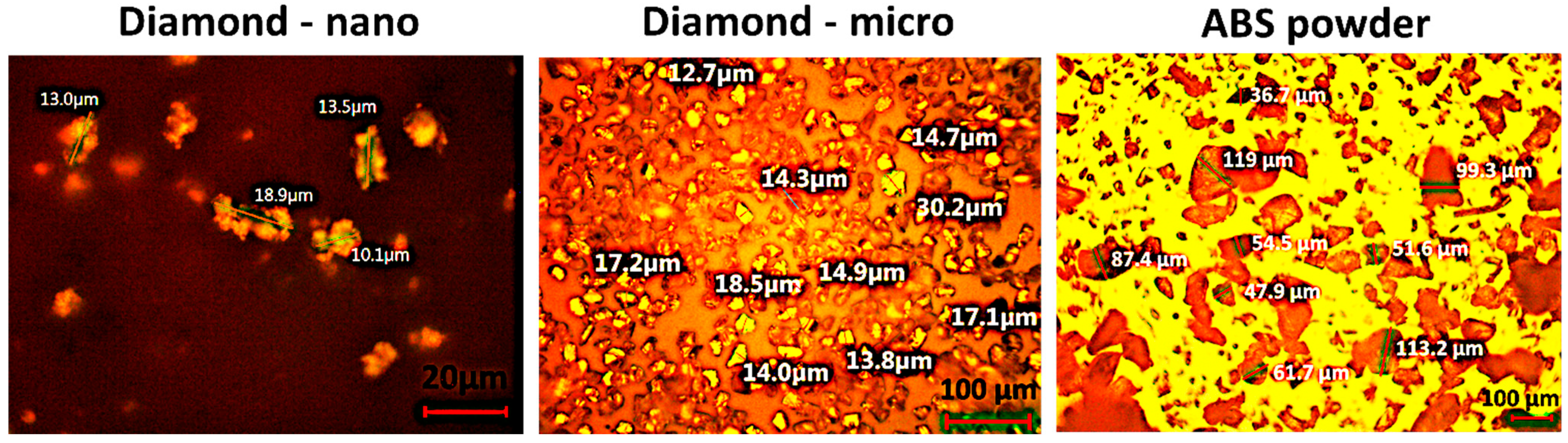
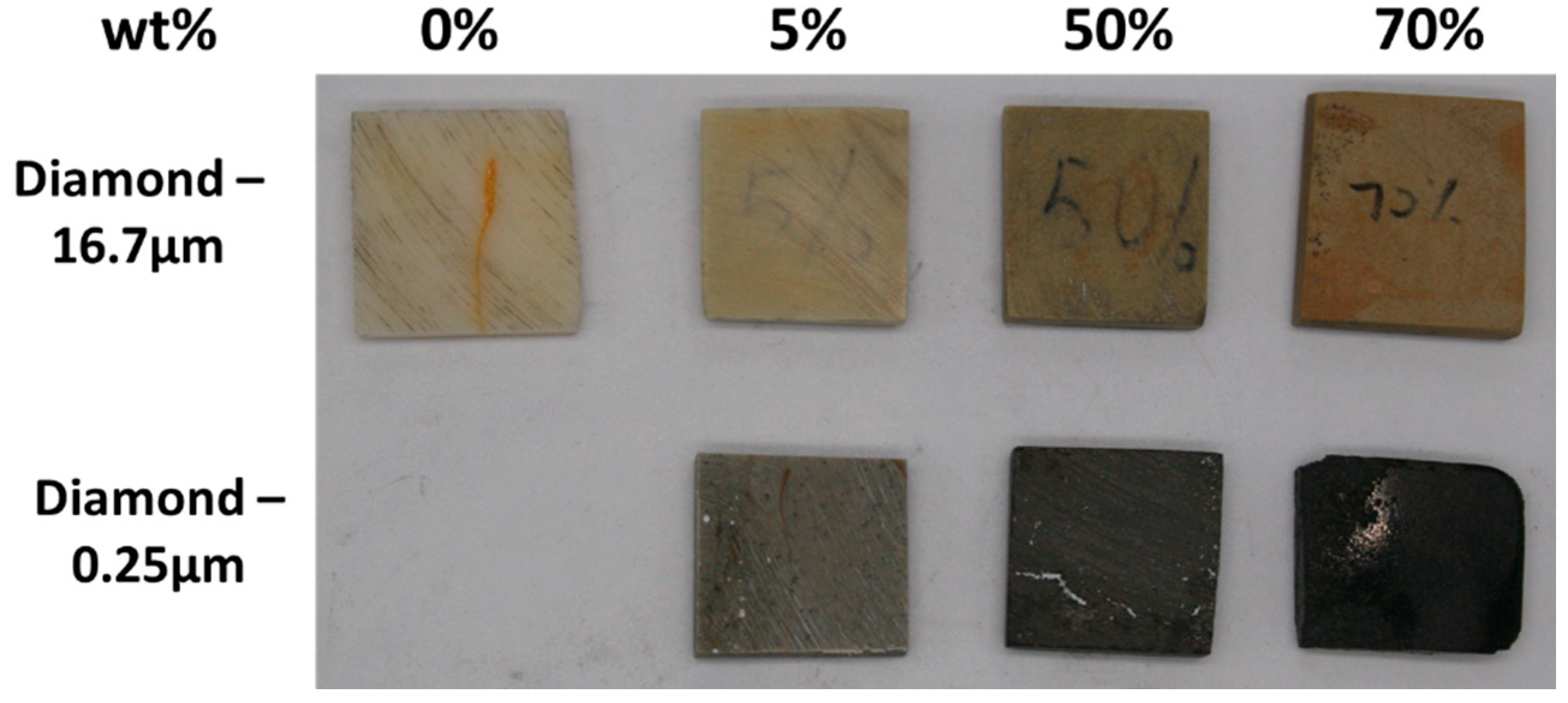
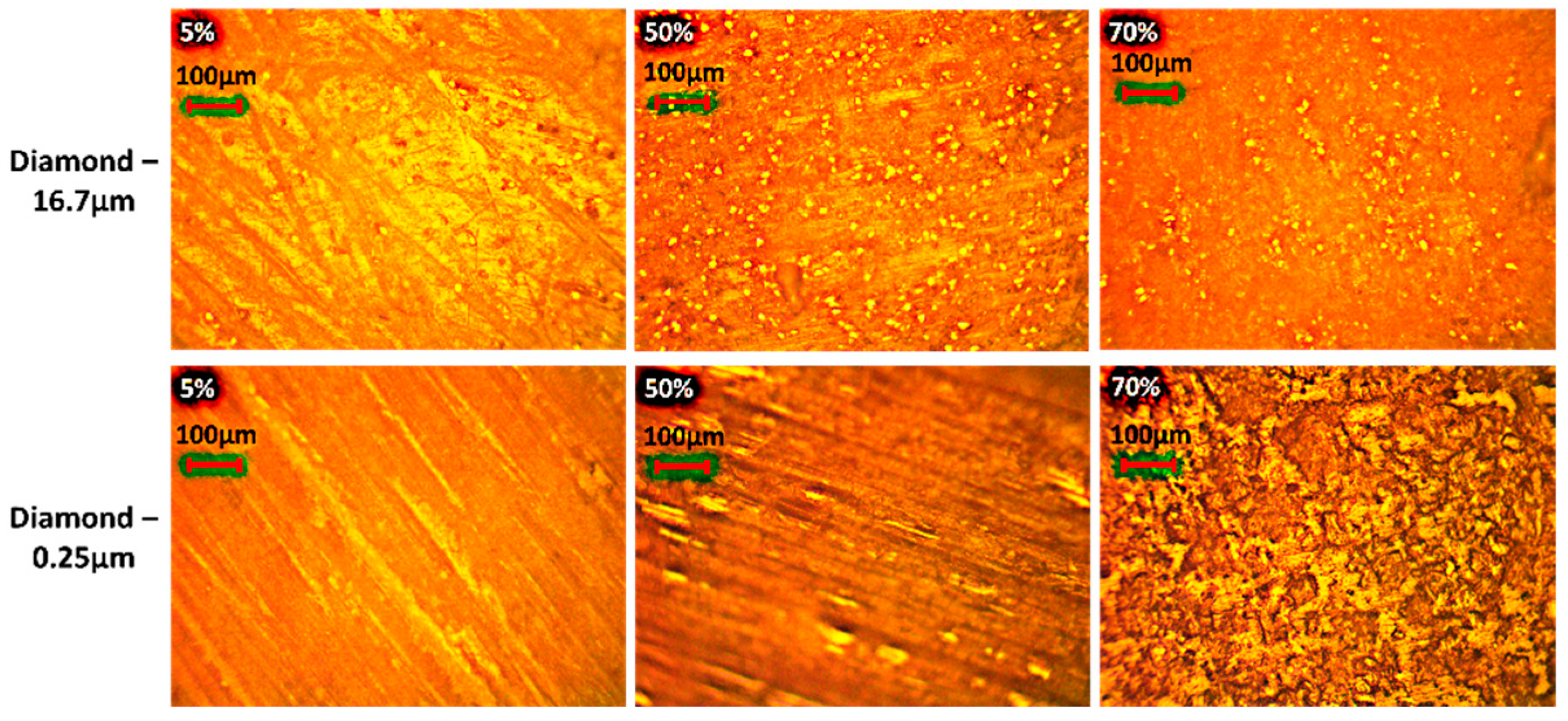
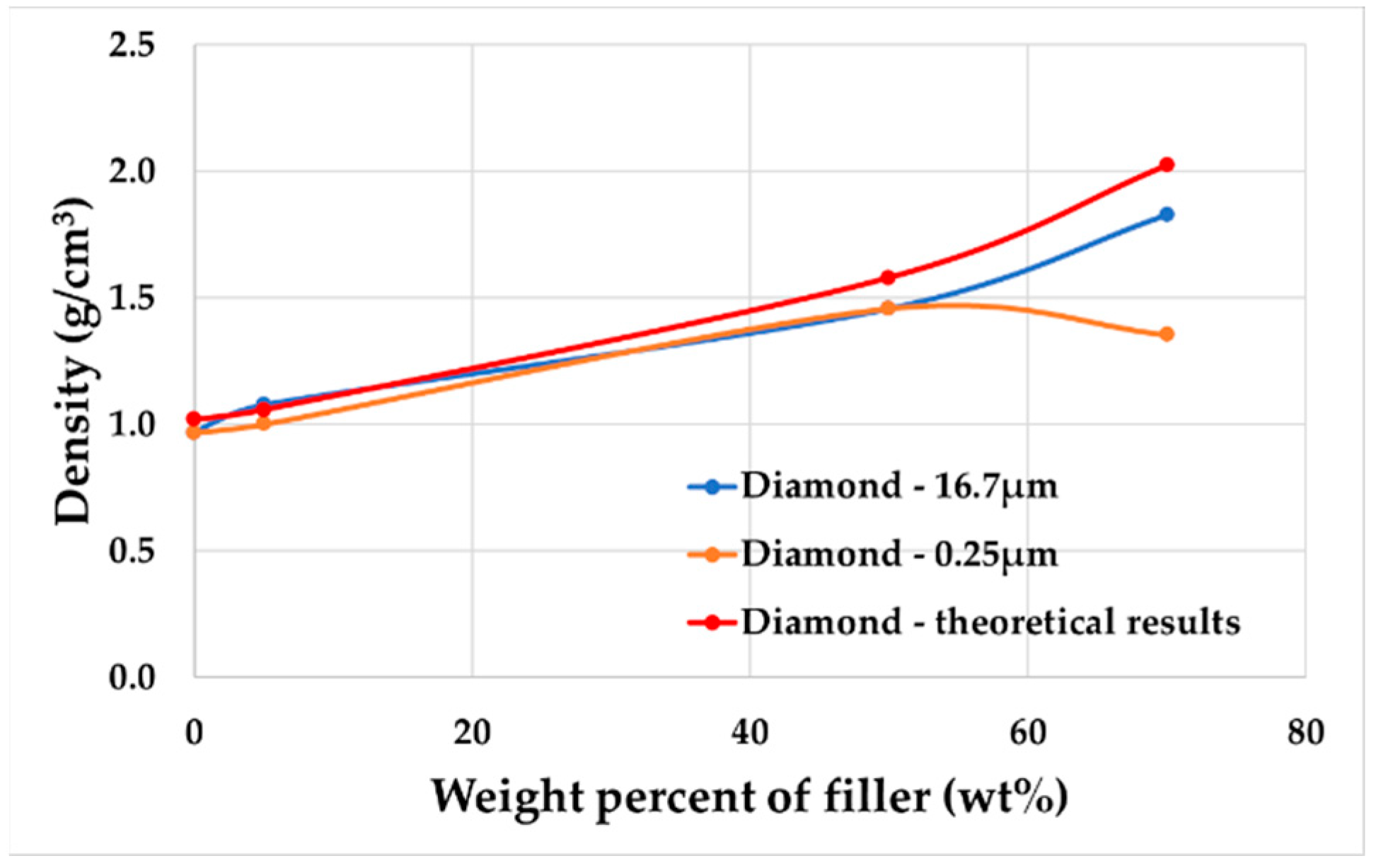
1. Introduction
Polymer heat exchangers (HXs) are lightweight and cost-effective due to the affordability of raw materials. They also offer excellent resistance to corrosion, making them ideal for use in harsh environments. Advanced polymer 3D printing technologies, such as fused filament fabrication (FFF) or fused deposition modeling (FDM), enable the production of highly customized polymer heat exchangers at lower costs and faster speeds. Moreover, 3D printing facilitates the integration of Triply Periodic Minimal Surface (TPMS) structures into the heat exchanger core. A TPMS structure enhances heat transfer efficiency from liquid to the solid wall and also strengthens the polymer heat exchanger, improving its overall performance [
1,
2,
3].
Despite these advantages, polymer heat exchangers (HXs) still face challenges. Polymers generally have lower thermal conductivity (TC) compared to metals like copper or aluminum. As a result, polymer heat exchangers may not be as efficient as their metal counterparts and are limited in applications where maximizing heat transfer is critical. Therefore, improving the thermal conductivity of polymer materials is essential.
To enhance thermal conductivity, high thermally conductive fillers such as diamond powder, carbon, or metal particles are added into 3D-printable polymers like Acrylonitrile Butadiene Styrene (ABS) to create thermally conductive polymer composites (TCPCs) [
4]. These fillers enhance the overall thermal conductivity by forming a percolating network that facilitates thermal transport [
5]. TCPCs combine the benefits of polymer matrices with the distinct properties of conductive fillers, offering a cost-effective and easy-to-process solution to increase polymer thermal conductivity. This approach has been gaining significant attention.
Several factors influence the properties of the composite, including the filler volume ratio, filler shape, alignment, the surface treatment of the filler, and its intrinsic conductivity [
6,
7]. Generally, the thermal conductivity of polymer composites increases with the filler volume fraction. A high filler aspect ratio can create a conductive network at a lower volume fraction due to a reduced percolation threshold [
8,
9].
Filler particle size also plays a significant role in determining thermal conductivity. Polydisperse fillers generally exhibit higher thermal conductivity compared to monodisperse fillers [
10]. Valeria et al. [
11] found that composites containing 5 µm copper particles tend to have lower thermal conductivity than those with 150 µm copper particles. Li et al. [
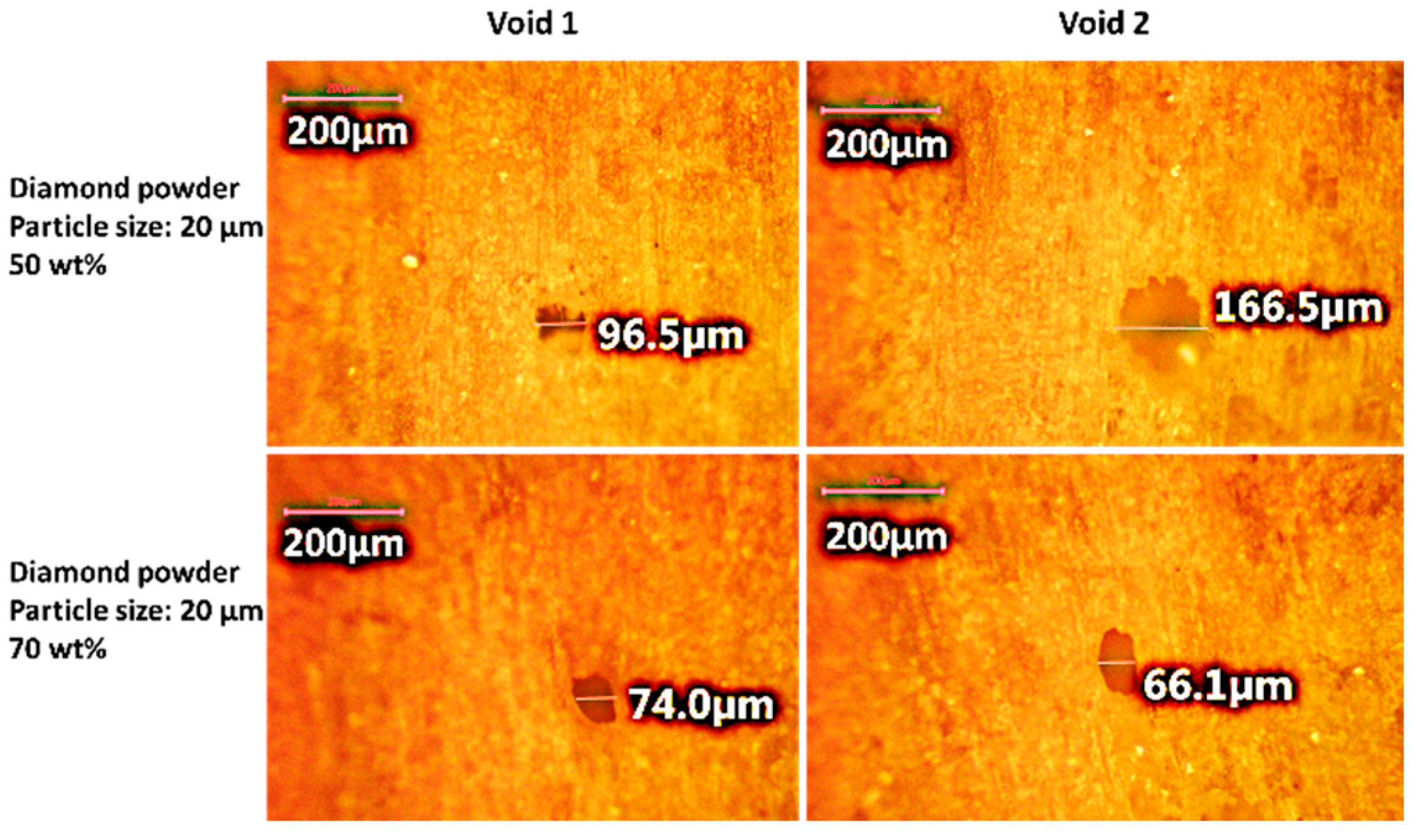
12] observed that smaller nanoparticles can lead to higher thermal conductivities due to their more widely spread aggregation structures in epoxy at the same concentration. The size effect of fillers at the nanoscale may differ from that of fillers at the microscale. While most studies focus on the effects of filler size at the micro level, further research on the impact of small filler sizes at the nanoscale is needed. Interfacial defects, such as voids or air gaps, increase thermal resistance by promoting phonon scattering [
13,
14,
15]. The high interfacial thermal resistance between the filler and the polymer matrix is a key factor limiting the thermal conductivity of polymer composites [
16]. The surface treatment of fillers can improve bonding with the polymer matrix, reducing interfacial flaws and thermal contact resistance.
Several theoretical models have been developed to calculate the thermal conductivity of polymer composites. Maxwell [
17] considered spherical particles with thermal conductivity
embedded in a continuous matrix with thermal conductivity
, assuming a small volume ratio where the particles are far enough apart relative to their size. Maxwell proposed a formula to determine the effective thermal conductivity of the composite material, as shown below:
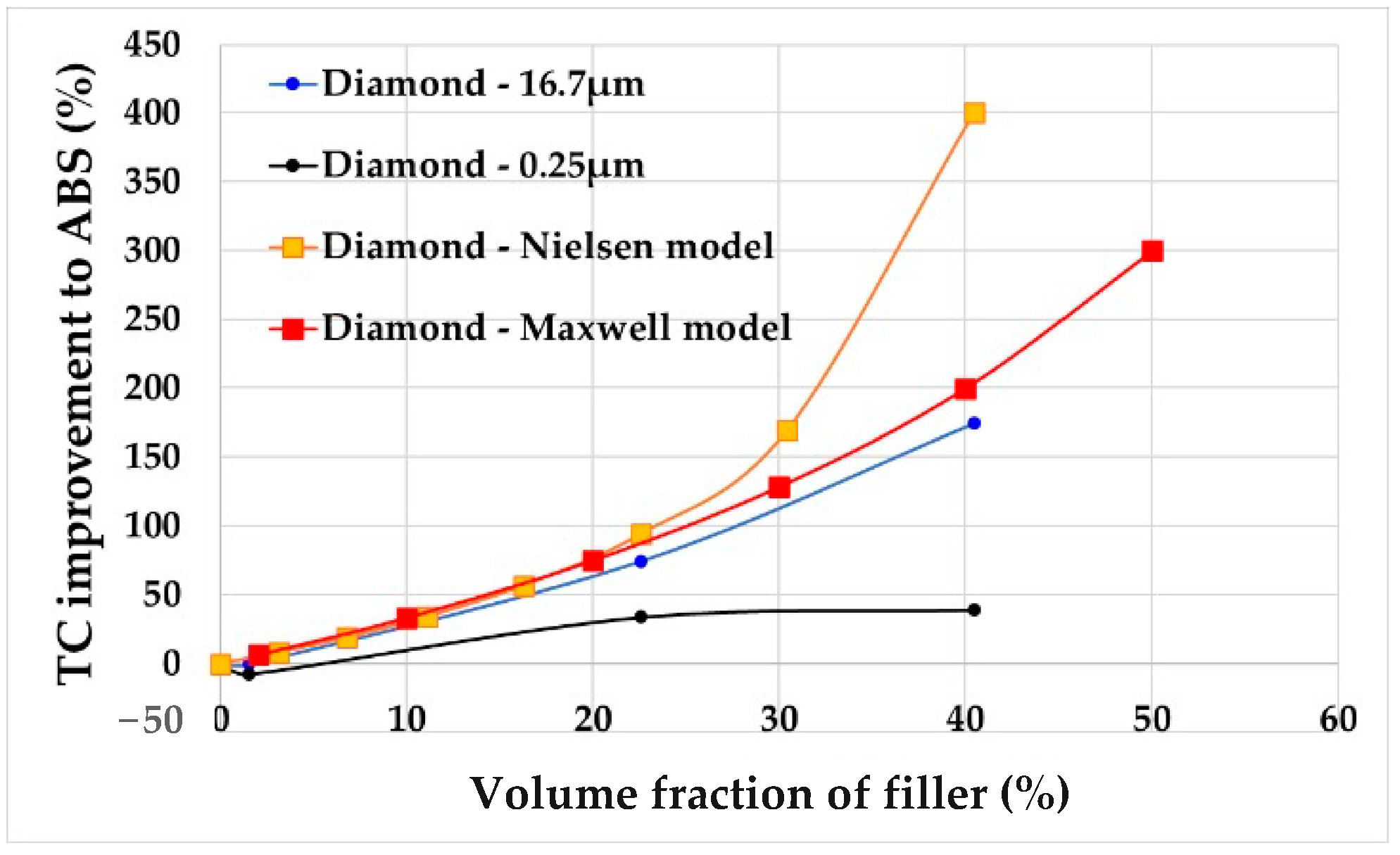
Maxwell’s theory is accurate for a low filler volume ratio, typically up to around 25%.
Percolation theory [
7,
18] can be applied to understand the impact of filler volume fraction on heat transfer in polymer composites. The effective thermal conductivity of filled polymer composites can be determined using the following equation derived from percolation theory:
where
is the effective thermal conductivity of composites when
, and percolation exponent
is dependent on the filler size, shape and distribution in the composites.
is the volume ratio. Once the filler volume ratio surpasses the percolation threshold, the thermal conductivity of the composite increases significantly with the filler volume ratio.
The thermal conductivity of polymer composites can also be estimated using the Nielsen theory [
19]. The Nielsen model provides an accurate prediction of thermal conductivity up to approximately a 40% filler volume fraction and considers the effect of particle size.
—thermal conductivity of the polymer composite;
—thermal conductivity of matrix material;
—thermal conductivity of filler;
—generalized Einstein coefficient;
—the true volume of the particles divided by the volume they appear to occupy when packed to their maximum extent;
—volume fraction.
According to these theories, factors such as the volume ratio, filler aspect ratio and filler thermal conductivity influence the thermal conductivity of polymer composites.
ABS (Acrylonitrile Butadiene Styrene) is a common polymer filament used in 3D printing. The glass transition temperature (Tg) of ABS typically ranges between 105 °C and 120 °C (221 °F to 248 °F). The high Tg of ABS allows it to be used in application involving heat transfer from high-temperature liquids, such as water. Diamond has the highest thermal conductivity, which is about 2000 W/(mK), and is an excellent heat conductor. Moreover, diamond has isotropic properties of thermal conductivity.
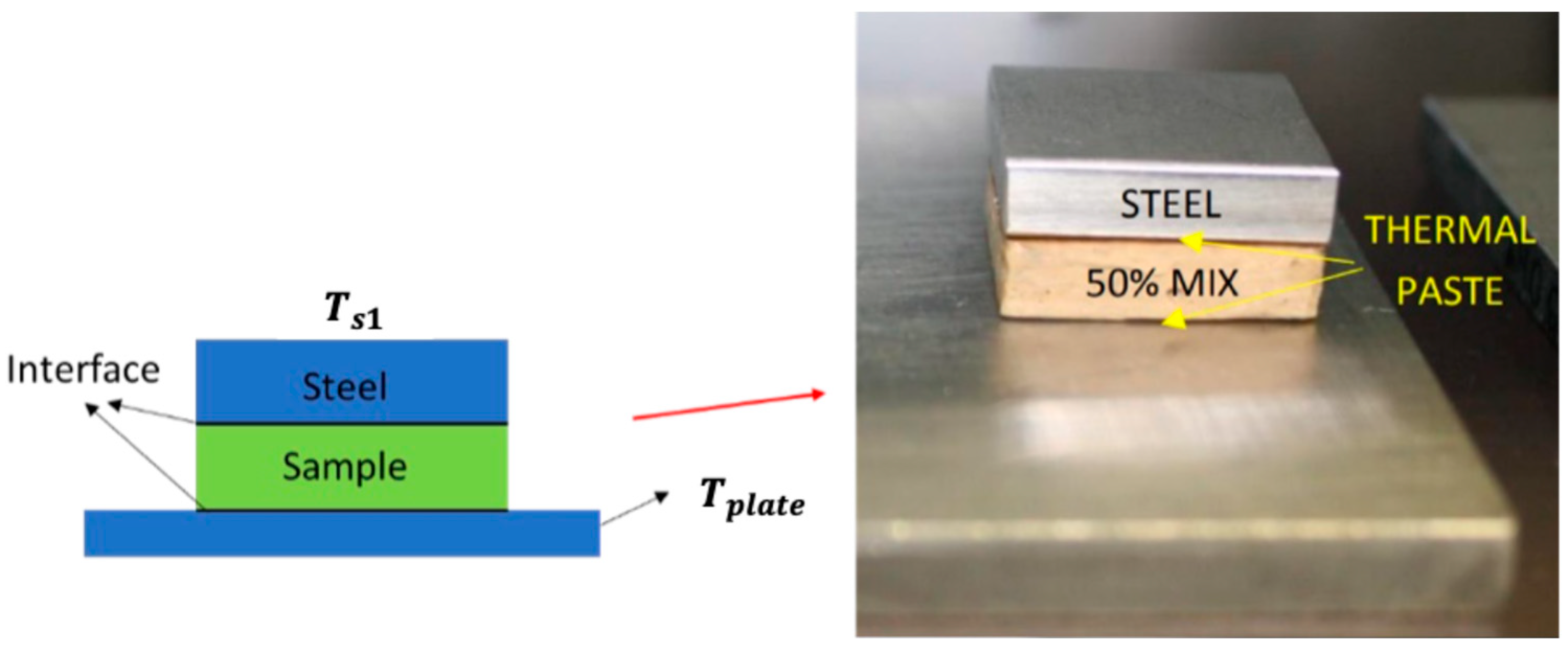
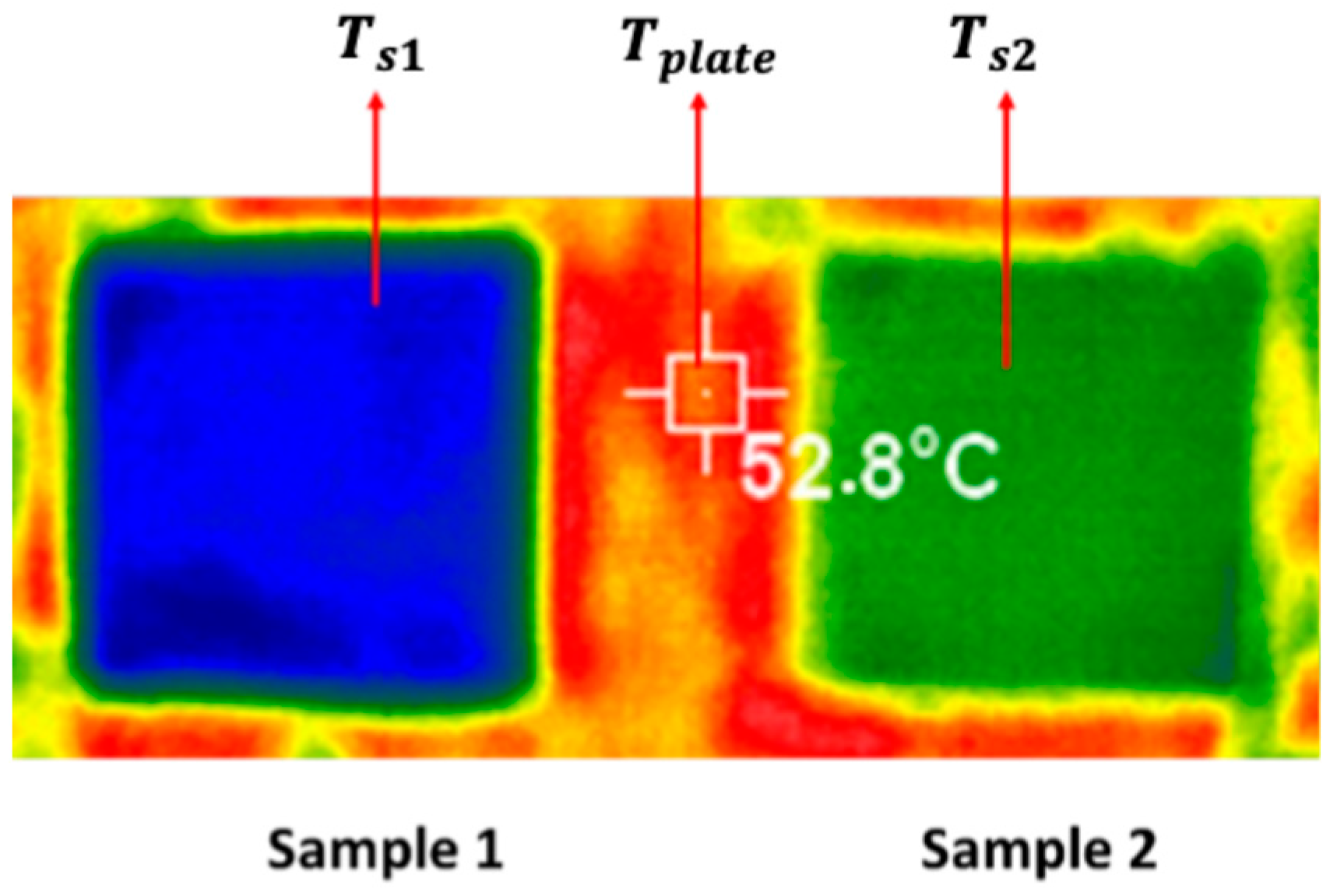
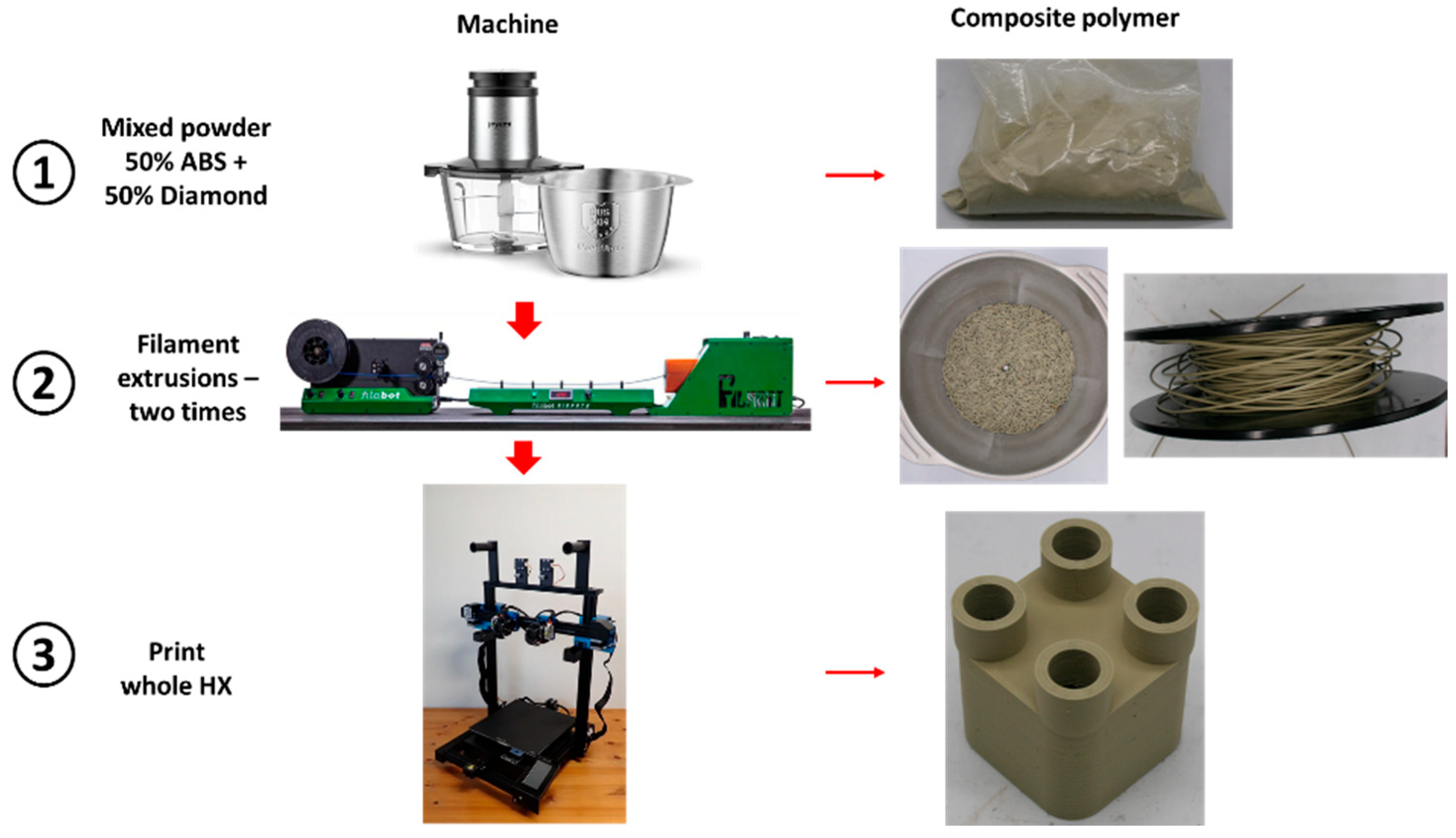
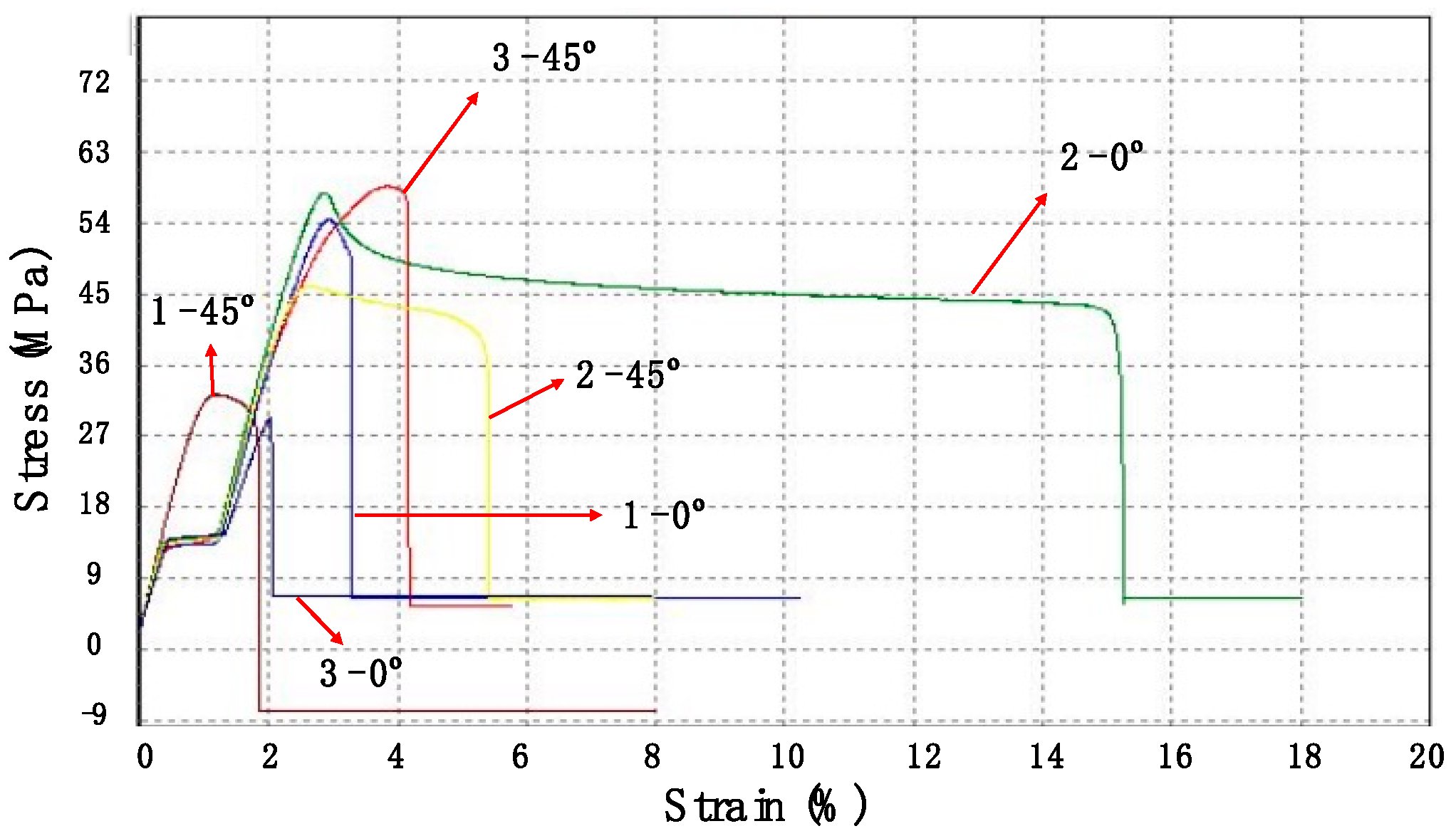
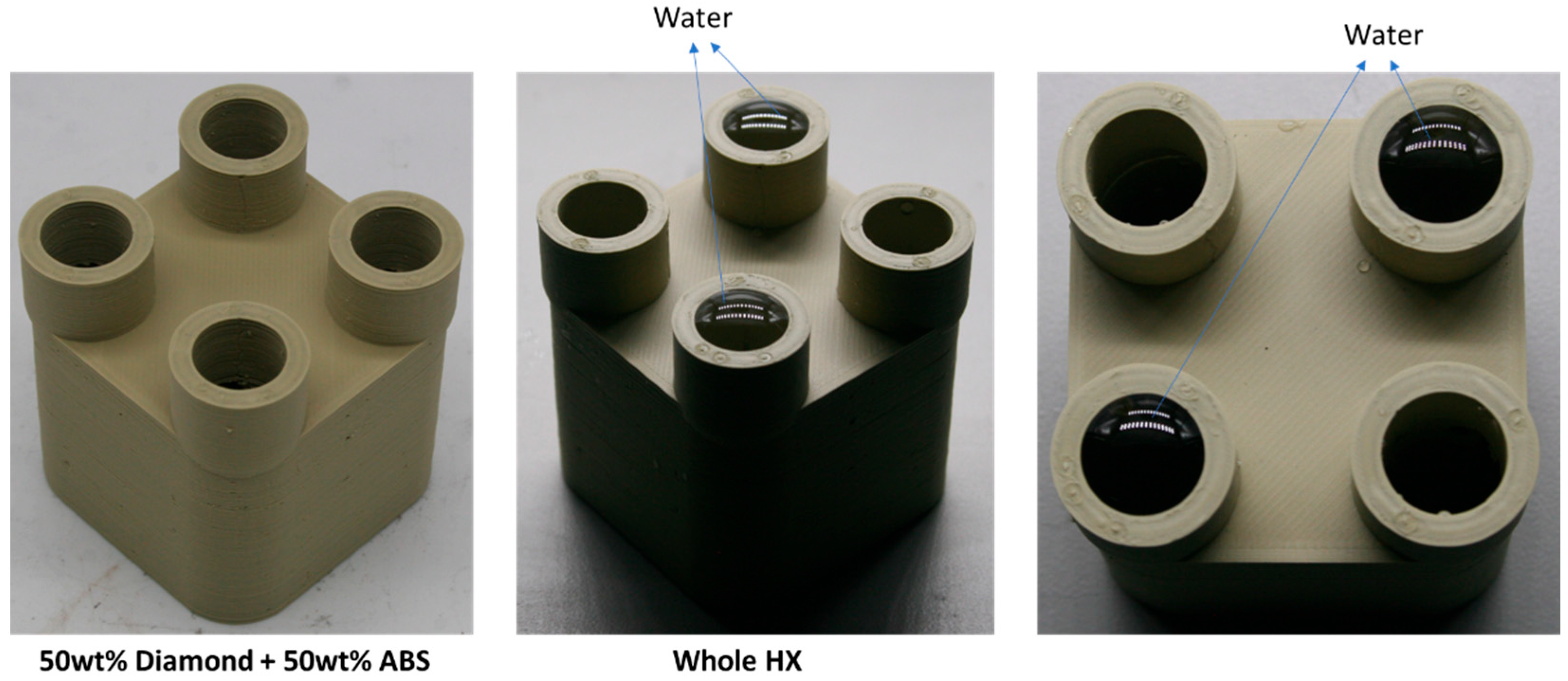
In this paper, diamond powder at both the nanoscale and microscale were used as fillers and added into ABS to create thermally conductive polymer composites (TCPCs). The density and thermal conductivity of two types of polymer composites were tested and compared. A heat exchanger (HX) model based on a gyroid lattice was designed and printed by a 3D printer. The heat transfer capacity of a polymer HX was evaluated through simulations and experimental testing. The results of the polymer HX were also compared with a metal HX. Key factors influencing the HX’s performance were analyzed. A mixed powder of 50 wt% ABS and 50 wt% microdiamond was extruded into filament. To enhance the filament quality, it was extruded twice. Using the extruded filament, a complete HX was successfully printed without any leakage. The mechanical strength of ABS polymer parts by 3D printing was tested.
Through the research in this paper, it has been demonstrated that adding fillers to the matrix can enhance the thermal conductivity of polymer composites. The experimental study also shows that nanometer-scale fillers do not significantly improve the thermal conductivity of polymer composites. The thermal conductivity of the polymer composites fabricated in this study still does not meet the practical requirements of heat exchangers. Further in-depth research on the relevant influencing factors is needed to improve the thermal conductivity of polymer composites. This study indicates that using a TPMS structure in polymer heat exchangers can improve heat transfer efficiency and reduce flow resistance. The internal flow channels of the polymer heat exchanger have a smoother surface, resulting in lower flow resistance compared to metal heat exchangers. This paper demonstrates the feasibility of the process by 3D printing a complete polymer heat exchanger using a polymer composite with a high weight fraction, proving the technological feasibility of this approach.
3. HX Modeling with Gyroid Lattice
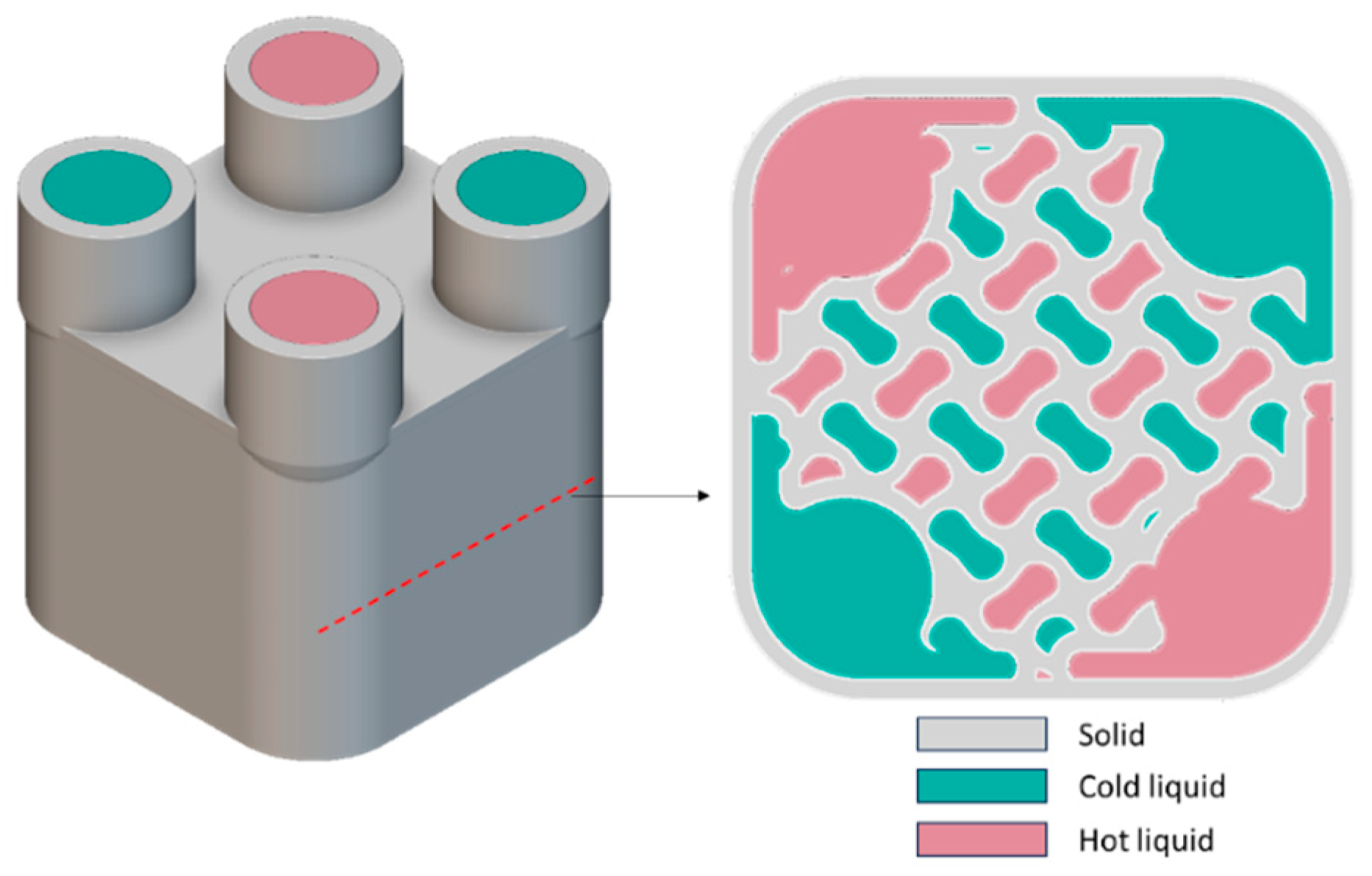
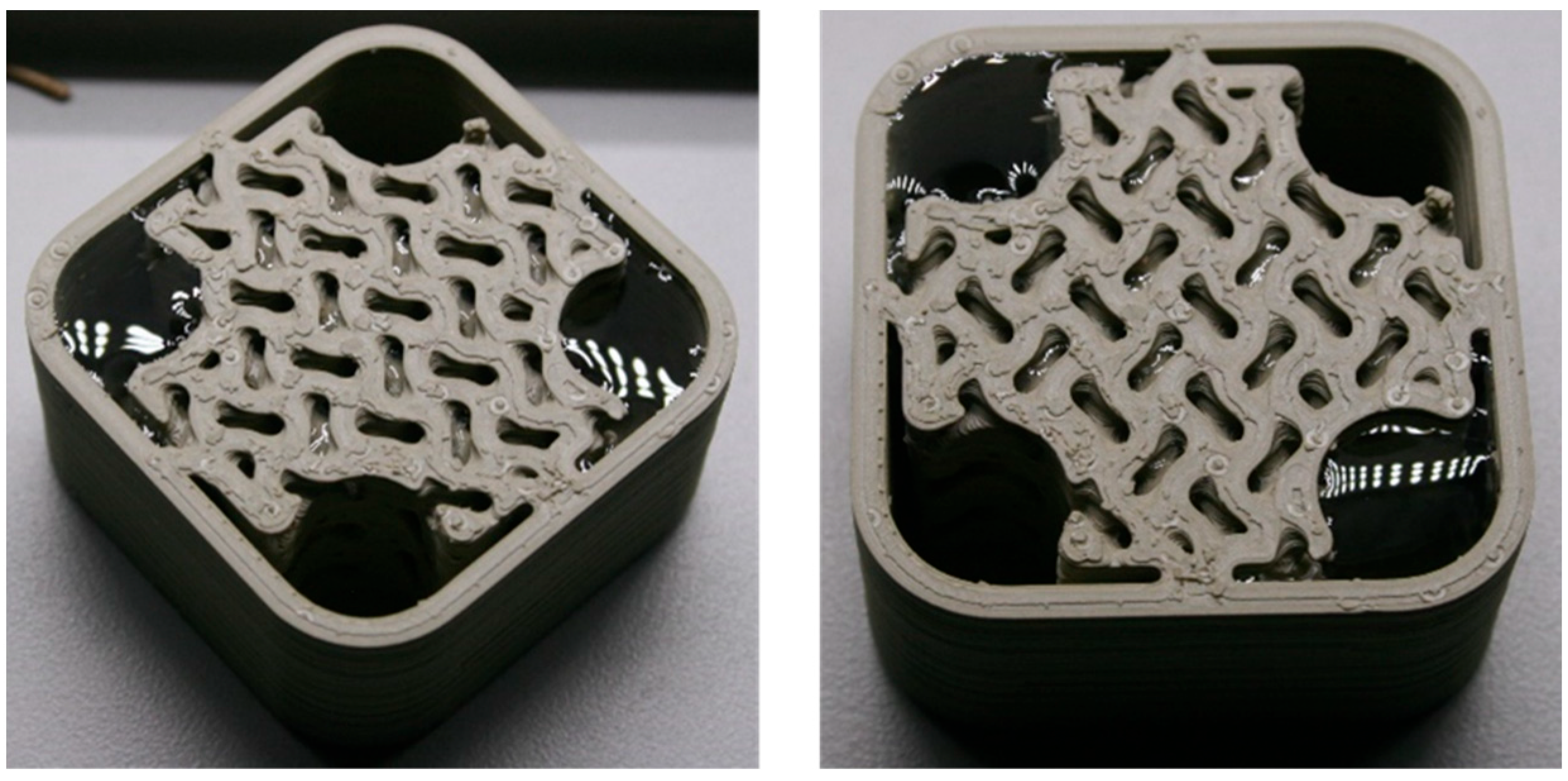
As shown in
Figure 9, a polymer heat exchanger (HX) based on a gyroid lattice design was created. The HX consists of two volumes: one for cold water and the other for hot water. Heat is transferred from the hot water to the cold water. The gyroid lattice has dimensions of 12 × 12 × 12 mm, with a wall thickness of 2 mm. This design ensures that there is no leakage between the two volumes after printing.
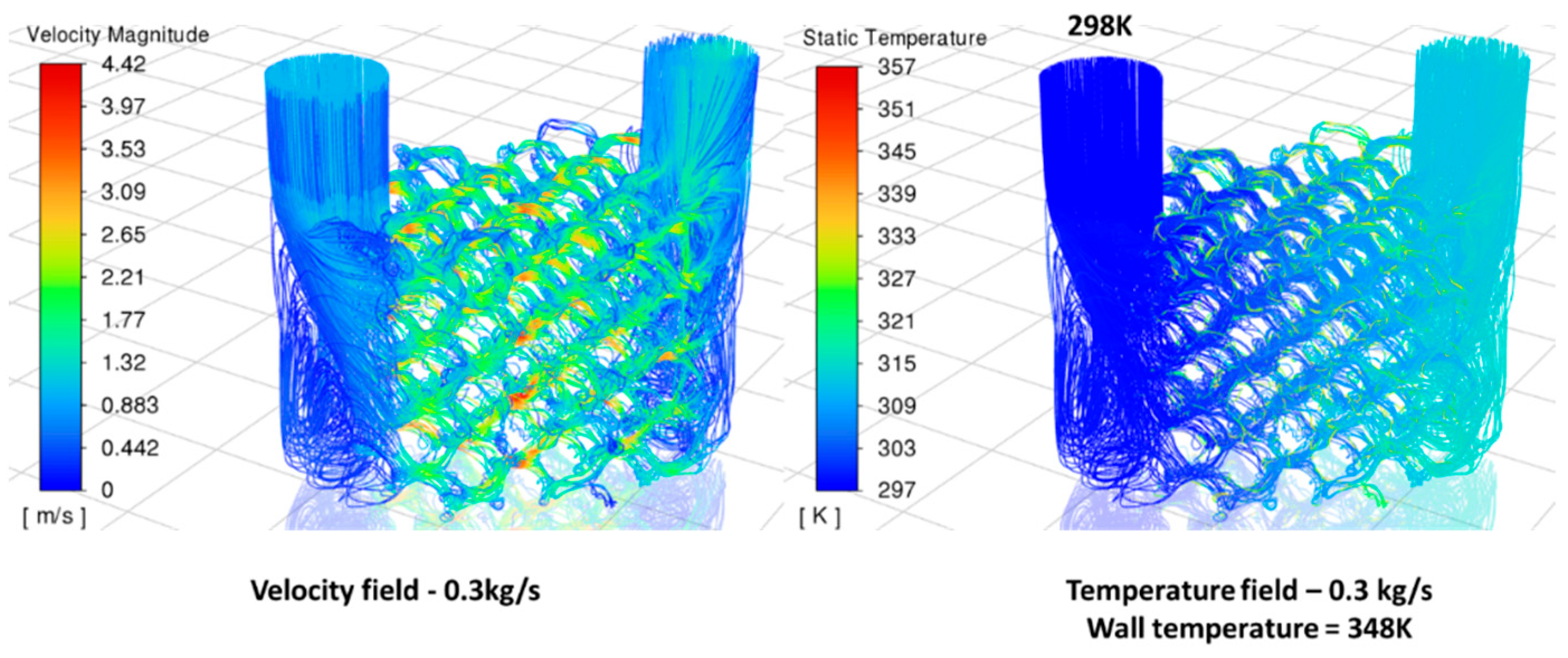
As shown in
Figure 10, one fluid volume was calculated based on the symmetry of the two volumes. The inlet temperature of the water is 298 K, while the wall temperature of the lattice structure is maintained at a constant 348 K. The pressure drop and overall heat transfer coefficient of the single volume (
) in the polymer HX were calculated through simulation.
The turbulence of the flow within the TPMS structure can be evaluated using the Reynolds number (Re), which represents the ratio of inertial forces to viscous forces and is defined as
where
is the fluid density (kg/m
3),
is velocity in porous media (m/s),
is the fluid viscosity (Pa.s), and
is the hydraulic diameter. The definition of the hydraulic diameter employed in the TPMS structures is
, where
is the specific surface area (m
2/m
3). Porosity
is defined as the fraction of the volume of voids over the total volume in the heat exchanger [
21].
To calculate velocity in porous media, a cross-sectional plane at the midpoint of the heat exchanger along the flow direction within a control volume in ANSYS Fluent 2024 is created and the mean velocity across the section is calculated.
The thermal and hydraulic performance of heat exchangers can be evaluated using the Nusselt number (Nu).
where
is the heat transfer coefficient of one volume,
is the surface area between the interface of fluid and solid or total wetted surface area (m
2),
is the fluid thermal conductivity (W/(m×K)).
For the simulation model, the heat transfer coefficient is calculated by the equation below:
is the heat transfer power;
is the surface area for heat transfer;
is the fluid inlet temperature;
is the fluid outlet temperature;
is the temperature of the wall, which is constant.
In the simulation, the temperature of the wall is set to be constant. The average temperature gap
is represented by the equation below:
For the test results, the heat transfer coefficient within a single volume can be calculated from the overall heat transfer coefficient using Equation (14), assuming that both volumes exhibit the same heat transfer coefficient due to the structural symmetry.
The friction factor (
) is commonly used to compare pressure loss in cooling channels and can be calculated using the following formula:
where
is the pressure loss in the system (Pa), and
is the channel length (m) [
21].
The Nu and f can be used together to evaluate the performance of the heat exchanger.
The metal heat exchanger (HX) in
Figure 11 has the same dimensions as the polymer heat exchanger. The metal HX contains a lattice (10 × 10 × 10 mm) with a wall thickness of 1 mm in the core and is printed with AlSi10Mg powder, while the polymer HX has a larger lattice (12 × 12 × 12 mm) with a wall thickness of 2 mm. The Nusselt number (Nu) and friction factor (f) of the metal HX was calculated by the test results. Further details about the testing of the metal HX can be found in reference paper [
1]. The Nusselt number (Nu) and friction factor (f) for the polymer HX was calculated by the simulation results. The pressure drop of the polymer HX at a flow rate of 0.2 kg/s was validated by the test results which are shown at
Table 4 and
Table 5. The simulation result regarding pressure drop at 0.2 kg/s matches the test results. No correction factor is applied on the simulation results of the polymer HX.
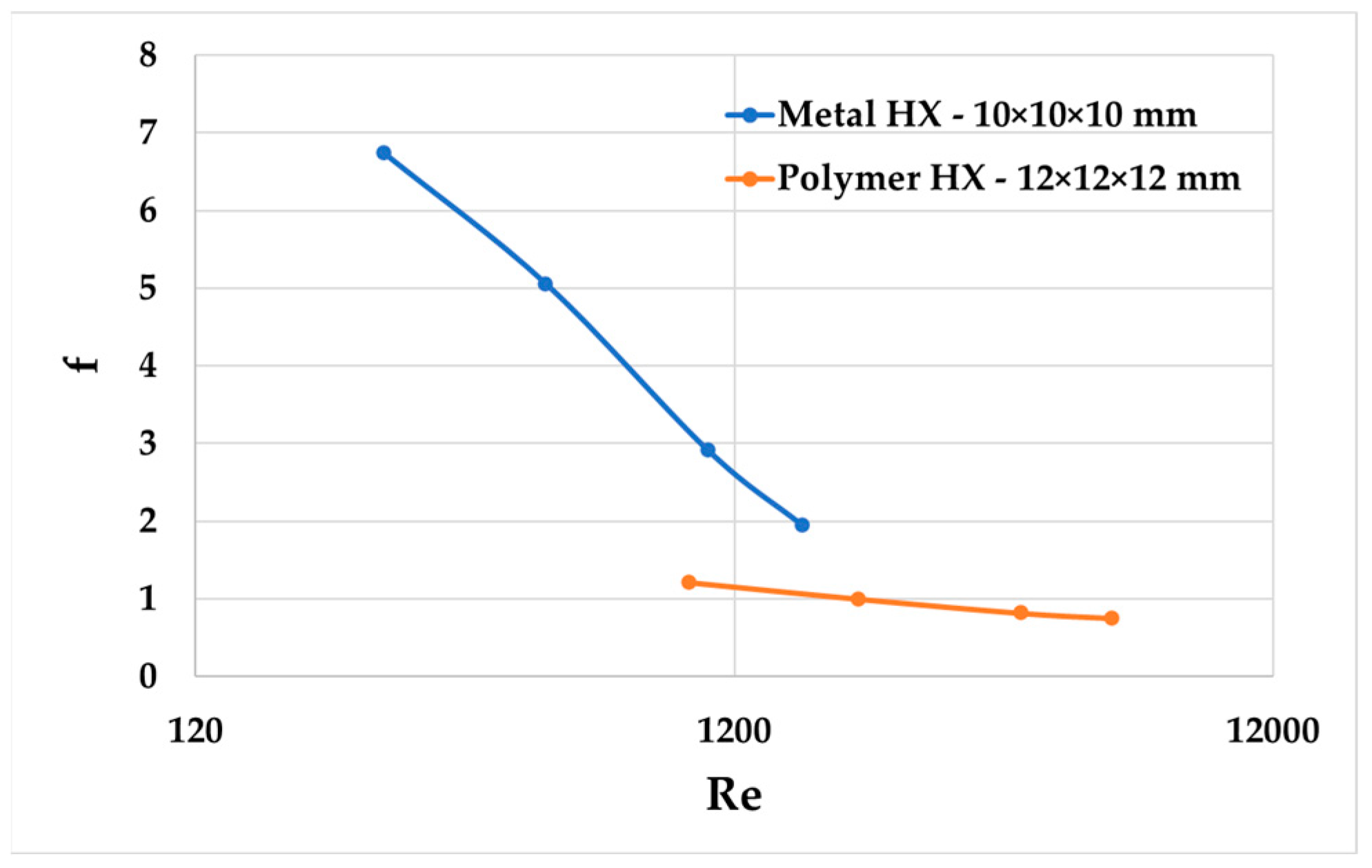
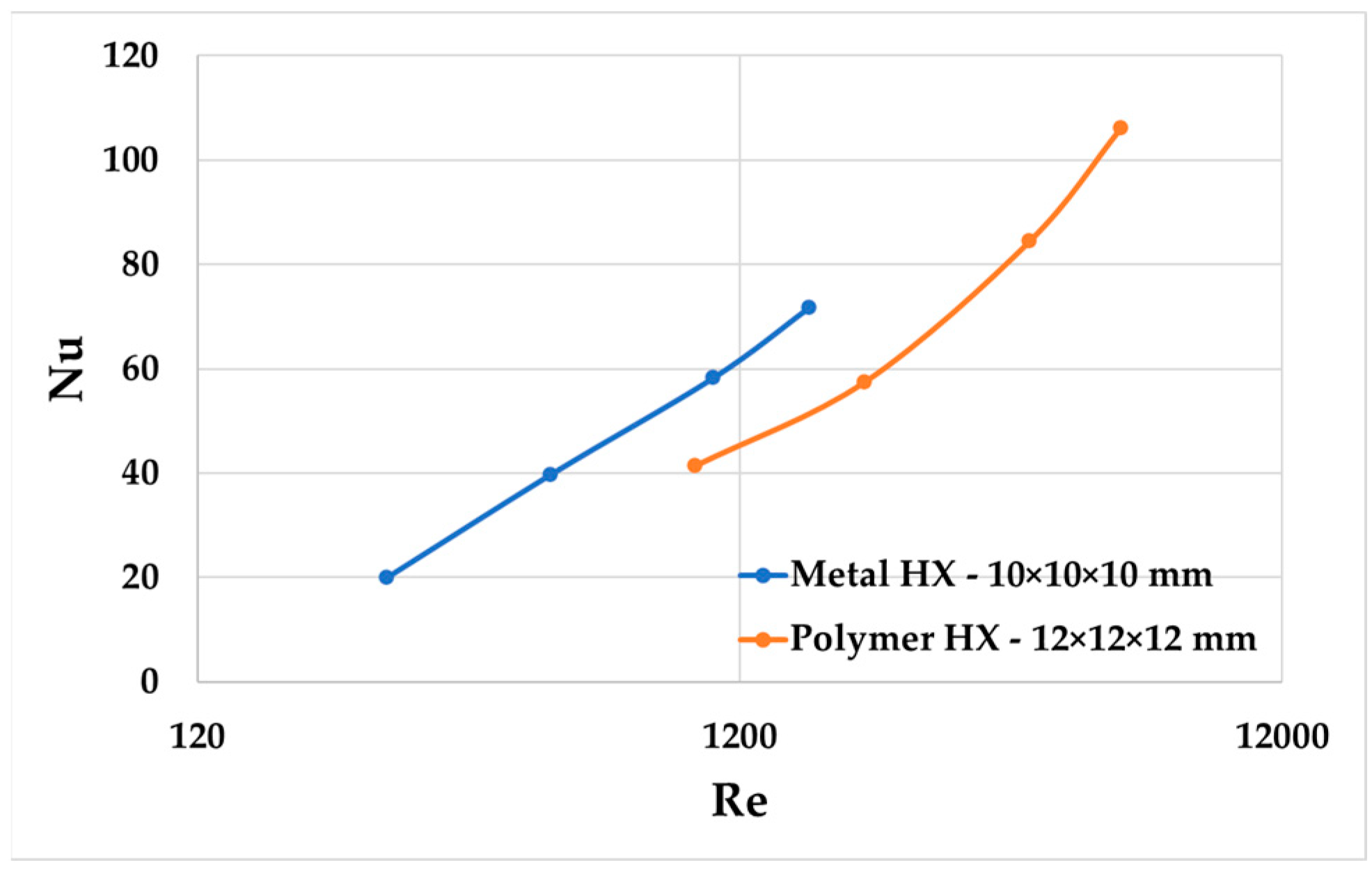
As shown in
Figure 11 and
Figure 12, an increase in friction factors at a low Re number in the metal HX was observed compared to the polymer HX. Nusselt numbers as functions of the Reynolds number in the metal HX had an upward and leftward shift across different Reynolds numbers compared to the polymer HX.
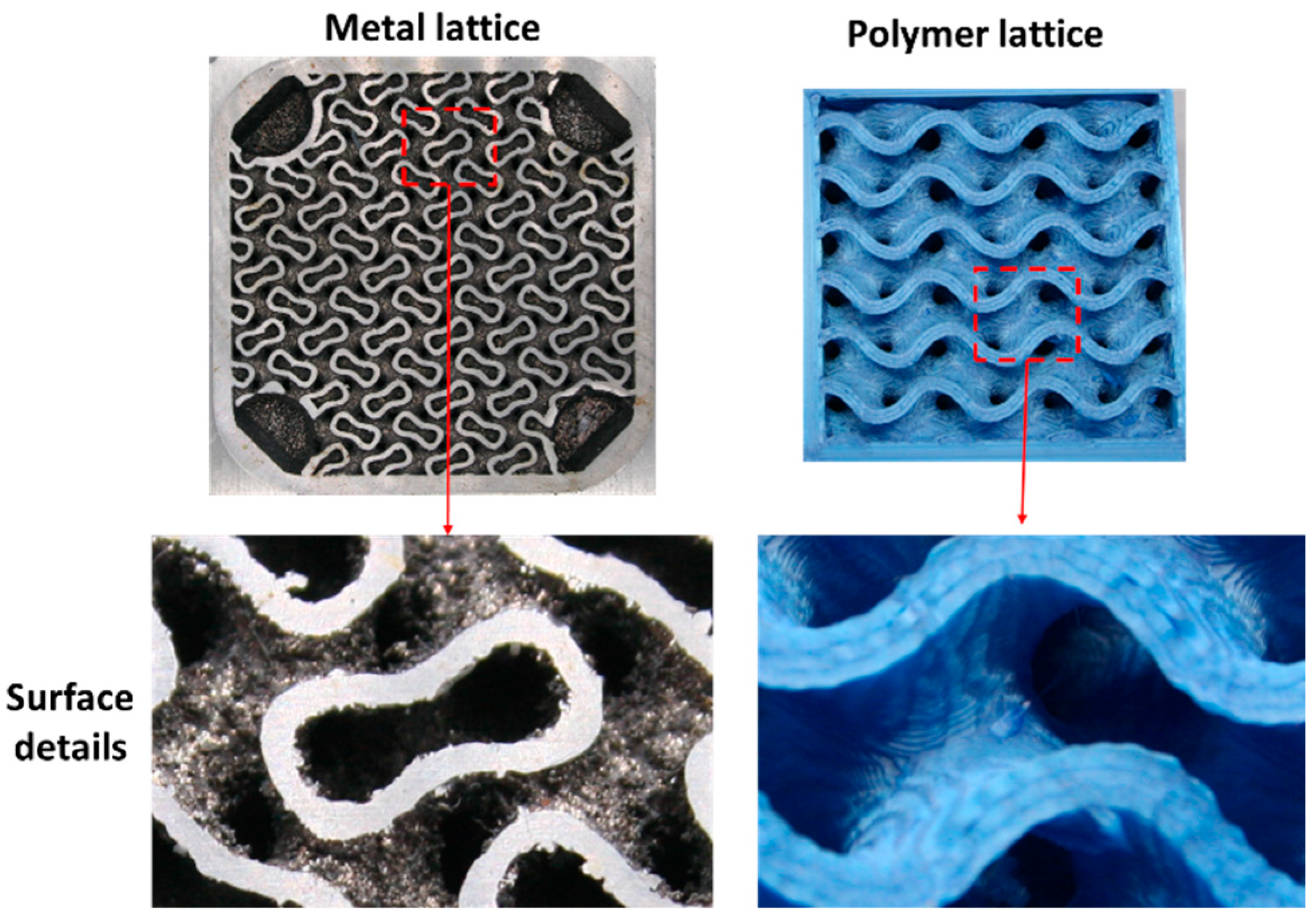
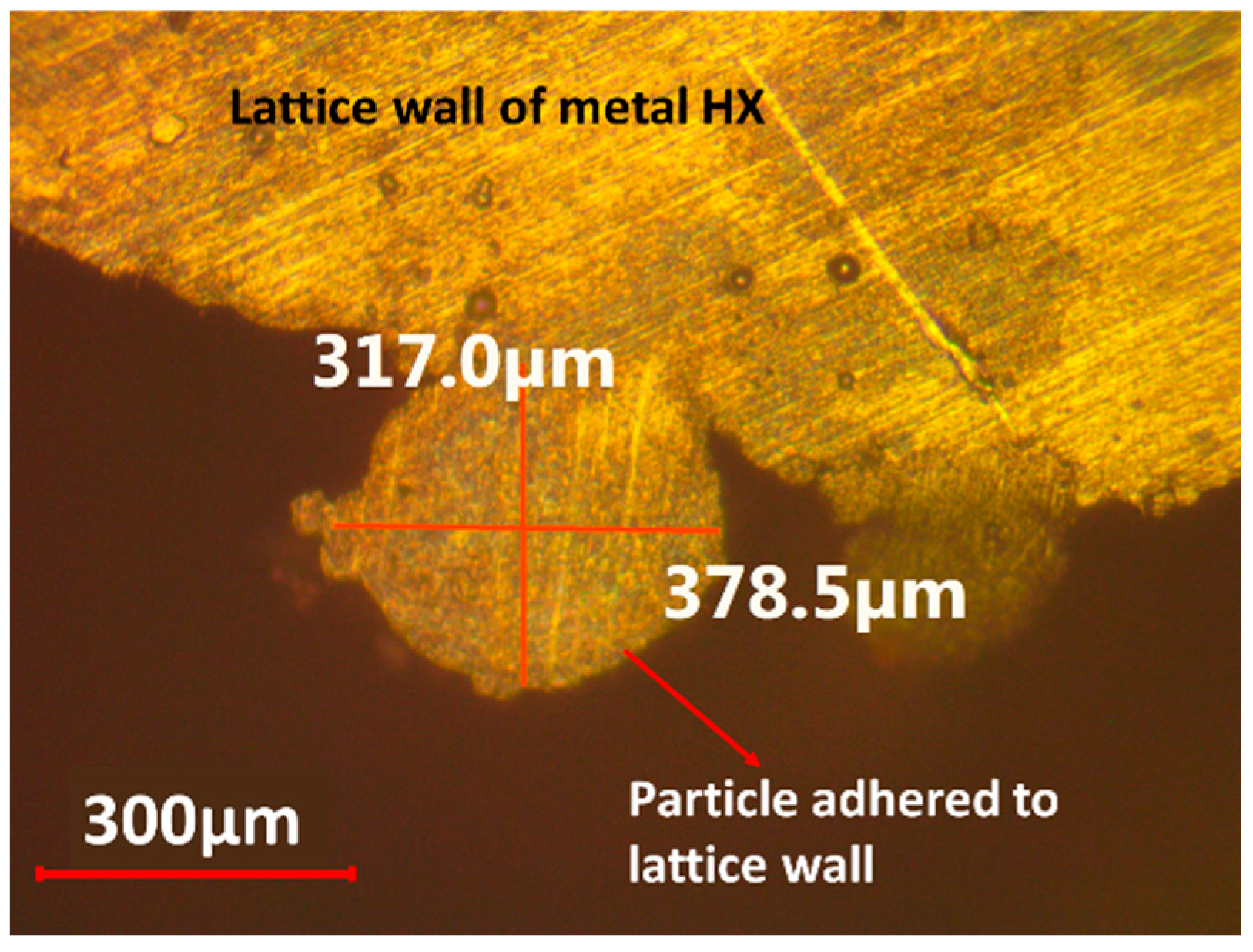
As shown in
Figure 13 and
Figure 14, a ball-shaped particle with a diameter of 350 µm was firmly attached to the lattice wall of the metal HX. Many particles of a similar size were adhered to the entire surface of metal lattice. Some of the attached particles resemble spikes, while others appear spherical. These particles are residual structures of 3D printing. The surface roughness of the metal lattice surface that do not have the attached particles is about 20 µm. The surface structure of metal lattice consists of areas with 20 µm roughness and the residual structures of 3D printing. The surface of the polymer lattice displayed several small stripes, but there were no residual structures on the surface.
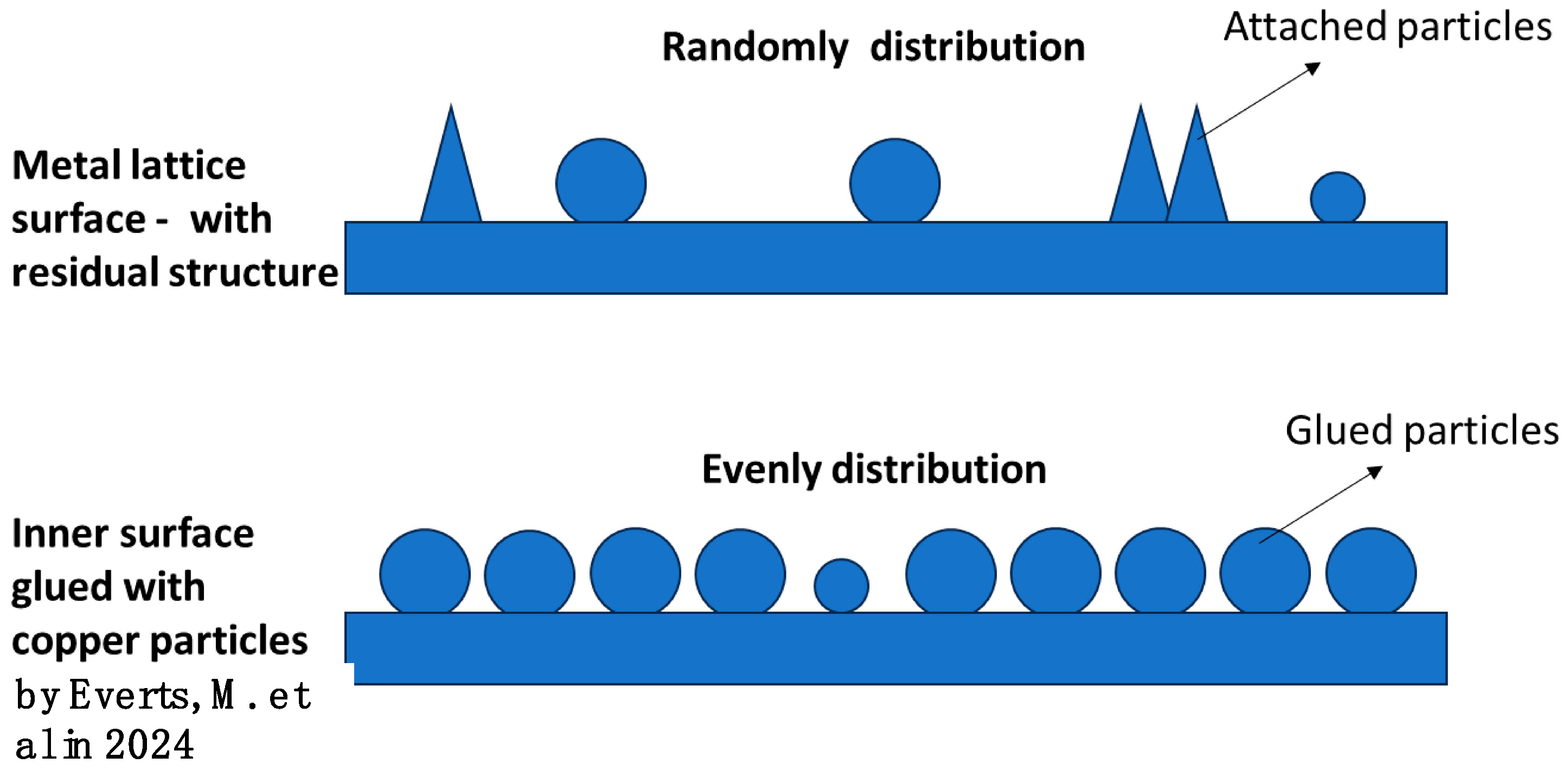
Everts, M. et al. [
22] investigated the impact of high relative surface roughness on heat transfer and pressure drop characteristics across different flow regimes through experimentation. To increase the relative surface roughness, Everts, M. et al. adhered small copper particles (150–300 μm) to the inner surface of the pipe, raising the surface roughness to 0.443 mm. The copper particles on the inner surface of the pipe are evenly distributed, as shown in
Figure 15. The residual structures on the metal lattice surface after 3D printing is randomly distributed. It may not be accurate to state that the surface roughness of the metal lattice is the same to that of the inner surface of the pipe coated with copper particles, as reported in [
22]. However, both the residual structures on the 3D-printed surface and the copper particles adhered to the inner surface of the pipe can disrupt the flow field around them, leading to an increased pressure drop and improved heat transfer efficiency. Some findings from the reference paper can help explain the variations in Nu and f with different Re values in the metal HX and polymer HX, as shown in
Figure 11 and
Figure 12.
Everts, M. et al. [
22] also found that both the friction factors and Nusselt numbers as functions of the Reynolds number showed a clear upward and leftward shift as the relative surface roughness increased from 0.04 to 0.11. As shown in
Figure 11 and
Figure 12, the Nusselt number and friction factors also have an upward and leftward shift. Considering the surface comparison in
Figure 13 and
Figure 15, it is possible that the difference in Nu and f in the metal HX and polymer HX was caused by the residual structures of 3D printing on the lattice surface for the metal HX. Ongoing research is being conducted to study the effect of the residual structures of 3D printing.
The wall thermal resistance in polymer HX is given by
where
is the thickness of the wall,
is the thermal conductivity of the wall and
is the heat transfer area of the wall, which is the same as
or
in this case [
23].
The heat exchanger overall heat transfer coefficient [
23]:
For the polymer HX, the pressure drop and
are provided in
Table 4 and the other volume yields the same results due to its symmetry. The overall heat transfer coefficient
of the entire heat exchanger is calculated using Equation (14). When the thermal conductivity is 0.20 W/(m×K),
is approximately 2.35 W/K, which is relatively low. The small
is attributed to the low thermal conductivity of the wall material. The polymer wall is the primary source of heat resistance.
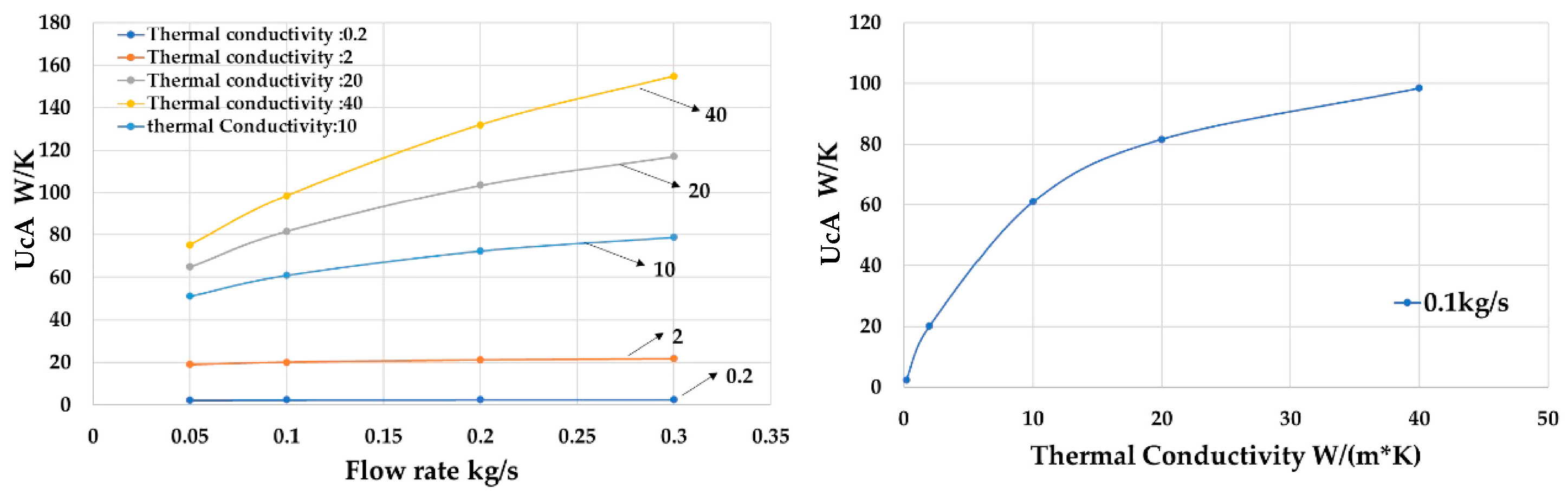
As shown in
Figure 16, when the thermal conductivity of the material increases from 0.2 to 10 W/(mK),
changes. However, when the thermal conductivity of the material increases from 20 to 40 W/(mK), the rate of increase in
slows down. This suggests that the impact of thermal conductivity on the overall heat transfer performance of the polymer heat exchanger diminishes at higher conductivity values.
The test rig for the HX is described in [
1]. During testing, the inlet temperature of hot water was 50 °C, and the inlet temperature of the cold water was 18 °C. The test results are provided in
Table 5. The pressure drop was close to the simulation results. The simulated heat transfer power at the tested flow rate is approximately 76 W, while the measured heat transfer power is 94 W, which is in good agreement with the simulation. To improve the efficiency of the plastic HX, it is crucial to improve the thermal conductivity of the polymer material.