Theoretical Study of the Halogen Concentration Effect on the 1,3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
- 1.
- Initiation stage:
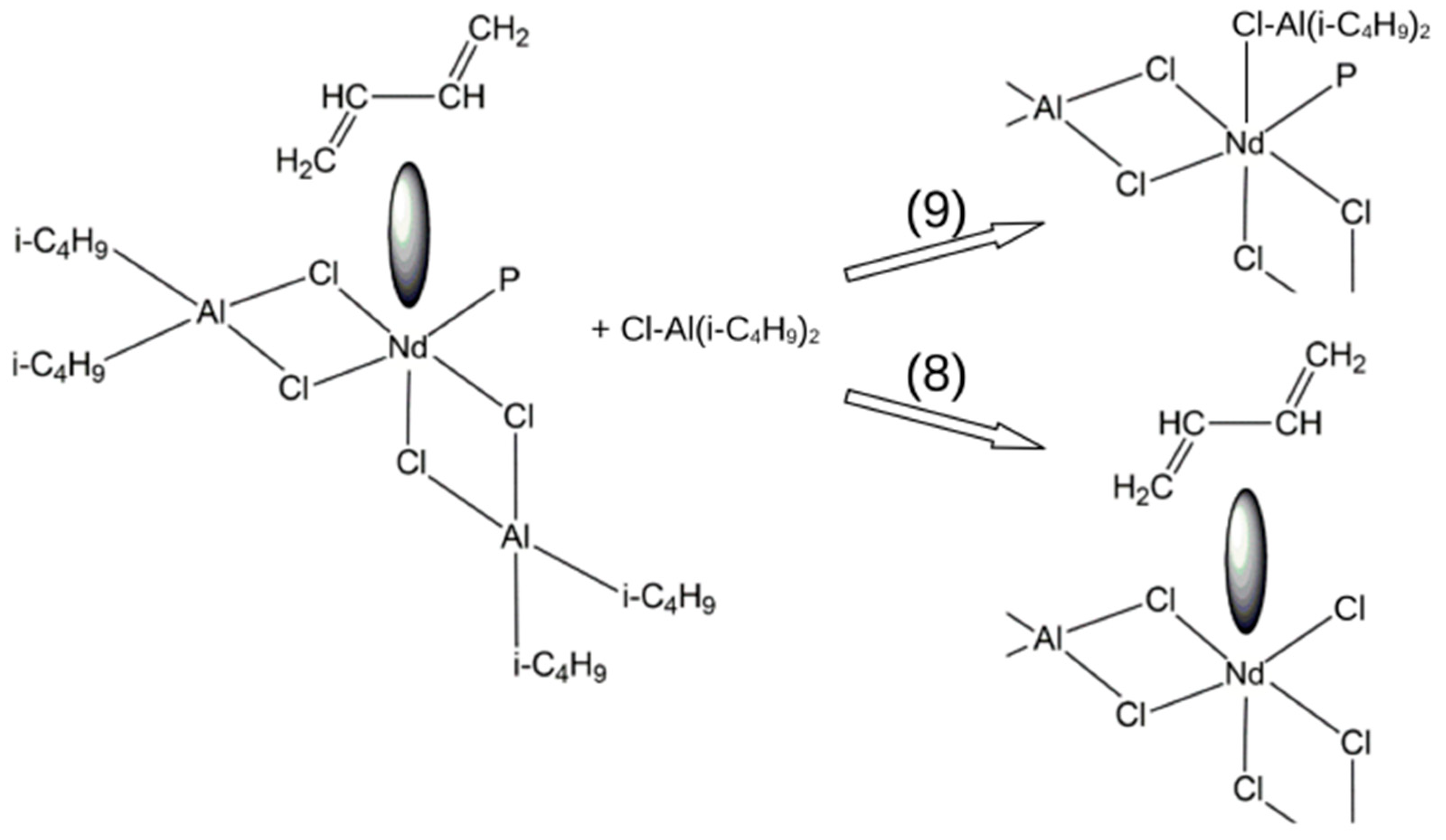
- 2.
- Growth stage (insertion of 1,3-butadiene into the reactive growing polymer chain):
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’amore, M.; Takasao, G.; Chikuma, H.; Wada, T.; Taniike, T.; Pascale, F.; Ferrari, A.M. Spectroscopic fingerprints of MgCl2/TiCl4 nanoclusters determined by machine learning and DFT. J. Phys. Chem. C 2021, 125, 20048–20058. [Google Scholar] [CrossRef]
- Piovano, A.; Zarupski, J.; Groppo, E. Disclosing the Interaction between Carbon Monoxide and Alkylated Ti3+ Species: A Direct Insight into Ziegler–Natta Catalysis. J. Phys. Chem. Lett. 2020, 11, 5632–5637. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, M.; Karkhaneh, F.; Nekoomanesh, M.; Sadjadi, S.; Emami, M.; Teimoury, H.; Posada-Pérez, S. Influence of the ethanol content of adduct on the Comonomer incorporation of related Ziegler–Natta catalysts in propylene (Co) polymerizations. Polymers 2023, 15, 4476. [Google Scholar] [CrossRef] [PubMed]
- Pernusch, D.C.; Spiegel, G.; Paulik, C.; Hofer, W. Influence of Poisons Originating from Chemically Recycled Plastic Waste on the Performance of Ziegler–Natta Catalysts. Macromol. React. Eng. 2022, 16, 2100020. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Ortega-Toro, R.; Castro-Suarez, J.R. Theoretical–experimental study of the action of trace amounts of formaldehyde, propionaldehyde, and butyraldehyde as inhibitors of the ziegler–natta catalyst and the synthesis of an ethylene–propylene copolymer. Polymers 2023, 15, 1098. [Google Scholar] [CrossRef]
- Blaakmeer, E.S.; Wensink, F.J.; van Eck, E.R.; de Wijs, G.A.; Kentgens, A.P. Preactive site in Ziegler–Natta catalysts. J. Phys. Chem. C 2019, 123, 14490–14500. [Google Scholar] [CrossRef]
- Cooper, W.; Vaughan, G. Recent developments in the polymerization of conjugated dienes. Prog. Polym. Sci. 1967, 1, 91–160. [Google Scholar] [CrossRef]
- Saltman, W.M. The Stereo Rubbers; Wiley-Interscience: New York, NY, USA, 1977; p. 897. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yeh, H.C. Polymerization of butadiene and isoprene with lanthanide catalysts; characterization and properties of homopolymers and copolymers. Rubber Chem. Technol. 1985, 58, 117–145. [Google Scholar] [CrossRef]
- Marina, N.G.; Monakov, Y.B.; Sabirov, Z.M.; Tolstikov, G.A. Lanthanide compounds—Catalysts of stereospecific polymerization of diene monomers. Rev. Polym. Sci. USSR 1991, 33, 387–417. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. In Neodymium Based Ziegler Catalysts–Fundamental Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–154. [Google Scholar] [CrossRef]
- Fischbach, A.; Anwander, R. Neodymium Based Ziegler Catalysts Fundamental Chemistry; Nuyken, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 204, pp. 155–281. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, D.; Wang, B.; Liu, B.; Yang, Y. Polymerization of 1, 3-conjugated dienes with rare-earth metal precursors. In Molecular Catalysis of Rare-Earth Elements. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2010; pp. 49–108. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Hu, Y.; Zhang, X. Lanthanide complexes mediated coordinative chain transfer polymerization of conjugated dienes. Sci. China Technol. Sci. 2018, 61, 1286–1294. [Google Scholar] [CrossRef]
- Göttker-Schnetmann, I.; Kenyon, P.; Mecking, S. Coordinative Chain Transfer Polymerization of Butadiene with Functionalized Aluminum Reagents. Angew. Chem. 2019, 131, 17941–17945. [Google Scholar] [CrossRef]
- González-Zapata, J.L.; Enríquez-Medrano, F.J.; González HR, L.; Revilla-Vázquez, J.; Carrizales, R.M.; Georgouvelas, D.; de León Gómez RE, D. Introducing random bio-terpene segments to high cis-polybutadiene: Making elastomeric materials more sustainable. RSC Adv. 2020, 10, 44096–44102. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante de Sá, M.C.; Córdova, A.M.T.; Díaz de León Gómez, R.E.; Pinto, J.C. Modeling of Isoprene Solution Coordinative Chain Transfer Polymerization. Macromol. React. Eng. 2021, 15, 2100005. [Google Scholar] [CrossRef]
- Córdova, T.; Enríquez-Medrano, F.J.; Cartagena, E.M.; Villanueva, A.B.; Valencia, L.; Álvarez EN, C.; Díaz-de-León, R. Coordinative Chain Transfer Polymerization of Sustainable Terpene Monomers Using a Neodymium-Based Catalyst System. Polymers 2022, 14, 2907. [Google Scholar] [CrossRef]
- Tereshchenko, K.A.; Ulitin, N.V.; Bedrina, P.S.; Shiyan, D.A.; Lifanov, A.D.; Madzhidov, T.I.; Volfson, S.I. Analysis of the Mechanism of Polybutadiene Synthesis in the Presence of the Neodymium Versatate+ Diisobutylaluminum Hydride+ Ethylaluminum Sesquichloride Catalytic System within the Solution of the Inverse Kinetic Problem. Ind. Eng. Chem. Res. 2022, 61, 15961–15969. [Google Scholar] [CrossRef]
- Romano, E.; Budzelaar, P.H.; De Rosa, C.; Talarico, G. Unconventional Stereoerror Formation Mechanisms in Nonmetallocene Propene Polymerization Systems Revealed by DFT Calculations. J. Phys. Chem. A 2022, 126, 6203–6209. [Google Scholar] [CrossRef]
- Quirk, R.P.; Kells, A.M.; Yunlu, K.; Cuif, J.P. Butadiene polymerization using neodymium versatate-based catalysts: Catalyst optimization and effects of water and excess versatic acid. Polymer 2000, 41, 5903–5908. [Google Scholar] [CrossRef]
- Kwag, G. A highly reactive and monomeric neodymium catalyst. Macromolecules 2002, 35, 4875–4879. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Q.; Dong, J.; Wang, F.; Luo, F.; Liu, H.; Zhang, X. Neodymium-based one-precatalyst/dual-cocatalyst system for chain shuttling polymerization to access cis-1, 4/trans-1, 4 multiblock polybutadienes. Mater. Today Commun. 2021, 27, 102453. [Google Scholar] [CrossRef]
- Wang, H.; Cue JM, O.; Calubaquib, E.L.; Kularatne, R.N.; Taslimy, S.; Miller, J.T.; Stefan, M.C. Neodymium catalysts for polymerization of dienes, vinyl monomers, and ε-caprolactone. Polym. Chem. 2021, 12, 6790–6823. [Google Scholar] [CrossRef]
- Iovu, H.; Hubca, G.; Simionescu, E.; Badea, E.G.; Dimonie, M. Polymerization of butadiene and isoprene with the NdCl3· 3TBP-TIBA catalyst system. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1997, 249, 59–77. [Google Scholar] [CrossRef]
- Srinivasa Rao, G.S.; Upadhyay, V.K.; Jain, R.C. Polymerization of 1, 3-butadiene using neodymium chloride tripentanolate–triethyl aluminum catalyst systems. J. Appl. Polym. Sci. 1999, 71, 595–602. [Google Scholar] [CrossRef]
- Ren, C.; Li, G.; Dong, W.; Jiang, L.; Zhang, X.; Wang, F. Soluble neodymium chloride 2-ethylhexanol complex as a highly active catalyst for controlled isoprene polymerization. Polymer 2007, 48, 2470–2474. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Liu, X.; Gao, K.; Cao, Y.; Zhang, C.; Zhang, X. Methylaluminoxane-activated neodymium chloride tributylphosphate catalyst for isoprene polymerization. J. Appl. Polym. Sci. 2014, 131, 40153. [Google Scholar] [CrossRef]
- Kularatne, R.N.; Yang, A.; Nguyen, H.Q.; McCandless, G.T.; Stefan, M.C. Neodymium catalyst for the polymerization of dienes and polar vinyl monomers. Macromol. Rapid Commun. 2017, 38, 1700427. [Google Scholar] [CrossRef]
- Oehme, A.; Gebauer, U.; Gehrke, K.; Beyer, P.; Hartmann, B.; Lechner, M.D. The influence of the catalyst preparation on the homo-and copolymerization of butadiene and isoprene. Macromol. Chem. Phys. 1994, 195, 3773–3781. [Google Scholar] [CrossRef]
- Boisson, C.; Barbotin, F.; Spitz, R. Polymerization of butadiene with a new catalyst based on a neodymium amide precursor. Macromol. Chem. Phys. 1999, 200, 1163–1166. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Windisch, H.; Obrecht, W. Polymerization of 1, 3-Butadiene Initiated by Neodymium Versatate/Diisobutylaluminium Hydride/Ethylaluminium Sesquichloride: Kinetics and Conclusions About the Reaction Mechanism. Macromol. Chem. Phys. 2002, 203, 1055–1064. [Google Scholar] [CrossRef]
- Quirk, R.P.; Kells, A.M. Polymerization of butadiene using neodymium versatate-based catalyst systems: Preformed catalysts with SiCl4 as halide source. Polym. Int. 2000, 49, 751–756. [Google Scholar] [CrossRef]
- Sigaeva, N.N.; Usmanov, T.S.; Budtov, V.P.; Monakov, Y.B. Effect of organoaluminum compound on kinetic nonuniformity and structure of active centers of neodymium catalytic systems in butadiene polymerization. Russ. J. Appl. Chem. 2001, 74, 1141–1146. [Google Scholar] [CrossRef]
- Monakov, Y.B.; Sabirov, Z.M.; Urazbaev, V.N.; Efimov, V.P. Relationship between the stereospecificity of lanthanide catalysts and the structures of active sites and dienes, the nature of a cocatalyst, and preparation conditions. Kinet. Catal. 2001, 42, 310–316. [Google Scholar] [CrossRef]
- Sigaeva, N.N.; Usmanov, T.S.; Budtov, V.P.; Spivak, S.I.; Zaikov, G.E.; Monakov, Y.B. The influence of the nature of organoaluminum compound on kinetic heterogeneity of active sites in lanthanide-based diene polymerization. J. Appl. Polym. Sci. 2003, 87, 358–368. [Google Scholar] [CrossRef]
- Monakov, Y.B.; Sabirov, Z.M.; Urazbaev, V.N.; Efimov, V.P. Diene polymerization initiated by NdCl3. 3TBP-based catalytic systems. Multiplicity of active centers and their structure and stereospecificity distributions. Polym. Science. Ser. A 2002, 44, 228–231. [Google Scholar]
- Urazbaev, V.N.; Efimov, V.P.; Sabirov, Z.M.; Monakov, Y.B. Structure of active centers, their stereospecificity distribution, and multiplicity in diene polymerization initiated by NdCl3-based catalytic systems. J. Appl. Polym. Sci. 2003, 89, 601–603. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. DFT and ONIOM Simulation of 1, 3-Butadiene Polymerization Catalyzed by Neodymium-Based Ziegler–Natta System. Polymers 2023, 15, 1166. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. Comparative ONIOM modeling of 1, 3-butadiene polymerization using Nd (III) and Gd (III) Ziegler–Natta catalyst systems. Int. J. Quantum Chem. 2024, 124, e27297. [Google Scholar] [CrossRef]
- Akhmetov, I.G.; Kozlov, V.G.; Salakhov, I.I.; Sakhabutdinov, A.G.; D‘yakonov, G.S. Polymerisation kinetics and molecular characteristics of “neodymium” polybutadiene: Influence of halogenating agent concentration. Int. Polym. Sci. Technol. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Akhmetov, I.G.; Kozlov, V.G. Polymerization of butadiene during the action of the catalytic system neodymium versatate-diisobutylaluminum hydride-hexachloro-p-xylene. Polym. Sci. Ser. B 2011, 53, 385–390. [Google Scholar] [CrossRef]
- Akhmetov, I.G. Synthesis of Diene Rubbers Using Modified Catalytic Systems Based on Neodymium and Lithium Compounds. Dr. Chem. Sci. Diss.; KNRTU Publisher: Kazan, Russia, 2013; 379p. (In Russian) [Google Scholar]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, 73–78. [Google Scholar] [CrossRef]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461, 1–21. [Google Scholar] [CrossRef]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Stoychev, G.L.; Auer, A.A.; Neese, F. Automatic generation of auxiliary basis sets. J. Chem. Theory Comput. 2017, 13, 554–562. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted abinitio pseudopotentials for the rare earth elements. J. Chem. Phys. 1989, 90, 1730–1734. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and efficient implicit solvation model for fast semiempirical methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
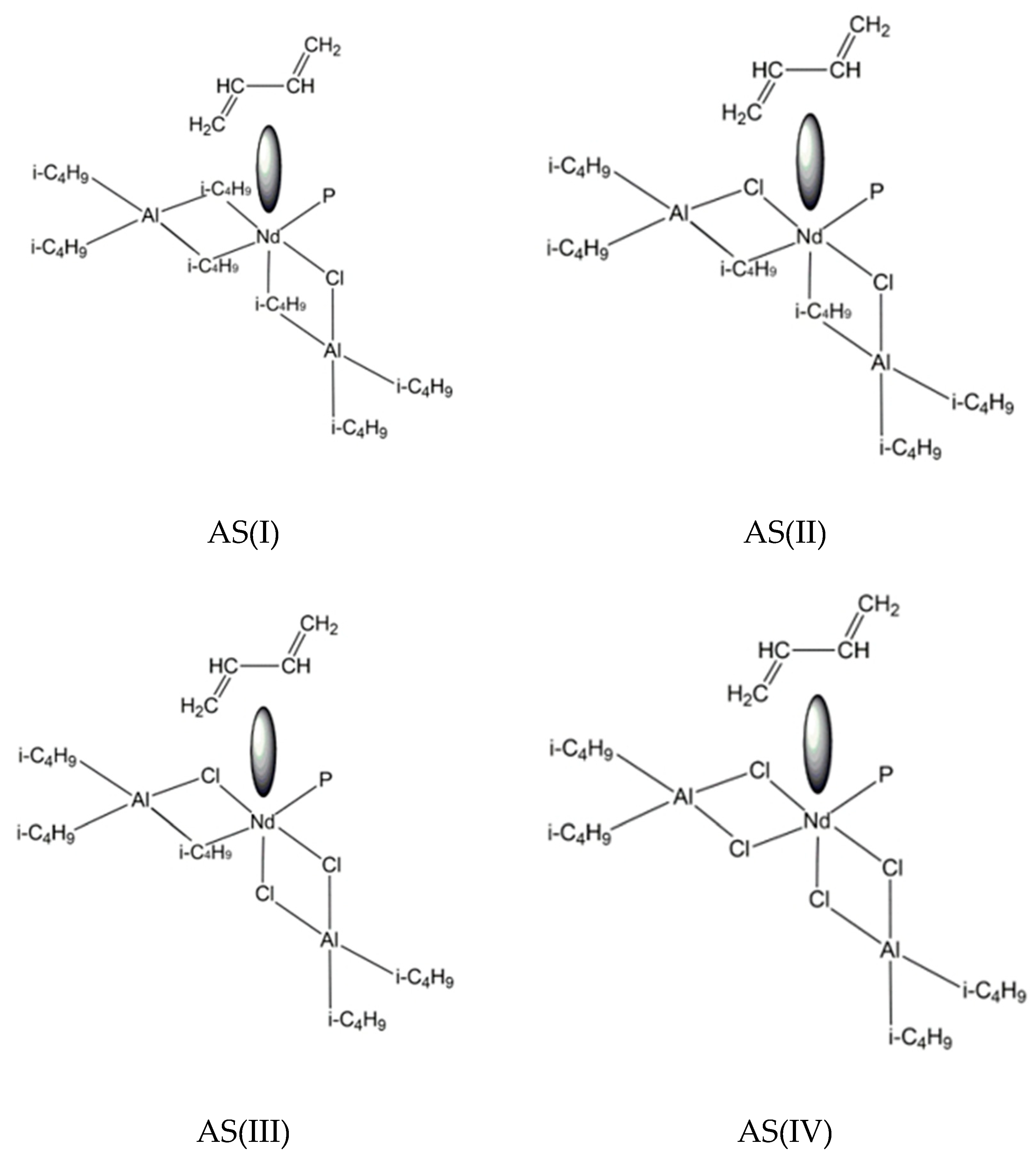
| [Cl]:[Nd] | Wp, mol/(l min) | kg, l/(mol min) | γNd, % | cis-1,4- Content, % | trans-1,4-Content, % | 1,2-Content, % |
|---|---|---|---|---|---|---|
| 1.0 | 0.03 | 520 | 18 | 95.5 | 3.4 | 1.1 |
| 2.0 | 0.18–0.20 | 2900–2950 | 32–37 | 96.0–96.5 | 2.5–3.0 | 1.0 |
| 3.0 | 0.25–0.28 | 4800–4950 | 27–29 | 97.1 | 1.9 | 1.0 |
| 4.0 | 0.14–0.16 | 4800–5187 | 13–17 | 98.1 | 0.9 | 1.0 |
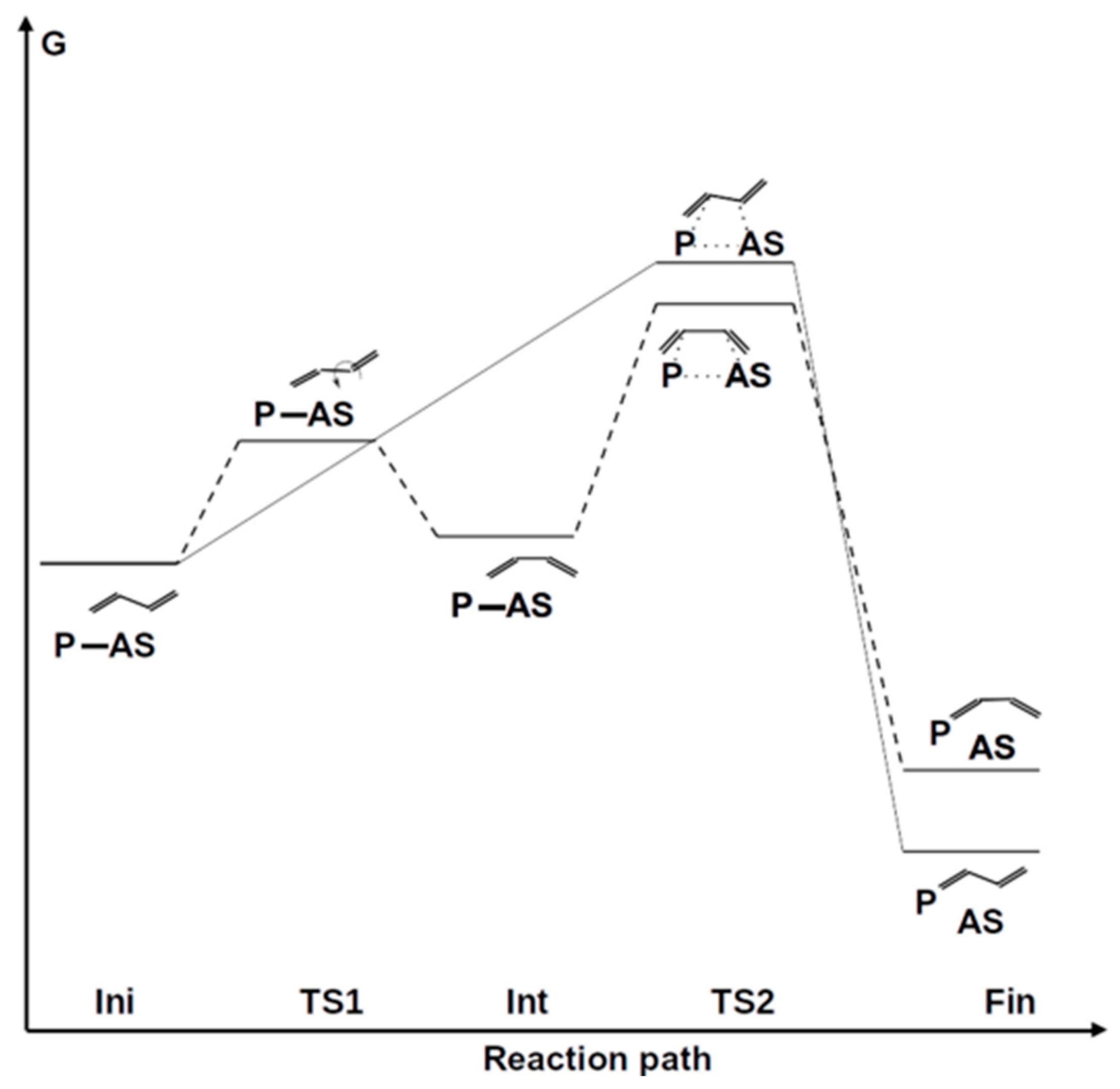
| Initial Structure | ΔG≠298 (trans-cis) | ΔG298 (trans-cis) | ΔG≠298 (Polymer) | ΔG≠298 (Total) | ΔG298 (Polymer) | Product |
|---|---|---|---|---|---|---|
| i-C4H9-AS(I)-T | 41.4 | 10.4 | 64.9 | 75.3 | −38.0 | i-C4H9-C-AS(I) |
| i-C4H9-AS(I)-T | 81.6 | 81.6 | −70.3 | i-C4H9-T-AS(I) | ||
| i-C4H9-AS(II)-T | 43.9 | 12.8 | 56.8 | 69.6 | −66.6 | i-C4H9-C-AS(II) |
| i-C4H9-AS(II)-T | 87.9 | 87.9 | −54.8 | i-C4H9-T-AS(II) | ||
| i-C4H9-AS(III)-T | 41.1 | 11.4 | 59.2 | 70.6 | −61.9 | i-C4H9-C-AS(III) |
| i-C4H9-AS(III)-T | 86.2 | 86.2 | −51.5 | i-C4H9-T-AS(III) | ||
| i-C4H9-AS(IV)-T | 43.0 | 6.6 | 54.3 | 60.9 | −51.7 | i-C4H9-C-AS(IV) |
| i-C4H9-AS(IV)-T | 67.0 | 67.0 | −55.1 | i-C4H9-T-AS(IV) |
| Reaction Parameter | Reaction No. | ||||
|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | |
| ΔH0298, kJ/mol | −33.5 | −41.6 | −89.1 | −76.8 | −8.4 |
| ΔS0298, J/(mol∙K) | 18.1 | 13.3 | 15.6 | −11.7 | −33.6 |
| ΔG0298, kJ/mol | −38.9 | −47.5 | −93.7 | −73.3 | 1.5 |
| Initial Structure | ΔG≠298 (trans-cis) | ΔG298 (trans-cis) | ΔG≠298 (Polymer) | ΔG≠298 (Total) | ΔG298 (Polymer) | Product |
|---|---|---|---|---|---|---|
| i-C4H9-CC-AS(I)-T | 29.5 | 23.5 | 63.9 | 87.4 | −15.0 | i-C4H9-CCC-AS(I) |
| i-C4H9-CCC-AS(I)-T | 28.9 | 23.3 | 64.0 | 87.3 | −35.1 | i-C4H9-CCCC-AS(I) |
| i-C4H9-CC-AS(I)-T | 102.8 | 102.8 | −58.7 | i-C4H9-CCT-AS(I) | ||
| i-C4H9-CT-AS(I)-T | 32.1 | 22.5 | 60.8 | 82.3 | −18.4 | i-C4H9-CTC-AS(I) |
| i-C4H9-CT-AS(I)-T | 90.9 | 90.9 | −56.6 | i-C4H9-CTT-AS(I) | ||
| i-C4H9-CC-AS(II)-T | 35.5 | 7.3 | 65.0 | 72.3 | −21.3 | i-C4H9-CCC-AS(II) |
| i-C4H9-CCC-AS(II)-T | 34.0 | 7.6 | 63.7 | 71.3 | −50.3 | i-C4H9-CCCC-AS(II) |
| i-C4H9-CC-AS(II)-T | 95.7 | 95.7 | −53.7 | i-C4H9-CCT-AS(II) | ||
| i-C4H9-CT-AS(II)-T | 23.2 | 17.6 | 60.4 | 78.0 | −29.0 | i-C4H9-CTC-AS(II) |
| i-C4H9-CT-AS(II)-T | 94.5 | 94.5 | −2.2 | i-C4H9-CTT-AS(II) | ||
| i-C4H9-CC-AS(III)-T | 29.9 | 14.8 | 51.5 | 66.8 | −27.3 | i-C4H9-CCC-AS(III) |
| i-C4H9-CCC-AS(III)-T | 29.5 | 7.6 | 51.8 | 59.4 | −35.7 | i-C4H9-CCCC-AS(III) |
| i-C4H9-CC-AS(III)-T | 104.0 | 104.0 | −51.7 | i-C4H9-CCT-AS(III) | ||
| i-C4H9-CT-AS(III)-T | 32.1 | 26.2 | 56.0 | 82.2 | −2.8 | i-C4H9-CTC-AS(III) |
| i-C4H9-CT-AS(III)-T | 99.2 | 99.2 | −25.3 | i-C4H9-CTT-AS(III) | ||
| i-C4H9-CC-AS(IV)-T | 24.6 | 14.6 | 51.7 | 66.3 | −35.0 | i-C4H9-CCC-AS(IV) |
| i-C4H9-CCC-AS(IV)-T | 24.7 | 6.3 | 54.2 | 60.5 | −60.4 | i-C4H9-CCCC-AS(IV) |
| i-C4H9-CC-AS(IV)-T | 102.0 | 102.0 | −52.9 | i-C4H9-CCT-AS(IV) | ||
| i-C4H9-CT-AS(IV)-T | 21.4 | 12.9 | 70.1 | 83.0 | −8.9 | i-C4H9-CTC-AS(IV) |
| i-C4H9-CT-AS(IV)-T | 77.5 | 77.5 | −40.3 | i-C4H9-CTT-AS(IV) |
| Characteristic Distances, (R, Å) | AS (I) | AS (II) | AS (III) | AS (IV) |
|---|---|---|---|---|
| i-C4H9-AS + η-trans-C4H6 * | ||||
| R(Nd-C(i-C4H9)) | 2.431 | 2.387 | 2.377 | 2.414 |
| R(Nd-C1(C4H6)) | 3.504 | 3.244 | 3.261 | 3.513 |
| R(Nd-C2(C4H6)) | 3.148 | 3.021 | 3.048 | 3.161 |
| R(Nd-C3(C4H6)) | 3.19 | 3.143 | 3.151 | 3.111 |
| R(Nd-C4(C4H6)) | 3.559 | 3.605 | 3.616 | 3.415 |
| R(C1(C4H6)-C(i-C4H9) | 3.634 | 3.476 | 3.46 | 3.571 |
| i-C4H9-CCC-AS + η-trans-C4H6 ** | ||||
| R(Nd-C1(C4H6)) | 3.295 | 3.38 | 3.309 | 3.161 |
| R(Nd-C2(C4H6)) | 3.552 | 3.469 | 3.364 | 3.32 |
| R(Nd-C3(C4H6)) | 4.31 | 4.19 | 4.014 | 4.147 |
| R(Nd-C4(C4H6)) | 5.144 | 4.945 | 4.739 | 4.908 |
| R(Nd-Cα(C4H6)) | 2.617 | 2.628 | 2.659 | 2.609 |
| R(Nd-Cβ(C4H6)) | 2.702 | 2.69 | 2.682 | 2.67 |
| R(Nd-Cγ(C4H6)) | 2.732 | 2.751 | 2.672 | 2.758 |
| R(Nd-Cδ(C4H6)) | 3.077 | 3.186 | 3.052 | 3.349 |
| R(C1(C4H6)-Cα(C4H6) | 3.302 | 3.324 | 3.427 | 3.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. Theoretical Study of the Halogen Concentration Effect on the 1,3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System. Reactions 2024, 5, 753-764. https://doi.org/10.3390/reactions5040037
Masliy AN, Akhmetov IG, Kuznetsov AM, Davletbaeva IM. Theoretical Study of the Halogen Concentration Effect on the 1,3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System. Reactions. 2024; 5(4):753-764. https://doi.org/10.3390/reactions5040037
Chicago/Turabian StyleMasliy, Alexey N., Ildar G. Akhmetov, Andrey M. Kuznetsov, and Ilsiya M. Davletbaeva. 2024. "Theoretical Study of the Halogen Concentration Effect on the 1,3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System" Reactions 5, no. 4: 753-764. https://doi.org/10.3390/reactions5040037
APA StyleMasliy, A. N., Akhmetov, I. G., Kuznetsov, A. M., & Davletbaeva, I. M. (2024). Theoretical Study of the Halogen Concentration Effect on the 1,3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System. Reactions, 5(4), 753-764. https://doi.org/10.3390/reactions5040037







