Abstract
Drylands in Brazil have been exploring sisal (Agave sisalana) as an essential source of income. However, the solid residues generated because of this activity still need suitable destinations; therefore, research has been carried out to transform them into added-value products. Therefore, the present study evaluated the potential of sisal or agave solid residue as a precursor feedstock for second-generation ethanol production. Acid and acid–alkaline pretreatments were carried out on sisal residues to enrich the biomass with cellulose and maximize enzymatic digestibility. Second-generation ethanol production was carried out using Semi-simultaneous saccharification and fermentation (SSSF). Regardless of catalyst dosage and incubation time, oxalic acid pretreatments generated samples with a similar chemical composition to those pretreated with sulfuric acid. However, samples pretreated with oxalic acid showed lower enzymatic digestibility. Samples pretreated with oxalic acid and sodium hydroxide obtained 14.28 g/L of glucose and cellulose conversion of 79.1% (at 5% solids), while 21.49 g/L glucose and 91.2% of cellulose conversion were obtained in the hydrolysis of pretreated samples with sulfuric acid and sodium hydroxide combined pretreatments. The pretreatment sequence efficiently reduced cellulase dosage from 20 to 10 FPU/g without compromising sugar release. SSSF achieved maximum production of 40 g/L ethanol and 43% ethanol conversion using 30% solids and gradually adding biomass and cellulases.
1. Introduction
Ethanol is a highly versatile product that is used as a disinfectant, a solvent for the chemical industry, and, mainly, as liquid fuel for light vehicles. Ethanol as a fuel is vital for the energy security of some countries [1]. For these reasons, ethanol production will likely grow in the coming decades, especially with a more significant share of second-generation ethanol. According to Consegic (2022), second-generation ethanol will have a CAGR (Compound Annual Growth Rate) of 14.9% until 2030, reaching a market worth U.S dollars. There is also the expectation of using second-generation ethanol to fuel vehicles powered by fuel cells. Ethanol is broken down into H2, CO2, and H2O. The hydrogen powers cells [2]. Second-generation ethanol is obtained using lignocellulosic biomass as feed and offers an advantage by avoiding land competition with food production [3]. However, second-generation ethanol technology has been frowned upon due to its high water consumption, in some cases rivaling the impact caused on gasoline production [4]. To alleviate this bottleneck, biomasses from arid and semi-arid regions have been investigated.
Crassulacean acid metabolism (CAM) plants are groups of plants common in arid and semi-arid regions which can survive with low irrigation due to an adaptation of their photosynthetic metabolism [5]. Sisal (Agave sisalana) is a CAM plant identified as a bet for second-generation ethanol production. Natural fibers, a cellulose-rich material, can be extracted from sisal and used directly as second-generation ethanol precursors [6,7]. Another possibility would be using a byproduct, the solid waste from defibration. In addition to representing a solution for inadequate disposal, using sisal solid residue for second-generation ethanol would not affect the demand for natural fibers for textile applications [8]. The solid sisal residue has holocellulose (cellulose + hemicellulose) in its composition, so it can be a potential precursor for ethanol production. According to Guerra et al. [8], sisal solid residue has a low cellulose content and a high content of extractives compared to conventional substrates such as food residues and wood samples. These differences impose additional effort in investigating second-generation ethanol, especially in the pretreatment step [9].
Different chemical pretreatments have been applied to sisal-derived biomass. Pretreatments with acid catalysts promote the extensive removal of hemicellulose, extractives, and ashes, increasing the cellulose content of the biomass [10,11]. Hydrothermal pretreatments, such as steam explosion, can perform the same function, but they require more complex reactors and higher operating temperatures [12]. Due to their high reactivity, mineral acids are commonly selected as catalysts in pretreatments. Rios-González et al. [13] and Julio-Altamiranda et al. [14] reported the success of sulfuric acid pretreatments to enhance the enzymatic hydrolysis of sisal fibers. However, they have the disadvantage of using expensive corrosion-resistant reactors, and concentrated acidic sulfuric pretreatment generates a high concentration of inhibitors and imposes greater effort on wastewater treatment [2]. Dicarboxylic acids, such as oxalic acid, are alternative catalysts for pretreatments. Compared to sulfuric acid, dicarboxylic acids exhibit higher pKa values (in theory, greater efficiency over a wide temperature and pH range) and lower toxicity to yeast [15]. Alkaline pretreatments have also been applied to improve the performance of enzymatic hydrolysis and fermentation, either as a single pretreatment or as a sequenced pretreatment. Sodium hydroxide is the main alkali catalyst for pretreatments due to its reactivity to cleave ether and ester bonds in lignin, promoting its intense depolymerization [15]. As a result, alkaline pretreated biomasses present high cellulose content, biomass swelling, and high surface area [16]. It is worth noting that reports on pretreatments of sisal biomass focus on derivatives of Agave tequilana or blue agave; however, to the authors’ knowledge, there are no findings on the effects of chemical pretreatments on solid sisal residue in the context of second-generation ethanol.
Therefore, the present study investigated the potential of sisal residue to produce sugars and ethanol from chemical pretreatments. Acid and acid–alkaline pretreatments were performed to enrich cellulose and boost the enzymatic hydrolysis of sisal residue. In acid pretreatment, oxalic acid and sulfuric acid were used as catalysts, and more conditions were tested for the former. Finally, ethanol production from sisal residue was conducted by Semi-simultaneous saccharification and fermentation under different solid loadings.
2. Materials and Methods
2.1. Chemicals
Acetic acid, glucose, yeast extract, sodium hydroxide, sulfuric acid, oxalic acid, and sodium citrate were purchased from Synth (São Paulo, Brazil). Meat peptone, 3,5-dinitro salicylic acid (DNS), and cellulase blend (Cellic Ctec 2) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Deionized water was purchased from the EASYpure RF system (Barnstead, NH, USA).
2.2. Lignocellulosic Biomass
Sisal residues from the Hybrid Bahia variety were kindly provided by Embrapa Algodão, whose unit is in Monteiro (Paraíba, Brazil). The sisal residue was stored in plastic bags and frozen before processing. After thawing, the sisal residues were dried in an air circulation oven, TE394/I (TECNAL, Piracicaba, Brazil), at 60 °C for 96 h. Then, the residue was crushed in a TE-680 knife mill (TECNAL, Brazil) to a particle size of 48 mesh (~0.8 mm). The sisal residue powders were also stored in plastic bags in a dry environment.
2.3. Chemical Pretreatments
Different pretreatments were performed to enrich cellulose and improve the enzymatic hydrolysis of sisal residues. All pretreatments were carried out in 1000 mL Erlenmeyer flasks containing 50 g of sisal residue (10% solids, w/v) and 500 mL of reagent solution. The vials were incubated in an AV autoclave (Phoenix, Brazil) at 121 °C, including heating for 15 min and cooling for 25 min. The reaction mixtures were cloth-filtered (mesh~40), and the solid fractions (pretreated biomass) were washed and squeezed 6 times to finally be dried in air circulation ovens at 60 °C for 24 h. All dry pretreated biomasses were stored in plastic pots before characterization, enzymatic hydrolysis, and fermentation.
Table 1 presents the operating conditions to be varied in the pretreatments. Acid pretreatments were carried out using oxalic acid and sulfuric acid as catalysts. In oxalic acid pretreatment, the residence time varied between 30 and 90 min, and the catalyst concentration varied between 1.0 and 2.0% (w/v). Pretreatment with sulfuric acid was operated under a single condition: a residence time of 30 min and a catalyst concentration of 1% (w/v). After choosing the best pretreatment, a subsequent alkaline pretreatment was carried out to promote delignification. The alkaline pretreatment was carried out with 10% (w/v) acid pretreated residue, a temperature of 121 °C (autoclave), a residence time of 60 min, and 2% (w/v) sodium hydroxide. Pretreated samples were coded as O1-O6 for pretreatments with oxalic acid, S for pretreatment with sulfuric acid, ON for sequential pretreatment with oxalic acid and sodium hydroxide, and OS for sequential pretreatment with sulfuric acid and sodium hydroxide. Untreated sisal residue was coded as UT.

Table 1.
Operating conditions for chemical pretreatments of sisal residues. All pretreatments were performed under 10% (w/v) solids and 121 °C (autoclave).
2.4. Biomass Characterization
The chemical composition of untreated and pretreated sisal residue was determined from the NREL protocol and Sluiter et al. [17,18]. All residues were characterized by cellulose, hemicellulose, Klason lignin, extractives, ashes, and moisture [19].
The samples’ crystallinity was determined from analyses using an XRD-6000 X-ray diffractometer (Shimadzu, Kyoto, Japan). The analysis was carried out with Kα radiation, a voltage of 40 kV, and an electric current of 30 mA. The crystallinity index value was calculated from the peak intensity of the 002 planes at 22.6° (I002) and the valley intensity at 17.5° (Iam), as shown in Equation (1):
2.5. Enzymatic Hydrolysis of Sisal Residue
The enzymatic hydrolysis experiments were carried out in 25 mL Erlenmeyer flasks containing a mixture of sisal residue, sodium citrate buffer (50 mM, pH 4.8), 0.05% (w/v) sodium azide, and cellulases. The flasks were placed in a shaker at 50 °C and 120 rpm for 48 h, and then the supernatant was recovered by centrifugation in SL-700 equipment (SOLAB, Piracicaba, Brazil). Supernatants were diluted and filtered with 0.45 µm membranes before sugar analysis in high-performance liquid chromatography (HPLC) (See Section 2.7). The enzymatic hydrolysis experiments were divided into two stages. Initially, the result of enzymatic hydrolysis was used as a criterion for choosing pretreatments, and the process was operated with 5% (w/v) solids and a cellulase dosage of 20 FPU/g. In the second moment, the experiments were conducted with cellulase dosages of 10, 15, and 20 FPU/g, and sugar analyses were carried out at times of 2, 6, 24, and 48 h. The following equation gives the cellulosic conversion calculation:
The terms Gf and Gi correspond to the mass of glucose at time i and at 0 h, respectively. The term C is the mass of cellulose used in enzymatic hydrolysis.
2.6. Saccharification and Fermentation of Sisal Residue
Saccharomyces cerevisiae PE-2 strain was provided by the Institute for Biotechnology and Bioengineering (University of Minho, Portugal), and it was used as a fermentation agent. Firstly, the inoculum of S. cerevisiae PE-2 was prepared by transferring aliquots of the strain from PDA (potato dextrose agar) plates to 50 mL Erlenmeyer flasks with culture medium containing 20 g/L glucose, 20 g/L peptone, and 10 g/L yeast extract. The flasks were placed on a shaker at 30 °C for 24 h, and then the cells were recovered by centrifugation at 1500× g and washed with saline water (0.9% sodium chloride, w/v). The concentration of cells in suspension was determined by counting under an optical microscope using a Neubauer chamber.
The ethanol production from sisal residues was evaluated in fed-batch saccharification and fermentation and Semi-simultaneous saccharification and fermentation (SSSF) separately. In 25 mL Erlenmeyer flasks, pretreated sisal residue was added along with sodium citrate buffer (50 mM and pH 4.8) and cellulases. The flasks were incubated in a shaker at 50 °C and 120 rpm for 8 h before adding S. cerevisiae PE-2 (for a final concentration of 108 cells per mL). After adding the inoculum, the shaker temperature was changed to 40 °C, and the process was evaluated for another 48 h. Supernatants were recovered by centrifugation, diluted with water, and filtered with 0.45 µm membranes before HPLC analysis. The SSSF experiments were carried out under the following solid loadings: 10%, 20%, and 30% (w/v). Particularly at the 30% (w/v) solid loading, another operational change was implemented due to the free water restriction. The addition of solids and cellulases was divided into two moments: 15% (w/v) solids and 5 FPU/g cellulases at 0 h and another 15% (w/v) solids and 5 FPU/g cellulases at 20 h (8 h pre-saccharification and 12 h after inoculum addition). All pretreated residues and cellulases were added at once in the control condition. The ethanol yield was calculated by Equation (3) from the ratio between the mass of ethanol and the maximum potential for ethanol production from biomass.
2.7. HPLC Analyses
HPLC analyses on a Shim-pack SCR 101-H column (7.9 × 300 mm) were used to determine the concentration of sugars (cellobiose, glucose, xylose, and arabinose), organic acids (formic acid, acetic acid, and levulinic acid), and ethanol. This analysis used 5 mM sulfuric acid as a mobile phase, a liquid flow rate of 0.6 mL/min, a temperature of 50 °C, and a refractive index detector. The concentrations of furfural and hydroxymethylfurfural were determined in analysis using a Shim-pack C18 column (4.5 × 150 mm) and SPD detector (with a wavelength of 280 nm). The elution conditions were carried out according to Padilha et al. [20]. Both analyses were performed on a Shimadzu VP HPLC system platform (Shimadzu, Japan).
2.8. Plotting and Statistical Analysis
Origin 8.0 software was used to plot crystallinity, enzymatic hydrolysis, and fermentation data. The significance of effects on sugar and ethanol production was assessed using the Tukey test using a 95% confidence level (p < 0.05). Tukey’s test was performed using Statistica 7.0 software.
3. Results
3.1. Effects of Chemical Pretreatments on the Composition of Sisal Residue
The sisal residue used in the present study is a byproduct of the processing of sisal leaves and, therefore, it is a material rich in extractives and poor in cellulose (Table 2). The UT material presents 50.2% extractives as the main fraction, while cellulose represents only 16.34%. This cellulose value is similar to the results reported by Sampaio et al. [ 44%] with the same biomass [21]. The remainder of the balance is closed with 12.24% hemicellulose, 10.68% Klason lignin, and 11.16% ashes. The high content of extractives is a critical factor for the accessibility of enzymes to cellulose and, therefore, indicates poor performance in enzymatic hydrolysis. Even if the biomass were completely hydrolyzed, the release of glucose would be considered low, which confirms the need for pretreatments.

Table 2.
Chemical composition of untreated and pretreated sisal residues. All pretreatments were carried out using 10% (w/v) solids, temperature 121 °C.
Pretreatments with oxalic acid were adequate for the proportional increase in cellulose content, although it was not possible to notice a clear trend. The lowest cellulose content was observed in sample O2 (20.85%), while the highest was in sample O3 (23.55%). These values are similar to the results with sulfuric acid (22.67%). The retention and enrichment of cellulose in biomass is guaranteed by the crystalline nature of the polysaccharide. The amount of catalyst in the present study is also far from the dosage used in the acid hydrolysis of cellulose, which should be greater than 41% (w/v), based on Lacerda et al. [22]. The enrichment of cellulose is due to removing other components from the biomass.
Extractives consist of substances not chemically bound in the lignocellulosic matrix, including phenolic compounds, proteins, sugars, and saponins. As reported by Padilha et al. [1], these substances are easily removed or degraded after pretreatments since pores are formed on the surface of pretreated biomasses. Extractives in samples pretreated with oxalic acid (O1–O6) reached 7.66–13.50%, while the content in sample S reached 7.16%. The effective loss of extractables in pretreated materials explains the low yield values. Ashes are also unbound components, but another behavior was observed. Samples O1–O6 presented ash content similar to sample NT, but sample S recorded a lower component content (5.75%). One hypothesis would be that the high temperature in an aqueous environment could remove ashes [20], but eventually the oxalic acid would convert into oxalates. Residues from sisal and other CAM plants are known to accumulate oxalate [23], [24]. In turn, using strong acids promotes the leaching of alkali and alkaline earth metals, as reported by Yishou Yang et al. [20].
The protons released in the acid pretreatment are highly reactive with the glycosidic bonds of hemicellulose, an amorphous polysaccharide. Hemicellulose fell from 12.24% to values below 8.3%. Unlike cellulose, it is possible to observe that hemicellulose removal is associated with pretreatment severity. Pretreatment O6 with the highest severity (2% oxalic acid and 60 min) achieved the lowest hemicellulose content (4.99%). This hemicellulose reduction is higher than that of sample S, which has 7.95% hemicellulose. Klowsowski et al. [12] observed strong hemicellulose removal after acid pretreatments. Cleavage of hemicellulose releases xylose and other sugars into the liquid bulk, which can eventually be dehydrated to furfural. Next, depending on the operating condition, furfural is a precursor of pseudo lignin, an aliphatic polymer that does not differ from Klason lignin in chemical characterization analysis [25]. This phenomenon may explain the significant increase in Klason lignin content observed in the pretreated samples in the present study [26,27]. The high content of Klason lignin in biomass is commonly associated with the low sugar release in enzymatic hydrolysis. Lignin not only affects the accessibility of enzymes as a physical obstacle but also promotes the non-productive adsorption of cellulases as an adverse effect. Lignins exposed to acidic environments present more significant condensation in their structure (higher content of C-C bonds) and, therefore, cause the loss of cellulases through adsorption [28].
An alkaline pretreatment was carried out after the acid pretreatments to improve the cellulose content and mitigate the effects of lignin. Material O1 was chosen among the other residues pretreated with oxalic acid, as it offers a higher yield than the rest (41.22%), and there were no significant differences in cellulose content. The combination of pretreatments led to obtaining the sample ON. Alkaline pretreatment was also applied to sample S; the resulting combination is sample SN. The samples ON and SN showed lower contents of extractives and hemicellulose than the precursor samples, as seen in Table 2. According to Yuyang et al. [29], the alkaline environment efficiently solubilizes tannins and glycolipids still present in biomass. Although most of the hemicellulose was removed in the first pretreatment, residues of this polysaccharide remain linked to the lignin by ester bonds, which are susceptible to disruption by alkali catalysts [30]. The loss of ether bonds between hemicellulose and residual lignin and the consequent rupture of the lignin–carbohydrate complex (LCC) is essential for the proper course of enzymatic hydrolysis [31].
Due to removing these components, a redistribution was recorded in both samples. The sample ON presented 32.50% cellulose and 41.09% Klason lignin, while the sample SN presented 42.25% cellulose and 38.50% Klason lignin. The increase in cellulose is a favorable aspect, as it implies a greater sugar release via enzymatic hydrolysis. The cellulose content in the SN sample is more than twice as high as that in the UT sample. However, it was expected that the sample would show higher cellulose values after two pretreatments. Biomass commonly used for second-generation ethanol production, such as corn stover, sugarcane bagasse, rice straw, and sawdust, already have a cellulose content of around 30% or higher in their untreated form [32,33]. The appeal for the valorization of sisal residue is due to its origin in dry areas. However, the energy effort required in pretreatment and its low overall yield (~20%) are challenges to the progress of technology. The increase in lignin after alkaline pretreatment was also unexpected. In theory, alkali catalysts promote the disruption of internal ether bonds in native lignin, releasing phenolic compounds into the liquid bulk during pretreatment [30]. In the present study, the lignin in the acid-pretreated biomass may be highly condensed and, therefore, it would be less affected. Ribeiro et al. [34] did not record a reduction in lignin after the alkaline pretreatment of coconut fiber previously treated with steam explosion.
3.2. Effects of Pretreatments on the Crystallinity of Sisal Residues
Crystallinity is another aspect that informs about the degree of disorganization of biomass after pretreatments. The nature of the biomass slightly hampered the CrI analysis in the present study. The untreated sisal residue (sample UT) accumulates inorganic materials and extractives, and, therefore, there is no well-defined formation of planes 001 and 002, as seen in Figure 1. Inorganics is also notable in the diffractograms, especially in pretreated samples. Impurities remained in the SN pretreated material (see Table 1), which explains the presence of peaks at 15.0°, 24.4°, 30.2°, and 38.3°. Kaur et al. [35] also recorded several peaks in XRD analysis of rice straw samples. It is noteworthy that although it was incubated with sodium hydroxide, the SN sample did not present cellulose II polymorphism in the diffractogram. The peak corresponding to plane 002 and typical of cellulose I only became more defined in the SN sample.
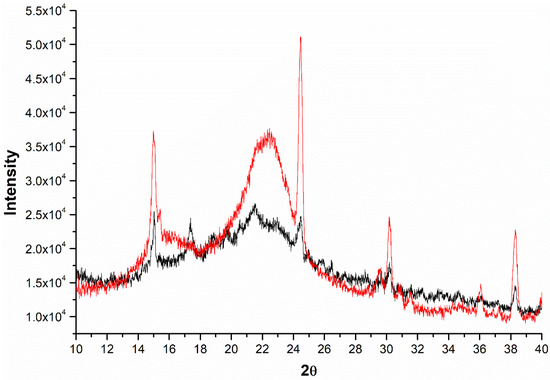
Figure 1.
X-ray diffraction diagram for untreated sisal residue (black line) and sisal residue pretreated with sulfuric acid and sodium hydroxide (red line).
For a quantitative assessment, CrI values were calculated for untreated and pretreated sisal residue, and the results are shown in Table 3. Higher CrI values mean that the biomass under study is richer in cellulose and, therefore, more attractive for ethanol production. The sample UT presented a CrI equal to 30.04%, while the CrI values of the samples O1 and S were equal to 40.78% and 38.48%, respectively. As reported in the previous section, pretreatments effectively remove extractives and hemicellulose, all amorphous components, which justifies the increase in response. Alkaline pretreatments slightly increased CrI values due to the excess removal of extractives and hemicellulose as well as a partial breakdown of lignin. In [20], CrI values did not increase strongly after sequential pretreatments.

Table 3.
CrI values for untreated and pretreated sisal residues.
3.3. Effects of Pretreatments on the Enzymatic Digestibility of Sisal Residues
The enzymatic hydrolysis of UT generated only 2.85 g/L glucose and 5.42 g/L xylose (Figure 2). This result can be explained by the fact that the lignocellulosic matrix is still organized and has a high content of extractives. In addition to reducing the availability of sugars, extractives can have adverse effects on the action of cellulases [36]. Therefore, after removing extractives and other amorphous components, a tendency for a greater sugar release was observed in the pretreated samples. Xylose release was reduced in experiments with acid-pretreated samples due to the removal of hemicellulose. In turn, glucose release more than doubled. Enzymatic hydrolysis of samples O1 and O4 generated 6.27 and 7.44 g/L glucose, respectively. The highest average glucose concentration among samples pretreated with oxalic acid (8.76 g/L) was also in the most severe condition, sample O6.
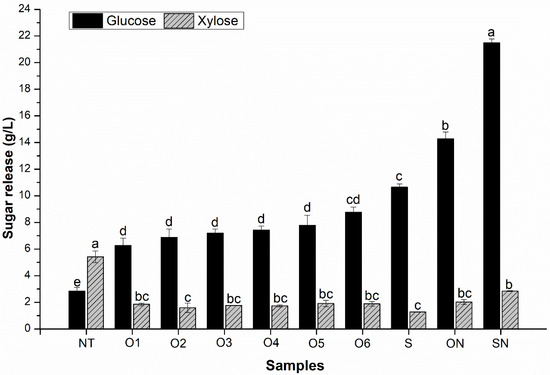
Figure 2.
Sugar release via enzymatic hydrolysis of untreated and pretreated sisal residues. Enzymatic hydrolysis was performed with 5% (w/v) solids, cellulases at 20 FPU/g, temperature of 50 °C, agitation of 120 rpm, and incubation time of 48 h. Equal lowercase letters mean no significant differences between responses of the same type. Tukey test was used with significance level of 5% (p < 0.05).
Here, it is already possible to see a slight advantage of using sulfuric acid. The hydrolysis of sample S released 10.64 g/L glucose and 1.28 g/L xylose. Even though there were no differences in chemical composition between the acid-pretreated samples, it is possible to suggest that sulfuric acid promoted more physical changes in the substrate, such as increased porosity [37,38]. Unfortunately, the sugar release with the sample SN was also higher than that with the sample ON. The sample SN released 21.49 g/L glucose and 2.85 g/L xylose, while the sample ON obtained only 14.28 g/L glucose and 2.03 g/L xylose. The cellulosic conversion values for the samples ON and SN were 79.2% and 91.2%, respectively. The present study aimed to understand the previously unknown role of oxalic acid in the pretreatments and enzymatic hydrolysis of sisal residue, which is indeed beneficial. This result aligns with the findings of Qiang et al. [28], who reported better performance of sulfuric acid as an acid catalyst for pretreatments than organic acids. However, this innovation already appears without much purpose compared to the performance of pretreatment with sulfuric acid, which is a conventional practice. Due to the greater sugar release generated in the samples ON and SN, both were chosen for a new investigation into enzymatic dosing. This decision-making was carried out to check whether performance is equivalent in another enzyme dosage.
Figure 3 shows the kinetic profile of glucose release from samples ON and SN at 10, 15, and 20 FPU/g. In all experiments, the abrupt increase in glucose in the first moments of the reaction is noticeable. As the reaction progresses, only the crystalline and less accessible portions of cellulose persist [39], which explains the slight increase in glucose after 6 h of the process. It is noteworthy that during enzymatic hydrolysis, cellobiose and glucose are inhibitors of cellulase, while cellulases are gradually adsorbed on lignin or deactivated by thermal stress and the air–liquid interface [40].
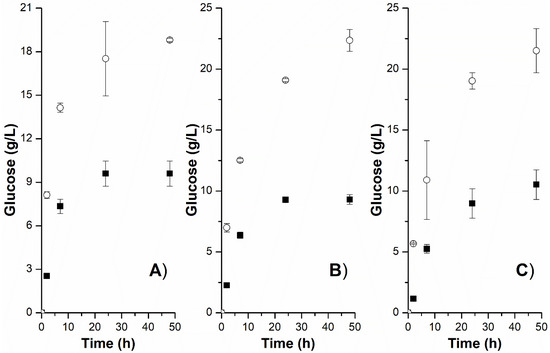
Figure 3.
Glucose release kinetics in the enzymatic hydrolysis of the samples ON (black square) and SN (white circle) under different enzymatic dosages (10 FPU/g—(A); 15 FPU/g—(B); 20 FPU/g—(C)). Enzymatic hydrolysis was performed with 5% (w/v) solids, a temperature of 50 °C, an agitation of 120 rpm, and a final incubation time of 48 h.
In both sisal residue samples, reducing the cellulase dosage did not compromise the sugar release. The glucose concentration in the hydrolysis of the sample ON reached 9.60 g/L for 10 FPU/g, 9.30 g/L for 15 FPU/g, and 10.52 g/L for 20 FPU/g, with no significant differences observed between the responses. Likewise, no significant differences were recorded between the results of the sample SN. The glucose concentration in hydrolysis with 10 FPU/g was 18.80 g/L, while 21.51 g/L of glucose was released in experiments with twice as many cellulases (20 FPU/g). Reducing the consumption of cellulases is a strategic action to reduce process costs and, consequently, move towards making the technology more competitive. According to Prasad et al. [41], cellulase costs can incur up to 48% of the minimum ethanol sales price. Considering the mitigation of the final costs of the process, the dosage of 10 FPU/g was maintained in the next stage. The comparison between the digestibility of the samples ON and SN again proved that the organic acid is not attractive. Although obtaining oxalic acid has less environmental impact than sulfuric acid, it is not effective as an acid catalyst in pretreatments of sisal residue. For this reason, only the sample SN was used in the fermentation tests.
3.4. Effects of Solid Loading on SSSF of Acid–Alkaline-Pretreated Sisal Residue
Understanding the effects of solid loading is crucial to maximizing ethanol production and observing any mass transfer limitations. In the present study, the increase in solid loading was linked to the increase in ethanol production since there is more substrate to be converted (Figure 4). Increasing the solid loading from 10% to 20% increased ethanol production from 17 to 21 g/L. Ethanol production reached 28 g/L at 30% solids without gradual addition. These findings are in agreement with results observed in the literature using agave-derived biomasses. Avila-Gaxiol [42] reported ethanol production of 12.2 g/L from agave tequilana powder using 10% solids. In Flores-Gómez et al. [43], ethanol production exceeded 35 g/L in hydrolysis and separate fermentation; however, the untreated biomasses (agave bagasse and leaves) in the study had higher cellulose contents and lower Klason lignin contents, which favored higher ethanol titers.
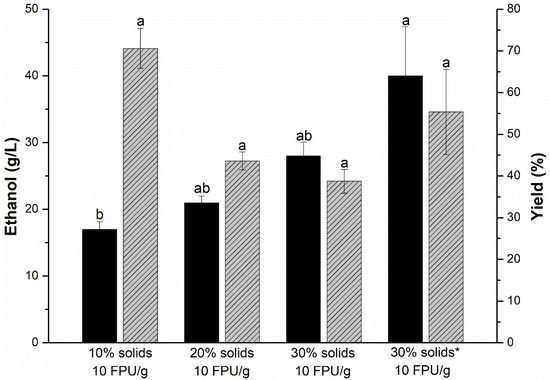
Figure 4.
Ethanol production (black bar) and ethanol yield (gray bar) after SSSF of SN sample. The experiments were carried out under the following conditions: pre-saccharification at 50 °C and 120 rpm for 8 h, final cellulase of 10 FPU/g, S. cerevisiae PE-2 inoculum of 108 cells per mL, fermentation temperature of 40 °C, and final incubation time of 56 h (8 h of pre-saccharification + 48 h of fermentation). The symbol * represents that the addition of solids and cellulases was carried out gradually. Equal lowercase letters mean no significant differences between responses of the same type. The Tukey test was used with a significance level of 5% (p < 0.05).
On the other hand, ethanol conversion decreased severely with the increase in solids due to the free water constraint. Lignocellulosic biomasses are porous materials that can retain water, so their increase implies diffusion problems for enzymes to access cellulose [44]. Water restriction also hinders the migration of hydrolysis products (monosaccharides and oligosaccharides) away from the reaction environment and the catalytic sites of cellulases, resulting in lower enzyme performance [45].
The gradual addition of biomass and cellulases was implemented to alleviate the limitation of free water in the 30% solids. The ethanol concentration obtained was 40 g/L, representing an increase of 43% compared to the one-time addition of 30% solids. According to [46], this value of ethanol produced (~5%, v/v) is already considered an interesting limit for downstream processing. The cellulosic conversion increased to 66% and is closer to the ethanol conversion obtained at 10% solids. In addition to solving free water problems, the gradual addition of cellulases implies less temperature and agitation stress. Nogueira et al. [47] and Ribeiro et al. [34] successfully gradually added cellulases to SSF with a high solid loading. Although the initial chemical composition of the sisal residue was not promising, the combination of pretreatments and the use of the fed-batch in the SSSF allowed the study to achieve similar results to other more traditional second-generation ethanol biomasses, such as softwoods [48], sugarcane bagasse [49], and food [50].
4. Conclusions
The sisal residue has potential for second-generation ethanol technology as long as it undergoes pretreatment. Oxalic acid can be used as an acid catalyst in pretreatment to improve the cellulose content in biomass and improve enzyme digestibility; its performance is lower than that of sulfuric acid. Samples of sisal residue from pretreatment with sulfuric acid, without and with subsequent alkaline pretreatment, showed higher cellulosic conversion regardless of the cellulase dosage. In SSSF, the pretreated acid–alkaline residue can be converted into ethanol at almost 5% (v/v), which is a desired level in the industry. Gradually adding biomass (substrate) and cellulases offered a notable ethanol production advantage over the conventional approach. Still, the low pretreatment yield and low cellulose values attracted attention and the search for varieties/species of sisal with better chemical compositions would be attractive. The valorization of compounds present in the extractives, such as fatty acids, fatty alcohols, and saponins, could be an additional income to the scheme in addition to ethanol and would contribute to the development of biorefineries from agave residue.
Author Contributions
H.Y.T.: Conceptualization, Experiment execution, Formal analysis, Writing—Original draft preparation, Reviewing, and Editing. J.D.N.C.: Experiment execution. W.M.d.S.: Reviewing and Editing. D.F.d.S.S.: Supervision, Reviewing, and Editing. C.E.d.A.P.: Conceptualization, Experiment execution, Writing—Original draft preparation, Reviewing, and Editing. R.S.C.M.: Resources and Supervision. R.B.d.S.: Supervision, Formal analysis, Reviewing, and Editing. E.D.D.: Conceptualization, Supervision, Resources, Reviewing, and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (Coordination for the Improvement of Higher Education Personnel–Finance code 001) and CNPq (National Council for Scientific and Technological Development). This article was funded by the Project Biotec-Semiárido APQ-1379-9.25/21), IPECTI Energias Renováveis e descarbonização: APQ-2274-24-72913, Facepe Universal: APQ-1287-24-72692 and is part of the National Observatory of Water and Carbon Dynamics in the Caatinga Biome—NOWCDCB—supported by the FACEPE (grants: APQ-0498-3.07/17 INCT 2014); CNPq (grants: INCT 465764/2014-2, 406202/2022-2, and 440444/2022-5); and CAPES (grant: 88887.136369/2017-00).
Data Availability Statement
Data and materials are available on request.
Acknowledgments
The authors would like to thank CAPES, CNPQ, PROTEN-UFPE and LEB-UFRN for support with the laboratory and materials to carry out this research.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Eduardo, C.; Padilha, D.A.; Nogueira, C.; Fabiano, D.; Souza, D.S.; Araújo, J.; Oliveira, D. Industrial Crops & Products Valorization of green coconut fibre: Use of the black liquor of organolsolv pretreatment for ethanol production and the washing water for production of rhamnolipids by Pseudomonas aeruginosa ATCC 27583. Ind. Crop Prod. 2019, 140, 111604. [Google Scholar] [CrossRef]
- Choudhary, P.; Sharma, R.; Kumar, V.; Singh, A.; Sharma, N. Synthesis, Characterization and Catalytic Activity of Bio-MCM-41 for Production of Bio Crude Oil via Pyrolysis of Rice Straw. Waste Biomass Valorization 2023, 14, 4173–4186. [Google Scholar] [CrossRef]
- Sandor, D.; Wallace, R. Understanding the Growth of the Cellulosic Ethanol Industry; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2008; pp. 1–49. [Google Scholar]
- Niechayev, N.A.; Pereira, P.N.; Cushman, J.C. Understanding trait diversity associated with crassulacean acid metabolism (CAM). Curr. Opin. Plant Biol. 2019, 49, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Buckland, C.E.; Thomas, D.S.G. Analysing the potential for CAM-fed bio-economic uses in sub-Saharan Africa. Appl. Geogr. 2021, 132, 102463. [Google Scholar] [CrossRef]
- Christin, P.A.; Wood, D. C4 and CAM Photosynthesis in Land Plants, Evolution and Diversification of. Encycl. Evol. Biol. 2016, 1, 254–259. [Google Scholar] [CrossRef]
- Lueangwattanapong, K.; Ammam, F.; Mason, P.M.; Whitehead, C.; McQueen-Mason, S.J.; Gomez, L.D.; Smith, J.A.C.; Thompson, I.P. Anaerobic digestion of Crassulacean Acid Metabolism plants: Exploring alternative feedstocks for semi-arid lands. Bioresour. Technol. 2020, 297, 122262. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.N.; Cabral Albuquerque, E.C.D.M.; Campos, L.M.A.; Pontes, L.A.M. Chemical and Physicochemical Characterization of Alkali Pretreated and in Natura Sisal Solid Waste. J. Nat. Fibers 2021, 18, 203–212. [Google Scholar] [CrossRef]
- Thomas, H.Y.; Cavalcante, J.D.N.; de Araújo Padilha, C.E.; dos Santos, E.S.; Gasparin, F.P.; da Silva Ries, L.A.; Sales, A.T.; Menezes, R.S.C.; Dutra, E.D. Valorization of lignocellulosic agave residues via pyrolysis and its use as adsorbent for methylene blue removal. Biomass Conv. Bioref. 2024. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. Bioresource Technology The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D. Impact of lignocellulose pretreatment by-products on S. cerevisiae strain ethanol red metabolism during aerobic and an-aerobic growth. Molecules 2021, 26, 806. [Google Scholar] [CrossRef] [PubMed]
- Ríos-González, L.J.; Medina-Morales, M.A.; Rodríguez-De la Garza, J.A.; Romero-Galarza, A.; Medina, D.D.; Morales-Martínez, T.K. Comparison of dilute acid pretreatment of agave assisted by microwave versus ultrasound to enhance enzymatic hydrolysis. Bioresour. Technol. 2021, 319, 124099. [Google Scholar] [CrossRef]
- Julio-Altamiranda, Y.T.; Mercado-Pacheco, J.D.; Sánchez-Tuirán, E.L.; González-Delgado, Á.D.; Ojeda, K.A. Evaluation of mechanical-green solvent pretreatment of oil palm wastes for reducing sugars production in North-Colombia. Sustain. Chem. Pharm. 2020, 16, 100256. [Google Scholar] [CrossRef]
- Prasad, S.; Malav, M.K.; Kumar, S.; Singh, A.; Pant, D.; Radhakrishnan, S. Enhancement of bio-ethanol production potential of wheat straw by reducing furfural and 5-hydroxymethylfurfural (HMF). Bioresour. Technol. Rep. 2018, 4, 50–56. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Bhattacharya, A.; Rashamuse, K. The Effects of Alkaline Pretreatment on Agricultural Biomasses (Corn Cob and Sweet Sorghum Bagasse). Agronomy 2020, 10, 1211. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples Laboratory Analytical Procedure (LAP) Issue Date: 12/08/2006 Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Proce. 2008. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 1 September 2024).
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, J.W. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. 2008, 3–5. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 analytical procedure-Determination of structural carbohydrates and lignin in Biomass. Lab. Anal. Proced. 2012, 17. [Google Scholar]
- De Padilha, C.E.A.; da Nogueira, C.C.; Alencar, B.R.A.; de Abreu, Í.B.S.; Dutra, E.D.; Ruiz, J.A.C.; Fabiano, D.; de Souza, S.; dos Santos, E.S. Production and Application of Lignin-Based Chemicals and Materials in the Cellulosic Ethanol Production: An Overview on Lignin Closed-Loop Production and Application of Lignin-Based Chemicals and Materials in the Cellulosic Ethanol Production: An Over. Waste Biomass Valorization 2022, 12, 6309–6337. [Google Scholar] [CrossRef]
- Sampaio, T.Q.S.; Cunha, F.S.; Campos, L.M.A.; Pires, C.A.M. Evaluation of the influence of hydrophilic extractives on the formation of bio-oil from the micro-pyrolysis of biomass waste. J. Anal. Appl. Pyrolysis 2024, 178, 106417. [Google Scholar] [CrossRef]
- Rigo, P.D.; Siluk, J.C.M.; Lacerda, D.P.; Rosa, C.B.; Rediske, G. Is the success of small-scale photovoltaic solar energy generation achievable in Brazil? J. Clean. Prod. 2019, 240, 118243. [Google Scholar] [CrossRef]
- Monje, P.V.; Baran, E.J. Characterization of calcium oxalate biominerals in some (non-cactaceae) succulent plant species. Z. Naturforsch. Sect. C J. Biosci. 2010, 65, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J.Y. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Marulanda, V.A.; Gutierrez, C.D.B.; Alzate, C.A.C. Thermochemical, biological, biochemical, and hybrid conversion methods of bio-derived molecules into renewable fuels. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Woodhead Publishing: Sawston, UK, 2019; pp. 59–81. [Google Scholar] [CrossRef]
- Frei, M. Lignin: Characterization of a multifaceted crop component. Sci. World J. 2013, 2013, 436517. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G.; Al-Ansari, T. Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers. Biorefinery 2020, 12, 669–681. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, J.; Zhao, X.; Hao, Z.; Zhuang, Q.; Zhu, J.; Wang, X.; Wei, X.; Zhou, M.; Pu, F.; et al. KOH activated carbon derived from biomass-banana fibers as an efficient negative electrode in high performance asymmetric. J. Energy Chem. 2017, 26, 56–62. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zhang, B.; Gong, F.; Feng, J.; Huang, H.; Vanka, S.; Fan, R.; Cao, Q.; Shen, M.; et al. Renewable formate from sunlight, biomass and carbon dioxide in a photoelectrochemical cell. Nat. Commun. 2023, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Virgínio e Silva, J.O.V.; Almeida, M.F.; da Conceição Alvim-Ferraz, M.; Dias, J.M. Integrated production of biodiesel and bioethanol from sweet potato. Renew. Energy 2018, 124, 114–120. [Google Scholar] [CrossRef]
- Tarasov, D.; Schlee, P.; Pranovich, A.; Moreno, A.; Wang, L.; Rigo, D.; Sipponen, M.H.; Xu, C.; Balakshin, M. AqSO biorefinery: A green and parameter-controlled process for the production of lignin-carbohydrate hybrid materials. Green Chem. 2022, 24, 6639–6656. [Google Scholar] [CrossRef]
- Yuan, H.; Song, X.; Guan, R.; Zhang, L.; Li, X.; Zuo, X. Effect of low severity hydrothermal pretreatment on anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 294, 122238. [Google Scholar] [CrossRef] [PubMed]
- Nava-Cruz, N.Y.; Contreras-Esquivel, J.C.; Aguilar-González, M.A.; Nuncio, A.; Rodríguez-Herrera, R.; Aguilar, C.N. Agave atrovirens fibers as substrate and support for solid-state fermentation for cellulase production by Trichoderma asperellum. 3 Biotech 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; de Souza, R.D.F.R.; da Aquino, K.A.S.; Morais, M.A.; Menezes, R.S.C. Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: Status and perspectives. Biomass Convers. Biorefinery 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Raya, F.T.; Marone, M.P.; Carvalho, L.M.; Rabelo, S.C.; de Paula, M.S.; Campanari, M.F.Z.; Freschi, L.; Mayer, J.L.S.; Silva, O.R.R.F.; Mieczkowski, P.; et al. Extreme physiology: Biomass and transcriptional profiling of three abandoned Agave cultivars. Ind. Crops Prod. 2021, 172, 114043. [Google Scholar] [CrossRef]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Ruiz, H.A. Enhancement and modeling of enzymatic hydrolysis on cellulose from agave bagasse hydrothermally pretreated in a horizontal bioreactor. Carbohydr. Polym. 2019, 211, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gu, Z.; Bjornson, B.E.; Muthukumarappan, A. Biochar based solid acid catalyst hydrolyze biomass. J. Environ. Chem. Eng. 2013, 1, 1174–1181. [Google Scholar] [CrossRef]
- Matei, J.C.; Soares, M.; Bonato, A.C.H.; de Freitas, M.P.A.; Helm, C.V.; Maroldi, W.V.; Magalhães, W.L.E.; Haminiuk, C.W.I.; Maciel, G.M. Enzymatic delignification of sugar cane bagasse and rice husks and its effect in saccharification. Renew. Energy 2020, 157, 987–997. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Du, J.; Liang, J.; Zhang, X.; Wang, J.; Li, W.; Song, P.; Feng, X. Identifying the negative cooperation between major inhibitors of cellulase activity and minimizing their inhibitory potential during hydrolysis of acid-pretreated corn stover. Bioresour. Technol. 2022, 343, 126113. [Google Scholar] [CrossRef]
- Prasad, B.R.; Padhi, R.K.; Ghosh, G. A review on key pretreatment approaches for lignocellulosic biomass to produce biofuel and value-added products. Int. J. Environ. Sci. Technol. 2023, 20, 6929–6944. [Google Scholar] [CrossRef]
- Avila-Gaxiol, J.C.A.-G. Ethanol production from Agave tequilana leaves powder by Saccharomyces cerevisiae yeast applying enzymatic saccharification without detoxification. Ind. Crops Prod. 2022, 177, 114515. [Google Scholar] [CrossRef]
- Flores-Gómez, C.A.; Escamilla Silva, E.M.; Zhong, C.; Dale, B.E.; Da Costa Sousa, L.; Balan, V. Conversion of lignocellulosic agave residues into liquid biofuels using an AFEXTM-based biorefinery. Biotechnol. Biofuels 2018, 11, 1–18. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, Y.G.; Song, Y.; Cho, E.J.; Bae, H.J. Hydrolysis Patterns of Xylem Tissues of Hardwood Pretreated with Acetic Acid and Hydrogen Peroxide. Front. Energy Res. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; Dos Santos, E.S.; Teixeira, J.A.; De Macedo, G.R. Bioethanol production from coconuts and cactus pretreated by autohydrolysis. Ind. Crops Prod. 2015, 77, 1–12. [Google Scholar] [CrossRef]
- Barbanera, M.; Lascaro, E.; Foschini, D.; Cotana, F.; Buratti, C. Optimization of bioethanol production from steam exploded hornbeam wood (Ostrya carpinifolia) by enzymatic hydrolysis. Renew. Energy 2018, 124, 136–143. [Google Scholar] [CrossRef]
- Nogueira, C.; Eduardo, C.; Padilha, D.A. Enzymatic hydrolysis and simultaneous saccharification and fermentation of green coconut fiber under high concentrations of ethylene oxide-based polymers. Renew. Energy 2021, 163, 1536–1547. [Google Scholar] [CrossRef]
- Guilherme, A.D.A.; Victor, P.; Dantas, F.; Eduardo, C.; Padilha, D.A.; Silvino, E.; Macedo, G.R. De Ethanol production from sugarcane bagasse: Use of different fermentation strategies to enhance an environmental-friendly process. J. Environ. Manag. 2019, 234, 44–51. [Google Scholar] [CrossRef]
- Meléndez-Hernández, P.A.; Hernández-Beltrán, J.U.; Hernández-Guzmán, A.; Morales-Rodríguez, R.; Torres-Guzmán, J.C.; Hernández-Escoto, H. Comparative of alkaline hydrogen peroxide pretreatment using NaOH and Ca(OH)2 and their effects on enzymatic hydrolysis and fermentation steps. Biomass Convers. Biorefinery 2019, 11, 1897–1907. [Google Scholar] [CrossRef]
- Michailos, S.E.; Webb, C. Biorefinery Approach for Ethanol Production from Bagasse; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128137666. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).