The Influence of the Structure of Organochlorine Compounds on Their Decomposition Process in a Dielectric Barrier Discharge
Abstract
1. Introduction
2. Materials and Methods
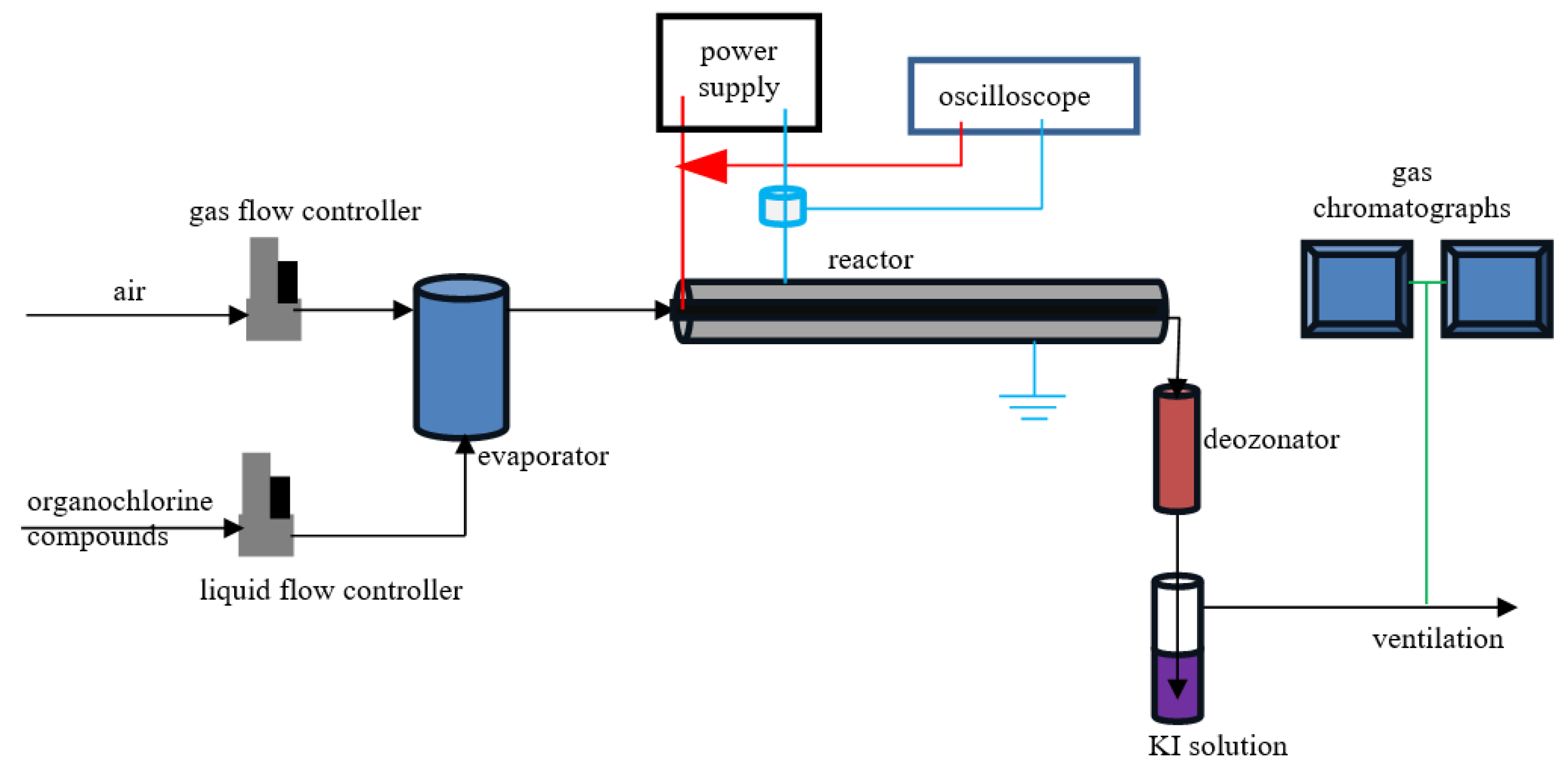
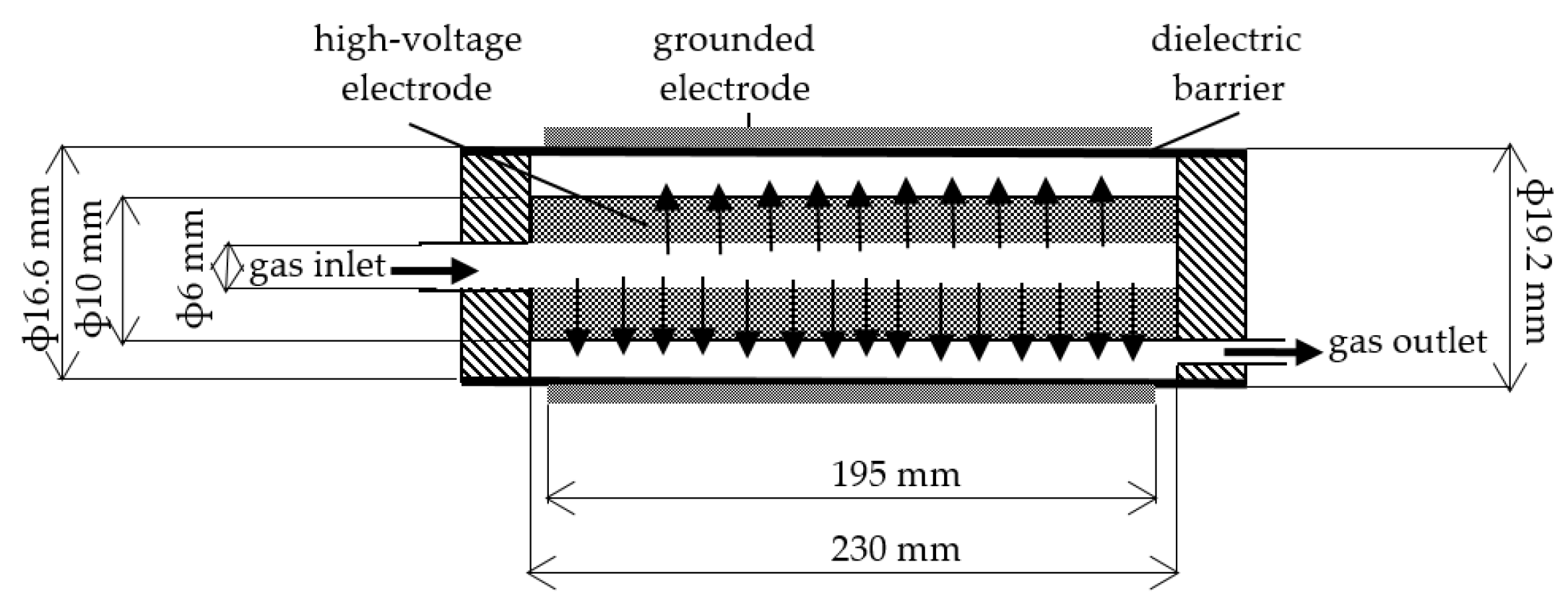
2.1. Reactor
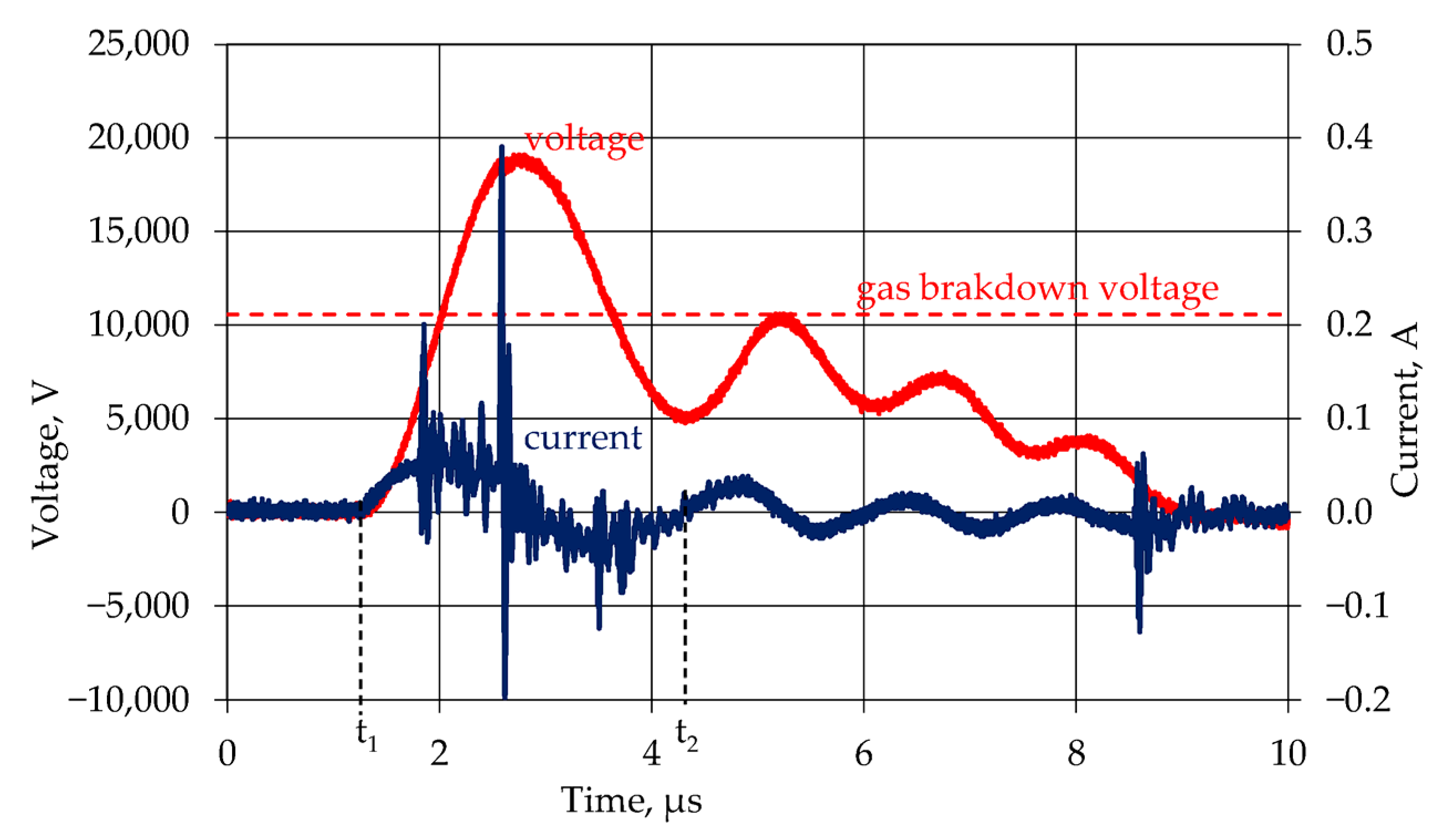
2.2. Electrical Measurements
2.3. Chemical Analysis
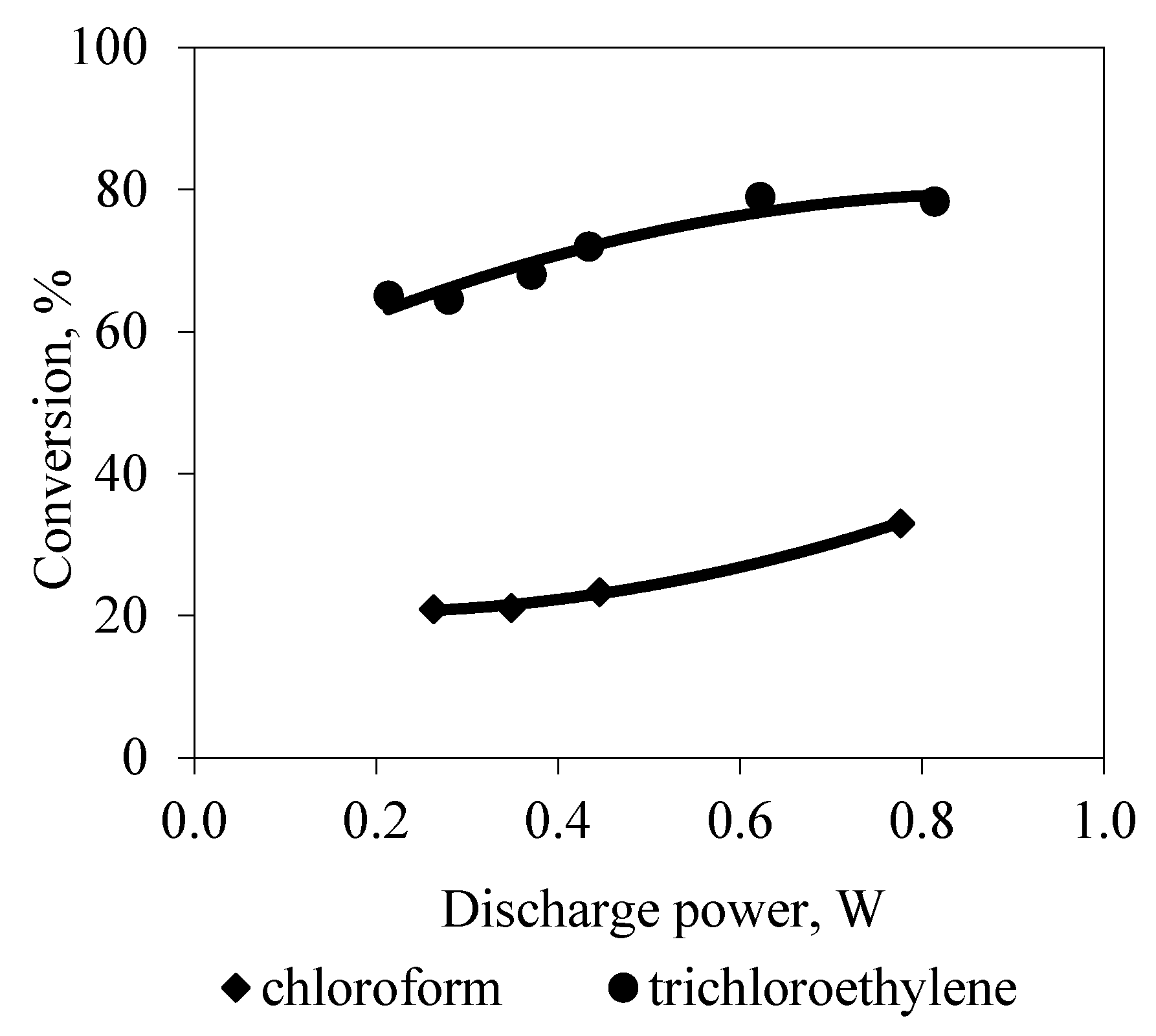
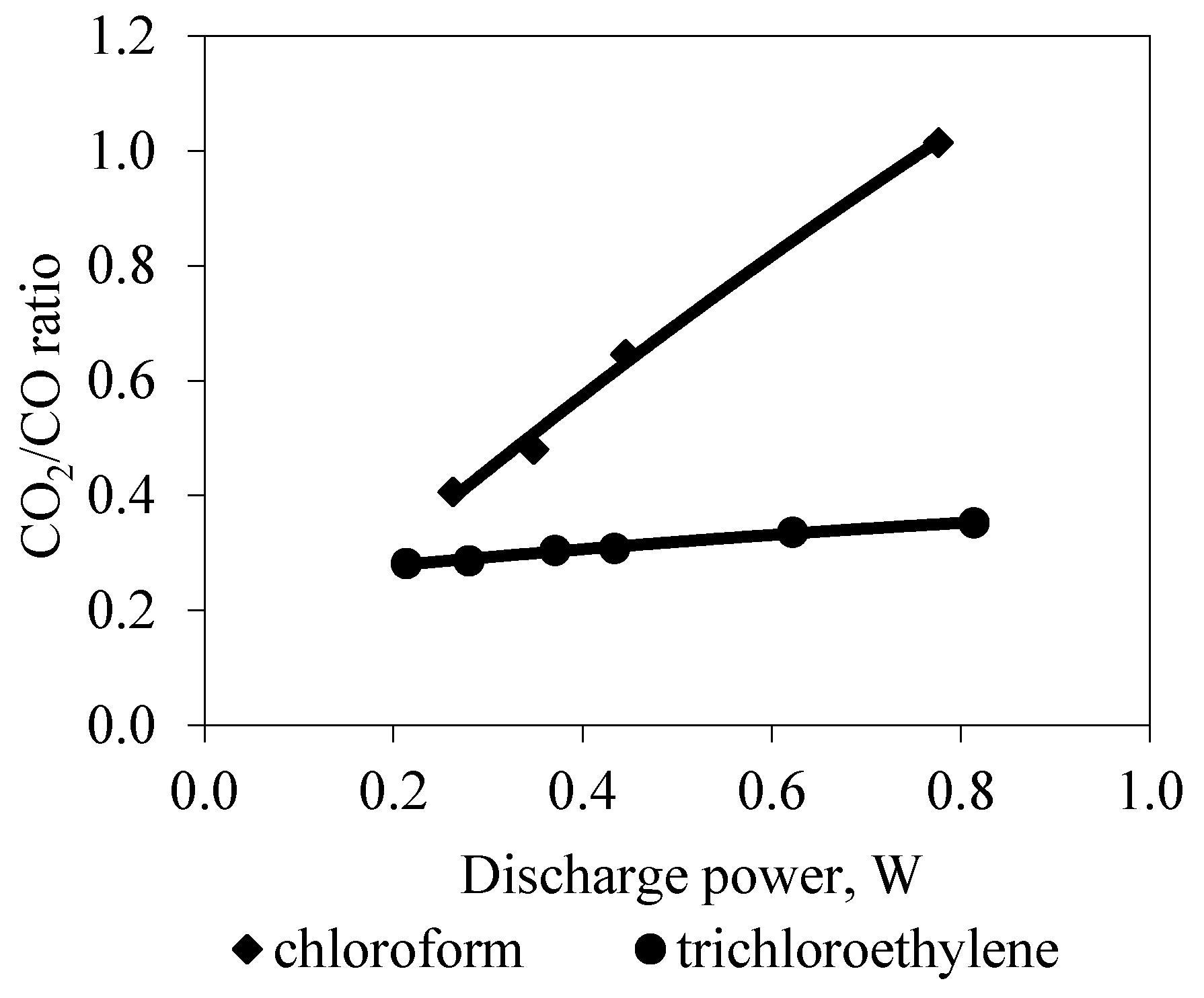
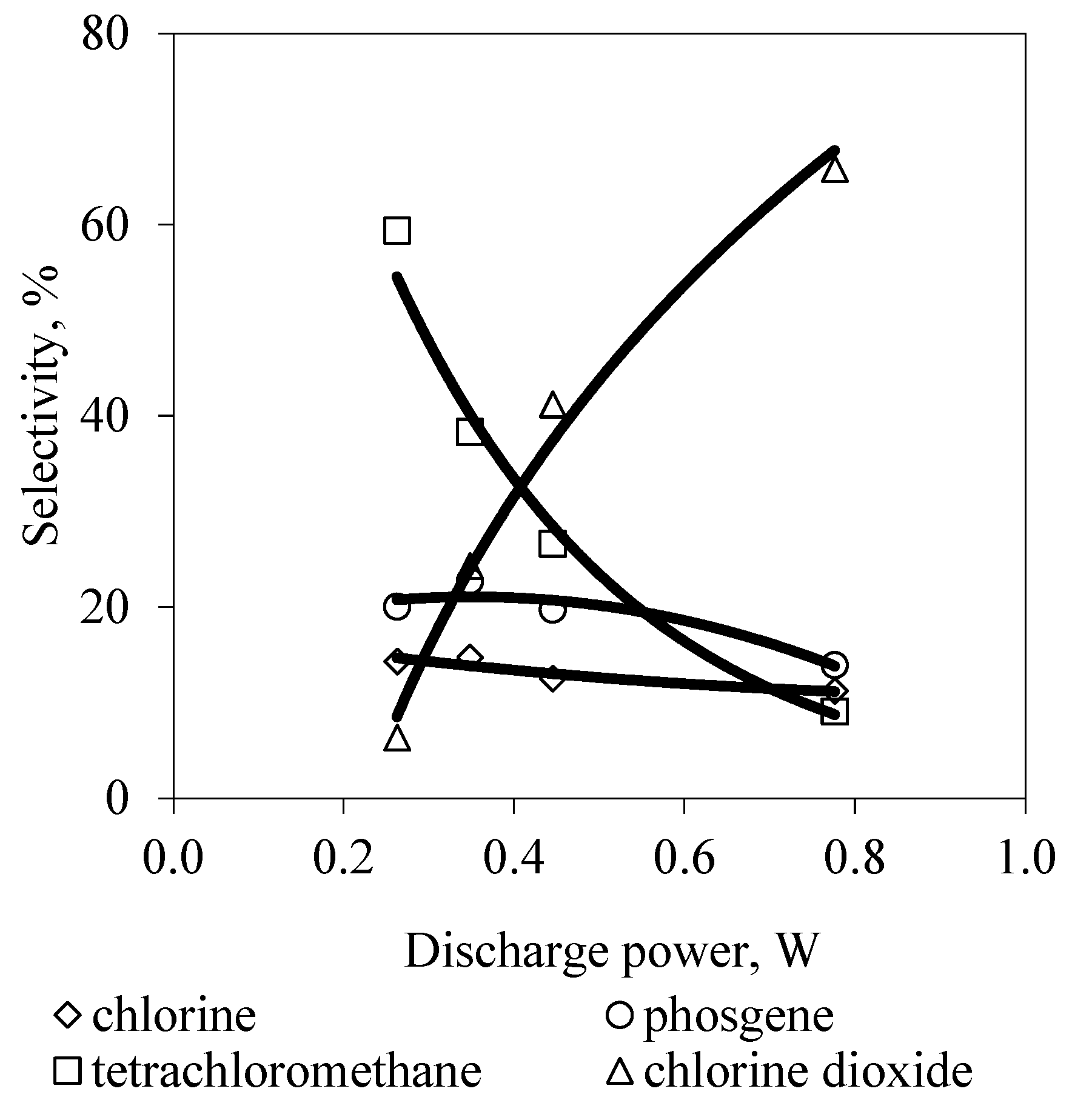
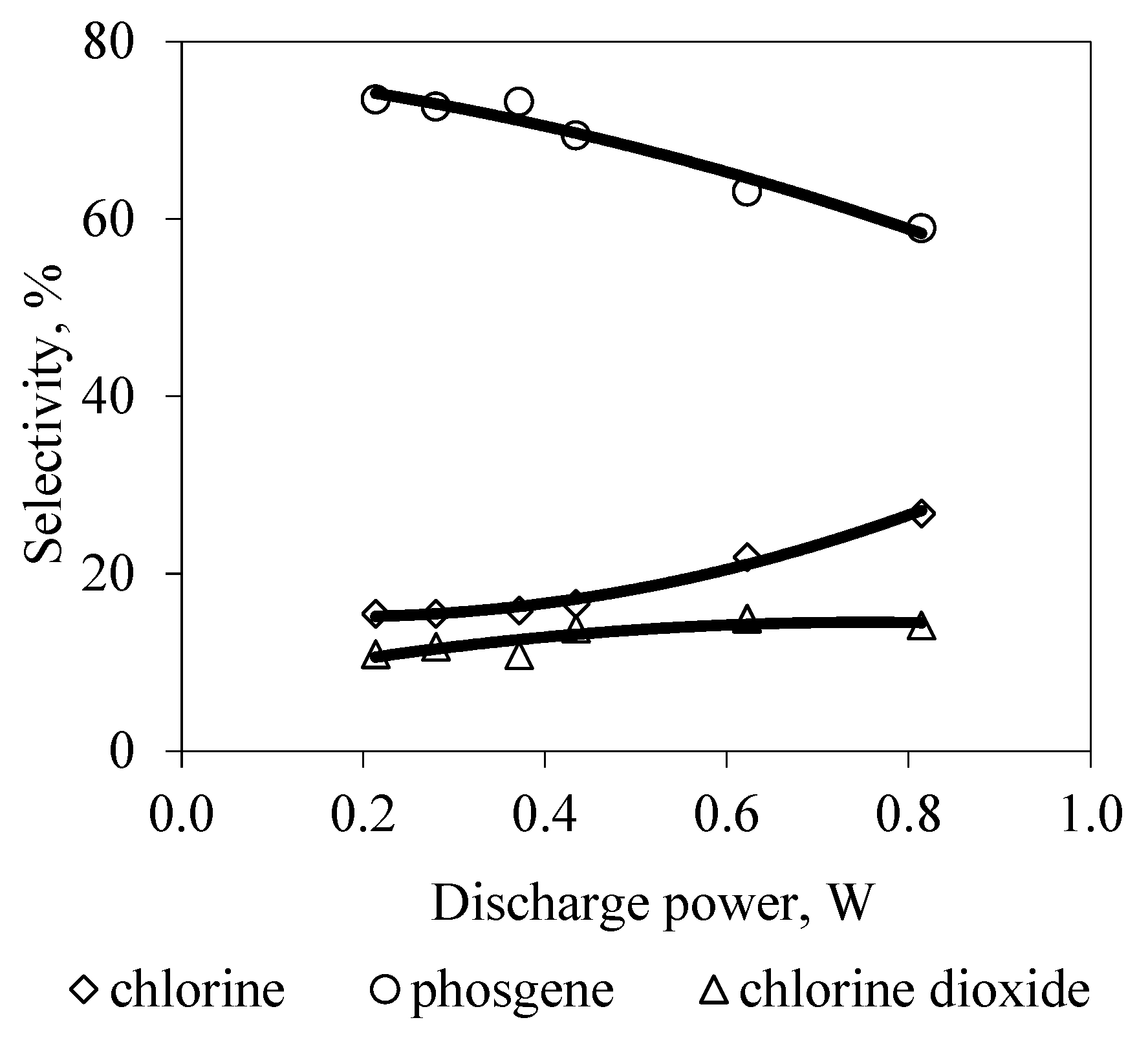
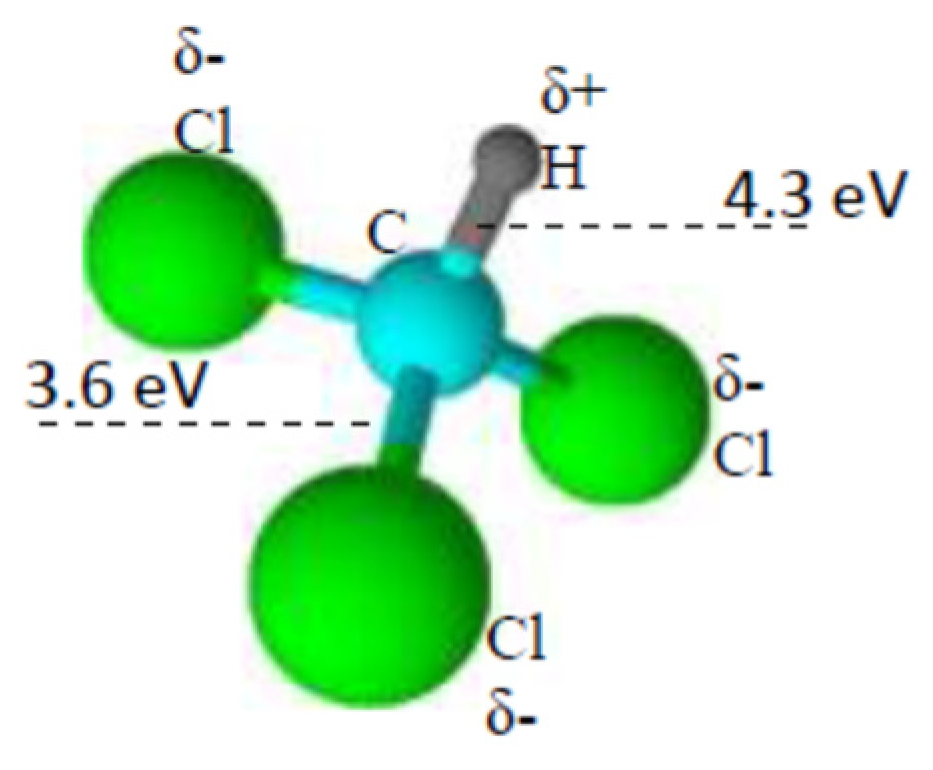
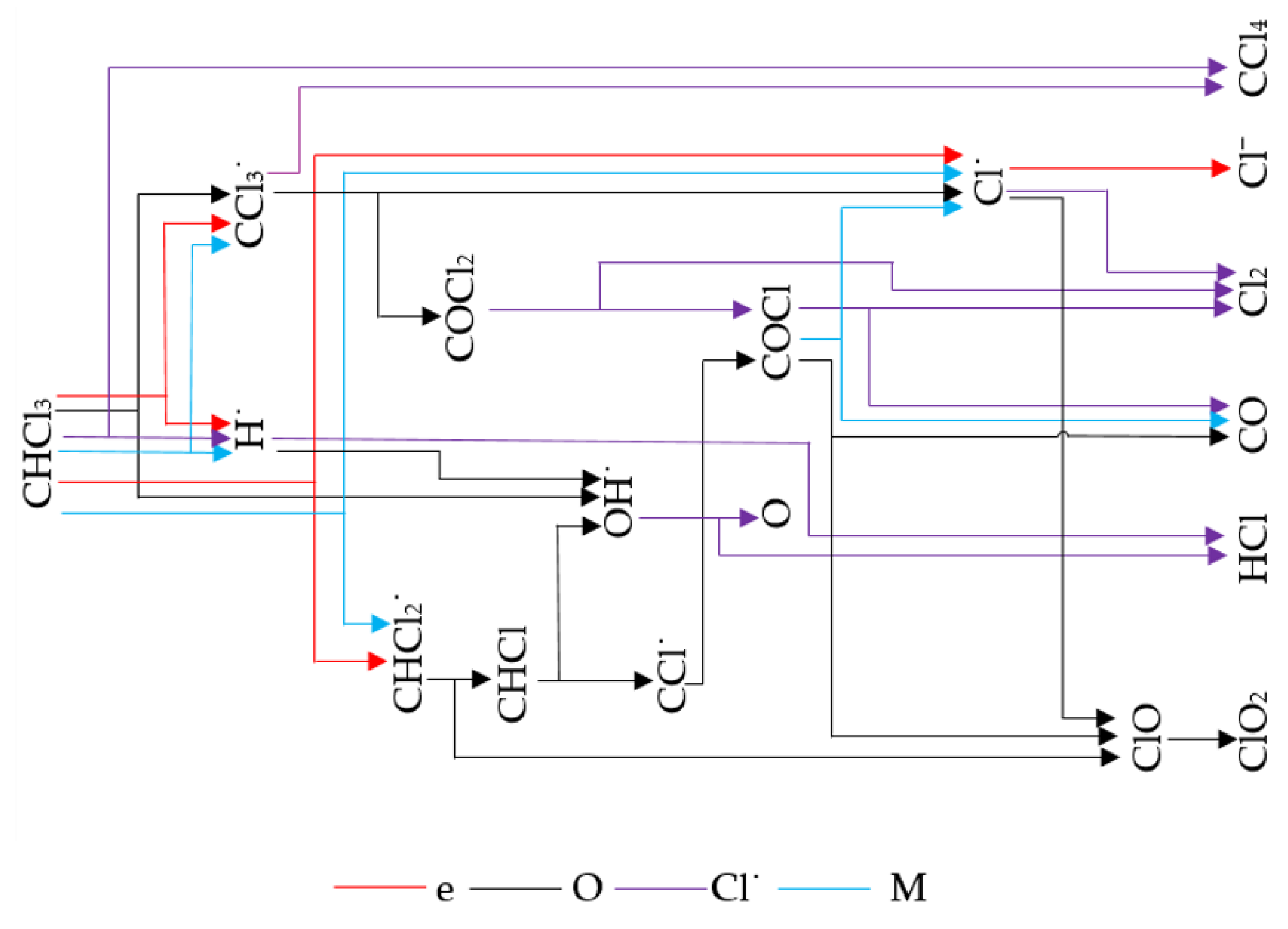
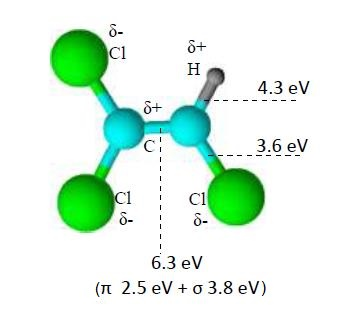
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krawczyk, K.; Jodzis, S.; Lamenta, A.; Kostka, K.; Ulejczyk, B.; Schmidt–Szałowski, K. Carbon tetrachloride decomposition by pulsed spark discharges in oxidative and nonoxidative conditions. IEEE Trans. Plasma Sci. 2011, 39, 3203–3210. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Krawczyk, K.; Młotek, M.; Schmidt–Szałowski, K.; Nogal, Ł.; Kuca, B. A comparison of carbon tetrachloride decomposition using spark and barrier discharges. Open Chem. 2015, 13, 509–516. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Chlorinated organic compounds decomposition in a dielectric barrier discharge. Plasma Chem. Plasma Process. 2007, 27, 679–690. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Krawczyk, K.; Młotek, M.; Schmidt–Szałowski, K.; Nogal, Ł.; Kuca, B. Decomposition of carbon tetrachloride in the reactor of dielectric barrier discharge with different power supplies. Eur. Phys. J. Appl. Phys. 2013, 61, 24324. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef]
- Gushchin, A.A.; Grinevich, V.I.; Kozlov, A.A.; Izvekova, T.V.; Kvitkova, E.Y.; Rybkin, V.V. Kinetics of 1,4-Dichlorobenzene decomposition in an atmospheric pressure dielectric barrier discharge in oxygen. High Energy Chem. 2020, 54, 64–68. [Google Scholar] [CrossRef]
- Gushchin, A.A.; Grinevich, V.I.; Izvekova, T.V.; Kvitkova, E.Y.; Tyukanova, K.A.; Rybkin, V.V. Decomposition of carbon tetrachloride under the action of a dielectric barrier discharge of atmospheric pressure in an oxygen atmosphere. Chemosphere 2021, 270, 129392. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Karuppiah, J.; Subrahmanyam, C. DBD plasma reactor for oxidative decomposition of chlorobenzene. Int. J. Chem. React. Eng. 2012, 10, A62. [Google Scholar] [CrossRef]
- Rubio, S.J.; Quintero, M.C.; Rodero, A. Application of microwave air plasma in the destruction of trichloroethylene and carbon tetrachloride at atmospheric pressure. J. Hazard. Mater. 2011, 186, 820–826. [Google Scholar] [CrossRef]
- Bo, Z.; Yan, J.H.; Li, X.D.; Chi, Y.; Cen, K.F.; Cheron, B.G. Effects of oxygen and water vapor on volatile organic compounds decomposition using gliding arc gas discharge. Plasma Chem. Plasma Process. 2007, 24, 155–167. [Google Scholar] [CrossRef]
- Krawczyk, K.; Ulejczyk, B. Influence of water vapor on CCl4 and CHCl3 conversion in gliding discharge. Plasma Chem. Plasma Process. 2004, 24, 155–167. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Aerts, R.; Van Gaens, W.; De Geyter, N.; Leys, C.; Morent, R.; Bogaerts, A. Modeling and experimental study of trichloroethylene abatement with a negative direct current corona discharge. Plasma Chem. Plasma Process. 2015, 35, 217–230. [Google Scholar] [CrossRef]
- Cucciniello, R.; Intiso, A.; Siciliano, T.; Palomares, A.E.; Martínez-Triguero, J.; Cerrillo, J.L.; Proto, A.; Rossi, F. Oxidative Degradation of Trichloroethylene over Fe2O3-doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance. Catalysts 2019, 9, 747. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Deng, J.; Zhang, K.; Yang, J.; Han, Z.; Dai, H. AuPd/3DOM TiO2 Catalysts: Good Activity and Stability for the Oxidation of Trichloroethylene. Catalysts 2018, 8, 666. [Google Scholar] [CrossRef]
- Schmidt, A.; Lerch, M.; Eufinger, J.-P.; Janek, J.; Tranca, I.; Islam, M.M.; Bredow, T.; Dolle, R.; Wiemhofer, H.-D.; Boysen, H.; et al. Chlorine ion mobility in Cl-mayenite (Ca12Al14O32Cl2): An investigation combining high-temperature neutron powder diffraction, impedance spectroscopy and quantum-chemical calculations. Solid State Ion. 2014, 254, 48–58. [Google Scholar] [CrossRef]
- Potylitsyna, A.R.; Rudneva, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A.; Shubin, Y.V.; Mishakov, I.V. Efficient Production of Segmented Carbon Nanofibers via Catalytic Decomposition of Trichloroethylene over Ni-W Catalyst. Materials 2023, 16, 845. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Potylitsyna, A.R.; Kadtsyna, A.S.; Chesnokov, V.V.; Nalivaiko, A.Y.; Gromov, A.A.; Buyanov, R.A. Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst. Catalysts 2020, 10, 1446. [Google Scholar] [CrossRef]
- He, Y.; Shen, J.; Alharbi, N.S.; Chen, C. Volatile organic compounds degradation by nonthermal plasma: A review. Environ. Sci. Pollut. Res. 2023, 30, 32123–32152. [Google Scholar] [CrossRef] [PubMed]
- Gomez–Ramirez, A.; Montoto–Damas, A.M.; Rodriguez, M.A.; Gonzalez–Elipe, A.R.; Cotrino, J. Improving the pollutant removal efficiency of packed-bed plasma reactors incorporating ferroelectric components. Chem. Eng. J. 2017, 314, 311–319. [Google Scholar] [CrossRef]
- Han, S.B.; Oda, T. Decomposition mechanism of trichloroethylene based on by-product distribution in the hybrid barrier discharge plasma process. Plasma Sources Sci. Technol. 2007, 16, 413–421. [Google Scholar] [CrossRef]
- Liu, L.; Shao, G.; Ma, C.; Nikiforov, A.; De Gryter, N.; Morent, R. Plasma-catalysis for VOCs docomposition: A review on micro- and macroscopic modeling. J. Hazard. Mater. 2023, 451, 131100. [Google Scholar] [CrossRef] [PubMed]
- Meloni, E.; Cafiero, L.; Renda, S.; Martino, M.; Pierro, M.; Palma, V. Ru- and Rh-Based catalysts for CO2 methanation assisted by non-thermal plasma. Catalysts 2023, 13, 488. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Zhu, S.; Yu, F.; Dai, B.; Yang, D. A Review of recent advances of dielectric barrier discharge plasma in catalysis. Nanomaterials 2019, 9, 1428. [Google Scholar] [CrossRef]
- Michielsen, I.; Uytdenhoiwen, Y.; Pype, J.; Michielsen, B.; Mertens, J.; Reniers, F.; Meynen, V.; Bogaerts, A. CO2 dissociation in a bed DBD reactors: First step towards a better understanding of plasma catalysis. Chem. Eng. J. 2017, 326, 477–488. [Google Scholar] [CrossRef]
- Van’t Veer, K.; Engelmann, Y.; Reniers, F.; Bogaerts, A. Plasma-Catalytic ammonia synthesis in a DBD plasma: Role of microdischarges and their afterglows. J. Phys. Chem. C 2020, 124, 22871–22883. [Google Scholar] [CrossRef]
- Jodzis, S. Effect of silica packing on ozone synthesis from oxygen-nitrogen mixtures. Ozone Sci. Eng. 2003, 25, 63–72. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Falkowski, P.; Krawczyk, K. Hydrogen production from ethanol using a special multi-segment plasma-catalytic reactor. J. Energy Inst. 2021, 95, 179–186. [Google Scholar] [CrossRef]
- Abdallah, G.; Giraudon, J.-M.; Bitar, R.; Geyter, N.D.; Morent, R.; Lamonier, J.-F. Post-Plasma Catalysis for Trichloroethylene Abatement with Ce-Doped Birnessite Downstream DC Corona Discharge Reactor. Catalysts 2021, 11, 946. [Google Scholar] [CrossRef]
- Shahna, F.G.; Ebrahimi, H.; Jahel, B.; Bahrami, A. Decomposition of gas-phase chloroform using nonoptotocatalyst downstream the novel non-thermal plasma reactor: By products elimination. Int. J. Environ. Sci. Technol. 2015, 12, 3489–3498. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Magureanu, M.; Renken, A.; Kiwi-Minsker, L. Catalytic abatement of volatile organic compounds assisted by non-thermal plasma: Part 1. A novel dielectric barrier discharge reactor containing catalytic electrode. Appl. Catal. B Environ. 2006, 65, 150–156. [Google Scholar] [CrossRef]
- Cheng, L.; Ghobeira, R.; Cools, P.; Luthringer, B.; Asadian, M.; De Geyter, N.; Liu, Z.; Yan, K.; Morent, R. Comparing medium pressure dielectric barrier discharge (DBD) plasmas and classic methods of surface cleaning/activation of pure Mg for biomedical applications. Surf. Coat. Technol. 2021, 410, 126934. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Hossain, M.M.; Kim, S.H.; Mok, Y.S. Tailoring the wettability of glass using a double-dielectric barrier discharge reactor. Heliyon 2018, 4, e00522. [Google Scholar] [CrossRef] [PubMed]
- Siemak, J.; Ulejczyk, B.; Mikołajczak, G.; Pęksiński, J.; Sreńscek-Nazzal, J.; Młotek, M.; Krawczyk, K.; Michalkiewicz, B. Cold Nitrogen Plasma: A Groundbreaking Eco-Friendly Technique for the Surface Modification of Activated Carbon Aimed at Elevating Its Carbon Dioxide Adsorption Capacity. Appl. Sci. 2024, 14, 6438. [Google Scholar] [CrossRef]
- Opalińska, T.; Ulejczyk, B.; Schmidt–Szałowski, K. Applications of pulsed discharge to thin-film deposition. IEEE Trans. Plasma Sci. 2009, 37, 934–940. [Google Scholar] [CrossRef]
- Wnukowski, M. Methane Pyrolysis with the use of plasma: Review of plasma reactors and process products. Energies 2023, 16, 6441. [Google Scholar] [CrossRef]
- Panda, N.R.; Sahu, D. Enhanced hydrogen generation efficiency of methanol using dielectric barrier discharge plasma methodology and conducting sea water as an electrode. Heliyon 2020, 6, e04717. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Hydrogen production from ethanol using dielectric barrier discharge. Energy 2019, 174, 261–268. [Google Scholar] [CrossRef]
- Kogelschatz, U.; Eliansson, B.; Egli, W. Dielectric-Barrier Discharges. Principle and Applications. J. De Phys. IV Fr. 1997, 7, C4-46–C4-66. [Google Scholar] [CrossRef]
- Jodzis, S. Temperature effects under ozone synthesis process conditions. Eur. Phys. J. Appl. Phys. 2013, 61, 24319. [Google Scholar] [CrossRef]
- Jodzis, S.; Baran, K. The influence of gas temperature on ozone generation and decomposition in ozone generator. How is ozone decomposed? Vacuum 2022, 195, 110647. [Google Scholar] [CrossRef]
- Minczewski, J.; Marczenko, Z. Chemia Analityczna 2 Chemiczne Metody Analizy Ilościowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2010; pp. 190’231, 285’297. [Google Scholar]
- Gushchin, A.A.; Grinevich, V.I.; Izvekova, T.V.; Kvitova, E.Y.; Tyukanova, K.A.; Rybkin, V.V. The Destruction of Carbon Tetrachloride Dissolved in Water in a Dielectric Barrier Discharge in Oxygen. Plasma Chem. Plasma Process. 2019, 39, 461–473. [Google Scholar] [CrossRef]
- Häggström, K.; Gunnarsson, M.; Bremert-Jirholm, K.; Simic, N. Method for analysis of ClO2 and Cl2 air emissions from pulp mill. Nord. Pulp Pap. Res. J. 2019, 34, 19–27. [Google Scholar] [CrossRef]
- Jodzis, S.; Petryk, J. Computer modeling of gas temperature in the ozonizer gap. Effects generated by a single microdischarge. IEEE Trans. Plasma Sci. 2011, 39, 2126–2127. [Google Scholar] [CrossRef]
- Elliason, B.; Hirth, M.; Kogelschatz, U. Ozone synthesis from oxygen in dielectric barrier discharges. J. Phys. D Appl. Phys. 1987, 20, 1421–1437. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Bibinov, N.; Awakowicz, P. Characterization of dielectric barrier discharge in air applying current measurement, numerical silulation and emission spectroscopy. arXiv 2012, arXiv:1202.3879. [Google Scholar]
- Razumovskii, S.D.; Zaikov, G.E. Kinetics and mechanism of the reaction of ozone with double bond. Russ. Chem. Rev. 1980, 49, 1163–1180. [Google Scholar] [CrossRef]
- Wu, Z.; Cravotto, G.; Ondruschka, B.; Stolle, A.; Li, W. Decomposition of chloroform and succinic acid by ozonation in a suction-cavitation system: Effect of gas flow. Sep. Purif. Technol. 2016, 161, 25–31. [Google Scholar] [CrossRef]
- Su, M.C.; Lim, K.P.; Michael, J.V.; Hranisavljevic, J.; Xun, Y.M.; Fontijn, A. Kinetics studies of the O(3P) + CH2Cl2 and CHCl3 reactions over the 468–1355 and 499–1090 K ranges using two techniques. J. Phys. Chem. 1994, 98, 8411–8418. [Google Scholar] [CrossRef]
- Ulejczyk, B. Decomposition of halocarbons in the pulsed dielectric barrier discharge. In Proceedings of the 2014 International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Bran, Romania, 22–24 May 2014; pp. 1053–1059. [Google Scholar] [CrossRef]
- Upadhyaya, H.P.; Kumar, A.; Naik, P.D.; Sapre, A.V. Discharge flow reaction kinetic studies of O(3P) with chloroethylenes CH2CCl2, CHClCCl2, CCl2CCl2. Chem. Phys. Lett. 2000, 321, 411–418. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/chemistry/ (accessed on 20 July 2024).
- Jiao, J.; Xiao, D.; Zhang, X.; Deng, Y. Analysis of the molecular structure and vertical electron affinity of organic gas impact on electric strength. Plasma Sci. Technol. 2016, 18, 554–559. [Google Scholar] [CrossRef]
- Choi, H.; Park, Y.C.; Im, Y.H.; Kwon, D.C.; Chung, S.Y. A DFT study on direct CF2 fragmentation mechanisms of 1,3-C4F6 and 1,3- C4F6+ in plasma. Plasma Chem. Plasma Process. 2023, 43, 47–66. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, A.; Zhang, Y.; Heroux, P.; Zhu, L.; Li, X.; Li, P.; Liu, Y. Remediation of polychlorinated biphenyls (PCBs) polluted soil using dielectric barrier discharge (DBD) plasma: Reactive nitrogen species (RNS) and produced dioxins. Chem. Eng. J. 2023, 459, 141632. [Google Scholar] [CrossRef]
- Boutadghart, T.; Ghailane, R. DFT mechanistic study of [4+2] cycloaddition reactions of 1-mathyl-1-H-pyrrole-2,5-dione with furoic acid, anticancer activity, molecular modeling and ADMET properties of new products from the norcantharimide family substituted by a carboxylic acid. Comput. Theor. Chem. 2023, 1230, 114384. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulejczyk, B. The Influence of the Structure of Organochlorine Compounds on Their Decomposition Process in a Dielectric Barrier Discharge. Reactions 2024, 5, 623-634. https://doi.org/10.3390/reactions5030031
Ulejczyk B. The Influence of the Structure of Organochlorine Compounds on Their Decomposition Process in a Dielectric Barrier Discharge. Reactions. 2024; 5(3):623-634. https://doi.org/10.3390/reactions5030031
Chicago/Turabian StyleUlejczyk, Bogdan. 2024. "The Influence of the Structure of Organochlorine Compounds on Their Decomposition Process in a Dielectric Barrier Discharge" Reactions 5, no. 3: 623-634. https://doi.org/10.3390/reactions5030031
APA StyleUlejczyk, B. (2024). The Influence of the Structure of Organochlorine Compounds on Their Decomposition Process in a Dielectric Barrier Discharge. Reactions, 5(3), 623-634. https://doi.org/10.3390/reactions5030031




