Polymerization Behavior and Rheological Properties of a Surfactant-Modified Reactive Hydrophobic Monomer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
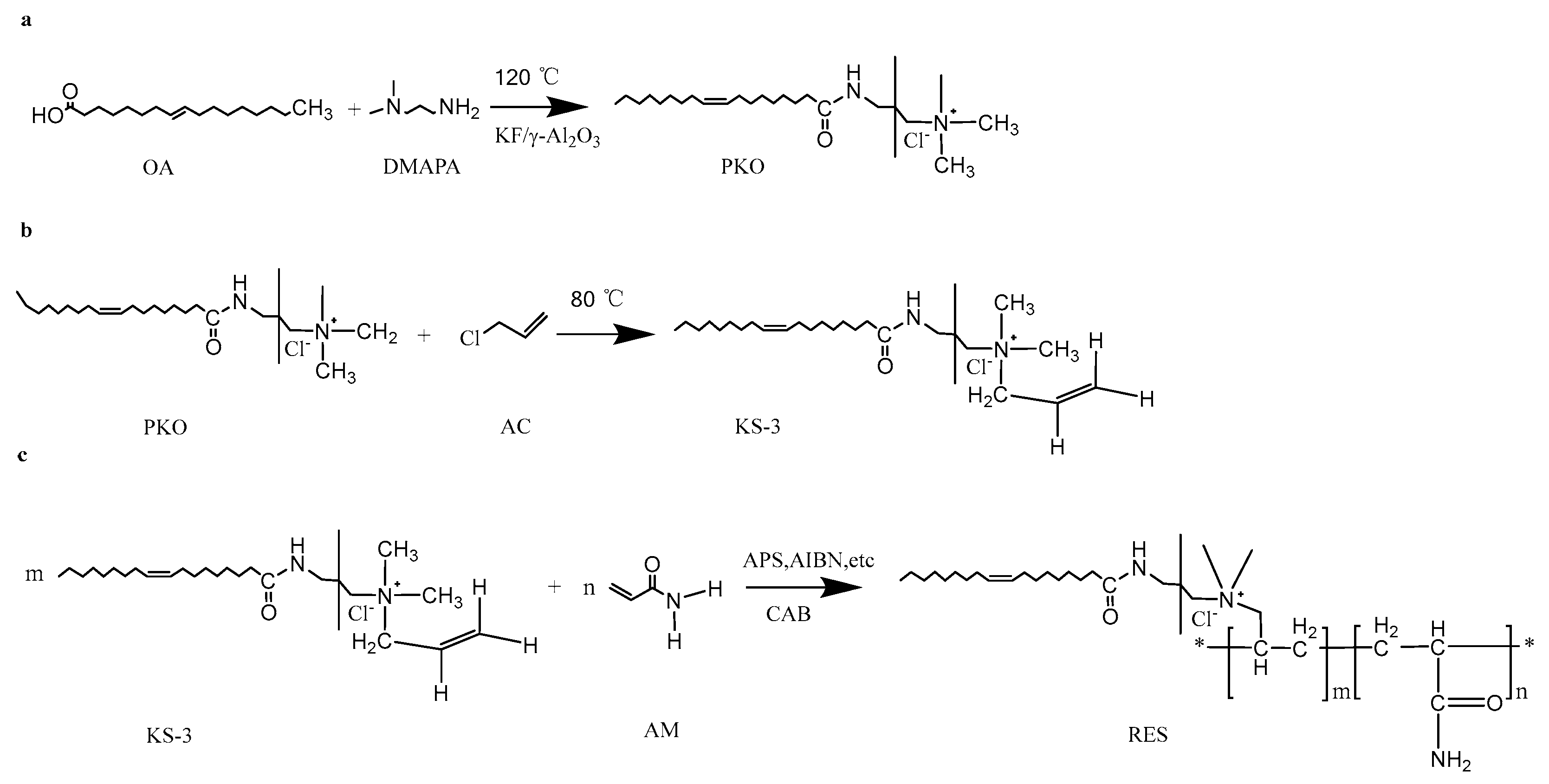
2.2.1. Synthesis of Surfactant
2.2.2. Polymer Synthesis
2.3. Characterization
2.3.1. Rheological Analysis
2.3.2. Microstructural Analysis
3. Results and Discussion
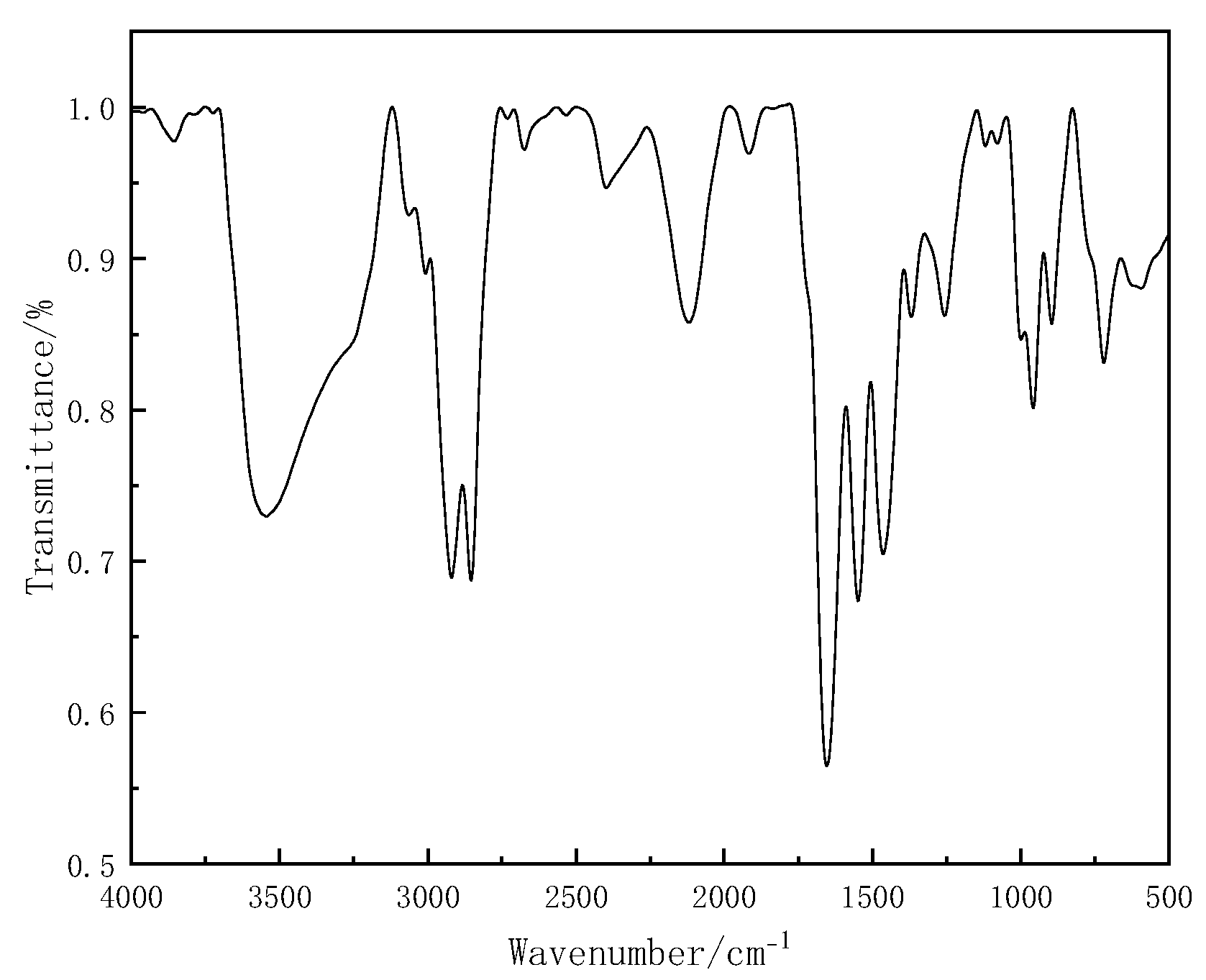
3.1. Infrared Analysis of KS-3
3.2. NMR Analysis of KS-3
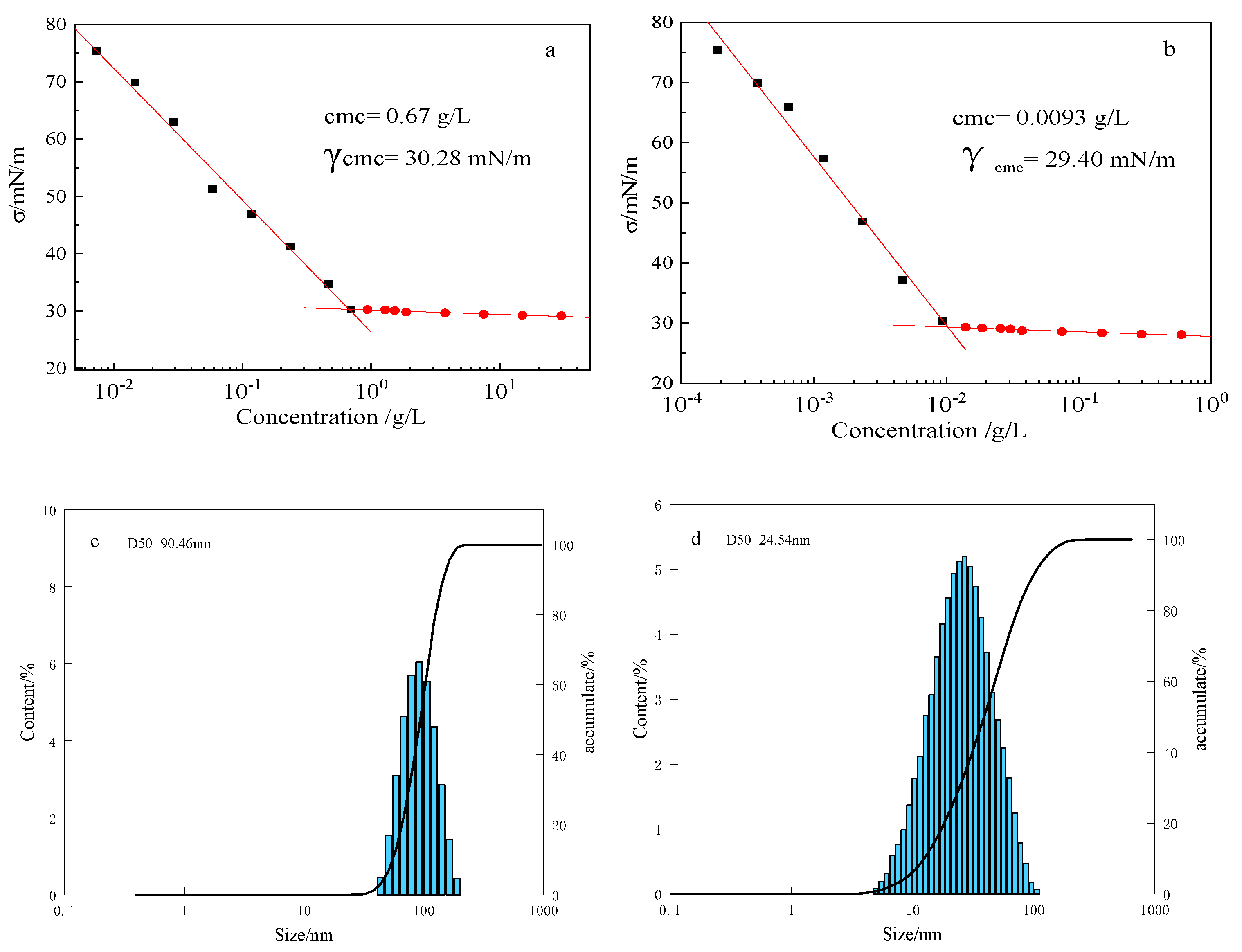
3.3. Critical Micelle Concentration of KS-3 in Aqueous Solution
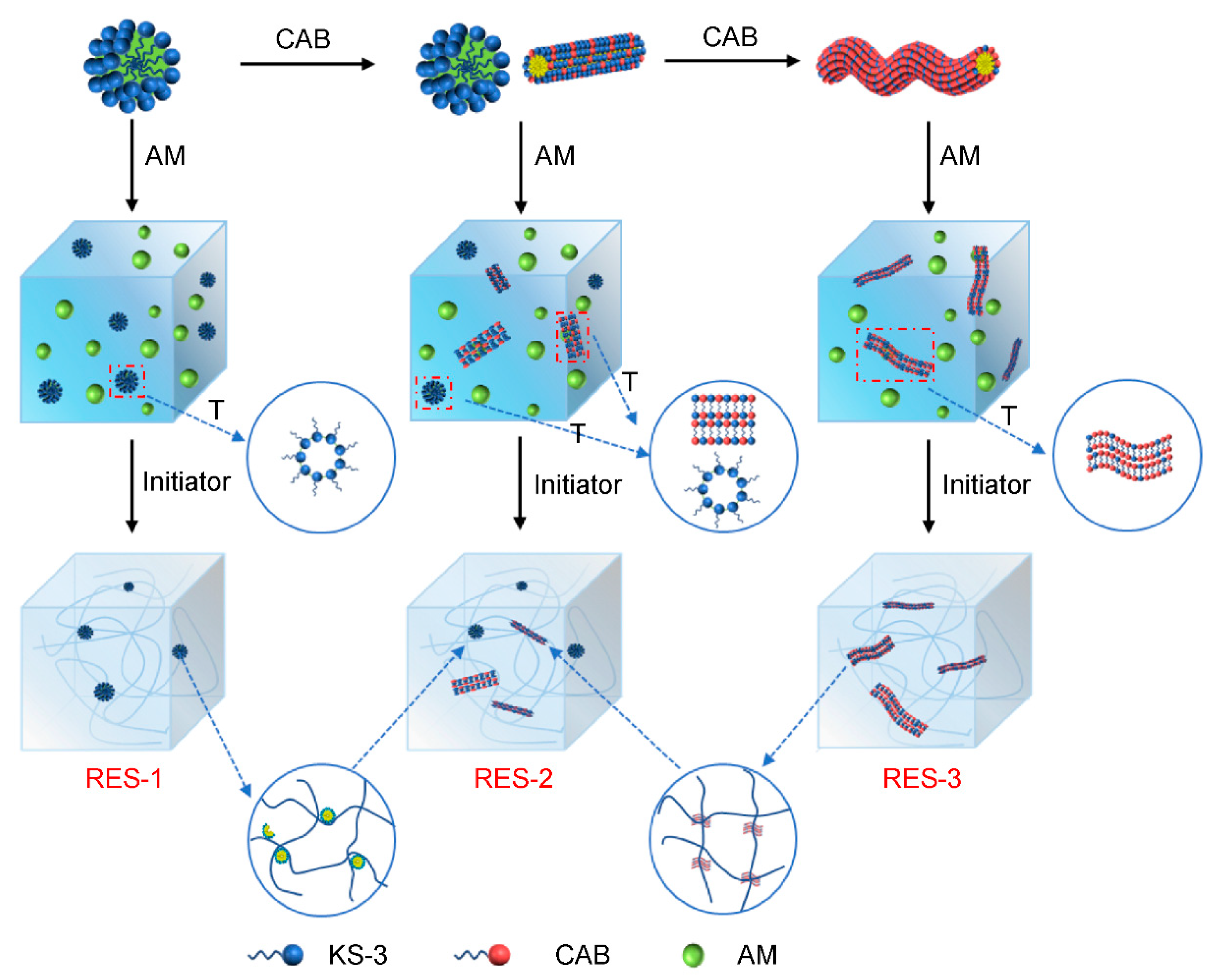
3.4. RES Polymerization
3.5. Behavior of KS-3 in Monomer Solutions
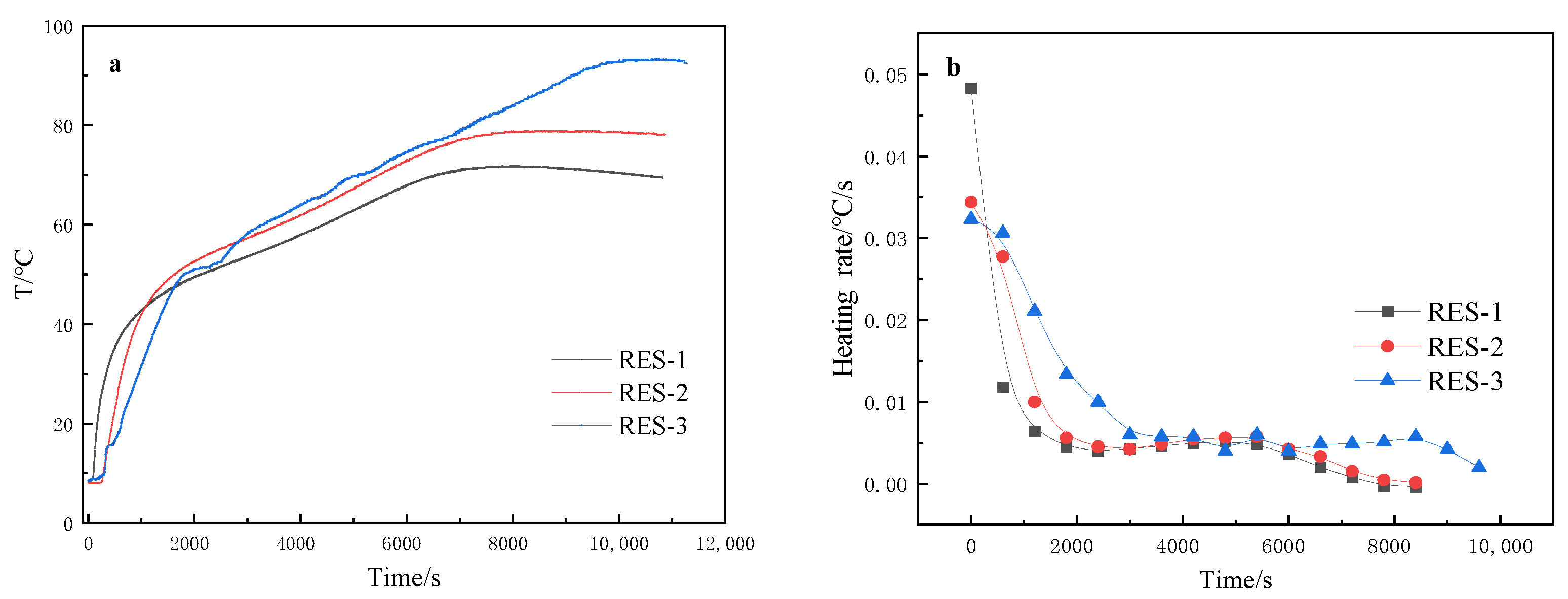
3.6. Temperature Changes during the Polymerization Process
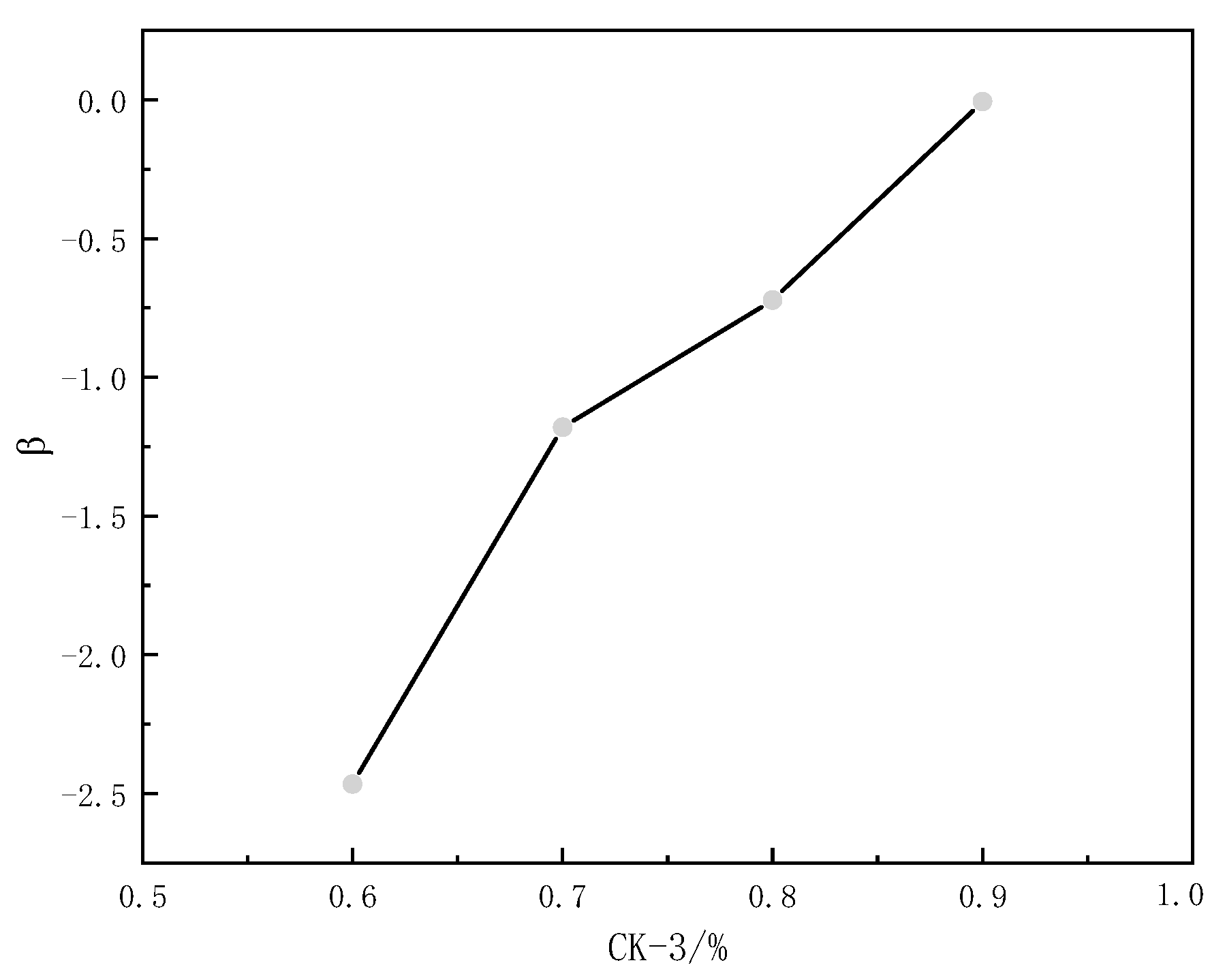
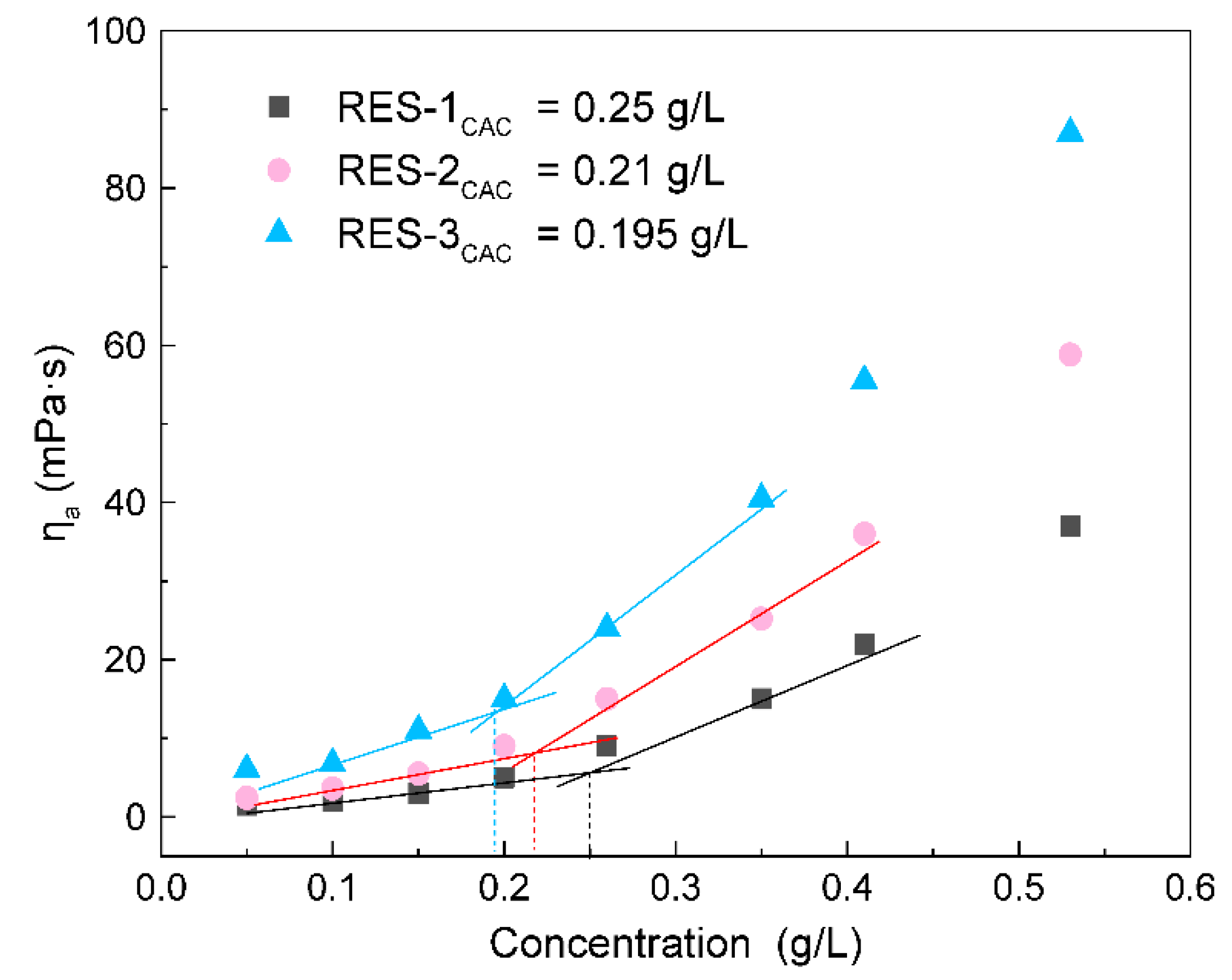
3.7. Critical Association Concentration of RES
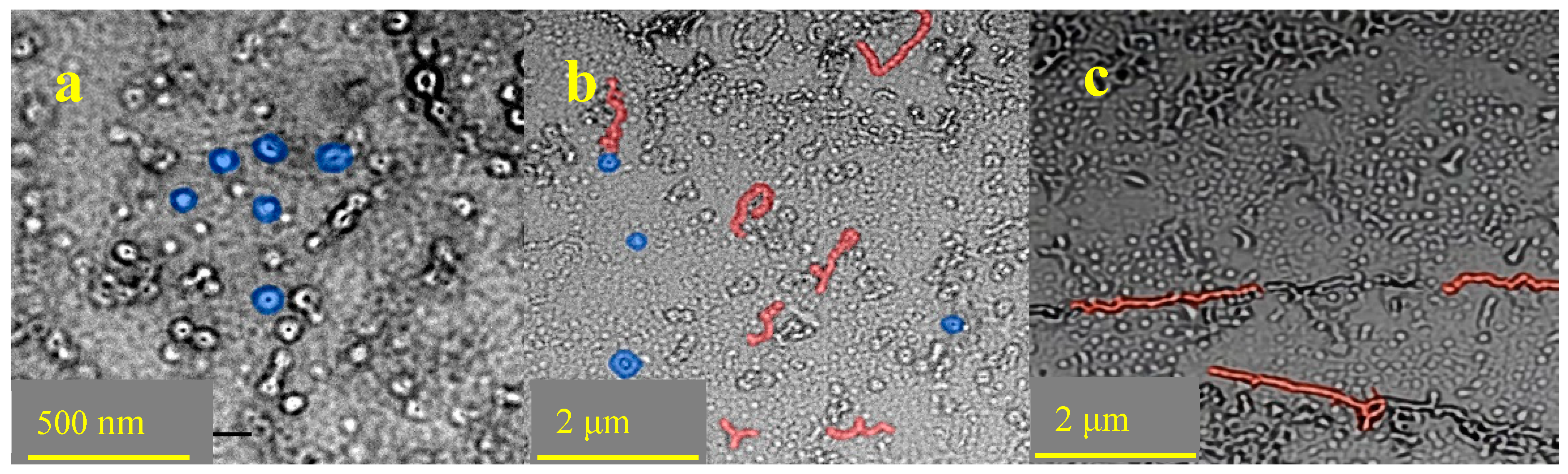
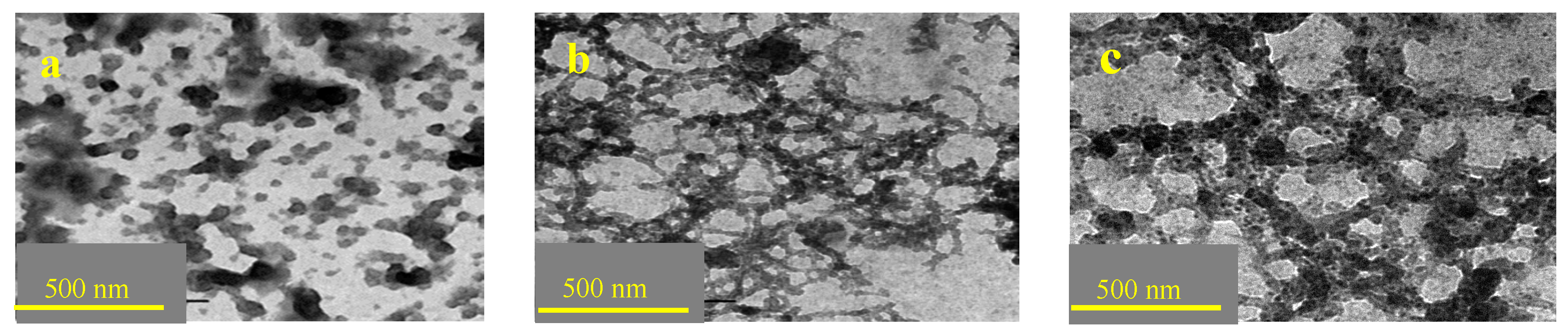
3.8. TEM Analysis of RES
3.9. SEM Analysis of RES
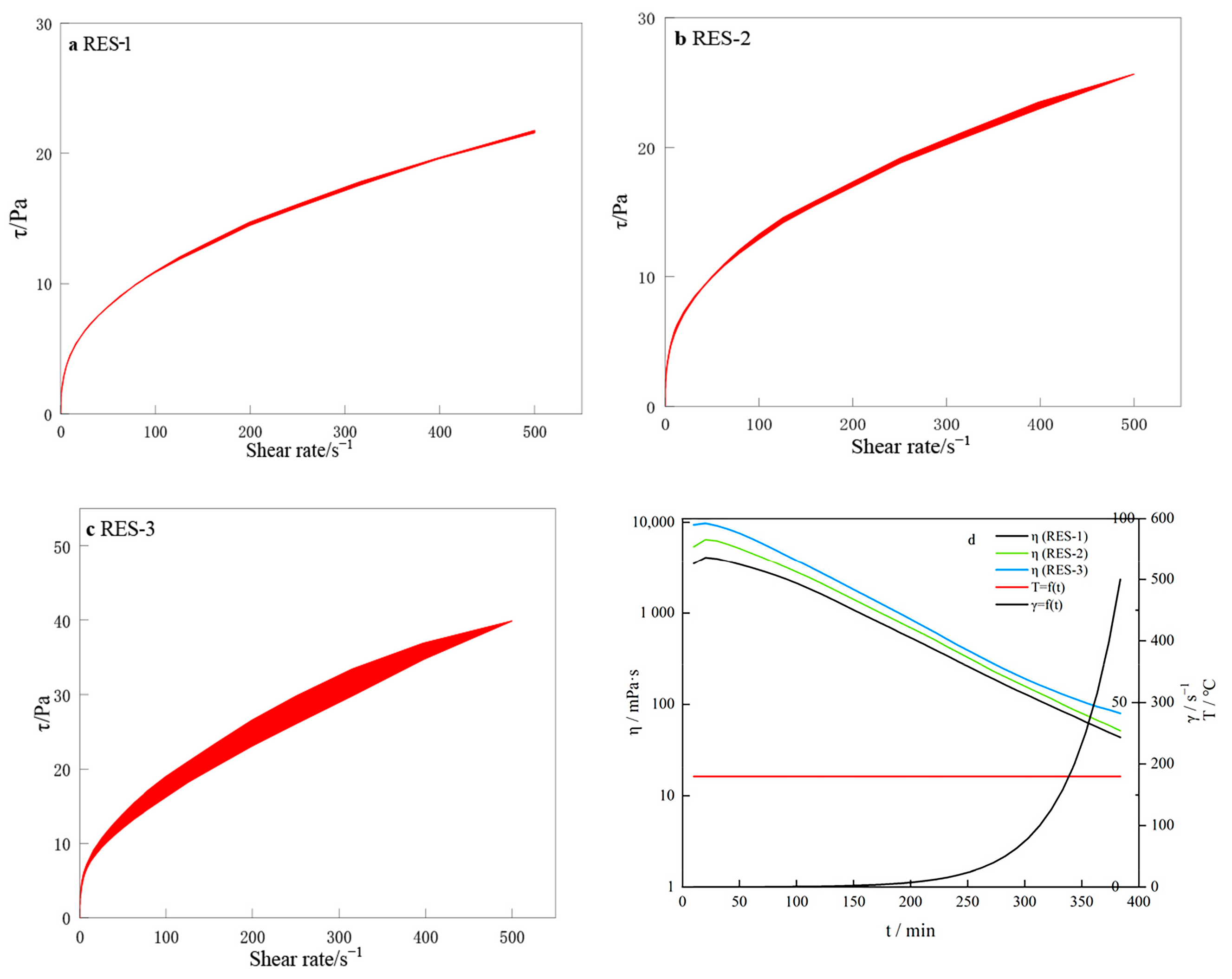
3.10. Thixotropic Analysis of RES
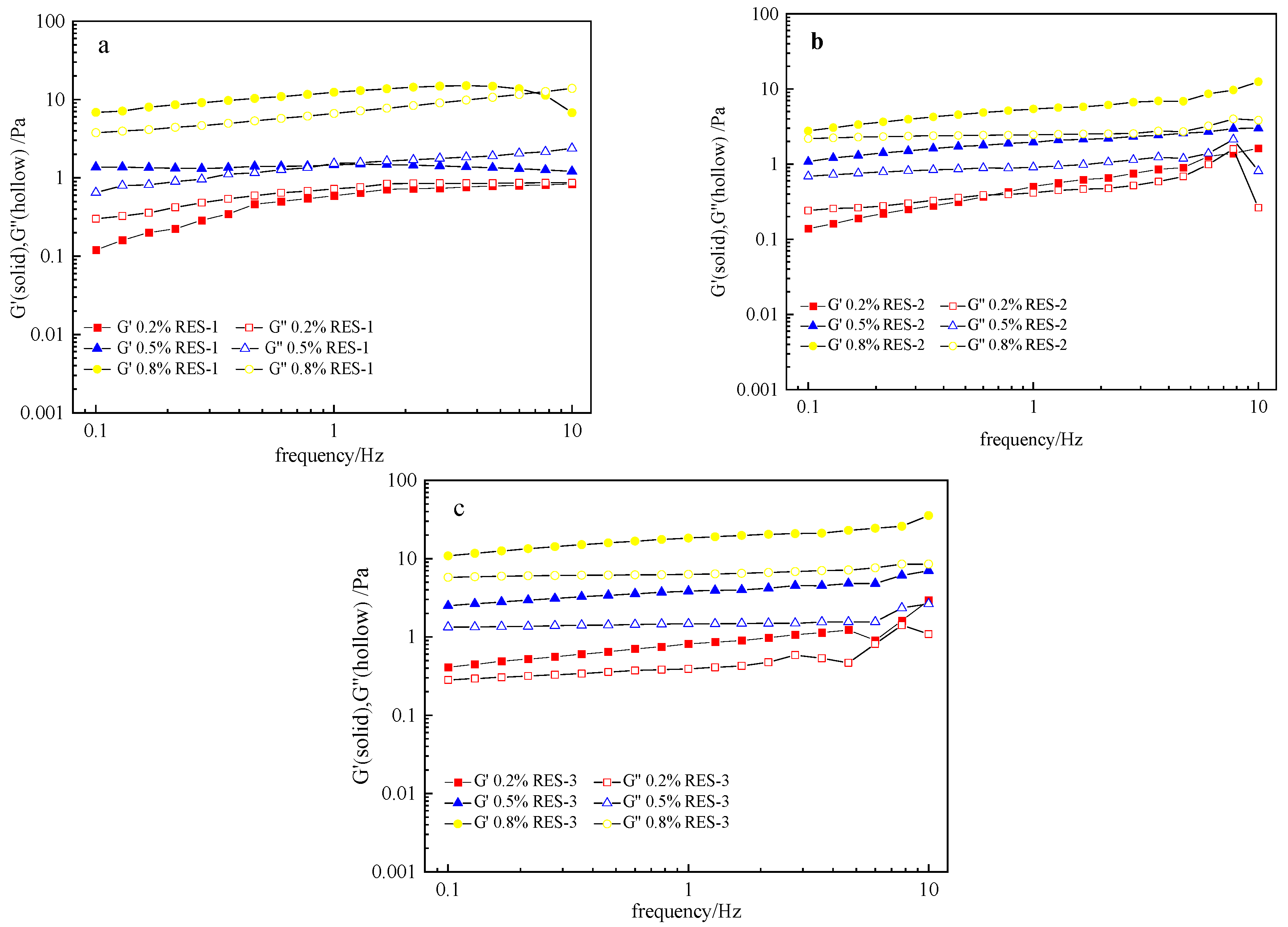
3.11. Viscoelasticity Analysis of RES
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, P.; Pei, X.; Li, C.; Li, R.; Chen, Z.; Song, B.; Cui, Z.; Xie, D. pH-switchable wormlike micelles with high viscoelasticity formed by pseudo-oligomeric surfactants. J. Mol. Liq. 2021, 334, 116499. [Google Scholar] [CrossRef]
- Zapf, A.; Beck, R.; Platz, G.; Hoffmann, H. Calcium surfactants: A review. Adv. Colloid Interface Sci. 2003, 100–102, 349–380. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheung, P.; Shen, A.Q. Microfluidic flows of wormlike micellar solutions. Adv. Colloid Interface Sci. 2014, 211, 34–46. [Google Scholar] [CrossRef]
- Theato, P.; Sumerlin, B.S.; O’Reilly, R.K.; Epps, I.T.H. Stimuli responsive materials. Chem. Soc. Rev. 2013, 42, 7055–7056. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Kuklin, A.I.; Torocheshnikov, V.N.; Orekhov, A.S.; Roland, S.; Miquelard-Garnier, G.; Matsarskaia, O.; Iliopoulos, I.; Philippova, O.E. Double dynamic hydrogels formed by wormlike surfactant micelles and cross-linked polymer. J. Colloid Interface Sci. 2022, 611, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Dreiss, C.A. Wormlike micelles: Where do we stand? Recent developments, linear rheology and scattering techniques. Soft Matter 2007, 3, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, G.; Wang, L.; Ding, L.; Shi, H.; Lai, X.; Wen, X.; Ma, S.; Huang, C. Rheological properties of an ultra-high salt hydrophobic associated polymer as a fracturing fluid system. RSC Adv. 2019, 9, 15246–15256. [Google Scholar] [CrossRef] [PubMed]
- Cadix, A.; Wilson, J.; Carouhy, T.; Harrisson, S.; Guichon, H. A new class of associative polymer for hydraulic fracturing applications. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015. [Google Scholar] [CrossRef]
- Richtering, W. Rheology and shear induced structures in surfactant solutions. Curr. Opin. Colloid Interface Sci. 2001, 6, 446–450. [Google Scholar] [CrossRef]
- de Molina, P.M.; Gradzielski, M. Gels Obtained by Colloidal Self-Assembly of Amphiphilic Molecules. Gels 2017, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Gradzielski, M. Polymer–surfactant interaction for controlling the rheological properties of aqueous surfactant solutions. Curr. Opin. Colloid Interface Sci. 2023, 63, 101662. [Google Scholar] [CrossRef]
- Kortemeier, U.; Venzmer, J.; Howe, A.; Grüning, B.; Herrwerth, S. Thickening agents for surfactant systems. SOFW J. 2010, 136, 30–36. [Google Scholar]
- de Lima, H.H.C.; Santos, G.M.; da Silva, C.T.P.; Mori, J.C.; de Carvalho Rinaldi, E.S.K.; Cotica, E.B.; Tambourgi, E.B.; Guilherme, M.R.; Rinaldi, A.W. Synthesis and characterization of a hydrophobic association hydrogel for drug delivery. J. Mol. Liq. 2023, 372, 120709. [Google Scholar] [CrossRef]
- Chen, H.; He, X.; Yang, X.; Li, P.; Wu, W. Preparation and investigations of PEG-AT-PEG organic nano-polymer photothermal material. Mater. Res. Express 2022, 9, 095101. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Jin, J.; Tin, Z.; Yang, W.; Graham, N.J.D.; Yang, Z. Enhanced removal of trace pesticides and alleviation of mem-brane fouling using hydrophobic-modified inorganic-organic hybrid flocculants in the flocculation-sedimentation-ultrafiltration process for surface water treatment. Water Res. 2023, 229, 119447. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, G.; Zhang, R.; Xia, W.; Nie, Y.; Kong, Y.; Jia, B.; Li, S. Emulsified oil removal from steel rolling oily wastewater by using magnetic chitosan-based flocculants: Flocculation performance, mechanism, and the effect of hydrophobic monomer ratio. Sep. Purif. Technol. 2023, 304, 122329. [Google Scholar] [CrossRef]
- Cai, S.; Wan, Y.; He, X. Molecular interaction of poly (acrylamide-co-2-acrylamido-dodecyl sulfonate) with dual responsiveness and application in oily emulsion wastewater. J. Appl. Polym. Sci. 2022, 139, 51528. [Google Scholar] [CrossRef]
- Liu, L.; Gou, S.; Ma, Y.; Zhou, L.; He, Y.; Liu, L.; Tang, L.; Fang, S. A thermal thickening system based on the self-assembly of a zwitterionic hydrophobic association polymer and surfactant. Aust. J. Chem. 2021, 74, 238–244. [Google Scholar] [CrossRef]
- Jiao, G.; Zhu, S.; Ye, Z.; Shu, Z.; Wang, X.; Wang, D. The effect of shear on the properties of an associated polymer solution for oil displacement. Polymers 2023, 15, 616. [Google Scholar] [CrossRef]
- Chu, Z.; Dreiss, C.A.; Feng, Y. Smart wormlike micelles. Chem. Soc. Rev. 2013, 42, 7174–7203. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, Y.; Chen, Q.; Wang, J.; Li, B.; Feng, Y. Synthesis and surface activities of Amidobetaine surfactants with ultra-long unsaturated hydrophobic chains. J. Surfactants Deterg. 2012, 15, 657–661. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, G.; Zhao, L. Surfactant Chemistry and Technology; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Zetian, H. Polymer Reaction Thermodynamics; Science Press: Beijing, China, 1985. [Google Scholar]
- Zhang, Q.; Mao, J.; Liao, Y.; Mao, J.; Yang, X.; Lin, C.; Wang, Q.; Huang, Z.; Xu, T.; Liu, B.; et al. Salt resistance study and molecular dynamics simulation of hydrophobic-association polymer with internal salt structure. J. Mol. Liq. 2022, 367, 120520. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, J. Polymer Material Rheology; Higher Education Press: Beijing, China, 2014. [Google Scholar]
- Zhang, Y.; Chen, A.; Mao, J.-C.; Qin, S.-H.; Li, J.; Yang, X.-J.; Lin, C.; Huang, Z.-Y.; Liu, Y.-F. Preparation of a functional fracturing fluid with temperature- and salt-resistance, and low damage using a double crosslinking network. Pet. Sci. 2023; in press. [Google Scholar]
- Xu, J.; Ren, X.; Gao, G. Salt-inactive hydrophobic association hydrogels with fatigue resistant and self-healing properties. Polymer 2018, 150, 194–203. [Google Scholar] [CrossRef]
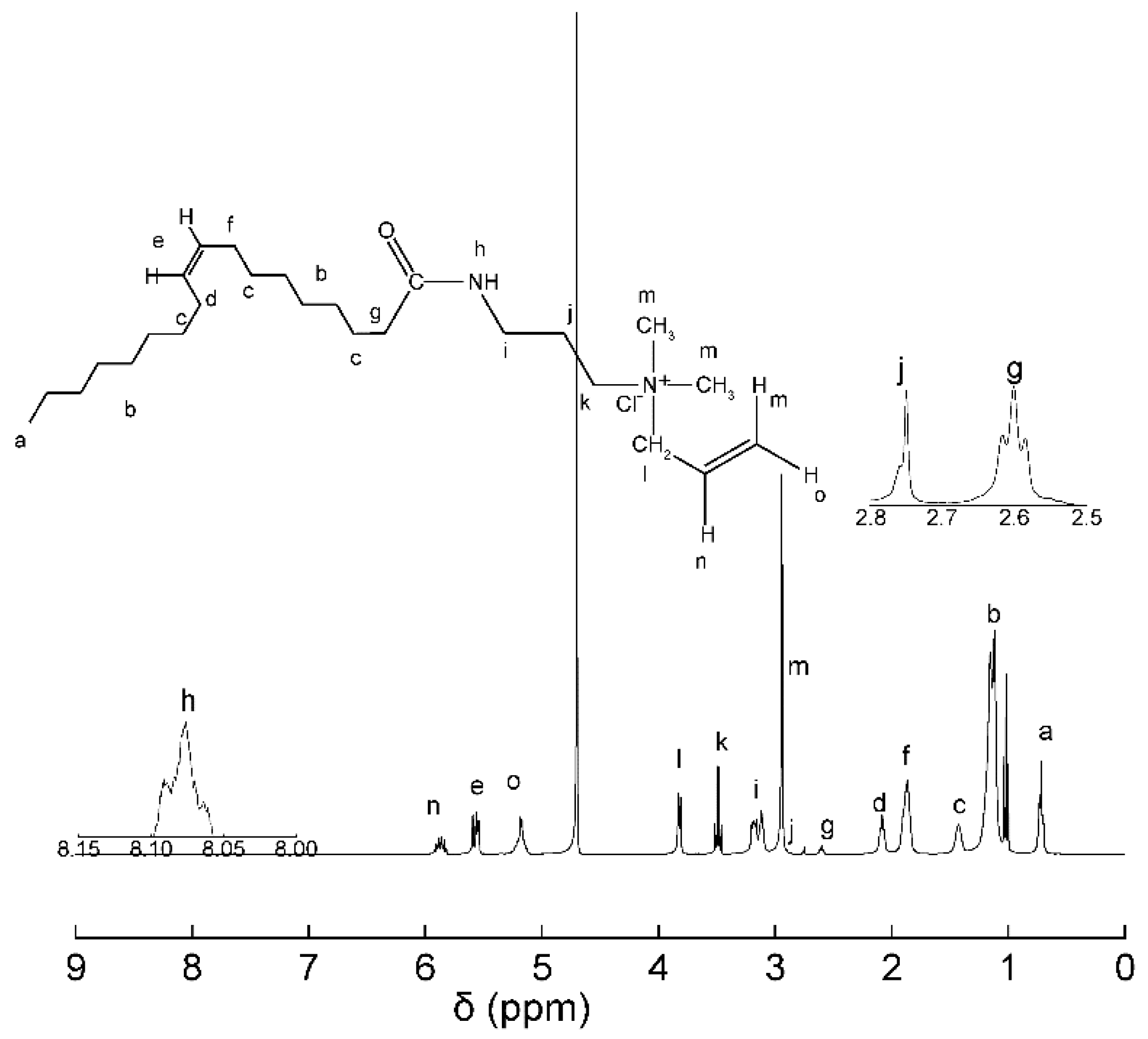




| Peak Number | δ | Atom Number |
|---|---|---|
| 1 | 0.66–0.80 | 3 |
| 2 | 0.99–1.26 | 10 |
| 3 | 1.35–1.48 | 2 |
| 4 | 2.04–2.13 | 2 |
| 5 | 5.51–5.62 | 2 |
| 6 | 1.80–1.93 | 2 |
| 7 | 2.51–2.67 | 2 |
| 8 | 8.05–8.10 | 1 |
| 9 | 3.07–3.22 | 2 |
| 10 | 2.70–2.80 | 2 |
| 11 | 3.45–3.52 | 2 |
| 12 | 3.78–3.86 | 2 |
| 13 | 2.91–2.99 | 3 |
| 14 | 5.81–5.95 | 1 |
| 15 | 2.51–2.67 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Wang, L.; Lai, X.; Liu, G.; Yang, W.; Gao, J.; Liu, Y.; Cui, W. Polymerization Behavior and Rheological Properties of a Surfactant-Modified Reactive Hydrophobic Monomer. Chemistry 2023, 5, 2598-2612. https://doi.org/10.3390/chemistry5040168
Wen X, Wang L, Lai X, Liu G, Yang W, Gao J, Liu Y, Cui W. Polymerization Behavior and Rheological Properties of a Surfactant-Modified Reactive Hydrophobic Monomer. Chemistry. 2023; 5(4):2598-2612. https://doi.org/10.3390/chemistry5040168
Chicago/Turabian StyleWen, Xin, Lei Wang, Xiaojuan Lai, Guiru Liu, Wenwen Yang, Jinhao Gao, Yameng Liu, and Wenyu Cui. 2023. "Polymerization Behavior and Rheological Properties of a Surfactant-Modified Reactive Hydrophobic Monomer" Chemistry 5, no. 4: 2598-2612. https://doi.org/10.3390/chemistry5040168
APA StyleWen, X., Wang, L., Lai, X., Liu, G., Yang, W., Gao, J., Liu, Y., & Cui, W. (2023). Polymerization Behavior and Rheological Properties of a Surfactant-Modified Reactive Hydrophobic Monomer. Chemistry, 5(4), 2598-2612. https://doi.org/10.3390/chemistry5040168





