The Second Protonation in the Bio-Catalytic Cycles of the Enzymes Cytochrome P450 and Superoxide Reductase
Abstract
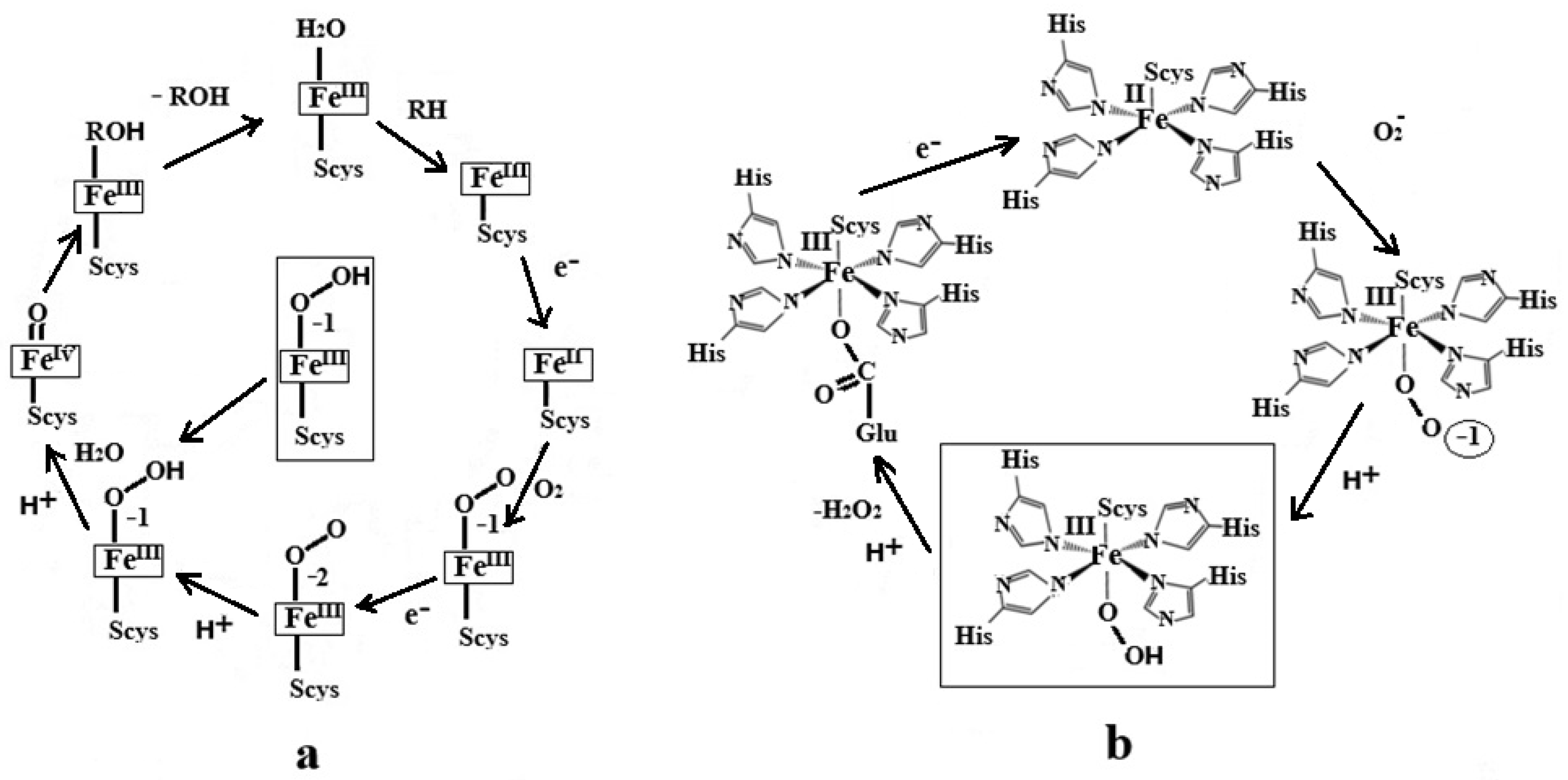
1. Introduction
2. Computational Details
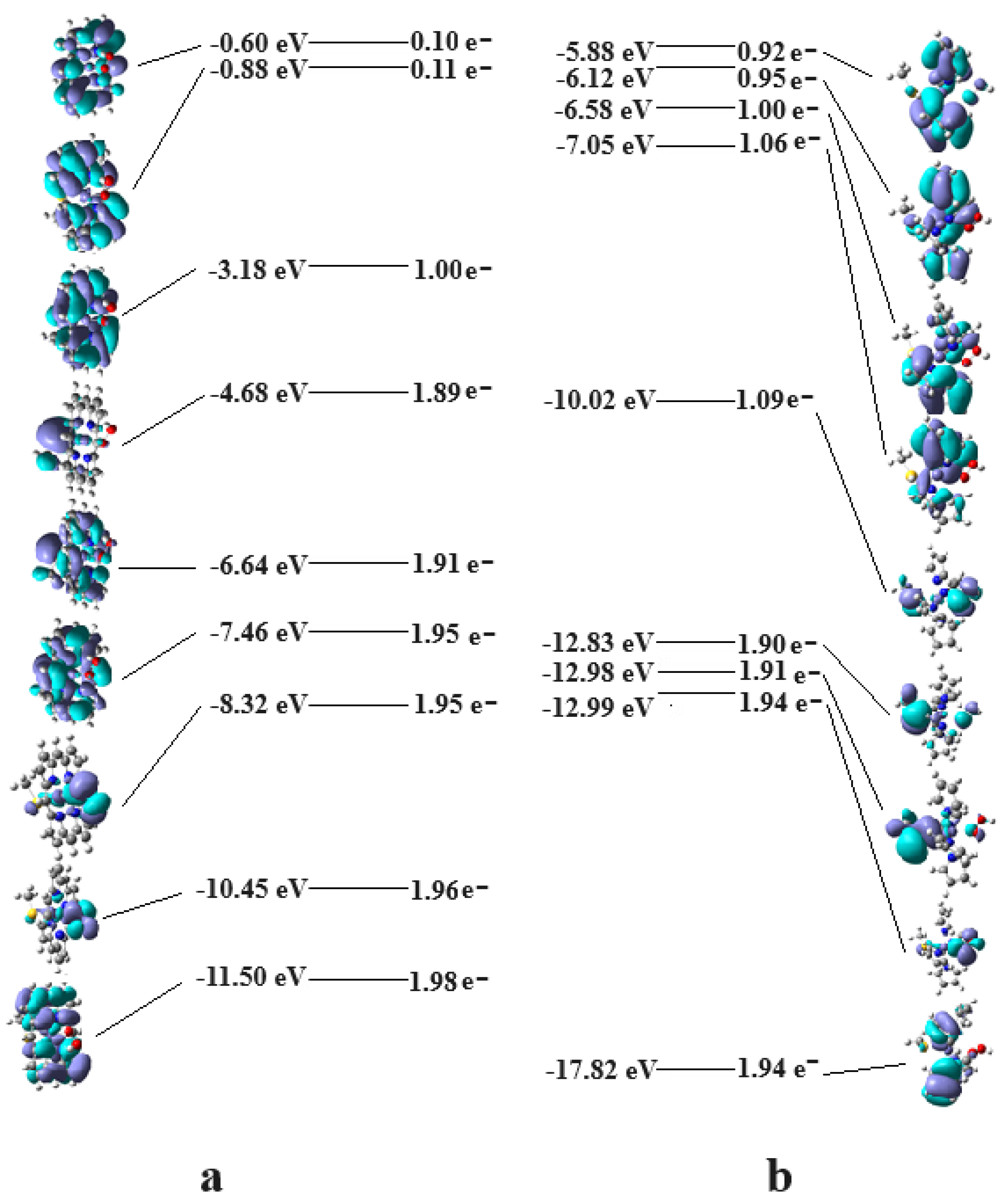
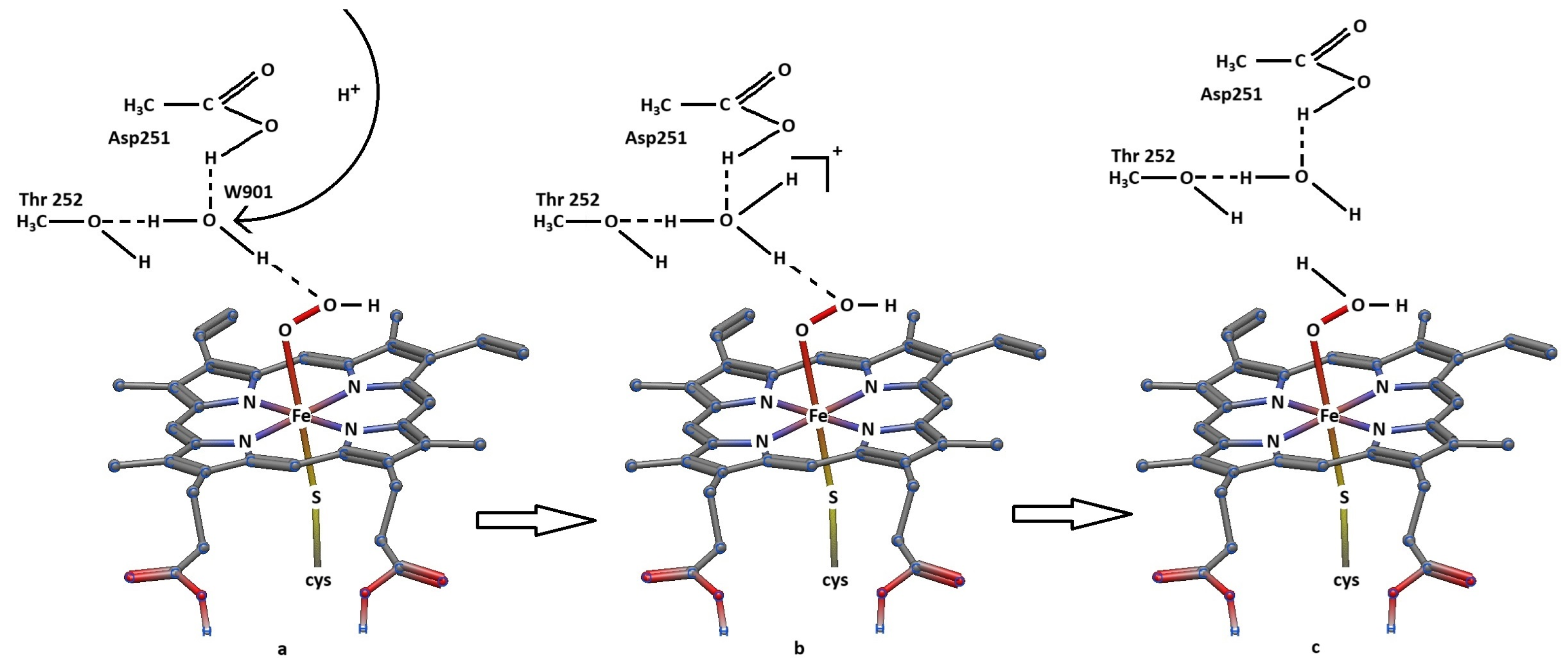
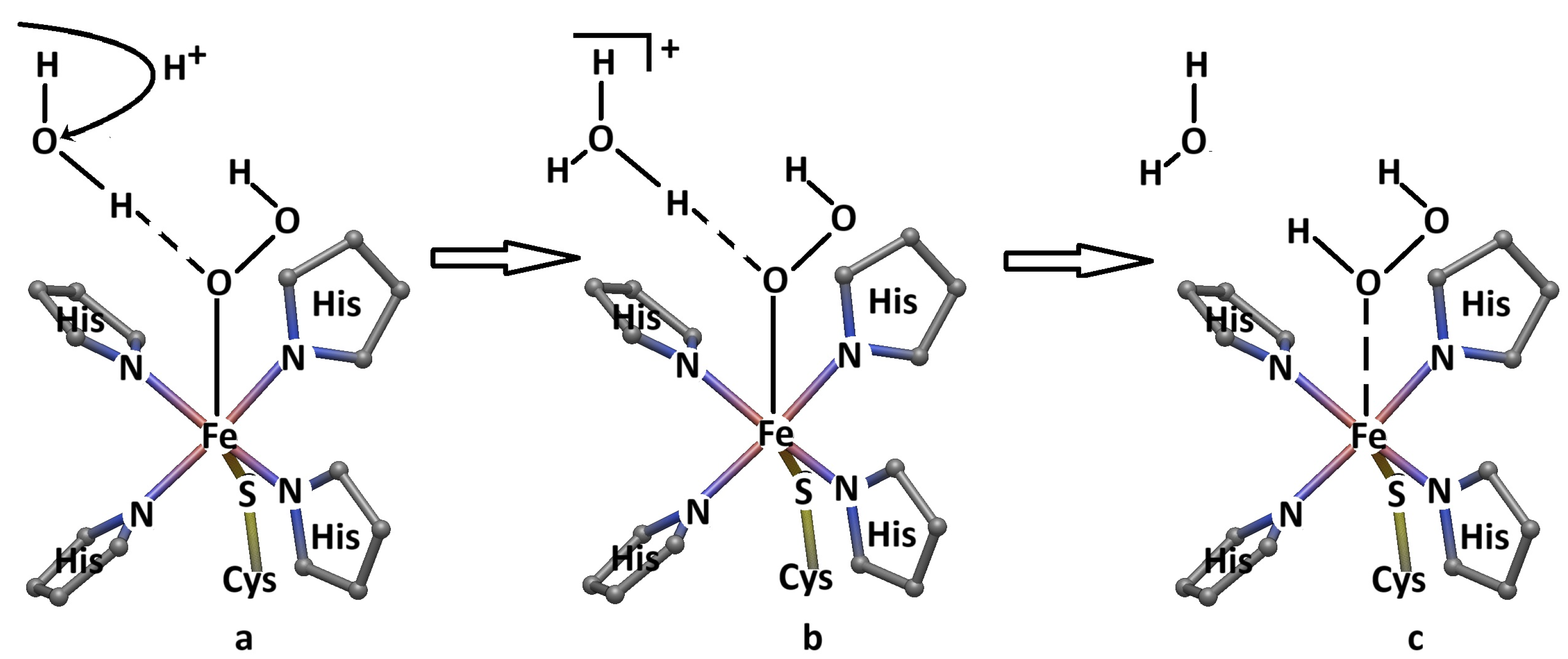
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovacs, J.A. Synthetic Analogues of Cysteinate-Ligated Non-Heme Iron and Non-Corrinoid Cobalt Enzymes. Chem. Rev. 2004, 104, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Makris, T.M.; Davydov, R.; Denisov, I.G.; Hoffman, B.M.; Sligar, S.G. Mechanistic Enzymology of Oxygen Activation by the Cytochrome P450. Drug Metab. Rev. 2002, 34, 691–708. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Cohen, S.; Wang, Y.; Chen, H.; Kumar, D.; Thiel, W. P450 Enzymes: Their Structure, Reactivity, and Selectivity-Modeled by QM/MM Calculations. Chem. Rev. 2010, 110, 949–1017. [Google Scholar] [CrossRef]
- Kovacs, J.A.; Brines, L.M. Understanding How the Thiolate Sulfur Contributes to the Function of the Non-Heme Iron Enzyme Superoxide Reductase. Acc. Chem. Res. 2007, 40, 501–509. [Google Scholar] [CrossRef]
- Kovacs, J.A. How Iron Activates O2. Science 2003, 299, 1024–1025. [Google Scholar] [CrossRef]
- Jenney, F.E.; Verhagen, M.F.J.M., Jr.; Cui, X.; Adams, M.W.W. Anaerobic Microbes: Oxygen Detoxification without Superoxide Dismutase. Science 1999, 286, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Mathé, C.; Mattioli, T.A.; Horner, O.; Lombard, M.; Latour, J.-M.; Fontecave, M.; Nivière, V. Identification of Iron (III) Peroxo Species in the Active Site of the Superoxide Reductase SOR from Desulfoarculus baarsii. J. Am. Chem. Soc. 2002, 124, 4966–4967. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D., Jr. Microbial Detoxification of Superoxide: The Non-Heme Iron Reductive Paradigm for Combating Oxidative Stress. Acc. Chem. Res. 2004, 37, 902–908. [Google Scholar] [CrossRef]
- Ogliaro, F.; de Visser, S.P.; Cohen, S.; Sharma, P.K.; Shaik, S. Searching for the Second Oxidant in the Catalytic Cycle of Cytochrome P450: A Theoretical Investigation of the Iron(III)-Hydroperoxo Species and Its Epoxidation Pathways. J. Am. Chem. Soc. 2002, 124, 2806–2817. [Google Scholar] [CrossRef]
- Liu, Y.; Denisov, I.G.; Grinkova, Y.V.; Sligar, S.G.; Kincaid, J.R. P450 CYP17A1 Variant with a Disordered Proton Shuttle Assembly Retains Peroxo-Mediated Lyase Efficiency. Chem. A Eur. J. 2020, 26, 16846–16852. [Google Scholar] [CrossRef]
- Yeh, A.P.; Hu, Y.; Jenney, F.E., Jr.; Adams, M.W.W.; Rees, D.C. Structures of the Superoxide Reductase from Pyrococcus furiosus in the Oxidized and Reduced States. Biochemistry 2000, 39, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Surawatanawong, P.; Tye, J.W.; Hall, M.B. Density Functional Theory Applied to a Difference in Pathways Taken by the Enzymes Cytochrome P450 and Superoxide Reductase: Spin States of Ferric Hydroperoxo Intermediates and Hydrogen Bonds from Water. Inorg. Chem. 2010, 49, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Altarsha, M.; Benighaus, T.; Kumar, D.; Thiel, W. How is the Reactivity of Cytochrome P450 can be Affected by Thr252X Mutation? A QM/MM Study for X = Serine, Valine, Alanine, Glycine. J. Am. Chem. Soc. 2009, 131, 4755–4763. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, M.; Sligar, S.G.; Li, T.H.; Poulos, T.L. Understanding the role of the essential Asp251 in cytochrome p450cam using site-directed mutagenesis, crystallography, and kinetic solvent isotope effect. Biochemistry 1998, 37, 9211–9219. [Google Scholar] [CrossRef] [PubMed]
- Taraphder, S.; Hummer, G. Protein Side-Chain Motion and Hydration in Proton-Transfer Pathways. Results for Cytochrome P450cam. J. Am. Chem. Soc. 2003, 125, 3931–3940. [Google Scholar] [CrossRef]
- Kamachi, T.; Yoshizawa, K. A Theoretical Study on the Mechanism of Camphor Hydroxylation by Compound I of Cytochrome P450. J. Am. Chem. Soc. 2003, 125, 4652–4661. [Google Scholar] [CrossRef]
- Schlichting, I.; Berendzen, J.; Chu, K.; Stock, A.M.; Shelley, A.; Maves, S.; Benson, S.D.; Sweet, R.M.; Ringe, D.; Petsko, G.A.; et al. The Catalytic Pathway of Cytochrome P450cam at Atomic Resolution. Science 2000, 287, 1615–1622. [Google Scholar] [CrossRef]
- Guallar, V.; Friesner, R.A. Cytochrome P450CAM Enzymatic Catalysis Cycle: A Quantum Mechanics/Molecular Mechanics Study. J. Am. Chem. Soc. 2004, 126, 8501–8508. [Google Scholar] [CrossRef]
- Oprea, T.I.; Hummer, G.; Garcia, A.E. Identification of a Functional Water Channel in Cytochrome P450 Enzymes. Proc. Nat. Acad. Sci. USA 1997, 94, 2133–2138. [Google Scholar] [CrossRef]
- Shaik, S.; Kumar, D.; de Visser, S.P.; Altun, A.; Thiel, W. Theoretical Perspective on the Structure and Mechanism of Cytochrome P450 Enzymes. Chem. Rev. 2005, 105, 2279–2328. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, D.; Thiel, W.; Sason Shaik, S. QM/MM Study of Mechanisms for Compound I Formation in the Catalytic Cycle of Cytochrome P450cam. J. Am. Chem. Soc. 2006, 128, 13204–13215. [Google Scholar] [CrossRef]
- Harris, D.L.; Loew, G.L. Theoretical Investigation of the Proton Assisted Pathway to Formation of Cytochrome P450 Compound I. J. Am. Chem. Soc. 1998, 120, 8941–8948. [Google Scholar] [CrossRef]
- Hata, M.; Hirano, Y.; Hoshino, T.; Nishida, R.; Tsuda, M. Theoretical Study on Compound I Formation in Monooxygenation Mechanism by Cytochrome P450. J. Phys. Chem. B 2004, 108, 11189–11195. [Google Scholar] [CrossRef]
- Bach, R.D.; Dmitrenko, O. The “Somersault” Mechanism for the P-450 Hydroxylation of Hydrocarbons. The Intervention of Transient Inverted Metastable Hydroperoxides. J. Am. Chem. Soc. 2006, 128, 1474–1488. [Google Scholar] [CrossRef]
- Altarsha, M.; Benighaus, T.; Kumar, D.; Thiel, W. Coupling and uncoupling mechanisms in the methoxythreonine mutant of cytochrome P450cam: A quantum echanical/molecular mechanical study. Biol. Inorg. Chem. 2010, 15, 361–372. [Google Scholar] [CrossRef][Green Version]
- Loida, P.J.; Sligar, S.G.; Paulsen, M.D.; Arnoldn, G.E.; Ornstein, R.L. Stereoselective Hydroxylation of Norcamphor by Cytochrome P450cam: Experimental verification of molecular dynamics simulations. J. Biol. Chem. 1995, 270, 5326–5330. [Google Scholar] [CrossRef]
- Silaghi-Dumitrescu, R.; Silaghi-Dumitrescu, I.; Coulter, E.D.; Kurtz, D.M., Jr. Computational study of the non-heme iron active site in superoxide reductase and its reaction with superoxide. Inorg. Chem. 2003, 42, 446–456. [Google Scholar] [CrossRef]
- Ali Attia, A.A.A.; Cioloboc, D.; Lupan, A.; Silaghi-Dumitrescu, R. Fe-O versus O-O bond cleavage in reactive iron peroxide intermediates of superoxide reductase. J. Biol. Inorg. Chem. 2013, 18, 95–101. [Google Scholar] [CrossRef]
- Ali Attia, A.A.A.; Cioloboc, D.; Lupan, A.; Silaghi-Dumitrescu, R. Multiconfigurational and DFT analyses of the electromeric formulation and UV–vis absorption spectra of the superoxide adduct of ferrous superoxide reductase. J. Inorg. Biochem. 2016, 165, 49–53. [Google Scholar] [CrossRef]
- Romão, C.V.; Matias, P.M.; Sousa, C.M.; Pinho, F.G.; Pinto, A.F.; Teixeira, M.; Bandeiras, T.M. Insights into the Structures of Superoxide Reductases from the Symbionts Ignicoccus hospitalis and Nanoarchaeum equitans. Biochemistry 2018, 57, 5271–5281. [Google Scholar] [CrossRef]
- Yang, T.-C.; McNaughton, R.L.; Clay, M.D.; Francis, E.; Jenney, J.; Krishnan, R.; Kurtz, D.M.; Adams, M.W.W.; Johnson, M.K.; Hoffman, B.M. Comparing the electronic properties of the low-spin cyano-ferric [Fe(N4)(Cys)] active sites of superoxide reductase and p450cam using ENDOR spectroscopy and DFT calculations. J. Am. Chem. Soc. 2006, 128, 16566–16578. [Google Scholar] [CrossRef]
- Silaghi-Dumitrescu, R.; Cioloboc, D. Comparative computational characterization of ferric Cytochrome P450 and Superoxide Reductase binding to cyanide. Stud. UBB Chem. 2016, 61, 45–54. [Google Scholar]
- Spataru, T. The Miracle of Vitamin B12 Biochemistry. Reactions 2024, 5, 20–76. [Google Scholar] [CrossRef]
- Spataru, T. The complete electronic structure and mechanism of the methionine synthase process as determined by the MCSCF method. J. Organomet. Chem. 2021, 942, 181211–181221. [Google Scholar] [CrossRef]
- Spataru, T. The electronic structure and mechanism of the adenosylcobalamin-dependent bio-processes as determined by the MCSCF method. J. Med. Chem. 2021, 11, 595–606. [Google Scholar]
- Spataru, T. The First Step and the Cob(II)alamin Cofactor Inactive Particles Reactivation in the Updated Mechanism of the Methionine Synthase Process. Reactions 2023, 4, 274–285. [Google Scholar] [CrossRef]
- Spataru, T. The Co-N bond cleavage in the adenosylcobalamin cofactor in advance to Glutamete Mutase and Methylmalonyl-Co-A Mutase processes. Chem. J. Mold. 2023, 18, 96–104. [Google Scholar] [CrossRef]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; van Dam, H.J.J.; Wang, D.; Nieplocha, D.; Apra, E.; Windus, T.L.; et al. “NwChem”: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477. [Google Scholar] [CrossRef]
- Bersuker, I.B. Limitations of Density Functional Theory in Application to the Degenerate States. J. Comp. Chem. 1997, 2, 260–267. [Google Scholar] [CrossRef]
- Spataru, T.; Birke, R.L. Carbon-Cobalt Bond Distance and Bond Cleavage in OneElectron Reduced Methylcobalamin: A Failure of the Conventional DFT Method. J. Phys. Chem. A 2006, 110, 8599–8604. [Google Scholar] [CrossRef] [PubMed]
- Bearpark, M.J.; Blancafort, L.; Robb, M.A. The pseudo-Jahn—Teller effect: A CASSCF diagnostic. Mol. Phys. 2002, 100, 1735–1739. [Google Scholar] [CrossRef]
- Katona, G.; Carpentier, P.; Niviere, V.; Amara, P.; Adam, V.; Ohana, J.; Tsanov, N.; Bourgeois, D. Raman-assisted crystallography reveals end-on peroxide intermediates in a non-heme iron enzyme. Science 2007, 316, 449–453. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spataru, T.; Dascalu, L.M.; Moraru, A.; Moraru, M. The Second Protonation in the Bio-Catalytic Cycles of the Enzymes Cytochrome P450 and Superoxide Reductase. Reactions 2024, 5, 778-788. https://doi.org/10.3390/reactions5040039
Spataru T, Dascalu LM, Moraru A, Moraru M. The Second Protonation in the Bio-Catalytic Cycles of the Enzymes Cytochrome P450 and Superoxide Reductase. Reactions. 2024; 5(4):778-788. https://doi.org/10.3390/reactions5040039
Chicago/Turabian StyleSpataru, Tudor, Lisa Maria Dascalu, Andreea Moraru, and Mariana Moraru. 2024. "The Second Protonation in the Bio-Catalytic Cycles of the Enzymes Cytochrome P450 and Superoxide Reductase" Reactions 5, no. 4: 778-788. https://doi.org/10.3390/reactions5040039
APA StyleSpataru, T., Dascalu, L. M., Moraru, A., & Moraru, M. (2024). The Second Protonation in the Bio-Catalytic Cycles of the Enzymes Cytochrome P450 and Superoxide Reductase. Reactions, 5(4), 778-788. https://doi.org/10.3390/reactions5040039



