Abstract
It is well known that beer is more than 90% water, and therefore, water can be one of the main sources of fluoride in beers. With this in mind, the goal of the present study was to determine the mass concentration of fluoride in 53 beer samples. Using the recently published standard addition method in potentiometry, the fluoride content of 28 samples of the most consumed beers in the Republic of Croatia was determined. The remaining 25 beer samples tested came from so-called microbreweries, which together account for less than 10% of the Croatian market. Fluoride concentrations in light beers ranged from 49 to 180 μg L−1, with a mean value of 95 ± 34 μg L−1, and from 52 to 164 μg L−1, with a mean value of 89 ± 29 μg L−1 in dark beers. The mean value of fluoride content in beers from large producers was 100 ± 38 μg L−1 and 89 ± 38 μg L−1 in beers from small producers. All values are within the recommended range and thus do not pose a risk to human health. The statistical analysis showed no correlation between the mass concentration of fluoride and pH, i.e., alcohol content in beers.
1. Introduction
Fluorides are the most chemically reactive and electronegative of all elements in the periodic table and the thirteenth most abundant element in the Earth’s crust [1,2]. Flouride occurs in the form of fluorides in a variety of minerals, such as fluorite (CaF2), cryolite (Na3AlF6), and fluorapatite (Ca5(PO4)3F) [1]. Fluoride ions are one of the most important elements in the human body and play an essential role in both human health and environmental protection. Unfortunately, environmental pollution by fluorine compounds from various sources such as aluminum factories, glass and brick manufacturing, mining companies, the use of mineral fertilizers, etc., is increasing [3].
The human body is constantly exposed to fluoride through the consumption of food, beverages, or the use of dental care products. A significant portion of the fluoride ingested in this way is found in beverages, including alcoholic beverages such as beer. The multiple effects of alcohol consumption on human health have gained increasing attention in the international scientific community. In particular, the question as to the role of moderate beer consumption, by far the most widely consumed alcoholic beverage in the world, has arisen [4].
Beer, a complex brewed beverage made from barley malt, water, hops, and yeast, is considered one of the oldest alcoholic beverages in history [5,6] and is a popular drink in many cultures (the most popular alcoholic beverage; after water and tea, the third-most consumed beverage in general [7]). It contains alcohol and carbohydrates and consists of over 90% water. Beer also contains various trace elements such as magnesium, iron, calcium, phosphorus, sodium, potassium, zinc, manganese, copper and selenium, fluoride, and silicon [4]. Beer consumption is of particular interest because of its organoleptic and health properties and because of its low price compared to other alcoholic beverages, such as wine. The alcohol content of beer varies depending on the type of beer and is often estimated to range from 2.5% to 13% v/v. Recently, new types of beer have been produced, such as low-alcohol and alcohol-free beers, low-calorie beers, beers high in vegetable proteins, beers with flavorings (e.g., fruits), gluten-free beers, and beers with functional substances [8,9].
Beer, wine, and other alcoholic beverages usually contain small amounts of fluoride. Since there are few scientific publications on the fluoride content in alcoholic beverages, we investigated them as a possible source of dietary fluoride.
A variety of methods have been developed for the determination of fluoride in various samples, and the most important techniques can be divided into six main categories: electrochemical methods (potentiometry and voltammetry), chromatographic methods (ion chromatography, high-performance liquid chromatography, and gas chromatography), spectroscopic methods (atomic and molecular spectroscopy), microfluidic analysis (flow injection analysis and sequential injection analysis), sensors, and titration [1]. The extent to which research on the determination of fluoride ions has become the focus of interest in the scientific community is best illustrated by the recent publication of a series of articles on the subject [10,11,12,13,14,15,16], including a series of methods for the determination of fluoride in real samples using more modern and powerful chemical analytical techniques.
The aim of this study was to determine the role of beer as a potential source of dietary fluoride by measuring the fluoride concentration in 53 selected beers from different producers in Croatia, differing in their reported alcohol content. More than half of the selected beers (28 samples) are produced by the seven largest Croatian beer producers, which dominate the Croatian beer market with a share of more than 90%, according to the latest report of the Financial Agency (the official Croatian provider of financial and electronic services).
Fluoride concentrations in each sample were measured using a fluoride ion selective electrode (FISE) according to a recently published standard potentiometric addition method [2,17,18]. The results obtained were compared with each other and with the fluoride content of some worldwide beer producers.
The results of this study provide new information, as no results on fluoride content in beers produced in Croatia were found in the literature. Based on the obtained results, the authors pay special attention to the question of the main source of fluorides in the studied beers.
2. Materials and Methods
2.1. Chemicals and Solutions
All chemicals and reagents used in this work were of analytical grade, and all solutions were prepared with redistilled water. Sodium fluoride, ethylenediaminetetraacetic acid disodium salt (EDTA), glacial acetic acid, and sodium hydroxide anhydrous were obtained from Kemika (Zagreb, Croatia), while sodium chloride was purchased from Merck (Darmstadt, Germany).
To prepare total ionic strength adjusting buffer solution (TISAB), 58.06 g of NaCl, 37.24 g of NaOH, and 57.0 mL of glacial acetic acid were first mixed with 1.0 L of a 0.03 mol/L EDTA solution. The pH of the prepared solution was then adjusted to 5.5 by adding small amounts of 1.5 mol/L acetic acid solution. Finally, the prepared solution was thoroughly transferred to a 2 L volumetric flask and made up to the mark with distilled water [2,19].
The 0.1000 mol/L sodium fluoride standard solution was prepared by dissolving 1.05 g of sodium fluoride (which was previously oven-dried at 110 °C for 2 h and then cooled to room temperature in a desiccator [20]) in 250 mL of TISAB (in polyethylene volumetric flask).
2.2. Aliquots of Beer Samples
Approximately 100 mL of each beer sample was placed in a bottle, which was loosely capped. After 12 h, each sample was mixed with a magnetic stirrer for 2 h. To prepare beer aliquots, 10.0 mL of each decarbonated beer sample was transferred to a 50 mL beaker and 10.0 mL of TISAB was added to keep the ionic strength and pH of the aliquot constant [21,22].
2.3. Apparatus
For the experiments, a combined FISE (model perfectIONTM combination F-, P/N 51344715, Mettler Toledo, GmbH, Greifensee, Switzerland), an InLab Expert Pro electrode (model P/N 51343101, Mettler Toledo, GmbH, Greifensee, Switzerland), a digital pH/mV meter (model Seven Easy, Mettler Toledo, GmbH, Schwerzenbach, Switzerland), an analytical weighing balance (model PA214C Ohaus Pioneer Plus (accuracy 0.0001 g), Ohaus Corp, Parsippany, NJ, USA), a premium hotplate stirrer (model MSH-20A, Witeg Labortechnik, GmbH, Wertheim, Germany), and a laboratory sterilizer and dryer (model ST -01/02, Instrumentaria, Zagreb, Croatia) were used as measuring devices and accessories.
2.4. Application of the Proposed Standard Addition Method for Determination of Mass Concentration of Fluoride in Beer
The mass concentration of fluoride in the studied beers was determined according to the recently proposed and applied standard addition method in potentiometry [2,17,18]. The expression was placed on the abscissa axis, while the expression was placed on the ordinate axis (where and are the concentration and the sum of increments in the sodium fluoride standard solution; is the initial volume of the aliquot under study, is the difference in the recorded potentials after each increment in the standard solution, and is the slope of the calibration curve). The value of the zero point of the straight line equation thus obtained represents a negative value of the fluoride concentration in each studied aliquot. In summary, the mass concentration of fluoride in beer () was calculated according to the following equation:
where is the negative value of the fluoride concentration in the analyte (zero point of the linear equation), is the volume of the analyte studied (20 mL), is the volume of the previously decarbonated beer sample (10 mL), and C.f. is the correction factor of 1 × 106. Six identical measurements were performed for each beer.
2.5. Statistical Analyzes
The data obtained from the combined FISE for the determination of fluorides in Croatian beers were statistically evaluated. Descriptive statistics was applied, and the correlation between numerical and categorical parameters was investigated. Correlation analysis was performed on 53 measured samples (six replicates). Pearson’s correlation coefficient was calculated for each of the two parameters. Statsoft Statistica 14 (Hamburg, Germany) was used for the statistical analysis.
3. Results and Discussion
A total of 53 beer samples (33 samples of light beer, 20 samples of dark beer) from different producers in Croatia were purchased at local markets and analyzed. The classification of beer into varieties and types is precisely defined in various regulations on the quality of beer. Therefore, the beer classification in this work was not carried out arbitrarily, but the beers were sorted by color based on the information provided by the producers. The beer manufacturers, the beer types, the values for alcohol content in percent by volume (each provided by the brewery), the measured pH values, and the mean and standard deviation of the fluoride mass concentration in beers are listed in Table 1.

Table 1.
A list of beer names, breweries, declared alcohol content, measured pH values, and fluoride contents.
The pH values for light beers ranged from 3.23 to 4.79, while for dark beers, they ranged from 3.53 to 4.58. The lowest pH value (3.23) was measured in the L24 beer sample, while the highest value (4.79) was measured in the L30 beer sample. The lowest pH for dark beers was 3.53 in sample D9, while the highest value was 4.58 in samples D6, D14, and D18. The highest alcohol content (6.0% vol.) for light beers was reported by the producers in two beer samples, L2 and L15, while the lowest alcohol content (0.0% vol.) was reported in L22. In general, the alcohol content values for light beers ranged from 0% to 6.0% vol. For dark beers, the highest alcohol content (7.5% vol.) was reported for beer samples D6 and D14, and the lowest (2.0% vol.) for beer sample D9. Dark beers generally have a higher alcohol content than light beers.
After calculation, it was found that the mass concentrations of fluoride in the studied beers ranged from 49 µg/L to 180 µg/L. The highest and lowest mass concentrations of fluoride were found in the light beers (samples L6 and L3).
Considering the very small relative standard deviations of fluoride content between six identical measurements for each sample with the generally low fluoride content (4.2% on average), the results of this work are an additional confirmation of the accuracy of the proposed new model of the standard addition method in potentiometry that has been recently published and applied [2,17,18]. The statements of various groups of authors that the potentiometric determination of fluoride in TISAB solution is recommendable [2,19,23,24,25] were also confirmed with regard to the aforementioned fact that there were negligible standard deviations of the results of fluoride content in the same samples.
Based on the obtained results, descriptive statistics was applied, and it was found that pH and alcohol concentration correlate with a correlation factor of 0.68, while the other variables do not correlate with each other. Although in the literature, it is claimed that beers with a higher alcohol content have a lower mass concentration of fluoride [26,27], it is evident from Table 2 that this is not in agreement with the results of the present work, i.e., that both pH and alcohol content have no influence on the fluoride content in the studied beers of Croatian producers.

Table 2.
Correlation analysis of variables.
Considering the process of beer production itself, it is logical to expect that pH and alcohol content do not correlate with fluoride content. Namely, during the alcoholic fermentation process, when the yeast converts the glucose (contained in the wort) into ethyl alcohol and carbon dioxide gas, the alcohol content and pH change [28,29], while the value of the total fluoride content remains constant.
Of the samples tested, 28 beers came from one of the seven largest breweries in the Republic of Croatia, which together produce more than 90% of the total beer sales in the country (Zagreb Brewery, Heineken Croatia Brewery, Carlsberg Croatia Brewery, Istrian Brewery, Daruvar Brewery, Medvedgrad Brewery, and Brewery of Lika). The remaining 25 samples were from various “smaller” breweries. When comparing the results, no significant differences were found in the fluoride content of beers from “large” and “small” breweries (Figure 1); more precisely, these values were 100 ± 37 μg/L for beers from large breweries and 89 ± 21 μg/L for those from small breweries.
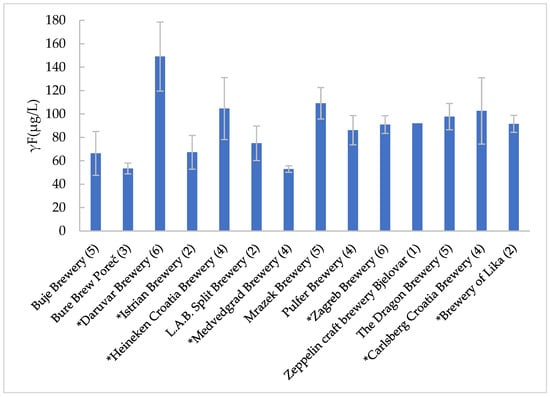
Figure 1.
Graphical description of the average mass concentrations of fluoride in the beers of Croatian breweries; *—“large” brewery; (number of different beer samples studied).
The wide variations in fluoride content between different beer samples from the same brewery can be clearly seen from Figure 1. If the reported deviations were presented as a relative standard, Buje Brewery, Carlsberg Croatia Brewery, Heineken Croatia Brewery, Istrian Brewery, Daruvar Brewery, and L.A.B. Split Brewery would have beers with deviations in fluoride content of 28.2, 27.6, 25.3, 21.4, 19.8, and 19.7%, respectively. These results of the relative standard deviation of fluoride content are not comparable, and the conclusions drawn from them would be incorrect (the same number of different samples from each brewery was not studied; moreover, only one beer sample from Zeppelin craft brewery Bjelovar was studied). To get a better insight, a scatter plot (Figure S1, in the Supplementary Materials) of the relationship between the number of different beer samples studied from one brewery and the relative standard deviation of the fluoride content in the beers studied (from that brewery) was also made. From this, it can be seen that these two parameters are also not correlated.
All beer samples tested have an allowable fluoride concentration of 1.5 mg/L in Croatia, which corresponds to the levels recommended by the World Health Organization. These results do not differ significantly from the results for beers from other European producers. In fact, low average fluoride levels were found in the literature for Polish, Ukrainian, German, and Armenian beers [27], while higher levels were found for beers from Thailand, Italy, Mexico, China, Belgium, France, the United Kingdom, and significantly for beers from Ireland and the United States [30]. This could mainly indicate large differences in the fluoride content of water from these countries. Comparing the average fluoride content in beers from Croatian producers with the fluoride content in beer samples from some global producers, it can be seen that Croatian beers have similar fluoride content to beers from Ukraine or Poland (Figure 2).
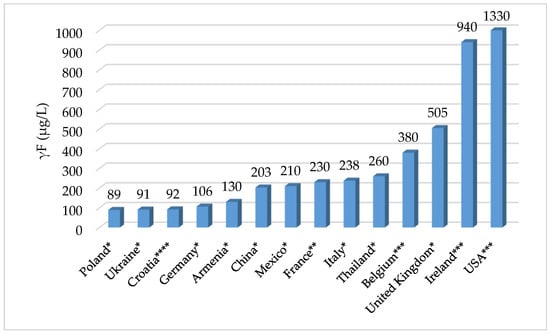
Figure 2.
Graphical representation of the comparison of the average values of fluoride in beers from Croatia and from other worldwide countries (* [27], ** [31], *** [30], **** present study).
Research by Warnakulasuriya et al. [31] shows that the fluoride content of a particular variety or even brand can vary depending on where it is produced (e.g., Heineken and Carlsberg brand beers produced in the United Kingdom were found to have lower fluoride content than Heineken and Carlsberg brand beers produced in Denmark and the Netherlands). Water, one of the most important raw materials for beer production, influences many properties of beer [32]. Technological water used for beer production must correspond to the quality of drinking water in physical, chemical, and microbiological aspects, the properties of which are prescribed by a number of regulations [33]. The chemical composition of water for beer production varies, so waters differ in terms of total hardness, permanent and temporary hardness, pH and alkalinity, microbes, presence of disinfection byproducts, content of individual salts, etc. [34,35]. Salts dissolved in water influence the taste of beer through their effect on enzymatic and colloidal chemical reactions that take place during beer production [35,36]. Various groups of authors explain the differences in the fluoride content of beer by the naturally occurring fluoride in water or the fluoridation of water in certain regions [26,37,38]. Such a conclusion is not compatible with the results of the present study. In fact, from Figure 1 and Figure S1, the fluoride content in different beers from the same brewery is very different (high standard deviation), which, similar to the observation of Tekle-Haimanot and Haile [39], leads to the conclusion that the fluoride content in Croatian beers is closely related to the other raw materials, i.e., barley malt (wheat malt) and hops, or to the fluoride content of the soil where they are grown, and not to the type of water. The results show no significant differences in fluoride content between light and dark beers and between beers from large and small producers. However, the fluoride content results show significant differences between different beers from the same producer. This indicates that drinking water is not a significant source of fluoride in Croatian beers. Considering that Croatian drinking water is not fluoridated, this was logically to be expected, even though such low fluoride levels were not found in beers in some countries that have also stopped the fluoridation of drinking water. However, if we take into account the fact that beers from breweries in the continental part of Croatia had higher fluoride concentrations (101 µg/L) than beers from breweries in the coastal region (64 µg/L), we could return to the original conclusion (water is the main source of fluoride in beer). But, based on everything that has been discussed here, that would lead us down the wrong path. Considering the very low fluoride levels in the studied beers, and assuming that water is not the main source of fluoride in the beers, it is obvious that the fluoride content in other raw materials can significantly influence the total fluoride content in the beers produced in Croatia.
Notwithstanding the fact that the determination of fluoride content in various samples is the subject of research by scientists around the world, very few papers were found in the literature dealing with the determination of fluoride content in beers. Comparing two similar studies from two different time periods [30,31] (Irish beers from 1120 to 940 μg/L and German beers from 250–330 to 106 μg/L) and considering the mean value of present work (92 μg/L), it can be concluded that the fluoride content in beers has generally decreased in the last 20 years. This is also logical, since many countries have stopped water fluoridation because they fear negative effects on human health from excessive fluoride consumption.
Since moderate beer consumption is associated in the literature with a significant reduction in the mortality rate from cardiovascular diseases [40] and beer contains components that provide some nutritional and medical benefits to the consumer (proteins, B-group vitamins and some minerals, phenolic compounds, dietary fiber) [8], the results of this study in relation to the fluoride content in the studied beers clearly show that the moderate consumption of beers produced in the Republic of Croatia is highly recommended.
4. Conclusions
In the present work, the fluoride content in Croatian beers was determined by the standard addition method in potentiometry. It was found that there are no significant differences in fluoride concentrations of dark and light beers, as well as beers from large and small breweries. However, it was also found that the fluoride concentration in beers produced in the continental part of the Republic of Croatia is different from that in beers produced in the coastal region, and that water is not the main source of fluoride in the studied beers. This suggests that the other raw materials are the main source of fluoride in the studied beers, i.e., the type of soil where the raw materials are grown. Also, no correlation was found between the fluoride content and the measured pH, i.e., the reported values of alcohol content in the studied beers. All values are within the recommended limits, so the moderate consumption of beers produced in the Republic of Croatia can be recommended for the prevention of certain diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5040167/s1, Figure S1: Scatter plot of the relationship between the number of different beer samples tested from one brewery and the relative standard deviation of the fluoride content in beers (from that brewery).
Author Contributions
Conceptualization, M.B. (Maša Buljac) and J.R.; methodology, M.B. (Marija Bralić); validation, M.B. (Maša Buljac) and J.R.; formal analysis, J.D. and J.R.; investigation, M.B. (Maša Buljac); resources, M.B. (Maša Buljac) and M.B. (Marija Bralić); data curation, N.V. and J.R.; writing—original draft preparation, M.B. (Maša Buljac), M.B. (Marija Bralić) and J.R.; writing—review and editing, M.B. (Maša Buljac) and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yahyavi, H.; Kaykhaii, M.; Mirmoghaddam, M. Recent Developments in Methods of Analysis for Fluoride Determination. Crit. Rev. Anal. Chem. 2016, 46, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Buljac, M.; Bralić, M.; Vrca, I.; Kolar, M.; Radić, J. Potentiometric Determination of Free Fluoride Content in Wines from Dalmatia Region (Croatia)—A Comparative Study of Direct Potentiometry and Standard Addition Method. Chemistry 2022, 5, 31–40. [Google Scholar] [CrossRef]
- Petrenko, D.B.; Marchenko, D.Y.; Vasil’ev, N.V. Zirconium gallocyanin MS complex as a highly selective reagent for the spectophotometric determination of fluoride. Microchem. J. 2021, 164, 106081. [Google Scholar] [CrossRef]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef]
- Nelson, M. The Barbarian’s Beverage; Routledge: London, UK, 2005; ISBN 9781134386727. [Google Scholar]
- Walklate, J. Uncorking the Past: The Quest for Wine, Beer, and Other Alcoholic Beverages. Time Mind 2011, 4, 111–114. [Google Scholar] [CrossRef]
- Fangel, J.U.; Eiken, J.; Sierksma, A.; Schols, H.A.; Willats, W.G.T.; Harholt, J. Tracking polysaccharides through the brewing process. Carbohydr. Polym. 2018, 196, 465–473. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Health-Related Aspects of Beer: A Review. Int. J. Food Prop. 2012, 15, 350–373. [Google Scholar] [CrossRef]
- Mellor, D.D.; Hanna-Khalil, B.; Carson, R. A review of the potential health benefits of low alcohol and alcohol-free beer: Effects of ingredients and craft brewing processes on potentially bioactive metabolites. Beverages 2020, 6, 25. [Google Scholar] [CrossRef]
- Guo, W.; Jin, L.; Hu, S.; Guo, Q. Method Development for the Determination of Total Fluorine in Foods by Tandem Inductively Coupled Plasma Mass Spectrometry with a Mass-Shift Strategy. J. Agric. Food Chem. 2017, 65, 3406–3412. [Google Scholar] [CrossRef]
- Du, J.; Sheng, C.; Wang, Y.; Zhang, H.; Jiang, K. Determination of trace fluoride in water samples by silylation and gas chromatography/mass spectrometry analysis. Rapid Commun. Mass Spectrom. 2021, 35, e9089. [Google Scholar] [CrossRef]
- Ozbek, N.; Akman, S. Determination of fluorine in Turkish wines by molecular absorbance of CaF using a high resolution continuum source atomic absorption spectrometer. LWT Food Sci. Technol. 2015, 61, 112–116. [Google Scholar] [CrossRef]
- Ünal, E.İ.; Kenar, A.; Aksu, M.L.; Tastekin, M. Spectrophotometric methods for the determination of fluoride ion using indole-3-acetic acid interaction with iron(III). Turk. J. Chem. 2019, 43, 415–423. [Google Scholar] [CrossRef]
- Moro, T.T.; Arcênio, P.P.; de Oliveira, F.J.S.; Chaves, E.S.; Bascuñan, V.L.A.F.; Maranhão, T. de A. Determination of extractable fluorine from residue of oil and gas industry by HR-CS MAS applying toxicity characteristic leaching procedure. J. Fluor. Chem. 2021, 252, 109917. [Google Scholar] [CrossRef]
- Moirana, R.L.; Kivevele, T.; Mkunda, J.; Mtei, K.; Machunda, R. Trends towards Effective Analysis of Fluorinated Compounds Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). J. Anal. Methods Chem. 2021, 2021, 8837315. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.H.; Liu, S.H.; Chen, P. Fluoride Determination by Ion Chromatography in Fluorocarbon Coatings. Key Eng. Mater. 2017, 726, 50–54. [Google Scholar] [CrossRef]
- Radić, J.; Perović, D.; Gričar, E.; Kolar, K. Potentiometric Determination of Maprotiline Hydrochloride in Pharmaceutical and Biological Matrices Using a Novel Modified Carbon Paste Electrode. Sensors 2022, 22, 9201. [Google Scholar] [CrossRef]
- Radić, J.; Buljac, M.; Genorio, B.; Gričar, E.; Kolar, M. A Novel Reduced Graphene Oxide Modified Carbon Paste Electrode for Potentiometric Determination of Trihexyphenidyl Hydrochloride in Pharmaceutical and Biological Matrices. Sensors 2021, 21, 2955. [Google Scholar] [CrossRef]
- Spano, N.; Guccini, V.; Ciulu, M.; Floris, I.; Nurchi, V.M.; Panzanelli, A.; Pilo, M.I.; Sanna, G. Free fluoride determination in honey by ion-specific electrode potentiometry: Method assessment, validation and application to real unifloral samples. Arab. J. Chem. 2018, 11, 492–500. [Google Scholar] [CrossRef]
- Radić, J.; Bralić, M.; Kolar, M.; Genorio, B.; Prkić, A.; Mitar, I. Development of the New Fluoride Ion-Selective Electrode Modified with FexOy Nanoparticles. Molecules 2020, 25, 5213. [Google Scholar] [CrossRef]
- Heilman, J.R.; Kiritsy, M.C.; Levy, S.M.; Wefel, J.S. Assessing fluoride levels of carbonated soft drinks. J. Am. Dent. Assoc. 1999, 130, 1593–1599. [Google Scholar] [CrossRef]
- Heilman, J.R.; Levy, S.M.; Wefel, J.S.; Patterson, K.Y.; Cutrufelli, R.; Pehrsson, P.R.; Holden, J.M. Fluoride assay methodology for carbonated beverages. J. Dent. Child. 2006, 73, 136–139. [Google Scholar]
- Patel, S.; Omid, N.; Zohoori, F.V.; Maguire, A.; Waldron, K.J.; Valentine, R.A. Comparison of total ionic strength adjustment buffers iii and iv in the measurement of fluoride concentration of teas. Nutr. Health 2018, 24, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tokalioǧlu, Ş.; Kartal, Ş.; Şahin, U. Determination of fluoride in various samples and some infusions using a fluoride selective electrode. Turk. J. Chem. 2004, 28, 203–211. [Google Scholar]
- Harhash, A.Y.; ElSayad, I.I.; Zaghloul, A.G.S. A comparative in vitro study on fluoride release and water sorption of different flowable esthetic restorative materials. Eur. J. Dent. 2017, 11, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Goschorska, M.; Gutowska, I.; Baranowska-Bosiacka, I.; Rać, M.E.; Chlubek, D. Fluoride Content in Alcoholic Drinks. Biol. Trace Elem. Res. 2016, 171, 468–471. [Google Scholar] [CrossRef]
- Styburski, D.; Baranowska-Bosiacka, I.; Goschorska, M.; Chlubek, D.; Gutowska, I. Beer as a Rich Source of Fluoride Delivered into the Body. Biol. Trace Elem. Res. 2017, 177, 404–408. [Google Scholar] [CrossRef]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Jaudenes, J.R.; Hardisson, A.; Paz, S.; Rubio, C.; Gutiérrez, A.J.; Burgos, A.; Revert, C. Potentiometric Determination of Fluoride Concentration in Beers. Biol. Trace Elem. Res. 2018, 181, 178–183. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Harris, C.; Gelbier, S.; Keating, J.; Peters, T. Fluoride content of alcoholic beverages. Clin. Chim. Acta 2002, 320, 1–4. [Google Scholar] [CrossRef]
- Cetó, X.; Gutiérrez-Capitán, M.; Calvo, D.; del Valle, M. Beer classification by means of a potentiometric electronic tongue. Food Chem. 2013, 141, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Witrick, K.A.; Pitts, E.R. Bicarbonate Inhibition and Its Impact on Brettanomyces bruxellensis Ability to Produce Flavor Compounds. J. Am. Soc. Brew. Chem. 2022, 80, 270–278. [Google Scholar] [CrossRef]
- Anderson, H.E.; Santos, I.C.; Hildenbrand, Z.L.; Schug, K.A. A review of the analytical methods used for beer ingredient and finished product analysis and quality control. Anal. Chim. Acta 2019, 1085, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Punčochářová, L.; Poří zka, J.; Diviš, P.; Štursa, V. Study of the influence of brewing water on selected analytes in beer. Potravin. Slovak J. Food Sci. 2019, 13, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Barth, R. The Chemistry of Beer: The Science in the Suds, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; ISBN 978-1-119-78333-6. [Google Scholar]
- Yitbarek, M.; Abdeta, K.; Beyene, A.; Astatkie, H.; Dadi, D.; Desalew, G.; Van der Bruggen, B. Experimental evaluation of sorptive removal of fluoride from drinking water using natural and brewery waste diatomite. Process Saf. Environ. Prot. 2019, 128, 95–106. [Google Scholar] [CrossRef]
- Pehrsson, P.R.; Patterson, K.Y.; Perry, C.R. The fluoride content of select brewed and microwave-brewed black teas in the United States. J. Food Compos. Anal. 2011, 24, 971–975. [Google Scholar] [CrossRef]
- Tekle-Haimanot, R.; Haile, G. Chronic Alcohol Consumption and the Development of Skeletal Fluorosis in a Fluoride Endemic Area of the Ethiopian Rift Valley. J. Water Resour. Prot. 2014, 6, 149–155. [Google Scholar] [CrossRef]
- Kondo, K. Beer and health: Preventive effects of beer components on lifestyle-related diseases. Biofactors 2004, 22, 303–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).