Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fungal Growth
2.3. Enzymatic Assays
2.4. Response Surface Methodology to Optimize Laccase Production
2.5. Immobilization on Ionic Adsorption Resins
2.6. Reuse of Immobilized Enzymes
2.7. Biochemical Characterization of Free and Immobilized Enzymes
2.8. Saccharification of Sugarcane Bagasse
2.9. Synthetic Dye Decolorization
2.10. Statistical Analysis
3. Results and Discussion
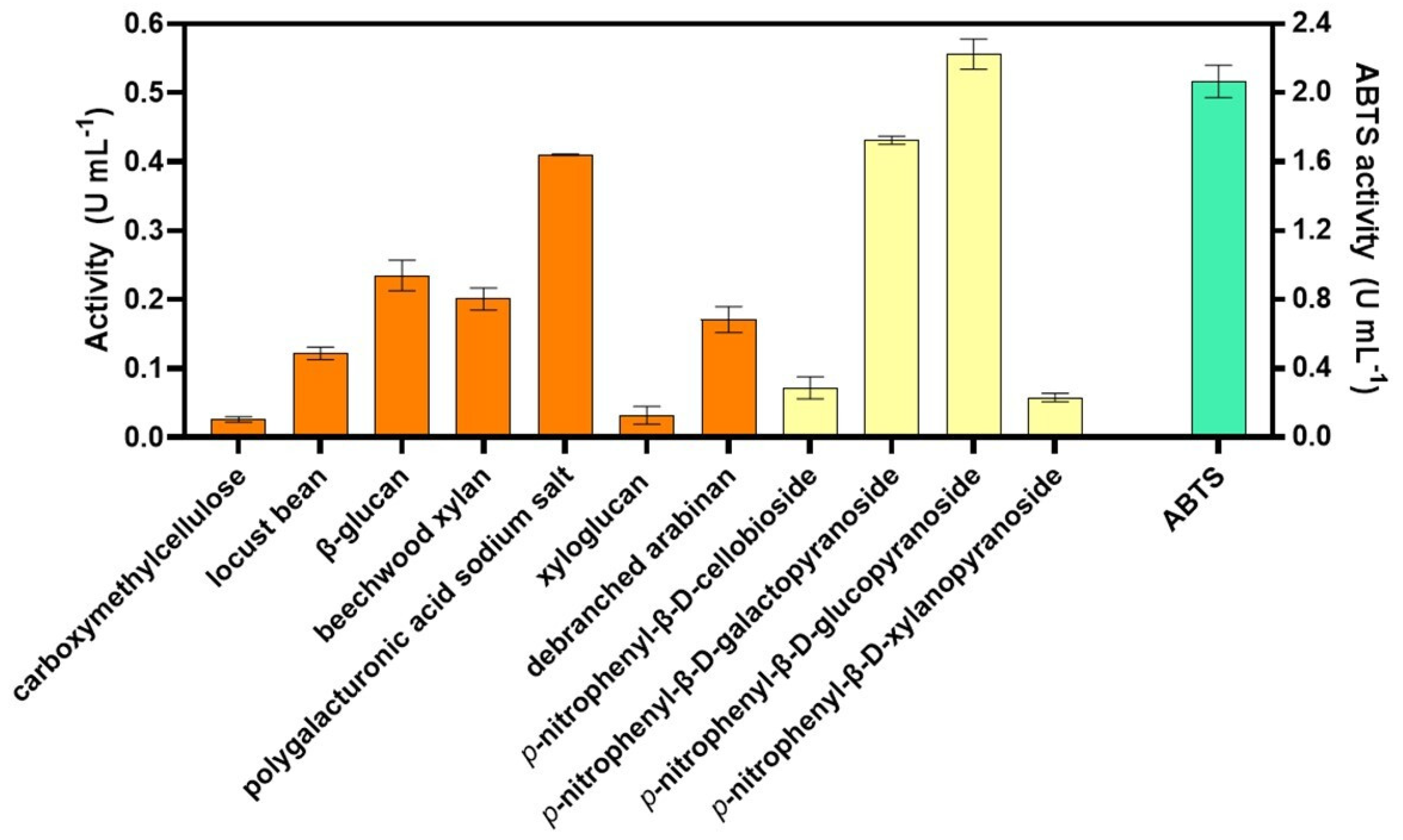
3.1. Enzymatic Screening
3.2. Optimization of Culture Conditions for Laccase Production
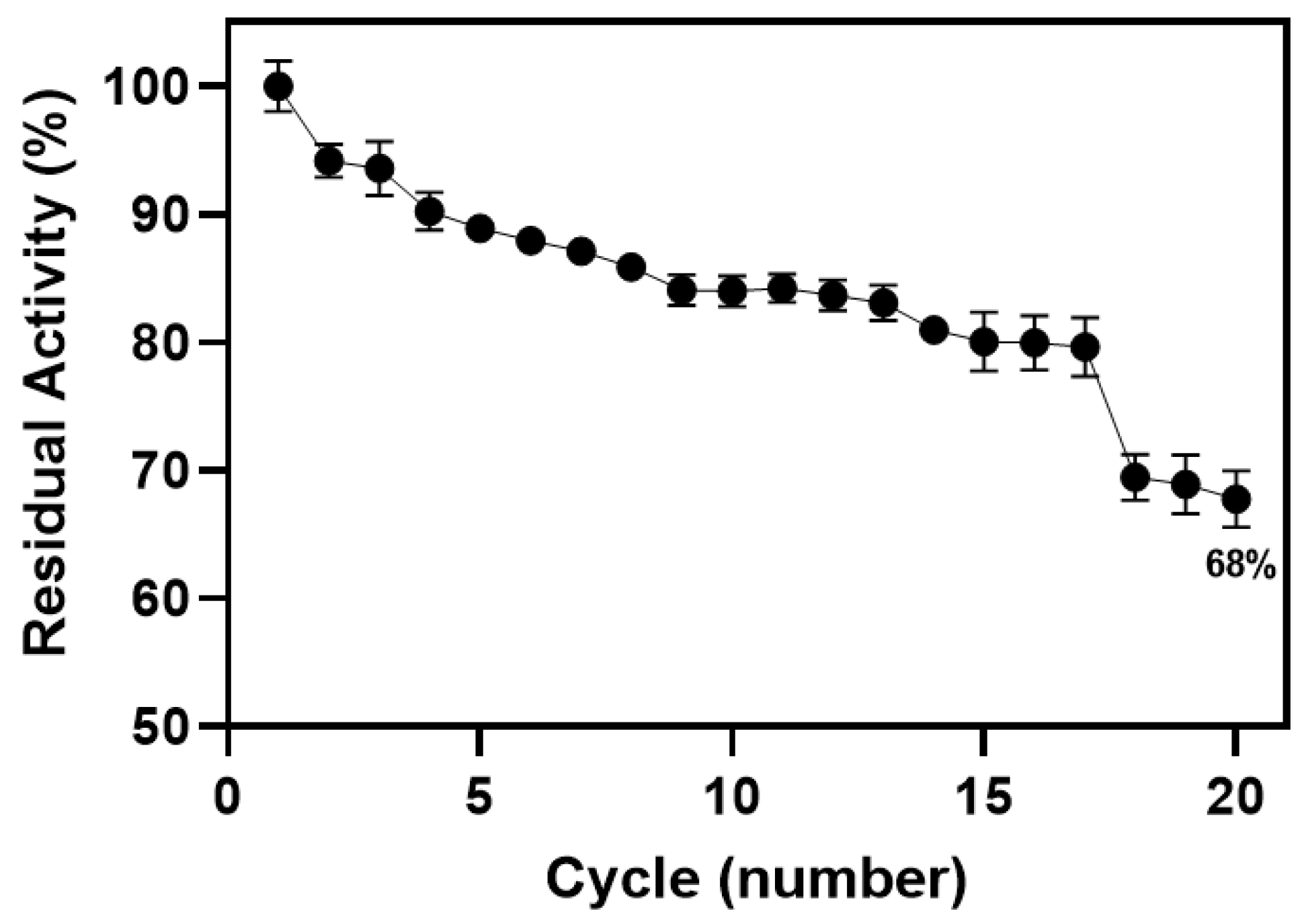
3.3. Ionic Immobilization
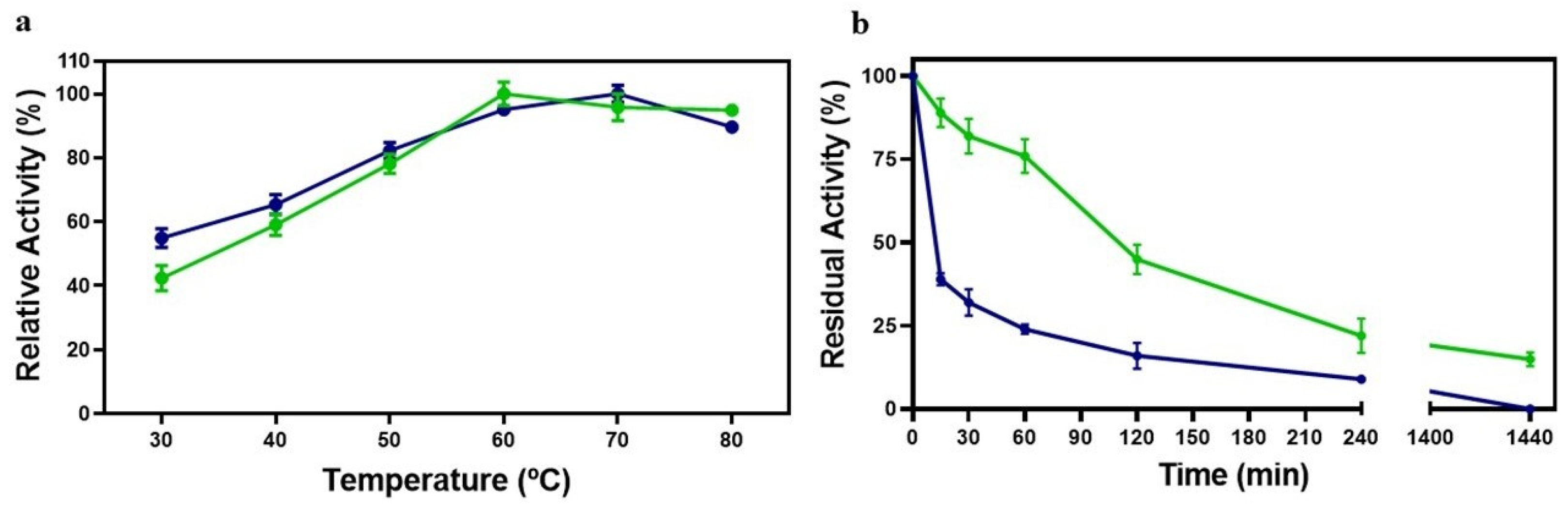
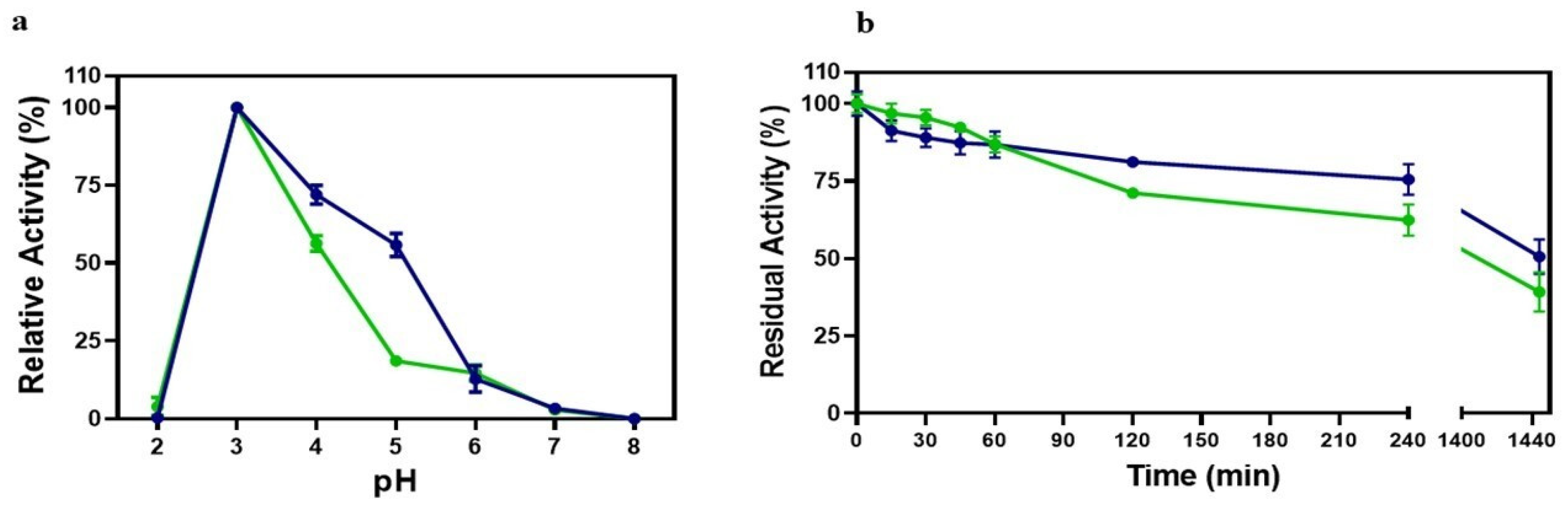
3.4. Influence of Temperature on Laccase Activity
3.5. Influence of pH on Laccase Activity
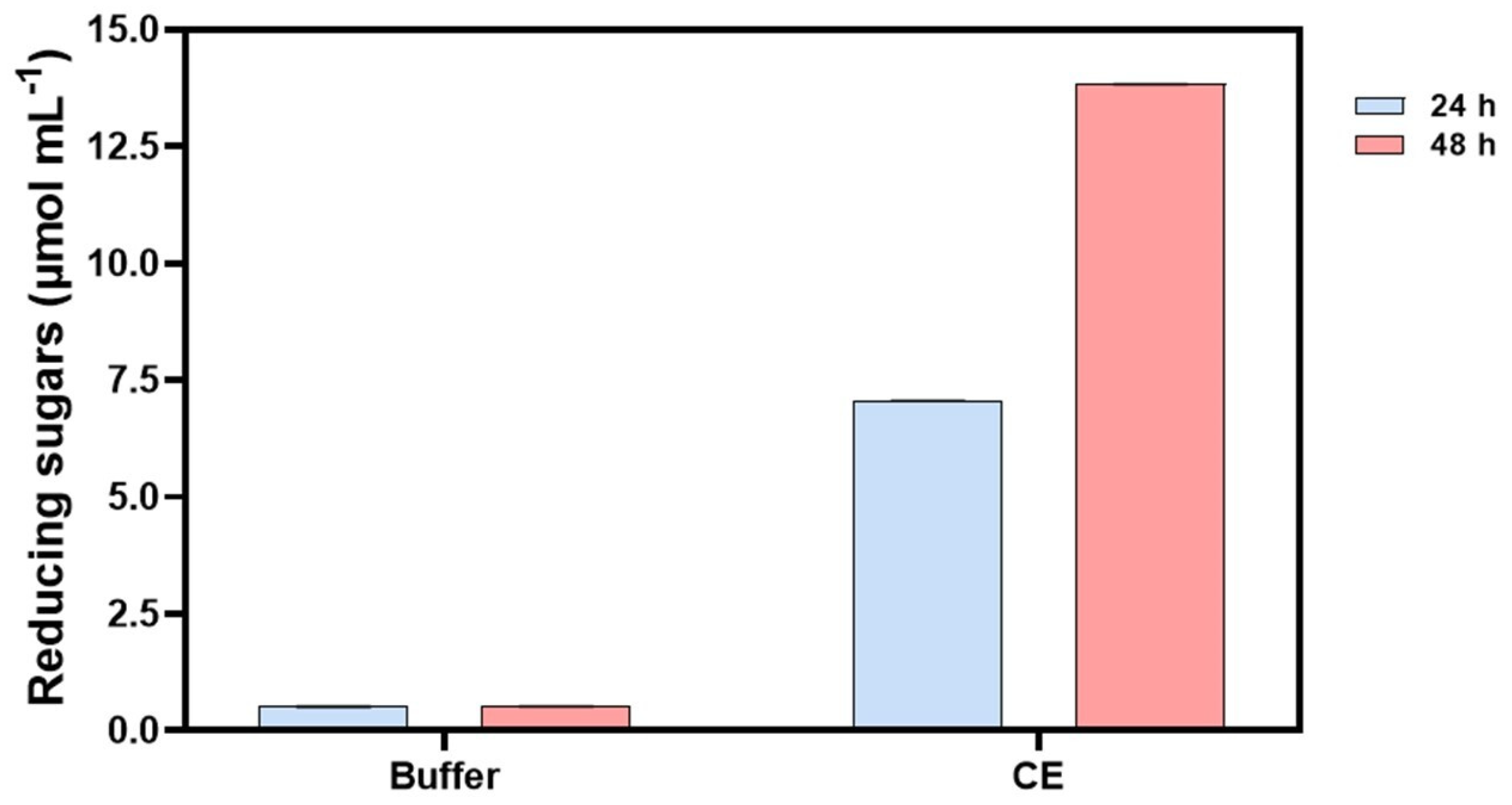
3.6. Sugarcane Bagasse Hydrolysis
3.7. Decolorization of Various Synthetic Dyes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tatta, E.R.; Imchen, M.; Moopantakath, J.; Kumavath, R. Bioprospecting of microbial enzymes: Current trends in industry and healthcare. Appl. Microbiol. Biotechnol. 2022, 106, 1813–1835. [Google Scholar] [CrossRef] [PubMed]
- Sulyman, A.O.; Aje, O.O.; Ajani, E.O.; Abdulsalem, R.A.; Balogun, F.O.; Sabiu, S. Bioprospection of selected plant secondary metabolites as modulators of the proteolytic activity of Plasmodium falciparum plasmepsin V. BioMed Res. Int. 2023, 2023, 6229503. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Song, W.; Gua, M. The integral role of bioproducts in the growing bioeconomy. Ind. Biotechnol. 2020, 16, 13–25. [Google Scholar] [CrossRef]
- Sharma, R.; Malaviya, P. Ecosystem services and climate action from a circular bioeconomy perspective. Renew. Sustain. Energy Rev. 2023, 175, 113164. [Google Scholar] [CrossRef]
- Muñoz-Castiblanco, T.; Mejia-Giraldo, J.C.; Puertas-Mejia, M.A. Trametes genus, a source of chemical compounds with anticancer activity in human osteosarcoma: A systematic review. J. Appl. Pharm. Sci. 2020, 10, 121–129. [Google Scholar]
- Pinheiro, V.E.; Almeida, P.Z.; Polizeli, M.L.T.M. Statistical optimization of cornmeal saccharification using various hydrolases. Biomass Convers. Biorefin. 2021, 13, 9011–9021. [Google Scholar] [CrossRef]
- Charpentier-Alfaro, C.; Benavides-Hernández, J.; Poggerini, M.; Crisci, A.; Mele, G.; Rocca, G.D.; Emiliani, G.; Frascella, A.; Torrigiani, T.; Palanti, S. Wood-decaying fungi: From timber degradation to sustainable insulating biomaterials production. Materials 2023, 16, 35–47. [Google Scholar] [CrossRef]
- Long, H.; Wu, Z. Immunoregulatory effects of Huaier (Trametes robiniophila Murr) and relevant clinical applications. Front. Immunol. 2023, 14, 1137098. [Google Scholar] [CrossRef]
- Alam, R.; Mahmoof, R.A.; Islam, S.; Ardiati, F.C.; Solihat, N.N.; Alam, M.B.; Lee, S.H.; Yanto, D.H.Y.; Kim, S. Understanding the biodegradation pathways of azo dyes by immobilized white-rot fungus, Trametes hirsuta D7, using UPLC-PDA-FTICR MS supported by in silico simulations and toxicity assessment. Chemosphere 2023, 313, 137505. [Google Scholar] [CrossRef]
- Savinova, O.S.; Shabaev, A.V.; Glazunova, O.A.; Moiseenko, K.V.; Fedorova, T.V. Benzyl butyl phthalate and diisobutyl phthalate biodegradation by white-rot fungus Trametes hirsuta. Appl. Biochem. Microbiol. 2022, 58, S113–S125. [Google Scholar] [CrossRef]
- Liu, J.; Sun, K.; Zhu, R.; Wang, X.; Waigi, M.G.; Li, S. Biotransformation of bisphenol A in vivo and in vitro by laccase-producing Trametes hirsuta La-7: Kinetics, products, and mechanisms. Environ. Pollut. 2023, 351, 121155. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; Oliveira, T.B.; Aranha, G.M.; Freitas, E.N.; Vici, A.C.; Nogueira, K.M.V.; Lucas, R.C.; Scarcella, A.S.A.; Buckeridge, M.S.; Silva, R.N.; et al. Prospection of fungal lignocellulolytic enzymes produced from jatoba (Hymenaea courbaril) and tamarind (Tamarindus indica) seeds: Scaling for bioreactor and saccharification profile of sugarcane bagasse. Microorganisms 2021, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Benatti, A.L.T.; Polizeli, M.L.T.M. Lignocellulolytic biocatalysts: The main players involved in multiple biotechnological processes for biomass valorization. Microorganisms. 2023, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Cai, Y.; Zhao, L.; Han, L.; Wang, F.; Yang, X.; Gao, X.; Lu, M.; Liu, W. Structure and function characterization of the α-L-arabinofuranosidase from the white-rot fungus Trametes hirsuta. Appl. Microbiol. Biotechnol. 2023, 107, 3967–3981. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef]
- Aza, P.; Camarero, S. Fungal laccases: Fundamentals, engineering and classification update. Biomolecules 2023, 13, 1716. [Google Scholar] [CrossRef]
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; Freitas, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; Souza, C.G.M.; Polizeli, M.d.L.T.d.M.; Bracht, A.; et al. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef]
- Brugnari, T.; Contato, A.G.; Pereira, M.G.; Freitas, E.N.; Bubna, G.A.; Aranha, G.M.; Bracht, A.; Polizeli, M.d.L.T.d.M.; Peralta, R.M. Characterisation of free and immobilised laccases from Ganoderma lucidum: Application on bisphenol a degradation. Biocatal. Biotransform. 2021, 39, 71–80. [Google Scholar] [CrossRef]
- Rostami, A.; Abdelrasoul, A.; Shokri, Z.; Shivarndi, Z. Application and mechanisms of free and immobilized laccase in detoxification of phenolic compounds—A review. Korean J. Chem. Eng. 2022, 39, 821–832. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Mahmoud, M.; Karim, G.S.A.A.; Abdelraof, M.; Othman, A.M. Purification and biochemical characterization of two laccase isoenzymes isolated from Trichoderma harzianum S7113 and its application for bisphenol A degradation. Microb. Cell Fact. 2023, 22, 1. [Google Scholar] [CrossRef]
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; Oliveira, D.; Soares, H.M. Laccase as an efficacious approach to remove anticancer drugs: A study of doxorubicin degradation, kinetic parameters, and toxicity assessment. J. Hazard. Mater. 2021, 409, 124520. [Google Scholar] [CrossRef] [PubMed]
- Nuryana, I.; Dewi, K.S.; Andriani, A.; Laksmi, F.A. Potential activity of recombinant laccase for biodegradation of ampicillin. IOP Conf. Ser. Earth Environ. Sci. 2023, 1201, 012071. [Google Scholar] [CrossRef]
- Serbent, M.P.; Magario, I.; Saux, C. Immobilizing white-rot fungi laccase: Toward bio-derived supports as a circular economy approach in organochlorine removal. Biotechnol. Bioeng. 2023, 121, 434–455. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Daverey, A.; Dutta, K.; Arunachalam, K. Bioremoval of toxic malachite green from water through simultaneous decolorization and degradation using laccase immobilized biochar. Chemosphere 2022, 297, 123126. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Rajendran, D.S.; Kumar, P.S.; Anand, S.S.; Kumar, V.V.; Rangasamy, G. Efficient decolorization and detoxification of triarylmethane and azo dyes by porous-cross-linked enzyme aggregates of Pleurotus ostreatus laccase. Chemosphere 2023, 356, 142016. [Google Scholar] [CrossRef]
- Sun, C.; Cheng, X.; Yuan, C.; Xia, X.; Zhou, Y.; Zhu, X. Carboxymethyl cellulose/Tween 80/Litsea cubeba essential oil nanoemulsion inhibits the growth of Penicillium digitatum and extends the shelf-life of ‘Shatangju’ mandarin. Food Control 2024, 160, 11032. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, N. Microbial laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Chaudhary, S.; Singh, A.; Varma, A.; Porwal, S. Recent advancements in biotechnological applications of laccase as a multifunctional enzyme. J. Pure Appl. Microbiol. 2022, 16, 1479–1491. [Google Scholar] [CrossRef]
- Ayodeji, F.D.; Shava, B.; Iqbal, H.M.N.; Ashraf, S.S.; Cui, J.; Franco, M.; Bilal, M. Biocatalytic versatilities and biotechnological prospects of laccase for a sustainable industry. Catal. Lett. 2023, 153, 1931–1956. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Nazar, M.; Xu, Q.; Zahoor; Ullah, M.W.; Khan, N.A.; Iqbal, B.; Zhu, D. Integrated laccase delignification with improved lignocellulose recalcitrance for enhancing enzymatic saccharification of ensiled rice straw. Ind. Crops Prod. 2023, 202, 116981. [Google Scholar] [CrossRef]
- Mustafa, A.H.; Rashid, S.S.; Rahim, M.H.A.; Roslan, R.; Musa, W.A.M.; Sikder, B.H.; Sasi, A.A. Enzymatic pretreatment of lignocellulosic biomass: An overview. J. Chem. Eng. Ind. Biotechnol. 2022, 8, 1–7. [Google Scholar] [CrossRef]
- Sawhney, D.; Vaid, S.; Bangotra, R.; Sharma, S.; Dutt, H.C.; Kapoor, N.; Mahajan, R.; Bajaj, B.K. Proficient bioconversion of rice straw biomass to bioethanol using a novel combinatorial pretreatment approach based on deep eutectic solvent, microwave irradiation and laccase. Bioresour. Technol. 2023, 373, 128791. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Chen, C.W.; Haldar, D.; Patel, A.K.; Dong, C.D.; Singhania, R.R. Laccase in biorefinery of lignocellulosic biomass. Appl. Sci. 2023, 13, 4673. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, J.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Casillo-Zacarias, C.; Sosa-Hernandéz, J.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Exploring current tendencies in techniques and materials for immobilization of laccases—A review. Int. J. Biol. Macromol. 2021, 181, 683–696. [Google Scholar] [CrossRef]
- Aranha, G.M.; Contato, A.G.; Salgado, J.C.S.; Oliveira, T.B.; Retamiro, K.M.; Ortolan, G.G.; Crevelin, E.J.; Nakamura, C.V.; Moraes, L.A.B.; Peralta, R.M.; et al. Biochemical characterization and biological properties of mycelium extracts from Lepista sordida GMA-05 and Trametes hirsuta GMA-01: New mushroom strains isolated in Brazil. Braz. J. Microbiol. 2023, 53, 349–358. [Google Scholar] [CrossRef]
- Contato, A.G.; Vici, A.C.; Pinheiro, V.E.; de Oliveira, T.B.; de Freitas, E.N.; Aranha, G.M.; Valvassora Junior, A.L.A.; Vargas-Rechia, C.G.; Buckeridge, M.S.; Polizeli, M.L.T.M. Comparison of Trichoderma longibrachiatum xyloglucanase production using tamarind (Tamarindus indica) and jatoba (Hymenaea courbaril) seeds: Factorial design and immobilization on ionic supports. Fermentation 2022, 8, 510. [Google Scholar] [CrossRef]
- Scarcella, A.S.A.; Pasin, T.M.; de Lucas, R.C.; Ferreira-Nozawa, M.S.; de Oliveira, T.B.; Contato, A.G.; Grandis, A.; Buckeridge, M.S.; Polizeli, M.L.T.M. Holocellulase production by filamentous fungi: Potential in the hydrolysis of energy cane and other sugarcane varieties. Biomass Convers. Biorefin. 2021, 13, 1163–1174. [Google Scholar] [CrossRef]
- Contato, A.G.; Brugnari, T.; Sibin, A.P.A.; Buzzo, A.J.R.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; Souza, C.G.M. Biochemical properties and effects of mitochondrial respiration of aqueous extracts of basidiomycete mushrooms. Cell Biochem. Biophys. 2020, 78, 111–119. [Google Scholar] [CrossRef]
- Vogel, H.J. A convenient growth medium for Neurospora crassa. Microb. Genet. Bull. 1956, 13, 42–47. [Google Scholar]
- Freitas, E.N.; Bubna, G.A.; Brugnari, T.; Kato, C.G.; Nolli, M.; Rauen, T.G.; Moreira, R.F.P.M.; Peralta, R.A.; Bracht, A.; Souza, C.G.M.; et al. Removal of bisphenol A by laccases from Pleurotus ostreatus and Pleurotus pulmonarius and evaluation of ecotoxicity of degradation products. Chem. Eng. J. 2017, 330, 1361–1369. [Google Scholar] [CrossRef]
- Freitas, E.N.; Alnoch, R.C.; Contato, A.G.; Nogueira, K.M.V.; Crevelin, E.D.; Moraes, L.A.B.; Silva, R.N.; Martínez, C.A.; Polizeli, M.L.T.M. Enzymatic pretreatment with laccases from Lentinus sajor-caju induces structural modification in lignin and enhances the digestibility of tropical forage grass (Panicum maximum) grown under future climate conditions. Int. J. Mol. Sci. 2021, 17, 9445. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Guedes-Júnior, J.G.E.; Mattor, F.R.; Sabi, G.J.; Carvalho, W.C.A.; Luiz, J.H.H.; Cren, E.C.; Fernandez-Lafuente, R.; Mendes, A.A. Design of a sustainable process for enzymatic production of ethylene glycol diesters via hydroesterification of used soybean cooking oil. J. Environ. Chem. Eng. 2022, 10, 107062. [Google Scholar] [CrossRef]
- Monteiro, L.M.O.; Pereira, M.G.; Vici, A.C.; Heinen, P.R.; Buckeridge, M.S.; Polizeli, M.L.T.M. Efficient hydrolysis of wine and grape juice anthocyanins by Malbranchea pulchella β-glucosidase immobilized on MANAE-agarose and ConA-Sepharose supports. Int. J. Biol. Macromol. 2019, 136, 1133–1141. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Andrades, D.; Graebin, N.G.; Kadowaki, M.K.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization and stabilization of different β-glucosidases using the glutaraldehyde chemistry: Optimal protocol depends on the enzyme. Int. J. Biol. Macromol. 2019, 129, 672–678. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- Contato, A.G.; Inácio, F.D.; Brugnari, T.; Araújo, C.A.V.; Maciel, G.M.; Haminiuk, C.W.I.; Peralta, R.M.P.; Souza, G.G.M. Solid-state fermentation with orange juice waste: Optimization of laccase production from Pleurotus pulmonarius CCB-20 and descolorization of synthetic dyes. Acta Sci. Biol. 2020, 42, e52699. [Google Scholar]
- Bentil, J.A.; Thygesen, A.; Mensah, M.; Lange, L.; Meyer, A.S. Cellulase production by white-rot basidiomycetous fungi: Solid-state versus submerged cultivation. Appl. Microbiol. Biotechnol. 2018, 102, 5827–5839. [Google Scholar] [CrossRef]
- Kameshwar, A.K.S.; Qin, W. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology 2018, 9, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The production of laccases by white-rot fungi under solid-state fermentation conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, C.; Zhu, L.; Zhang, D.; Pan, C. Purification, characterization, and gene cloning of two laccase isoenzymes (Lac1 and Lac2) from Trametes hirsuta MX2 and their potential in dye decolorization. Mol. Biol. Rep. 2020, 47, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Quintero, C.; Merino-Restrepo, A.; Hormaza-Anaguano, A. Production, extraction, and quantification of laccase obtained from an optimized solid-state fermentation of corncob with white-rot fungi. J. Clean. Prod. 2022, 370, 133598. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Laccase immobilization for water purification: A comprehensive review. J. Chem. Eng. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahum, A.H.; Azzazy, H.M.E.S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5194–5196. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.B.; Rios, N.S.; Zanatta, G.; Pessela, B.C.; Gonçalves, L.R.B. Aminated laccases can improve and expand the immobilization protocol on agarose-based supports by ion exchange. Process Biochem. 2023, 133, 292–302. [Google Scholar] [CrossRef]
- Vieira, M.F.; Vieira, A.M.S.; Zanin, G.M.; Tardioli, P.W.; Mateo, C.; Guisán, J.M. β-Glucosidase immobilized and stabilized on agarose matrix functionalized with distinct reactive groups. J. Mol. Catal. B Enzym. 2011, 69, 47–53. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; Muteeb, G.; Elgarawany, A.; Aatif, M.; Farhan, M.; Allam, S.; Almatar, B.A.; Radwan, M.G. Green nanobiocatalysts: Enhancing enzyme immobilization for industrial and biomedical applications. PeerJ 2024, 12, e17589. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, U.M.; Norizan, M.N.; Hamzah, F. ; Mat, HB Recent advances in enzyme immobilisation strategies: An overview of techniques and composite carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Talekar, S.; Pandharbale, A.; Ladole, M.; Nadar, S.; Mulla, M.; Japhalekar, K.; Pattankude, K.; Arage, D. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates (combi-CLEAs): A tri-enzyme biocatalyst with one pot starch hydrolytic activity. Bioresour. Technol. 2013, 147, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Venegas, M.; Tellez-Cruz, M.M.; Solorza-Feria, O.; López-Munguía, A.; Castillo, E.; Juaristi, E. Thermal and mechanical stability of immobilized Candida antarctica Lipase B: An approximation to mechanochemical energetics in enzyme catalysis. ChemCatChem 2020, 12, 803–811. [Google Scholar] [CrossRef]
- Sun, K.; Hong, D.; Liu, J.; Latif, A.; Li, S. Trametes versicolor laccase-assisted oxidative coupling of estrogens: Conversion kinetics, linking mechanisms, and practical applications in water purification. Sci. Total Environ. 2021, 782, 146917. [Google Scholar] [CrossRef]
- Wang, C.; Jia, Y.; Luo, J.; Chen, B.; Pan, C. Characterization of thermostable recombinant laccase F from Trametes hirsuta and its application in delignification of rice straw. Bioresour. Technol. 2024, 395, 130382. [Google Scholar] [CrossRef]
- Shabaev, A.V.; Moiseenko, K.V.; Glazunova, O.G.; Savinova, O.S.; Fedorova, T.V. Comparative analysis of Peniophora lycii and Trametes hirsuta exoproteomes demonstrates “shades of gray” in the concept of white-rotting fungi. Int. J. Mol. Sci. 2022, 23, 10322. [Google Scholar] [CrossRef]
- Moya, E.B.; Syhler, B.; Manso, J.O.; Dragone, G.; Mussatto, S.I. Enzymatic hydrolysis cocktail optimization for the intensification of sugar extraction from sugarcane bagasse. Int. J. Biol. Macromol. 2023, 242, 125051. [Google Scholar] [CrossRef]
- Ren, F.; Wu, F.; Wu, X.; Bao, T.; Jie, Y.; Gao, L. Fungal systems for lignocellulose deconstruction: From enzymatic mechanisms to hydrolysis optimization. Glob. Chang. Bio. Bioenergy 2024, 16, e13130. [Google Scholar] [CrossRef]
- Shafagh, I.; Shepley, P.; Shepherd, W.; Loveridge, F.; Schellart, A.; Tait, S.; Rees, S.J. Thermal energy transfer around buried pipe infrastructure. Geomech. Energy Environ. 2022, 29, 100273. [Google Scholar] [CrossRef]
- Sybuia, P.A.; Contato, A.G.; de Araújo, C.A.V.; Zanzarin, D.M.; Maciel, G.M.; Pilau, E.J.; Peralta, R.M.; de Souza, C.G.M. Application of the white-rot fungus Trametes sp. (C3) laccase in the removal of acetaminophen from aqueous solutions. J. Water Process Eng. 2024, 57, 104677. [Google Scholar] [CrossRef]
- de Araújo, C.A.V.; Contato, A.G.; Aranha, G.M.; Maciel, G.M.; Haminiuk, C.W.I.; Inácio, F.D.; Rodrigues, J.H.S.; Peralta, R.M.; de Souza, C.G.M. Biodiscoloration, detoxification and biosorption of Reactive Blue 268 by Trametes sp. M3: A strategy for the treatment of textile effluents. Water Air Soil Pollut. 2020, 231, 349. [Google Scholar] [CrossRef]
- Bettin, F.; Cousseau, F.; Marins, K.; Zaccaria, S.; Girardi, V.; Silveira, M.M.; Dillon, A.J.P. Effects of pH, temperature and agitation on the decolourisation of dyes by laccase-containing enzyme preparation from Pleurotus sajor-caju. Braz. Arch. Biol. Techn. 2019, 62, e19180338. [Google Scholar] [CrossRef]
- Jawale, J.P.; Nandre, V.S.; Latpate, R.V.; Kulkarni, M.V.; Doshi, P.J. Isolation, characterization and optimizations of laccase producer from soil: A comprehensive study of application of statistical approach to enhance laccase productivity in Myrothecium verrucaria NFCCI 4363. Bioresour. Technol. Rep. 2021, 15, 100751. [Google Scholar] [CrossRef]
- Almansa, E.; Kandelbauer, A.; Pereira, L.; Cavaco-Paulo, A.; Guebitz, G.M. Influence of structure on dye degradation with laccase mediator systems. Biocatal. Biotransform. 2004, 22, 315–324. [Google Scholar] [CrossRef]
- Maruthamuthu, M.; Dhandavel, R. Binding of bromocresol green onto poly (N-vinyl-2-pyrrolidone). Macromol. Rapid Commun. 1980, 1, 633–636. [Google Scholar] [CrossRef]
- Moiseenko, K.V.; Savinova, O.S.; Vasina, D.V.; Kononikhin, A.S.; Tyazhelova, T.V.; Fedorova, T.V. Laccase isoenzymes of Trametes hirsuta LE-BIN072: Degradation of industrial dyes and secretion under the different induction conditions. Appl. Biochem. Microbiol. 2018, 54, 834–841. [Google Scholar] [CrossRef]
- Jathanna, N.N.; Krishnamurthy, G.K.; Paithankar, J.G.; Hegde, S.; Goveas, L.C.; Ravindranath, B.S.; Gowdru, M. Phyto-bacterial biosorption of basic fuchsine: A self-sustainable approach towards biomitigation of contaminant of emerging concern. J. Environ. Chem. Eng. 2023, 11, 109330. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, A.; Singh, S.S.; Srivastava, S.; Thakur, I.S. Expression and characterization of novel laccase gene from Pandoraea sp. ISTKB and its application. Int. J. Biol. Macromol. 2018, 115, 308–316. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ulaganathan, P.; Sivasubramanian, S.; Esakkimuthu, S.; Krishnaswamy, S.; Subramaniam, S. Trichoderma asperellum laccase mediated crystal violet degradation—Optimization of experimental conditions and characterization. J. Environ. Chem. Eng. 2017, 5, 222–231. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
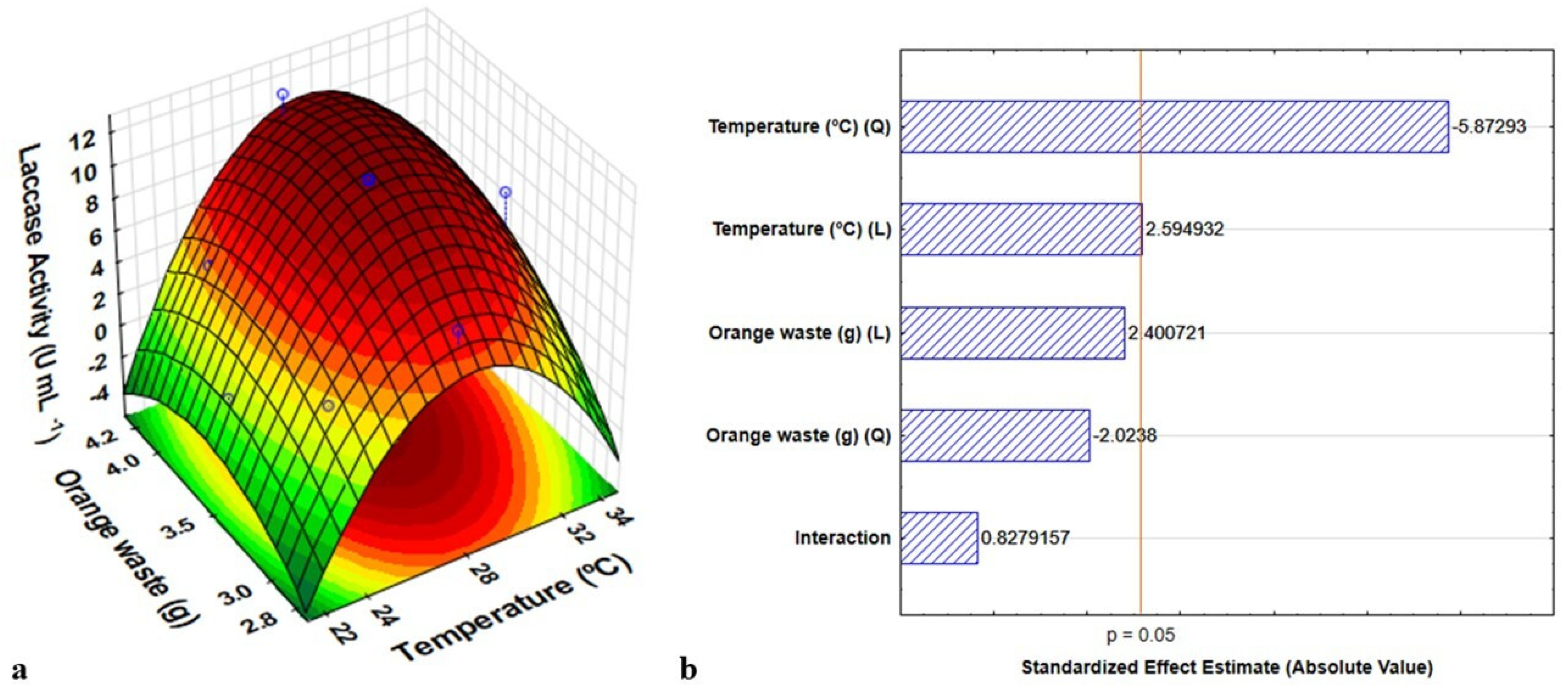
| Run | Temperature °C (x1) | Orange Waste g (x2) | Experimental Activity (U mL−1) | Predicted Activity (U mL−1) | Residue |
|---|---|---|---|---|---|
| 1 | −1 (24) | −1 (3.0) | 3.575 | 3.655 | −0.08042 |
| 2 | 1 (32) | −1 (3.0) | 3.656 | 5.284 | −1.62817 |
| 3 | −1 (24) | 1 (4.0) | 4.692 | 5.062 | −0.37032 |
| 4 | 1 (32) | 1 (4.0) | 7.444 | 9.362 | −1.91808 |
| 5 | −1.41 (22) | 0 (3.5) | 1.083 | 1.175 | −0.09247 |
| 6 | 1.41 (34) | 0 (3.5) | 7.458 | 5.355 | 2.10293 |
| 7 | 0 (28) | −1.41 (2.8) | 7.347 | 6.547 | 0.79962 |
| 8 | 0 (28) | 1.41 (4.2) | 11.625 | 10.414 | 1.21083 |
| 9 | 0 (28) | 0 (3.5) | 11.208 | 11.223 | −0.01497 |
| 10 | 0 (28) | 0 (3.5) | 11.222 | 11.223 | −0.000 |
| 11 | 0 (28) | 0 (3.5) | 11.215 | 11.223 | −0.00797 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | Fcalc | Ftab |
|---|---|---|---|---|---|
| Model | 717.212 | 10 | 71.712 | 15.56 | 3.35 |
| Residual | 36.871 | 8 | 4.609 | ||
| Lack of fit | 34.147 | 6 | 5.691 | 4.18 | 19.33 |
| Error | 2.723 | 2 | 1.361 | ||
| Total | 754.082 | 10 |
| Ionic Supports | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | |||||||
| Iy | Ef | Ra | Iy | Ef | Ra | Iy | Ef | Ra | |
| DEAE-cellulose | 9.0 ± 0.7 | 12.9 ± 0.4 | 6.5 ± 0.3 | 7.9 ± 0.6 | 10.2 ± 0.1 | 5.6 ± 0.4 | 9.1 ± 0.7 | 9.9 ± 0.6 | 6.5 ± 0.2 |
| MANAE-agarose | 20.6 ± 0.2 | 32.5 ± 1.1 | 18.0 ± 0.7 | 20.9 ± 0.3 | 29.8 ± 0.2 | 18.1 ± 0.3 | 21.0 ± 0.1 | 27.3 ± 0.2 | 17.9 ± 0.1 |
| PEI-agarose | 6.2 ± 0.3 | 19.3 ± 0.1 | 5.8 ± 0.3 | 4.9 ± 0.5 | 19.6 ± 0.2 | 4.5 ± 0.3 | 4.2 ± 0.7 | 19.0 ± 0.2 | 3.8 ± 0.3 |
| DEAE-Sephacel | 12.0 ± 0.4 | 23.2 ± 0.7 | 7.6 ± 0.3 | 9.4 ± 0.1 | 20.8 ± 0.5 | 6.6 ± 0.4 | 10.0 ± 0.3 | 17.6 ± 0.1 | 7.1 ± 0.4 |
| CM-cellulose | 93.1 ± 3.4 | 62.7 ± 0.8 | 91.9 ± 1.1 | 65.7 ± 1.3 | 61.9 ± 0.6 | 62.5 ± 0.8 | 58.4 ± 0.2 | 58.7 ± 0.5 | 56.0 ± 0.9 |
| MANAE-cellulose | 13.7 ± 0.4 | 15.8 ± 0.2 | 6.3 ± 0.4 | 9.7 ± 0.1 | 14.3 ± 0.1 | 4.1 ± 0.1 | 10.4 ± 0.1 | 14.5 ± 0.7 | 4.3 ± 0.4 |
| DEAE-agarose | 19.5 ± 0.1 | 55.3 ± 0.4 | 15.5 ± 0.1 | 20.1 ± 0.6 | 53.1 ± 0.7 | 15.5 ± 0.6 | 14.7 ± 2.1 | 52.8 ± 1.9 | 10.4 ± 0.8 |
| Dye | λmax (nm) | 0.05% (w/v) | 0.01% (w/v) | 0.005% (w/v) |
|---|---|---|---|---|
| Bromophenol blue | 610 | 71.9 ± 2.4 | 65.7 ± 3.4 | 33.9 ± 7.1 |
| Methylene Blue | 665 | 2.9 ± 1.0 | 6.3 ± 4.8 | 52.4 ± 9.8 |
| Trypan blue | 580 | 35.9 ± 8.6 | 83.4 ± 0.3 | 78.5 ± 5.2 |
| Orcein | 579 | 70.8 ± 2.1 | 36.9 ± 9.2 | 49.0 ± 2.8 |
| Ponceau S | 515 | 0 | 46.6 ± 0.2 | 48.2 ± 1.9 |
| Bromocresol green | 640 | 96.6 ± 2.7 | 89.7 ± 1.2 | 84.6 ± 1.8 |
| Methyl Red | 520 | 43.6 ± 0.8 | 34.6 ± 2.0 | 2.0 ± 0.9 |
| Congo red | 498 | 14.8 ± 5.9 | 73.2 ± 0.6 | 72.3 ± 2.1 |
| Victoria Blue B | 615 | 0 | 69.3 ± 0.7 | 62.2 ± 3.9 |
| Gentian violet | 587 | 0 | 59 ± 2.1 | 72.1 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortolan, G.G.; Contato, A.G.; Aranha, G.M.; Salgado, J.C.S.; Alnoch, R.C.; Polizeli, M.d.L.T.d.M. Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization. Reactions 2024, 5, 635-650. https://doi.org/10.3390/reactions5030032
Ortolan GG, Contato AG, Aranha GM, Salgado JCS, Alnoch RC, Polizeli MdLTdM. Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization. Reactions. 2024; 5(3):635-650. https://doi.org/10.3390/reactions5030032
Chicago/Turabian StyleOrtolan, Guilherme Guimarães, Alex Graça Contato, Guilherme Mauro Aranha, Jose Carlos Santos Salgado, Robson Carlos Alnoch, and Maria de Lourdes Teixeira de Moraes Polizeli. 2024. "Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization" Reactions 5, no. 3: 635-650. https://doi.org/10.3390/reactions5030032
APA StyleOrtolan, G. G., Contato, A. G., Aranha, G. M., Salgado, J. C. S., Alnoch, R. C., & Polizeli, M. d. L. T. d. M. (2024). Enhancing Laccase Production by Trametes hirsuta GMA-01 Using Response Surface Methodology and Orange Waste: A Novel Breakthrough in Sugarcane Bagasse Saccharification and Synthetic Dye Decolorization. Reactions, 5(3), 635-650. https://doi.org/10.3390/reactions5030032










