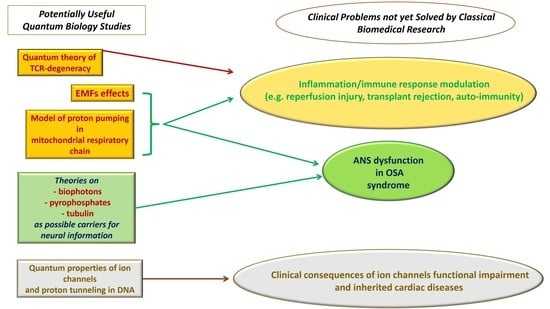
Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective
Abstract
1. Introduction
2. Methods
3. Results
3.1. Low Frequency Electromagnetic Fields
3.2. Proton Pumping in Mitochondrial Respiratory Chain and Quantum Theory of TCR-Degeneracy
- -
- Mitochondrial respiratory chain.
- -
- Quantum theory of TCR-degeneracy.
3.3. Theories on Biophotons, Pyrophosphates, or Tubulin as Possible Carriers for Neural Information
- pyrophosphate in the “Posner molecule”
- microtubules, present in the cell structural skeleton
3.4. Quantum Properties of Ion Channels and Proton Tunneling in the DNA
- -
- Ion Channels.
- (1)
- The studies focused on the selectivity filter of voltage-gated channels (the narrowest part of the conduction pathway through the pore of the channels), which discriminates between different ions.
- (2)
- The studies focused on the intracellular hydrophobic gate, which regulates the ion flow and the overall conductance of the channel.
- -
- Proton tunneling.
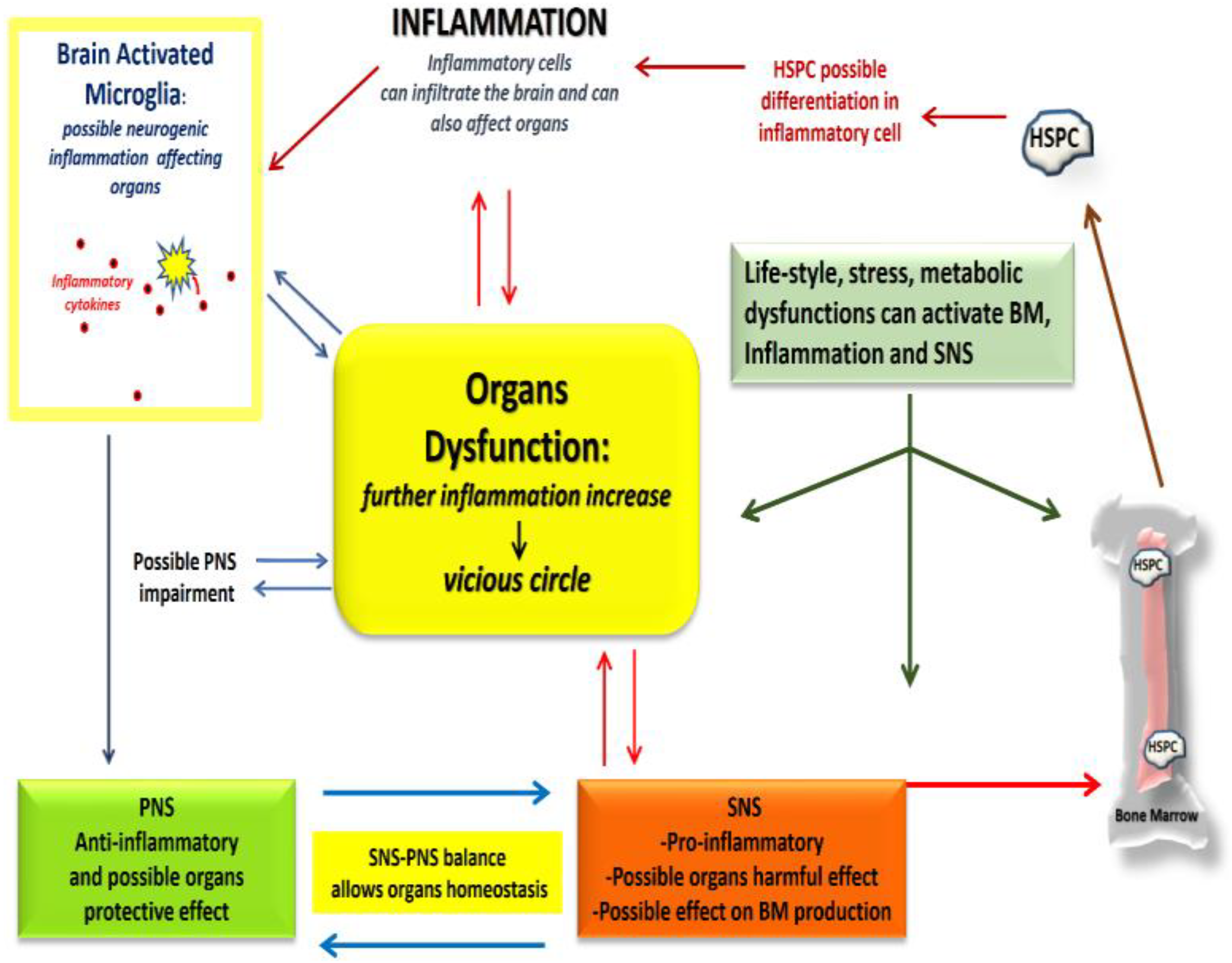
3.5. Problems Related to Inflammation and Immune Response
3.5.1. Stem Cells Therapy
3.5.2. Anti-Inflammatory Agents and Transplants for Organ Failures
3.6. OSA and Alterered Cardiovascular Autonomic Regulation
- (1)
- despite numerous studies demonstrating the association between OSA and hypertension, it is still unclear why effective OSA treatment with continuous positive airway pressure (CPAP) does not consistently improve blood pressure;
- (2)
- it is unclear why a percentage of OSA patients, who are adherent to OSA treatment, continue to suffer from residual excessive daytime sleepiness.
3.7. Ion Channels Dysfunction and Inherited Cardiac Diseases
- -
- Ion channels dysfunction.
- -
- Inherited Cardiac Diseases.
4. Conclusions and Perspectives
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Al-Khalili, J. Life on the Edge: The Coming of Age of Quantum Biology; Bantam Press: London, UK, 2014; ISBN 0593069323. [Google Scholar]
- Dicke, R.H. Coherence in Spontaneous Radiation Processes. Phys. Rev. 1954, 93, 99–110. [Google Scholar] [CrossRef]
- Del Giudice, E.; Doglia, S.; Milani, M. A Collective Dynamics in Metabolically Active Cells. Phys. Scr. 1982, 26, 232–238. [Google Scholar] [CrossRef]
- Del Giudice, E.; Preparata, G.; Vitiello, G. Water as a Free Electric Dipole Laser. Phys. Rev. Lett. 1988, 61, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Jibu, M.; Yasue, K. Volume 18, Numbers 2 and 3, Spring and Summer (special issue): Understanding Tomorrow’s Mind: Advances in Chaos Theory, Quantum, Theory, and Consciousness in Psychology by Larry Vandervert (Editor) American Nonlinear Systems. J. Mind Behav. 1990, 11, 247–258. [Google Scholar]
- Kobe, S.; Dražić, G.; Cefalas, A.C.; Sarantopoulou, E.; Stražišar, J. Nucleation and crystallization of CaCO3 in applied magnetic fields. Cryst. Eng. 2002, 5, 243–253. [Google Scholar] [CrossRef]
- Kobe, S.; Dražić, G.; McGuiness, P.J.; Meden, T.; Sarantopoulou, E.; Kollia, Z.; Cefalas, A.C. Control over nanocrystalization in turbulent flow in the presence of magnetic fields. Mater. Sci. Eng. C 2003, 23, 811–815. [Google Scholar] [CrossRef]
- Cefalas, A.C.; Kobe, S.; Dražic, G.; Sarantopoulou, E.; Kollia, Z.; Stražišar, J.; Meden, A. Nanocrystallization of CaCO3 at solid/liquid interfaces in magnetic field: A quantum approach. Appl. Surf. Sci. 2008, 254, 6715–6724. [Google Scholar] [CrossRef]
- Cefalas, A.C.; Sarantopoulou, E.; Kollia, Z.; Riziotis, C.; Dražic, G.; Kobe, S.; Stražišar, J.; Meden, A. Magnetic Field Trapping in Coherent Antisymmetric States of Liquid Water Molecular Rotors. J. Comput. Theor. Nanosci. 2010, 7, 1800–1805. [Google Scholar] [CrossRef]
- Cefalas, A.C.; Gavriil, V.; Ferraro, A.; Kollia, Z.; Sarantopoulou, E. Dynamics and Physics of Integrin Activation in Tumor Cells by Nano-Sized Extracellular Ligands and Electromagnetic Fields. Methods Mol. Biol. 2021, 2217, 197–233. [Google Scholar] [PubMed]
- Marais, A.; Adams, B.; Ringsmuth, A.K.; Ferretti, M.; Gruber, J.M.; Hendrikx, R.; Schuld, M.; Smith, S.L.; Sinayskiy, I.; Krüger, T.P.J.; et al. The future of quantum biology. J. R. Soc. Interface 2018, 15, 20180640. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.S.; Calhoun, T.R.; Read, E.L.; Ahn, T.K.; Mančal, T.; Cheng, Y.C.; Blankenship, R.E.; Fleming, G.R. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 2007, 446, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Collini, E.; Wong, C.Y.; Wilk, K.E.; Curmi, P.M.G.; Brumer, P.; Scholes, G.D. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 2010, 463, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Masgrau, L.; Roujeinikova, A.; Johannissen, L.O.; Hothi, P.; Basran, J.; Ranaghan, K.E.; Mulholland, A.J.; Sutcliffe, M.J.; Scrutton, N.S.; Leys, D. Atomic description of an enzyme reaction dominated by proton tunneling. Science 2006, 312, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Gauger, E.M.; Rieper, E.; Morton, J.J.L.; Benjamin, S.C.; Vedral, V. Sustained Quantum Coherence and Entanglement in the Avian Compass. Phys. Rev. Lett. 2011, 106, 040503. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Physics of life: The dawn of quantum biology. Nature 2011, 474, 272–274. [Google Scholar] [CrossRef]
- Melkikh, A.V.; Khrennikov, A. Nontrivial quantum and quantum-like effects in biosystems: Unsolved questions and paradoxes. Prog. Biophys. Mol. Biol. 2015, 119, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Dusi, V.; Ghidoni, A.; Ravera, A.; De Ferrari, G.M.; Calvillo, L. Chemokines and Heart Disease: A Network Connecting Cardiovascular Biology to Immune and Autonomic Nervous Systems. Mediat. Inflamm. 2016, 2016, 5902947. [Google Scholar] [CrossRef]
- Calvillo, L.; Gironacci, M.M.; Crotti, L.; Meroni, P.L.; Parati, G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat. Rev. Cardiol. 2019, 16, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, L.; Parati, G. Immune System and Mind-Body Medicine—An Overview. In Brain and Heart Dynamics; Springer: Cham, Switzerland, 2019; pp. 97–115. [Google Scholar] [CrossRef]
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement from the American Heart Association. Circulation 2021, 143, E763–E783. [Google Scholar] [CrossRef] [PubMed]
- Usselman, R.J.; Chavarriaga, C.; Castello, P.R.; Procopio, M.; Ritz, T.; Dratz, E.A.; Singel, D.J.; Martino, C.F. The Quantum Biology of Reactive Oxygen Species Partitioning Impacts Cellular Bioenergetics. Sci. Rep. 2016, 6, 38543. [Google Scholar] [CrossRef]
- Ventura, C.; Bianchi, F.; Cavallini, C.; Olivi, E.; Tassinari, R. The use of physical energy for tissue healing. Eur. Heart J. Suppl. 2015, 17, A69–A73. [Google Scholar] [CrossRef][Green Version]
- Gaetani, R.; Ledda, M.; Barile, L.; Chimenti, I.; De Carlo, F.; Forte, E.; Ionta, V.; Giuliani, L.; D’Emilia, E.; Frati, G.; et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc. Res. 2009, 82, 411–420. [Google Scholar] [CrossRef]
- Albaqami, M.; Hammad, M.; Pooam, M.; Procopio, M.; Sameti, M.; Ritz, T.; Ahmad, M.; Martino, C.F. Arabidopsis cryptochrome is responsive to Radiofrequency (RF) electromagnetic fields. Sci. Rep. 2020, 10, 11260. [Google Scholar] [CrossRef] [PubMed]
- Sherrard, R.M.; Morellini, N.; Jourdan, N.; El-Esawi, M.; Arthaut, L.D.; Niessner, C.; Rouyer, F.; Klarsfeld, A.; Doulazmi, M.; Witczak, J.; et al. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 2018, 16, e2006229. [Google Scholar] [CrossRef]
- Usselman, R.J.; Hill, I.; Singel, D.J.; Martino, C.F. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS ONE 2014, 9, e93065. [Google Scholar] [CrossRef]
- Means, A.R. Calcium, calmodulin and cell cycle regulation. FEBS Lett. 1994, 347, 1–4. [Google Scholar] [CrossRef]
- Liboff, A.R. Electric-field ion cyclotron resonance. Bioelectromagnetics 1997, 18, 85–87. [Google Scholar] [CrossRef]
- Lednev, V.V. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1991, 12, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Bianchi, F.; Tremolada, C.; Fontani, V.; Ventura, C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: A novel approach to multipotency. Cell Transplant. 2014, 23, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Lints, T.J.; Parsons, L.M.; Hartley, L.; Lyons, I.; Harvey, R.P. Nkx-2.5: A novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 1993, 119, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Maioli, M. Opioid Peptide Gene Expression Primes Cardiogenesis in Embryonal Pluripotent Stem Cells. Circ. Res. 2000, 87, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Zinellu, E.; Maninchedda, E.; Maioli, M. Dynorphin B is an agonist of nuclear opioid receptors coupling nuclear protein kinase C activation to the transcription of cardiogenic genes in GTR1 embryonic stem cells. Circ. Res. 2003, 92, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Mourokh, L.; Vittadello, M. Mechanism of proton pumping in complex i of the mitochondrial respiratory chain. Quantum Rep. 2021, 3, 425–434. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef] [PubMed]
- Cohn, M. An in depth analysis of the concept of “polyspecificity” assumed to characterize TCR/BCR recognition. Immunol. Res. 2008, 40, 128–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayala García, M.A.; González Yebra, B.; López Flores, A.L.; Guaní Guerra, E. The Major Histocompatibility Complex in Transplantation. J. Transplant. 2012, 2012, 842141. [Google Scholar] [CrossRef]
- Antipas, G.S.E.; Germenis, A.E. The quantum chemical causality of pMHC-TCR biological avidity: Peptide atomic coordination data and the electronic state of agonist N termini. Data Br. 2015, 3, 180–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antipas, G.S.E.; Germenis, A.E. Atomic Coordination Reflects Peptide Immunogenicity. Front. Mol. Biosci. 2016, 2, 77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antipas, G.S.E.; Germenis, A.E. Quantum chemical calculations predict biological function: The case of T cell receptor interaction with a peptide/MHC class I. Front. Chem. 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Germenis, A.E.; Manoussakis, M.N.; Georgios, S.E. The Dawn of Quantum Immunology. SRL Immunol. Immunother. 2016, 1, 3–6. [Google Scholar]
- Kobayashi, M.; Kikuchi, D.; Okamura, H. Imaging of ultraweak spontaneous photon emission from human body displaying diurnal rhythm. PLoS ONE 2009, 4, e0006256. [Google Scholar] [CrossRef]
- Cifra, M.; Pospíšil, P. Ultra-weak photon emission from biological samples: Definition, mechanisms, properties, detection and applications. J. Photochem. Photobiol. B Biol. 2014, 139, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Boone, K.; Tuszyński, J.; Barclay, P.; Simon, C. Possible existence of optical communication channels in the brain. Sci. Rep. 2016, 6, 36508. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.S.; Ahn, S.; Mescher, M.; Garwood, M.; Ugurbil, K.; Richter, W.; Rizi, R.R.; Hopkins, J.; Leigh, J.S. MR imaging contrast enhancement based on intermolecular zero quantum coherences. Science 1998, 281, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Dolde, F.; Jakobi, I.; Naydenov, B.; Zhao, N.; Pezzagna, S.; Trautmann, C.; Meijer, J.; Neumann, P.; Jelezko, F.; Wrachtrup, J. Room-temperature entanglement between single defect spins in diamond. Nat. Phys. 2013, 9, 139–143. [Google Scholar] [CrossRef]
- Shi, L.; Galvez, E.J.; Alfano, R.R. Photon Entanglement through Brain Tissue. Sci. Rep. 2016, 6, 37714. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.P.A. Quantum cognition: The possibility of processing with nuclear spins in the brain. Ann. Phys. 2015, 362, 593–602. [Google Scholar] [CrossRef]
- Swift, M.W.; Van de Walle, C.G.; Fisher, M.P.A. Posner molecules: From atomic structure to nuclear spins. Phys. Chem. Chem. Phys. 2018, 20, 12373–12380. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.A. Inorganic pyrophosphate generation and disposition in pathophysiology. Am. J. Physiol. Physiol. 2001, 281, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimo, D.A.; Wyler, S.C.; Romani, A.M.; O’Neill, W.C.; Dubyak, G.R. Regulation of vascular smooth muscle cell calcification by extracellular pyrophosphate homeostasis: Synergistic modulation by cyclic AMP and hyperphosphatemia. Am. J. Physiol. Physiol. 2010, 298, C702–C713. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, C.P.; Doraiswamy, P.M.; Fisher, M.P.A. A New Spin on Neural Processing: Quantum Cognition. Front. Hum. Neurosci. 2016, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Ghosh, S.; Hirata, K.; Fujita, D.; Bandyopadhyay, A. Multi-level memory-switching properties of a single brain microtubule. Appl. Phys. Lett. 2013, 102, 123701. [Google Scholar] [CrossRef]
- Penrose, R. The Emperor’s New Mind: Concerning Computers, Minds, and the Laws of Physics; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Hameroff, S.; Penrose, R. Consciousness in the universe. Phys. Life Rev. 2014, 11, 39–78. [Google Scholar] [CrossRef]
- Summhammer, J.; Salari, V.; Bernroider, G. A quantum-mechanical description of ion motion within the confining potentials of voltage-gated ion channels. J. Integr. Neurosci. 2012, 11, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Salari, V.; Naeij, H.; Shafiee, A. Quantum Interference and Selectivity through Biological Ion Channels. Sci. Rep. 2017, 7, 41625. [Google Scholar] [CrossRef]
- Summhammer, J.; Sulyok, G.; Bernroider, G. Quantum dynamics and non-local effects behind ion transition states during permeation in membrane channel proteins. Entropy 2018, 20, 558. [Google Scholar] [CrossRef]
- Summhammer, J.; Sulyok, G.; Bernroider, G. Quantum mechanical coherence of K+ ion wave packets increases conduction in the KcsA ion channel. Appl. Sci. 2020, 10, 4250. [Google Scholar] [CrossRef]
- Qaswal, A.B. Quantum tunneling of ions through the closed voltage-gated channels of the biological membrane: A mathematical model and implications. Quantum Rep. 2019, 1, 219–225. [Google Scholar] [CrossRef]
- Qaswal, A.B.; Ababneh, O.; Khreesha, L.; Al-Ani, A.; Suleihat, A.; Abbad, M. Mathematical modeling of ion quantum tunneling reveals novel properties of voltage-gated channels and quantum aspects of their pathophysiology in excitability-related disorders. Pathophysiology 2021, 28, 116–154. [Google Scholar] [CrossRef]
- Qaswal, A.B. Quantum electrochemical equilibrium: Quantum version of the Goldman–Hodgkin–Katz equation. Quantum Rep. 2020, 2, 266–277. [Google Scholar] [CrossRef]
- Ababneh, O.; Qaswal, A.B.; Alelaumi, A.; Khreesha, L.; Almomani, M.; Khrais, M.; Khrais, O.; Suleihat, A.; Mutleq, S.; Al-Olaimat, Y.; et al. Proton quantum tunneling: Influence and relevance to acidosis-induced cardiac arrhythmias/cardiac arrest. Pathophysiology 2021, 28, 400–436. [Google Scholar] [CrossRef] [PubMed]
- Qaswal, A.B. The Role of Quantum Tunneling of Ions in the Pathogenesis of the Cardiac Arrhythmias Due to Channelopathies, Ischemia, and Mechanical Stretch. Biophysics 2021, 66, 637–641. [Google Scholar] [CrossRef]
- Qaswal, A.B. Lithium Stabilizes the Mood of Bipolar Patients by Depolarizing the Neuronal Membrane via Quantum Tunneling through the Sodium Channels. Clin. Psychopharmacol. Neurosci. 2020, 18, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Qaswal, A.B. Magnesium ions depolarize the neuronal membrane via quantum tunneling through the closed channels. Quantum Rep. 2020, 2, 57–63. [Google Scholar] [CrossRef]
- Khreesha, L.; Qaswal, A.B.; Al Omari, B.; Albliwi, M.A.; Ababneh, O.; Albanna, A.; Abunab, A.; Iswaid, M.; Alarood, S.; Guzu, H.; et al. Quantum Tunneling-Induced Membrane Depolarization Can Explain the Cellular Effects Mediated by Lithium: Mathematical Modeling and Hypothesis. Membranes 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, L.; Al-Khalili, J.S.; Sacchi, M. Quantum and classical effects in DNA point mutations: Watson–Crick tautomerism in AT and GC base pairs. Phys. Chem. Chem. Phys. 2021, 23, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- Kryachko, E.S. The origin of spontaneous point mutations in DNA via Löwdin mechanism of proton tunneling in DNA base pairs: Cure with covalent base pairing. Int. J. Quantum Chem. 2002, 90, 910–923. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Quantum Genetics and the Aperiodic Solid: Some Aspects on the Biological Problems of Heredity, Mutations, Aging, and Tumors in View of the Quantum Theory of the DNA Molecule. Adv. Quantum Chem. 1966, 2, 213–360. [Google Scholar]
- Çelebi, G.; Özçelik, E.; Vardar, E.; Demir, D. Time delay during the proton tunneling in the base pairs of the DNA double helix. Prog. Biophys. Mol. Biol. 2021, 167, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lu, K.; Zhu, J.; Wang, J. Stem cell therapy for ischemic heart diseases. Br. Med. Bull. 2017, 121, 135–154. [Google Scholar] [CrossRef]
- Hao, L.; Zou, Z.; Tian, H.; Zhang, Y.; Zhou, H.; Liu, L. Stem cell-based therapies for ischemic stroke. Biomed. Res. Int. 2014, 2014, 468748. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.; Guo, S.; Lang, H.; Zhang, C.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef]
- Danieli, P.; Malpasso, G.; Ciuffreda, M.C.; Cervio, E.; Calvillo, L.; Copes, F.; Pisano, F.; Mura, M.; Kleijn, L.; de Boer, R.A.; et al. Conditioned Medium from Human Amniotic Mesenchymal Stromal Cells Limits Infarct Size and Enhances Angiogenesis. Stem Cells Transl. Med. 2015, 4, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Cova, L.; Silani, V. Amyotrophic lateral sclerosis: Applications of stem cells—An update. Stem Cells Cloning Adv. Appl. 2010, 3, 145–156. [Google Scholar] [CrossRef]
- Kasai-Brunswick, T.H.; Carvalho, A.B.; Campos de Carvalho, A.C. Stem cell therapies in cardiac diseases: Current status and future possibilities. World J. Stem Cells 2021, 13, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Hochedlinger, K. Induced pluripotency: History, mechanisms, and applications. Genes Dev. 2010, 24, 2239–2263. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, R.J.; Engle, S.J. Human inducible pluripotent stem cells: Realization of initial promise in drug discovery. Cell Stem Cell 2021, 28, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Bastaroli, F.; Corli, M.; Ginevrino, M.; Calabrò, F.; Boni, M.; Crotti, L.; Valente, E.M.; Schwartz, P.J.; Gnecchi, M. Generation of the human induced pluripotent stem cell (hiPSC) line PSMi006-A from a patient affected by an autosomal recessive form of long QT syndrome type 1. Stem Cell Res. 2020, 42, 101658. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 1. [Google Scholar] [CrossRef]
- Muller, F. The nature and mechanism of superoxide production by the electron transport chain: Its relevance to aging. Age 2000, 23, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.D.; Norrito, R.L.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory mechanisms in ischemic stroke: Focus on cardioembolic stroke, background, and therapeutic approaches. Int. J. Mol. Sci. 2020, 21, 6454. [Google Scholar] [CrossRef]
- Calvillo, L.; Vanoli, E.; Andreoli, E.; Besana, A.; Omodeo, E.; Gnecchi, M.; Zerbi, P.; Vago, G.; Busca, G.; Schwartz, P.J. Vagal Stimulation, through its Nicotinic Action, Limits Infarct Size and the Inflammatory Response to Myocardial Ischemia and Reperfusion. J. Cardiovasc. Pharmacol. 2011, 58, 500–507. [Google Scholar] [CrossRef]
- Swirski, F.K. From clonal haematopoiesis to the CANTOS trial. Nat. Rev. Cardiol. 2018, 15, 79–80. [Google Scholar] [CrossRef]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wen, A.; Lin, J. Pro-inflammatory cytokines in the formation of the pre-metastatic niche. Cancers 2020, 12, 3752. [Google Scholar] [CrossRef] [PubMed]
- Béland, L.-C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in amyotrophic lateral sclerosis: Blurred lines between excessive inflammation and inefficient immune responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef] [PubMed]
- Papismadov, N.; Krizhanovsky, V. Natural killers of cognition. Nat. Neurosci. 2021, 24, 2–4. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Youker, K.A.; Rossen, R.D.; Gwechenberger, M.; Lindsey, M.H.; Mendoza, L.H.; Michael, L.H.; Ballantyne, C.M.; Wayne Smith, C.; Entman, M.L. Cytokines and the Microcirculation in Ischemia and Reperfusion. J. Mol. Cell Cardiol. 1998, 30, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Shi, H. Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front. Biosci. 2007, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, L.; Masson, S.; Salio, M.; Pollicino, L.; De Angelis, N.; Fiordaliso, F.; Bai, A.; Ghezzi, P.; Santangelo, F.; Latini, R. In vivo cardioprotection by N-acetylcysteine and isosorbide 5-mononitrate in a rat model of ischemia-reperfusion. Cardiovasc. Drugs Ther. 2003, 17, 199–208. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Chemokines in the ischemic myocardium: From inflammation to fibrosis. Inflamm. Res. 2004, 53, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and Oxidative Stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Pietro Maggioni, A.; Ghezzi, P.; Bertini, R.; Latini, R.; Calvillo, L. Cardiac protection by pharmacological modulation of inflammation. Expert Opin. Investig. Drugs 2001, 10, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Mcmurray, J.J.V.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.S.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Clinical Investigation and Reports Targeted Anticytokine Therapy in Patients with Chronic Heart Failure. Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide—Identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Valenzuela, N.M.; Reed, E.F. Antibody-mediated rejection across solid organ transplants: Manifestations, mechanisms, and therapies. J. Clin. Investig. 2017, 127, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Kenta, I.; Takaaki, K. Molecular Mechanisms of Antibody-Mediated Rejection and Accommodation in Organ Transplantation. Nephron 2021, 144 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Siu, J.H.Y.; Surendrakumar, V.; Richards, J.A.; Pettigrew, G.J. T cell allorecognition pathways in solid organ transplantation. Front. Immunol. 2018, 9, 2548. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.; Sachs, D.H. Transplanting organs from pigs to humans. Sci. Immunol. 2019, 4, eaau6298. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2020, 10, 3060. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Fayad, Z.A.; Madsen, J.C.; Netea, M.G.; Mulder, W.J.M. Trained immunity in organ transplantation. Am. J. Transplant. 2020, 20, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Yuan, G.; Peng, Y.-J.; Khan, S.A.; Nanduri, J.; Singh, A.; Vasavda, C.; Semenza, G.L.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci. Signal. 2016, 9, ra80. [Google Scholar] [CrossRef] [PubMed]
- Teresa, M.; Rovere, L., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J.; Commentary, S. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998, 351, 478–484. [Google Scholar]
- Cortelli, P.; Lombardi, C.; Montagna, P.; Parati, G. Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Auton. Neurosci. Basic Clin. 2012, 169, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.F.; Chappell, M.J.; Bartlett, W.A.; Malhotra, A.; Beattie, J.M.; Cayton, R.M. The sleep apnoea/hypopnoea syndrome depresses waking vagal tone independent of sympathetic activation. Eur. Respir. J. 2001, 17, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Bisogni, V.; Pengo, M.F.; Maiolino, G.; Rossi, G.P. The sympathetic nervous system and catecholamines metabolism in obstructive sleep apnoea. J. Thorac. Dis. 2016, 8, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, W.; Liu, Y.; Fan, H.; Bajpai, R.P.; Fu, J.; Pang, J.; Zhao, X.; Han, J. Ultra-weak photon emission in healthy subjects and patients with type 2 diabetes: Evidence for a non-invasive diagnostic tool. Photochem. Photobiol. Sci. 2017, 16, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Fels, D. Physical Non-Contact Communication between Microscopic Aquatic Species: Novel Experimental Evidences for an Interspecies Information Exchange. J. Biophys. 2016, 2016, 7406356. [Google Scholar] [CrossRef]
- Schwartz, J.; Roth, T. Neurophysiology of Sleep and Wakefulness: Basic Science and Clinical Implications. Curr. Neuropharmacol. 2008, 6, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Abdul Kadir, L.; Stacey, M.; Barrett-Jolley, R. Emerging Roles of the Membrane Potential: Action Beyond the Action Potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Mathie, A.; Peters, J. Ion channels. Br. J. Phamacol. 2011, 164, S137–S174. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Gnecchi, M.; Dagradi, F.; Castelletti, S.; Parati, G.; Spazzolini, C.; Sala, L.; Crotti, L. From patient-specific induced pluripotent stem cells to clinical translation in long QT syndrome Type 2. Eur. Heart J. 2019, 40, 1832–1836. [Google Scholar] [CrossRef]
- Conte Camerino, D. Grand challenge for ion channels: An underexploited resource for therapeutics. Front. Pharmacol. 2010, 1, 113. [Google Scholar] [CrossRef] [PubMed]
- Conte Camerino, D.; Tricarico, D.; Desaphy, J.F. Ion Channel Pharmacology. Neurotherapeutics 2007, 4, 184–198. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Wang, D.W.; Desai, R.R.; Crotti, L.; Arnestad, M.; Insolia, R.; Pedrazzini, M.; Ferrandi, C.; Vege, A.; Rognum, T.; Schwartz, P.J.; et al. Cardiac sodium channel dysfunction in sudden infant death syndrome. Circulation 2007, 115, 368–376. [Google Scholar] [CrossRef]
- Calvillo, L.; Spazzolini, C.; Vullo, E.; Insolia, R.; Crotti, L.; Schwartz, P.J. Propranolol prevents life-threatening arrhythmias in LQT3 transgenic mice: Implications for the clinical management of LQT3 patients. Heart Rhythm 2014, 11, 126–132. [Google Scholar] [CrossRef] [PubMed]
- LoRusso, E.; Hickman, J.J.; Guo, X. Ion channel dysfunction and altered motoneuron excitability in ALS. Neurol. Disord. Epilepsy J. 2019, 3, 124. [Google Scholar]
- Yamamoto, Y.; Makiyama, T.; Harita, T.; Sasaki, K.; Wuriyanghai, Y.; Hayano, M.; Nishiuchi, S.; Kohjitani, H.; Hirose, S.; Chen, J.; et al. Allele-specific ablation rescues electrophysiological abnormalities in a human iPS cell model of long-QT syndrome with a CALM2 mutation. Hum. Mol. Genet. 2017, 26, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Kanai, K.; Kuwabara, S.; Misawa, S.; Tamura, N.; Ogawara, K.; Nakata, M.; Sawai, S.; Hattori, T.; Bostock, H. Altered axonal excitability properties in amyotrophic lateral sclerosis: Impaired potassium channel function related to disease stage. Brain 2006, 129, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Kotta, M.C.; Sala, L.; Ghidoni, A.; Badone, B.; Ronchi, C.; Parati, G.; Zaza, A.; Crotti, L. Calmodulinopathy: A Novel, Life-Threatening Clinical Entity Affecting the Young. Front. Cardiovasc. Med. 2018, 5, 175. [Google Scholar] [CrossRef]
- Corrado, D.; Mark, S.; Link, H.C. Arrhythmogenic right ventricular cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic cardiomyopathy. Circ. Res. 2017, 121, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Ghidoni, A.; Elliott, P.M.; Syrris, P.; Calkins, H.; James, C.A.; Judge, D.P.; Murray, B.; Barc, J.; Probst, V.; Schott, J.J.; et al. Cadherin 2-Related Arrhythmogenic Cardiomyopathy. Circ. Genom. Precis. Med. 2021, 14, e003097. [Google Scholar] [CrossRef] [PubMed]
- De Bortoli, M.; Postma, A.V.; Poloni, G.; Calore, M.; Minervini, G.; Mazzotti, E.; Rigato, I.; Ebert, M.; Lorenzon, A.; Vazza, G.; et al. Whole-Exome Sequencing Identifies Pathogenic Variants in TJP1 Gene Associated with Arrhythmogenic Cardiomyopathy. Circ. Genom. Precis. Med. 2018, 11, e002123. [Google Scholar] [CrossRef] [PubMed]
- Ben-Johny, M.; Dick, I.E.; Sang, L.; Limpitikul, W.B.; Kang, P.W.; Niu, J.; Banerjee, R.; Yang, W.; Babich, J.S.; Issa, J.B.; et al. Towards a Unified Theory of Calmodulin Regulation (Calmodulation) of Voltage-Gated Calcium and Sodium Channels. Curr. Mol. Pharmacol. 2015, 8, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Crotti, L.; Spazzolini, C.; Tester, D.J.; Ghidoni, A.; Baruteau, A.E.; Beckmann, B.M.; Behr, E.R.; Bennett, J.S.; Bezzina, C.R.; Bhuiyan, Z.A.; et al. Calmodulin mutations and life-threatening cardiac arrhythmias: Insights from the International Calmodulinopathy Registry. Eur. Heart J. 2019, 40, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Limpitikul, W.B.; Dick, I.E.; Tester, D.J.; Boczek, N.J.; Limphong, P.; Yang, W.; Choi, M.H.; Babich, J.; Disilvestre, D.; Kanter, R.J.; et al. A Precision Medicine Approach to the Rescue of Function on Malignant Calmodulinopathic Long-QT Syndrome. Circ. Res. 2017, 120, 39–48. [Google Scholar] [CrossRef]
- Hwang, H.S.; Nitu, F.R.; Yang, Y.; Walweel, K.; Pereira, L.; Johnson, C.N.; Faggioni, M.; Chazin, W.J.; Laver, D.; George, A.L.; et al. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ. Res. 2014, 114, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.E.; Huikuri, H.; et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013, 15, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Priori, S.G.; Cerrone, M.; Spazzolini, C.; Odero, A.; Napolitano, C.; Bloise, R.; De Ferrari, G.M.; Klersy, C.; Moss, A.J.; et al. Left Cardiac Sympathetic Denervation in the Management of High-Risk Patients Affected by the Long-QT Syndrome. Circulation 2004, 109, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.M.; Dusi, V.; Spazzolini, C.; Bos, J.M.; Abrams, D.J.; Berul, C.I.; Crotti, L.; Davis, A.M.; Eldar, M.; Kharlap, M.; et al. Clinical Management of Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation 2015, 131, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Bohr, N. Light and life. Nature 1933, 131, 421–423. [Google Scholar] [CrossRef]
- Fröhlich, H. Long-range coherence and energy storage in biological systems. Int. J. Quantum Chem. 1968, 2, 641–649. [Google Scholar] [CrossRef]
- Schrodinger, E. What is Life? With Mind and Matter and Autobiographical Sketches; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Asano, M.; Basieva, I.; Khrennikov, A.; Ohya, M.; Tanaka, Y.; Yamato, I. Quantum Information Biology: From Information Interpretation of Quantum Mechanics to Applications in Molecular Biology and Cognitive Psychology. Found. Phys. 2015, 45, 1362–1378. [Google Scholar] [CrossRef]
- Lloyd, S. A quantum of natural selection. Nat. Phys. 2009, 5, 164–166. [Google Scholar] [CrossRef]
- Yang, J.; Dettori, R.; Nunes, J.P.F.; List, N.H.; Biasin, E.; Centurion, M.; Chen, Z.; Cordones, A.A.; Deponte, D.P.; Heinz, T.F.; et al. Direct observation of ultrafast hydrogen bond strengthening in liquid water. Nature 2021, 596, 531–535. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Pereira, C.; Fonseca, A.C.R.G.; Pinto-do-Ó, P.; Nascimento, D.S. Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response. Front. Cell Dev. Biol. 2021, 8, 621644. [Google Scholar] [CrossRef] [PubMed]
- Brookes, J.C. Quantum effects in biology: Golden rule in enzymes, olfaction, photosynthesis and magnetodetection. Proc. R. Soc. A Math. Phys. Eng. Sci. 2017, 473, 20160822. [Google Scholar] [CrossRef] [PubMed]
- Vardi-Kilshtain, A.; Nitoker, N.; Major, D.T. Nuclear quantum effects and kinetic isotope effects in enzyme reactions. Arch. Biochem. Biophys. 2015, 582, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, M.; Ogryzko, V. Quantum biology at the cellular level—Elements of the research program. Biosystems 2013, 112, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Tegmark Max Importance of quantum decoherence in brain processes. Phys. Rev. 2000, E61, 4194–4206.
- Davies, P.C.W. Does quantum mechanics play a non-trivial role in life? Biosystems 2004, 78, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.; Gea-Banacloche, J.; Davies, P.C.W.; Hameroff, S.; Zeilinger, A.; Eisert, J.; Wiseman, H.; Bezrukov, S.M.; Frauenfelder, H. Plenary debate: Quantum effects in biology: Trivial or not? Fluct. Noise Lett. 2008, 08, C5–C26. [Google Scholar] [CrossRef]

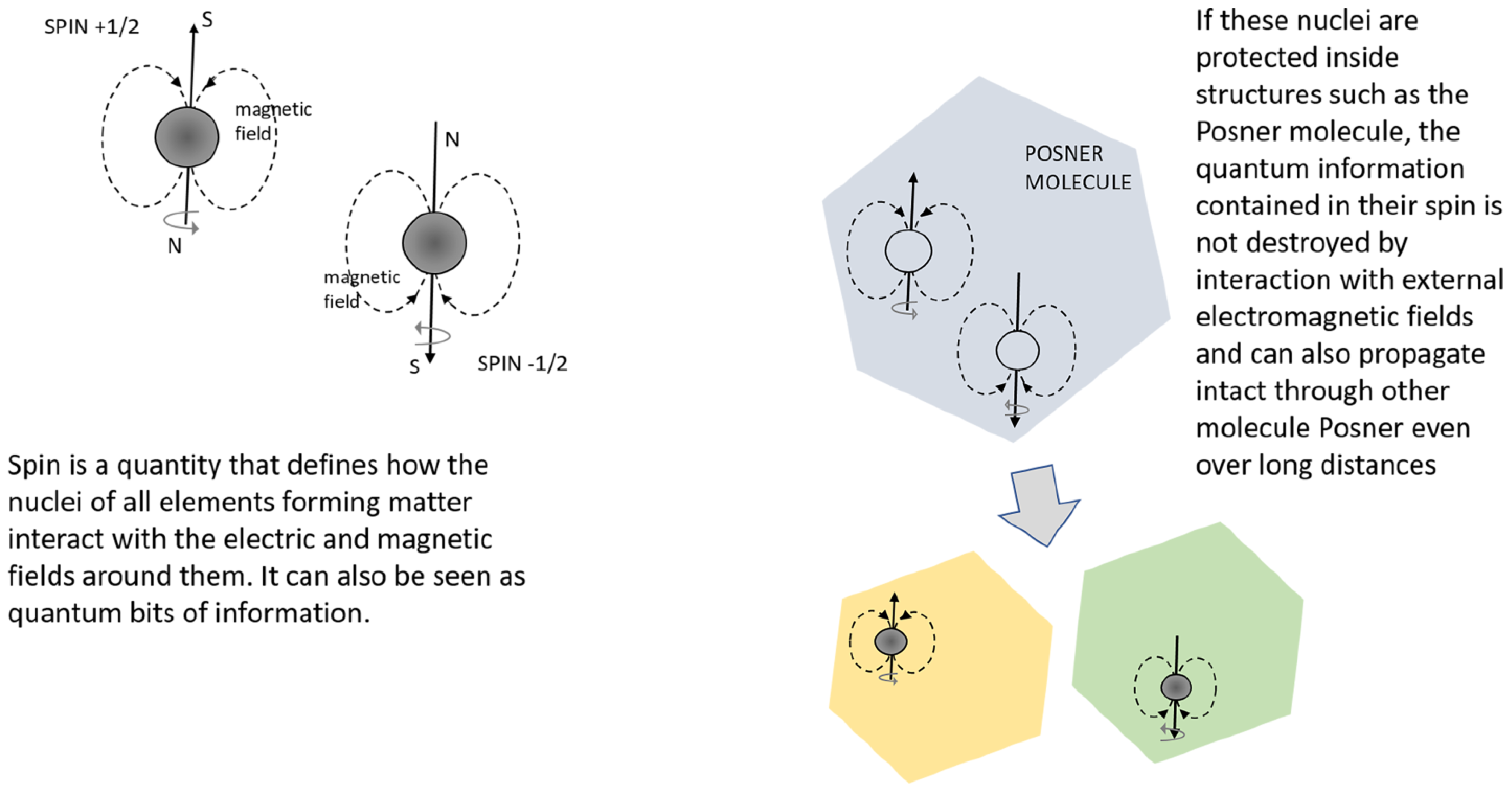
| Quantum particle is an object that behaves both as a particle and as a wave. Typically, subatomic particles like electrons have this property. For these objects, classical physics (e.g., Newton’s and Maxwell’s laws) cannot describe classical parameters (for example, position and speed) and a completely new physic (quantum mechanics) is needed. Quantum mechanics provides, with a good approximation, a description of the physical properties of such objects. |
| Quantum coherence is the condition necessary to a particle for maintaining its quantum behaviour (for example, the counter-intuitive state of being in two states at the same time). Quantum coherence is related to the concept that sub-atomic particles have wave-like properties. In order to maintain coherence, environmental conditions around the particle must be very stable and meet specific requirements. |
| Quantum Superposition is a quantum mathematical description that represents the non-locality of the particle based on its wave-like properties. Accordingly, the particle can be present at multiple locations at the same time. As an intuitive image, it is like having one stone able to hit many birds simultaneously with one shot. |
| Quantum Tunnelling is the phenomenon where a quantum object tunnels through a barrier that it cannot surmount, for example, for adverse thermodynamic conditions. This is counterintuitive because it is like spookily passing through a thick and tall wall instead of overcoming it. |
| Quantum Entanglement is a quantum phenomenon that describes the instantaneous interaction between two particles, which were previously in contact, when they are pushed apart from each other. Regardless of the distance between them, the two objects (e.g., electrons in a previously covalent bond), remain “in contact” in a so-called entangled state. It has been observed that the two particles change their spin in response to the spin changing of the other instantaneously. For example, given that a particle A has entangled with a particle B, if particle A is found to have spin-up, then particle B must have spin-down. Later, if particle A is found to have spin-down, regardless of distance, particle B immediately changes in a spin-up particle. |
| Avidity | The measure of the total binding strength of an antibody at every binding site |
| Tax peptides | Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of an aggressive form of T-cell disorders leading to cancer. Tax peptides are the oncoproteins inside the HTLV-1 which play a crucial role in the immortalization of malignant T-cells. |
| CVDs | Cardiovascular diseases |
| ANS | Autonomic Nervous System |
| SNS | Sympathetic Nervous System |
| PNS | Parasympathetic Nervous System |
| HSPCs | Haematopoietic Stem and Progenitor Cells |
| BRS | The Baroreflex Sensitivity index is defined as the change in interbeat interval in milliseconds per unit change in blood pressure. It measures the control on the heart rate. Alterations of the BRS contribute to the reduction of parasympathetic activity and to the increase of sympathetic activity with possible harmful effect on cardiovascular system. |
| CV | Cardiovascular |
| ATP | Adenosine Triphosphate |
| ACM | Arrhythmogenic Cardiomyopathy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvillo, L.; Redaelli, V.; Ludwig, N.; Qaswal, A.B.; Ghidoni, A.; Faini, A.; Rosa, D.; Lombardi, C.; Pengo, M.; Bossolasco, P.; et al. Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective. Quantum Rep. 2022, 4, 148-172. https://doi.org/10.3390/quantum4020011
Calvillo L, Redaelli V, Ludwig N, Qaswal AB, Ghidoni A, Faini A, Rosa D, Lombardi C, Pengo M, Bossolasco P, et al. Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective. Quantum Reports. 2022; 4(2):148-172. https://doi.org/10.3390/quantum4020011
Chicago/Turabian StyleCalvillo, Laura, Veronica Redaelli, Nicola Ludwig, Abdallah Barjas Qaswal, Alice Ghidoni, Andrea Faini, Debora Rosa, Carolina Lombardi, Martino Pengo, Patrizia Bossolasco, and et al. 2022. "Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective" Quantum Reports 4, no. 2: 148-172. https://doi.org/10.3390/quantum4020011
APA StyleCalvillo, L., Redaelli, V., Ludwig, N., Qaswal, A. B., Ghidoni, A., Faini, A., Rosa, D., Lombardi, C., Pengo, M., Bossolasco, P., Silani, V., & Parati, G. (2022). Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective. Quantum Reports, 4(2), 148-172. https://doi.org/10.3390/quantum4020011