Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review
Abstract
1. Introduction
2. Overview of TiO2′s Characteristics
2.1. Structural Properties
2.2. Energy Band-Gap (Eg)
2.3. Robustness and Phase Shift
2.4. Other Features
3. Methods Utilized in the Synthesis of Nanoparticles
3.1. Physical Methods
3.2. Chemical Methods
3.3. Green/Biosynthesis Methods
3.4. Synthesis of Nano-TiO2 Materials
3.4.1. Sol–Gel Synthetic Approach
Precursor:H2O Molar Ratio
Solution’s pH
Annealing Procedure’s Time and Temperature
Other Parameters
3.4.2. Hydrothermal Synthetic Approach
3.4.3. Chemical Vapor Deposition (CVD) Synthetic Approach
3.4.4. Physical Vapor Deposition (PVD) Synthetic Approach
3.4.5. Green Synthetic Approach
Synthesis of TiO2 Nanoparticles Using Microorganisms
Synthesis of TiO2 Nanoparticles Using Plants
Other Approaches
4. Photocatalytic Activity Mechanism of TiO2
5. Environmental Applications of TiO2-Based Photocatalytic Nanostructures
5.1. TiO2-Based Photocatalysts for Effective Elimination of Pharmaceutical Pollutants from Water and Wastewater
5.2. TiO2-Based Photocatalysts for Effective Elimination of Heavy Metals from Water and Wastewater
5.3. TiO2-Based Photocatalysts for Effective Elimination of Organic Dyes from Water and Wastewater
5.4. TiO2-Based Photocatalysts for Effective Elimination of Pesticides from Water and Wastewater
5.5. TiO2-Based Photocatalysts for Effective Elimination of Microbes from Water and Wastewater
5.6. TiO2-Based Photocatalysts for Effective Elimination of Hormones and Endocrine Disrupting Compounds (EDCs) from Water and Wastewater
6. TiO2 Nanoparticles’ Fate in the Atmosphere, Aqueous Environments, and Sediments
7. Potential Toxicity of TiO2 Nanoparticles
7.1. Biodistribution and Systemic Toxicity
7.2. TiO2 Nanoparticle-Induced Oxidative Stress
7.3. TiO2 Nanoparticles’ Cellular Uptake
7.4. Potential Approaches for Addressing the Potential Toxicity of TiO2 Nanoparticles
8. Conclusions and Future Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNESCO. The United Nations World Water Development Report 2020 Water and Climate Change; UNESCO: Paris, France, 2020. [Google Scholar]
- Chart, H. VTEC enteropathogenicity. Symp. Ser. Soc. Appl. Microbiol. 2000, 29, 12S–23S. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.C.; Galán, J.E. Decoding a Salmonella typhi regulatory network that controls typhoid toxin expression within human cells. Cell Host Microbe 2018, 23, 65–76. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Mekalanos, J.J. Cholera toxin promotes pathogen acquisition of host-derived nutrients. Nature 2019, 572, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Wang, Y.-H.; Chang, H.-J.; Chen, H.-L.; Huang, Y.-C.; Lin, T.-Y.; Ozer, E.A.; Allen, J.P.; Hauser, A.R.; Chiu, C.-H. Shanghai fever: A distinct Pseudomonas aeruginosa enteric disease. Gut 2014, 63, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Rajasurya, V.; Surani, S. Legionnaires Disease in Immunocompromised Host; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Vaez, Z.; Javanbakht, V. Synthesis, characterization and photocatalytic activity of ZSM-5/ZnO nanocomposite modified by Ag nanoparticles for methyl orange degradation. J. Photochem. Photobiol. A 2020, 388, 112064. [Google Scholar] [CrossRef]
- Khataee, A.R.; Pons, M.N.; Zahraa, O. Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: Influence of dye molecular structure. J. Hazard. Mater. 2009, 168, 451–457. [Google Scholar] [CrossRef]
- Liberatore, L.; Bressan, M.; Belli, C.; Lustrato, G.; Ranalli, G. Chemical and biological combined treatments for the removal of pesticides from wastewaters. Water Air Soil Pollut. 2012, 223, 4751–4759. [Google Scholar] [CrossRef]
- Ballesteros Martín, M.M.; Sánchez Pérez, J.A.; Casas López, J.L.; Oller, I.; Malato Rodríguez, S. Degradation of a four-pesticide mixture by combined photo-Fenton and biological oxidation. Water Res. 2009, 43, 653–660. [Google Scholar] [CrossRef]
- Bressan, M.; Liberatore, L.; d’Alessandro, N.; Tonucci, L.; Belli, C.; Ranalli, G. Improved combined chemical and biological treatments of olive oil mill wastewaters. J. Agric. Food Chem. 2004, 52, 1228–1233. [Google Scholar] [CrossRef]
- Tahir, M.S.; Saleem, M.; Malik, S.R.; Khan, J.R.; Siebenhofer, M. An innovative and advanced oxidation process for effluent treatment through wet tube-type electrostatic precipitation. Chem. Eng. Process. Process Intensif. 2012, 52, 16–20. [Google Scholar] [CrossRef]
- Ben Mansour, L.; Kesentini, I. Treatment of effluents from cardboard industry by coagulation-electroflotation. J. Hazard. Mater. 2008, 153, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Hanay, Ö.; Hasar, H. Effect of anions on removing Cu2+, Mn2+ and Zn2+ in electrocoagulation process using aluminum electrodes. J. Hazard. Mater. 2011, 189, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Centi, G.; Perathoner, S.; Torre, T.; Verduna, M.G. Catalytic wet oxidation with H2O2 of carboxylic acids on homogeneous and heterogeneous Fenton-type catalysts. Catal. Today 2000, 55, 61–69. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Lin, S.H.; Chang, C.C. Treatment of landfill leachate by combined electro-Fenton oxidation and sequencing batch reactor method. Water Res. 2000, 34, 4243–4249. [Google Scholar] [CrossRef]
- Ayodele, O.B.; Hameed, B.H. Synthesis of copper pillared bentonite ferrioxalate catalyst for degradation of 4-nitrophenol in visible light assisted Fenton process. J. Ind. Eng. Chem. 2013, 19, 966–974. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; San Martín, I.; Aguirre, M. Effect of light source on the catalytic degradation of protocatechuic acid in a ferrioxalate-assisted photo-Fenton process. Appl. Catal. B 2010, 96, 486–495. [Google Scholar] [CrossRef]
- John Peter, I.; Praveen, E.; Vignesh, G.; Nithiananthi, P. ZnO nanostructures with different morphology for enhanced photocatalytic activity. Mater. Res. Express 2017, 4, 124003. [Google Scholar] [CrossRef]
- Zhang, D.E.; Ren, L.Z.; Hao, X.Y.; Pan, B.B.; Wang, M.Y.; Ma, J.J.; Li, F.; Li, S.A.; Tong, Z.W. Synthesis and photocatalytic property of multilayered Co3O4. Appl. Surf. Sci. 2015, 355, 547–552. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Tanji, K.; Navio, J.A.; Chaqroune, A.; Naja, J.; Puga, F.; Hidalgo, M.C.; Kherbeche, A. Fast photodegradation of rhodamine B and caffeine using ZnO-hydroxyapatite composites under UV-light illumination. Catal. Today 2022, 388–389, 176–186. [Google Scholar] [CrossRef]
- Lagopati, N.; Tsilibary, E.P.; Falaras, P.; Papazafiri, P.; Pavlatou, E.A.; Kotsopoulou, E.; Kitsiou, P. Effect of nanostructured TiO2 crystal phase on photoinduced apoptosis of breast cancer epithelial cells. Int. J. Nanomed. 2014, 9, 3219–3230. [Google Scholar]
- Lagopati, N.; Kitsiou, P.; Kontos, A.; Venieratos, P.; Kotsopoulou, E.; Kontos, A.; Dionysiou, D.; Pispas, S.; Tsilibary, E.; Falaras, P. Photo-induced treatment of breast epithelial cancer cells using nanostructured titanium dioxide solution. J. Photochem. Photobiol. A Chem. 2010, 214, 215–223. [Google Scholar] [CrossRef]
- Anitha, B.; Khadar, M.A. Anatase-rutile phase transformation and photocatalysis in peroxide gel route prepared TiO2 nanocrystals: Role of defect states. Solid State Sci. 2020, 108, 106392. [Google Scholar] [CrossRef]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Biological effect of silver-modified nanostructured titanium dioxide in cancer. Cancer Genom. Proteom. 2021, 18 (Suppl. S3), 425–439. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process. Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef]
- Akimoto, J.; Gotoh, Y.; Oosawa, Y.; Nonose, N.; Kumagai, T.; Aoki, K.; Takei, H. Topotactic oxidation of ramsdellite-type Li0.5TiO2, a new polymorph of titanium dioxide: TiO2(R). J. Solid State Chem. 1994, 113, 27–36. [Google Scholar] [CrossRef]
- Latroche, M.; Brohan, L.; Marchand, R.; Tournoux, M. New hollandite oxides: TiO2(H) and K0.06TiO2. J. Solid State Chem. 1989, 81, 78–82. [Google Scholar] [CrossRef]
- Marchand, R.; Brohan, L.; Tournoux, M. TiO2(B) a new form of titanium dioxide and the potassium octatitanate K2Ti8O17. Mater. Res. Bull. 1980, 15, 1129–1133. [Google Scholar] [CrossRef]
- Simons, P.Y.; Dachille, F. The structure of TiO2II, a high-pressure phase of TiO2. Acta Cryst. 1967, 23, 334–336. [Google Scholar] [CrossRef]
- Tang, J.; Endo, S. P–T Boundary of α-PbO2 type and baddeleyite type high-pressure phases of titanium dioxide. J. Am. Ceram. Soc. 1993, 76, 796–798. [Google Scholar] [CrossRef]
- Staun Olsen, J.; Gerward, L.; Jiang, J.Z. On the rutile/α-PbO2-type phase boundary of TiO2. J. Phys. Chem. Solids 1999, 60, 229–233. [Google Scholar] [CrossRef]
- Hanaor, D.A.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Gamboa, J.A.; Pasquevich, D.M. Effect of chlorine atmosphere on the anatase-rutile transformation. J. Am. Ceram. Soc. 1992, 75, 2934–2938. [Google Scholar] [CrossRef]
- Ding, X.Z.; He, Y.Z. Study of the room temperature ageing effect on structural evolution of gel-derived nanocrystalline titania powders. J. Mater. Sci. Lett. 1996, 15, 320–322. [Google Scholar] [CrossRef]
- Muscat, J.; Swamy, V.; Harrison, N.M. First-principles calculations of the phase stability of TiO2. Phys. Rev. B 2002, 65, 224112. [Google Scholar] [CrossRef]
- Arlt, T.; Bermejo, M.; Blanco, M.A.; Gerward, L.; Jiang, J.Z.; Staun Olsen, J.; Recio, J.M. High-pressure polymorphs of anatase TiO2. Phys. Rev. B 2000, 61, 14414–14419. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Zhang, Q. Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl. Catal. B 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Haidry, A.A.; Yucheng, W.; Fatima, Q.; Raza, A.; Zhong, L.; Chen, H.; Rutendo Mandebvu, C.; Ghani, F. Synthesis and characterization of TiO2 nanomaterials for sensing environmental volatile compounds (VOCs): A review. TrAC Trends Anal. Chem. 2024, 170, 117454. [Google Scholar] [CrossRef]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Alves Junior, R.; Alves, H.P.A.; Cartaxo, J.M.; Rodrigues, A.M.; Neves, G.A.; Menezes, R.R. Use of nanostructured and modified TiO2 as a gas sensing agent. Ceramica 2021, 67, 316–326. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Hakkoum, H.; Comini, E. Recent advancements in TiO2 nanostructures: Sustainable synthesis and gas sensing. Nanomaterials 2023, 13, 1424. [Google Scholar] [CrossRef]
- Covaliu-Mierlă, C.I.; Matei, E.; Stoian, O.; Covaliu, L.; Constandache, A.-C.; Iovu, H.; Paraschiv, G. TiO2-Based nanofibrous membranes for environmental protection. Membranes 2022, 12, 236. [Google Scholar] [CrossRef]
- Wendt, S.; Sprunger, P.T.; Lira, E.; Madsen, G.K.; Li, Z.; Hansen, J.Ø.; Matthiesen, J.; Blekinge-Rasmussen, A.; Laegsgaard, E.; Hammer, B.; et al. The role of interstitial sites in the Ti3d defect state in the band gap of titania. Science 2008, 320, 1755–1759. [Google Scholar] [CrossRef]
- Tisdale, W.A.; Williams, K.J.; Timp, B.A.; Norris, D.J.; Aydil, E.S.; Zhu, X.Y. Hot-electron transfer from semiconductor nanocrystals. Science 2010, 328, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Sharma, M.U.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sensor. Actuators B Chem. 2013, 178, 636–647. [Google Scholar] [CrossRef]
- Skubal, L.R.; Meshkov, N.K.; Vogt, M.C. Detection and identification of gaseous organics using a TiO2 sensor. J. Photochem. Photobiol. Chem. 2002, 148, 103–108. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A Mater. 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Memon, S.F.; Wang, R.; Strunz, B.; Chowdhry, B.S.; Pembroke, J.T.; Lewis, E. A Review of optical fibre ethanol sensors: Current state and future prospects. Sensors 2022, 22, 950. [Google Scholar] [CrossRef]
- Subha, P.P.; Vikas, L.S.; Jayaraj, M.K. Solution-processed CuO/TiO2 heterojunction for enhanced room temperature ethanol sensing applications. Phys. Scripta 2018, 93, 055001. [Google Scholar] [CrossRef]
- Shannon, R.D.; Pask, J.A. Kinetics of the anatase-rutile transformation. J. Am. Ceram. Soc. 1965, 48, 391–398. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Oskam, G.; Nellore, A.; Penn, R.L.; Searson, P.C. The growth kinetics of TiO2 nanoparticles from titanium (IV) alkoxide at high water/titanium ratio. J. Phys. Chem. B 2003, 107, 1734–1738. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Bin, X.L.; Li, J.L.; Yang, B.; Yu, Y. Ti3+ in the surface of titanium dioxide: Generation, properties and photocatalytic application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef]
- Janotti, A.; Varley, J.B.; Rinke, P.; Umezawa, N.; Kresse, G.; Van De Walle, C.G. Hybrid functional studies of the oxygen vacancy in TiO2. Phys. Rev. B Condens. Matter. 2010, 81, 085212. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Infrared absorption of reduced rutile TiO2 single crystals. Phys. Rev. 1959, 113, 1222. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Nampoothiri, J.; Pullithadathil, B. Wearable, flexible ethanol gas sensor based on TiO2 nanoparticles-grafted 2D-titanium carbide nanosheets. ACS Appl. Nano Mater. 2019, 2, 1152–1163. [Google Scholar] [CrossRef]
- Nunes, D.; Fortunato, E.; Martins, R. Flexible nanostructured TiO2-based gas and UV sensors: A review. Discov. Mater. 2022, 2, 2. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Shafei, A.; Salarpour, M.E.; Sheibani, S. Effect of intermediate ball milling on the synthesis of Cu-doped TiO2 nano-photocatalyst by sol-gel method. J. Sol-Gel. Sci. Technol. 2019, 92, 173–185. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, S.; Zong, H.; Huang, T.; Li, F.; Pan, J.; Cui, J. Continuous synthesis of Ag/TiO2 nanoparticles with enhanced photocatalytic activity by pulsed laser ablation. J. Nanomater. 2017, 2017, 4604159. [Google Scholar] [CrossRef]
- Gupta, V.; Sarkar, S.; Aftenieva, O.; Tsuda, T.; Kumar, L.; Schletz, D.; Schultz, J.; Kiriy, A.; Fery, A.; Vogel, N.; et al. Nanoimprint lithography facilitated plasmonic-photonic coupling for enhanced photoconductivity and photocatalysis. Adv. Funct. Mater. 2021, 31, 2105054. [Google Scholar] [CrossRef]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef]
- Zhang, B.; He, X.; Ma, X.; Chen, Q.; Liu, G.; Zhou, Y.; Ma, D.; Cui, C.; Ma, J.; Xin, Y. In situ synthesis of ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst with enhanced photocatalytic activity. Sep. Purif. Technol. 2020, 247, 116932. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 46, 115–126. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lejeune, L.; Haynes, T.; Lambert, S.D.; Marcilli, R.H.M.; Fustin, C.-A.; Hermans, S. Eco-friendly colloidal aqueous sol-gel process for TiO2 synthesis: The peptization method to obtain crystalline and photoactive materials at low temperature. Catalysts 2021, 11, 768. [Google Scholar] [CrossRef]
- Song, M.; Yang, Y.; Xiang, M.; Zhu, Q.; Zhao, H. Synthesis of nano-sized TiC powders by designing chemical vapor deposition system in a fluidized bed reactor. Powder Technol. 2021, 380, 256–264. [Google Scholar] [CrossRef]
- Esparza-Contro, C.; Berthomé, G.; Renou, G.; Robaut, F.; Coindeau, S.; Vachey, C.; Cambin, J.; Mantel, M.; Latu-Romain, L. Microstructures of titanium oxide thin films grown continuously on stainless steel wires by PVD in an inverted cylindrical magnetron: Towards an industrial process. Surf. Coat. Technol. 2020, 389, 125643. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Patil, N.R.; Nataraj, S.K.; Sannaikar, M.S.; Inamdar, S.R. Synthesis and photoluminescence properties of titanium oxide (TiO2) nanoparticles: Effect of calcination temperature. Optik 2019, 194, 163070. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 synthesis by chemical reduction methods for photocatalysis applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, X.; Yang, C.; Lei, Q.; Zhao, J.; Zhao, X. Ultrafast synthesis of anatase TiO2 microspheres doped with rare-earth by one-step microwave method. Inorg. Chem. Commun. 2021, 127, 108532. [Google Scholar] [CrossRef]
- Leong, C.Y.; Lo, Y.S.; Koh, P.W.; Lee, S.L. Synthesis of titanium dioxide nanotubes with different N-containing ligands via hydrothermal method. Sci. Technol. Indones. 2021, 6, 67–73. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2015, 38, 545–560. [Google Scholar] [CrossRef]
- Robert, D.; Weber, J.V. Titanium dioxide synthesis by sol gel methods and evaluation of their photocatalytic activity. J. Mater. Sci. Lett. 1999, 18, 97–98. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 15, 82–89. [Google Scholar] [CrossRef]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Edmundson, M.C.; Capeness, M.; Horsfall, L. Exploring the potential of metallic nanoparticles within synthetic biology. New Biotechnol. 2014, 31, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol-gel method. Angew Chem. Int. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Barringer, E.A.; Bowen, H.K. Formation, packing, and sintering of monodisperse TiO2 powders. J. Am. Ceram. Soc. 1982, 65, C199–C201. [Google Scholar] [CrossRef]
- Yu, H.F.; Wang, S.M. Effects of water content and pH on gel-derived TiO2-SiO2. J. Non-Cryst. Solids 2000, 261, 260–267. [Google Scholar] [CrossRef]
- Matijević, E.; Budnik, M.; Meites, L. Preparation and mechanism of formation of titanium dioxide hydrosols of narrow size distribution. J. Colloid Interface Sci. 1977, 61, 302–311. [Google Scholar] [CrossRef]
- Castrejón-Sánchez, V.H.; López, R.; Ramón-González, M.; Enríquez-Pérez, Á.; Camacho-López, M.; Villa-Sánchez, G. Annealing control on the anatase/rutile ratio of nanostructured titanium dioxide obtained by sol-gel. Crystals 2019, 9, 22. [Google Scholar] [CrossRef]
- Kim, D.J.; Hahn, S.H.; Oh, S.H.; Kim, E.J. Influence of calcination temperature on structural and optical properties of TiO2 thin films prepared by sol-gel dip coating. Mater. Lett. 2002, 57, 355–360. [Google Scholar] [CrossRef]
- Acharyulu, N.P.S.; Srinivasu, C.; Babavali, S.K.F. Synthesis of carbon nano spherical structures and nano composite oxide [TiO2/SnO2 (2:1)] hollow spheres by hydrothermal method and study of characterization with photo catalytic activity. Mater. Today Proc. 2020, 27, 1282–1288. [Google Scholar] [CrossRef]
- Trinh, T.T.; Giang, N.T.H.; Huong, L.M.; Thinh, D.B.; Dat, N.M.; Trinh, D.N.; Hai, N.D.; Oanh, D.T.Y.; Nam, H.M.; Phong, M.T.; et al. Hydrothermal synthesis of titanium dioxide/graphene aerogel for photodegradation of methylene blue in aqueous solution. J. Sci. Adv. Mater. Devices 2022, 7, 100433. [Google Scholar] [CrossRef]
- Paajanen, J.; Pettilä, L.; Lönnrot, S.; Heikkilä, M.; Hatanpää, T.; Ritala, M.; Koivula, R. Electroblown titanium dioxide and titanium dioxide/silicon dioxide submicron fibers with and without titania nanorod layer for strontium(II) uptake. Chem. Eng. J. Adv. 2023, 13, 100434. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Nikfarjam, A.; Hajghassem, H.; Salehifar, N. Hierarchical dense array of ZnO nanowires spatially grown on ZnO/TiO2 nanofibers and their ultraviolet activated gas sensing properties. J. Phys. Chem. C 2020, 124, 322–335. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhowmik, B. A highly stable room temperature titania nanostructure-based thin film transistor (TFT) alcohol sensor. Sens. Diagn. 2023, 2, 225–235. [Google Scholar] [CrossRef]
- Prathan, A.; Sanglao, J.; Wang, T.; Bhoomanee, C.; Ruankham, P.; Gardchareon, A.; Wongratanaphisan, D. Controlled structure and growth mechanism behind hydrothermal growth of TiO2 nanorods. Sci. Rep. 2020, 10, 8065. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Anwar, M.K.; Ainuddin, A.R.; Hariri, A.; Rus, A.Z.M.; Kamdi, Z.; Yunos, M.Z.; Harun, Z. Synthesis and characterization of visible light active Fe-TiO2 using hydrothermal method. Int. J. Integr. Eng. 2019, 11, 80–85. [Google Scholar] [CrossRef]
- Reilly, K.; Adeli, B.; Fang, B.; Wilkinson, D.P.; Taghipour, F. Advanced titanium dioxide fluidizable nanowire photocatalysts. RSC Adv. 2022, 12, 4240–4252. [Google Scholar] [CrossRef]
- Hameed, H.G.; Abdulrahman, N.A. Synthesis of TiO2 nanoparticles by hydrothermal method and characterization of their antibacterial activity: Investigation of the impact of magnetism on the photocatalytic properties of the nanoparticles. Phys. Chem. Res. 2023, 11, 771–782. [Google Scholar]
- Ahn, K.H.; Park, Y.B.; Park, D.W. Kinetic and mechanistic study on the chemical vapor deposition of titanium dioxide thin films by in situ FT-IR using TTIP. Surf. Coat. Technol. 2003, 171, 198–204. [Google Scholar] [CrossRef]
- Djerdj, I.; Tonejc, A.M.; Bijelić, M.; Vraneša, V.; Turković, A. Transmission electron microscopy studies of nanostructured TiO2 films on various substrates. Vacuum 2005, 80, 371–378. [Google Scholar] [CrossRef]
- Astinchap, B.; Ghanbaripour, H.; Amuzgar, R. Multifractal study of TiO2 thin films deposited by MO-CVD method: The role of precursor amount and substrate temperature. Optik 2020, 222, 165384. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. Pure anatase phase titanium dioxide films prepared by mist chemical vapor deposition. Nanomaterials 2018, 8, 827. [Google Scholar] [CrossRef]
- Ghufran, M.; Uddin, G.M.; Khan, A.A.; Hussein, H.; Khurshid, K.; Arafat, S.M. Comparative Experimental Investigation of Mechanical Properties and Adhesion of Low Temperature PVD Coated TiO2 Thin Films. In Advances in Manufacturing II. Manufacturing; Lecture Notes in Mechanical Engineering; Gapiński, B., Szostak, M., Ivanov, V., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Artoshina, O.V.; Rossouw, A.; Semina, V.K.; Nechaev, A.N.; Apel, P.Y. Structural and physicochemical properties of titanium dioxide thin films obtained by reactive magnetron sputtering, on the surface of track-etched membranes. Pet. Chem. 2015, 55, 759–768. [Google Scholar] [CrossRef]
- Supriadi, S.; Suharno, B.; Nugraha, N.K.; Yasinta, A.O.; Annur, D. Adhesiveness of TiO2 PVD coating on electropolished stainless steel 17-4 PH orthodontic bracket. Mater. Res. Express 2019, 6, 094003. [Google Scholar] [CrossRef]
- Maziarz, W. TiO2/SnO2 and TiO2/CuO thin film nano-heterostructures as gas sensors. Appl. Surf. Sci. 2019, 480, 361–370. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic nanoparticles: Synthesis, characterisation and applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Salem, N.F.A.; Abouelkheir, S.S.; Yousif, A.M.; Meneses-Brassea, B.P.; Sabry, S.A.; Ghozlan, H.A.; El-Gendy, A.A. Large scale production of superparamagnetic iron oxide nanoparticles by the haloarchaeon Halobiforma sp. N1 and their potential in localized hyperthermia cancer therapy. Nanotechnology 2020, 32, 09LT01. [Google Scholar] [CrossRef] [PubMed]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Nethi, S.K. Biological Synthesis of Nanoparticles Using Bacteria. In Nanotechnology for Agriculture; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2019; pp. 37–51. [Google Scholar] [CrossRef]
- Babitha, S.; Korrapati, P.S. Biosynthesis of titanium dioxide nanoparticles using a probiotic from coal fly ash effluent. Mater. Res. Bull. 2013, 48, 4738–4742. [Google Scholar] [CrossRef]
- Salman, J.A.S.; Ibrahem, K.H.; Ali, F.A. Effect of culture media on biosynthesis of titanium dioxide nanoparticles using Lactobacillus crispatus. Int. J. Adv. Res. 2014, 2, 1014–1021. [Google Scholar]
- Órdenes-Aenishanslins, N.A.; Saona, L.A.; Durán-Toro, V.M.; Monrás, J.P.; Bravo, D.M.; Pérez-Donoso, J.M. Use of titanium dioxide nanoparticles biosynthesized by Bacillus mycoides in quantum dot sensitized solar cells. Microb. Cell Fact. 2014, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, S.; Nachiyar, C.V.; Lerensha, R.; Renugadevi, K. Biogenesis of TiO2 nanoparticles using endophytic Bacillus cereus. J. Nanopart. Res. 2014, 16, 2681. [Google Scholar] [CrossRef]
- Suriyaraj, S.P.; Selvakumar, R. Room temperature biosynthesis of crystalline TiO2 nanoparticles using Bacillus licheniformis and studies on the effect of calcination on phase structure and optical properties. RSC Adv. 2014, 4, 39619–39624. [Google Scholar] [CrossRef]
- Dheeba, B.; Marikani, K.; Rajarathinam, K.; Nageswari, K.; Kannan, K. Biosynthesis and characterization of intrcellular TiO2 nanoparticles by Lactobacillus sp: And its potential application in decolourization of methyl orange dyes. Int. J. Pharm. Pharmaceut. Sci. 2015, 7, 225–229. [Google Scholar]
- Jha, A.K.; Prasad, K.; Kulkarni, A.R. Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surf. B Biointerfaces 2009, 71, 226–229. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. Bioimpacts 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Ağçeli, G.K.; Hammachi, H.; Kodal, S.P.; Cihangir, N.; Aksu, Z. A novel approach to synthesize TiO2 nanoparticles: Biosynthesis by using Streptomyces sp. HC1. J. Inorg. Organomet. Polym. 2020, 30, 3221–3229. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef]
- Chinnaperumal, K.; Govindasamy, B.; Paramasivam, D.; Dilipkumar, A.; Dhayalan, A.; Vadivel, A.; Sengodan, K.; Pachiappan, P. Bio-pesticidal effects of Trichoderma viride formulated titanium dioxide nanoparticle and their physiological and biochemical changes on Helicoverpa armigera (Hub.). Pestic. Biochem. Physiol. 2018, 149, 26–36. [Google Scholar] [CrossRef]
- Rehman, S.; Jermy, R.; Mousa Asiri, S.; Shah, M.A.; Farooq, R.; Ravinayagam, V.; Azam Ansari, M.; Alsalem, Z.; Al Jindan, R.; Reshi, Z.; et al. Using Fomitopsis pinicola for bioinspired synthesis of titanium dioxide and silver nanoparticles, targeting biomedical applications. RSC Adv. 2020, 10, 32137–32147. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Sonawane, H.; Math, S.; Tambade, P.; Chaskar, M.; Shinde, D. Biogenic titanium nanoparticles (TiO2 NPs) from Tricoderma citrinoviride extract: Synthesis, characterization and antibacterial activity against extremely drugresistant Pseudomonas aeruginosa. Int. Nano Lett. 2020, 11, 35–42. [Google Scholar] [CrossRef]
- Peiris, M.; Gunasekara, T.; Jayaweera, P.M.; Fernando, S. TiO2 nanoparticles from baker’s yeast: A potent antimicrobial. J. Microbiol. Biotechnol. 2018, 28, 1664–1670. [Google Scholar] [CrossRef]
- Sumerel, J.L.; Yang, W.; Kisailus, D.; Weaver, J.C.; Choi, J.H.; Morse, D.E. Biocatalytically templated synthesis of titanium dioxide. Chem. Mater. 2003, 15, 4804–4809. [Google Scholar] [CrossRef]
- Johnson, J.M.; Kinsinger, N.; Sun, C.; Li, D.; Kisailus, D. Urease-mediated room-temperature synthesis of nanocrystalline titanium dioxide. J. Am. Chem. Soc. 2012, 134, 13974–13977. [Google Scholar] [CrossRef]
- Nabi, G.; Aain, Q.; Khalid, N.R.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Hussain, S.; Iqbal, T.; Majid, A. A Review on novel eco-friendly green approach to synthesis TiO2 nanoparticles using different extracts. J. Inorg. Organomet. Polym. 2018, 28, 1552–1564. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, K.Z.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Gowri, S. Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett. 2011, 8, 447–451. [Google Scholar]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Syn. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Koca, F.D.; Duman, F. Genotoxic and cytotoxic activity of green synthesized TiO2 nanoparticles. Appl. Nanosci. 2018, 9, 815–823. [Google Scholar] [CrossRef]
- Pushpamalini, T.; Keerthana, M.; Sangavi, R.; Nagaraj, A.; Kamaraj, P. Comparative analysis of green synthesis of TiO2 nanoparticles using four different leaf extract. Mater. Today Proceed. 2021, 40, S180–S184. [Google Scholar] [CrossRef]
- Rao, K.G.; Ashok, C.; Rao, K.V.; Chakra, C.S.; Tambur, P. Green synthesis of TiO2 nanoparticles using Aloe Vera extract. Int. J. Adv. Res. Phys. Sci. 2015, 2, 28–34. [Google Scholar]
- Rajkumari, J.; Magdalane, C.M.; Siddhardha, B.; Madhavan, J.; Ramalingam, G.; Al-Dhabi, N.A.; Arasu, M.V.; Ghilan, A.K.M.; Duraipandiayan, V.; Kaviyarasu, K. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B 2019, 201, 111667. [Google Scholar] [CrossRef] [PubMed]
- Hameed, R.S.; Fayyad, R.J.; Nuaman, R.S.; Hamdan, N.T.; Maliki, S.A.J. Synthesis and characterization of a novel titanium nanoparticals using banana peel extract and investigate its antibacterial and insecticidal activity. J. Pure Appl. Microbiol. 2019, 13, 2241–2249. [Google Scholar] [CrossRef]
- Ali, O.M.; Hasanin, M.S.; Suleiman, W.B.; Helal, E.E.H.; Hashem, A.H. Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: Antimicrobial, antioxidant, and anticancer activities. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Madadi, Z.; Soltanieh, M.; Lotfabad, T.B.; Nazari, S. Green synthesis of titanium dioxide nanoparticles with Glycyrrhiza glabra and their photocatalytic activity. Asian J. Green Chem. 2019, 4, 256–268. [Google Scholar] [CrossRef]
- Bekele, E.T.; Gonfa, B.A.; Zelekew, O.A.; Belay, H.H.; Sabir, F.K. Synthesis of titanium oxide nanoparticles using root extract of Kniphofia foliosa as a template, characterization, and its application on drug resistance bacteria. J. Nanomater. 2020, 2020, 2817037. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Qais, F.A.; Ahmad, N.; Khan, A.; Alyousef, A.A.; Arshad, M.; Noor, S.; Khan, J.M.; Alam, P.; et al. Phyto-mediated synthesis of porous titanium dioxide nanoparticles from Withania somnifera root extract: Broad-spectrum attenuation of biofilm and cytotoxic properties against HepG2 cell lines. Front. Microbiol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M.; et al. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac. J. Trop. Med. 2013, 6, 682–688. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Devikala, S.; Abisharani, J.M.; Bharath, M. Biosynthesis of TiO2 nanoparticles from Caesalpinia pulcherrima flower extracts. Mater. Today Proceed. 2021, 40, S185–S188. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Alomair, N.A.; Akhtar, S.; Youssef, T.E. Eco-friendly synthesized α-Fe2O3/TiO2 heterojunction with enhanced visible light photocatalytic activity. J. Photochem. Photobiol. A Chem. 2019, 382, 111951. [Google Scholar] [CrossRef]
- Aslam, M.; Abdullah, A.Z.; Rafatullah, M.; Fawad, A. Abelmoschus esculentus (Okra) seed extract for stabilization of the biosynthesized TiO2 photocatalyst used for degradation of stable organic substance in water. Environ. Sci. Pollut. Res. 2022, 29, 41053–41064. [Google Scholar] [CrossRef]
- Sunny, N.E.; Mathew, S.S.; Venkat Kumar, S.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Vasseghian, Y. Effect of green synthesized nano-titanium synthesized from Trachyspermum ammi extract on seed germination of Vigna radiate. Chemosphere 2022, 300, 134600. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, M.; Mehrvar, M. Photocatalytic degradation of aqueous Methyl Orange using nitrogen-doped TiO2 photocatalyst prepared by novel method of ultraviolet-assisted thermal synthesis. J. Environ. Sci. 2018, 66, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Bielan, Z.; Bartkowiak, A.; Gabała, E.; Piasecki, A.; Zalas, M.; Zielińska-Jurek, A.; Janczarek, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Synthesis of titanium dioxide via surfactant-assisted microwave method for photocatalytic and dye-sensitized solar cells applications. Catalysts 2020, 10, 586. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Z.; Zhong, W.; Liu, J.; Li, J.; Duan, A.; Jiang, G. Synthesis of 3D ordered macroporous TiO2-supported Au nanoparticle photocatalysts and their photocatalytic performances for the reduction of CO2 to methane. Catal. Today 2015, 258, 319–326. [Google Scholar] [CrossRef]
- Kubiak, A.; Wojciechowska, W.; Kurc, B.; Pigłowska, M.; Synoradzki, K.; Gabała, E.; Moszyński, D.; Szybowicz, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. Highly crystalline TiO2-MoO3 composite materials synthesized via a template-assisted microwave method for electrochemical application. Crystals 2020, 10, 493. [Google Scholar] [CrossRef]
- Coromelci, C.; Neamtu, M.; Ignat, M.; Samoila, P.; Zaltariov, M.F.; Palamaru, M. Ultrasound assisted synthesis of heterostructured TiO2/ZnFe2O4 and TiO2/ZnFe1.98La0.02O4 systems as tunable photocatalysts for efficient organic pollutants removal. Ceram. Int. 2022, 48, 4829–4840. [Google Scholar] [CrossRef]
- López-Mayán, J.J.; del-Ángel-Monroy, S.; Peña-Vázquez, E.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Titanium dioxide nanoparticles assessment in seaweeds by single particle inductively coupled plasma-mass spectrometry. Talanta 2022, 236, 122856. [Google Scholar] [CrossRef] [PubMed]
- Mauchauffé, R.; Kang, S.; Moon, S.Y. Fast formation of amorphous titanium dioxide thin films using a liquid-assisted plasma-enhanced deposition process in open air. Surf. Coat. Technol. 2019, 376, 84–89. [Google Scholar] [CrossRef]
- Guo, C.; Kong, M. Fabrication of ultralow stress TiO2/SiO2 optical coatings by plasma ion-assisted deposition. Coatings 2020, 10, 720. [Google Scholar] [CrossRef]
- Sugahara, T.; Alipour, L.; Hirose, Y.; Ekubaru, Y.; Nakamura, J.; Ono, H.; Harada, N.; Suganuma, K. Formation of metal-organic decomposition derived nanocrystalline structure titanium dioxide by heat sintering and photosintering methods for advanced coating process, and its volatile organic compounds’ gas-sensing properties. ACS Appl. Electron. Mater. 2020, 2, 1670–1678. [Google Scholar] [CrossRef]
- Xin, J.H.; Daoud, W.A.; Kong, Y.Y. A new approach to UV-blocking treatment for cotton fabrics. Textil. Res. J. 2004, 74, 97–100. [Google Scholar] [CrossRef]
- Martinez, U.; Hammer, B. Adsorption properties versus oxidation states of rutile TiO2. J. Chem. Phys. 2011, 134, 194703. [Google Scholar] [CrossRef]
- Mendive, C.B.; Hansmann, D.; Bredow, T.; Bahnemann, D. New insights into the mechanism of TiO2 photocatalysis: Thermal processes beyond the electron–hole creation. J. Phys. Chem. C 2011, 115, 19676–19685. [Google Scholar] [CrossRef]
- Boroski, M.; Rodrigues, A.C.; Garcia, J.C.; Sampaio, L.C.; Nozaki, J.; Hioka, N. Combined electrocoagulation and TiO2 photo-assisted treatment applied to wastewater effluents from pharmaceutical and cosmetic industries. J. Hazard. Mater. 2009, 162, 448–454. [Google Scholar] [CrossRef]
- Yao, J.; Chen, H.; Jiang, F.; Jiao, Z.; Jin, M. Titanium dioxide and cadmium sulfide co-sensitized graphitic carbon nitride nanosheets composite photocatalysts with superior performance in phenol degradation under visible-light irradiation. J. Colloid Interface Sci. 2017, 490, 154–162. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y.; Li Puma, G.; Wang, C.; Wang, P.; Zhang, W.; Wang, Q. Mechanism and experimental study on the photocatalytic performance of Ag/AgCl@chiral TiO2 nanofibers photocatalyst: The impact of wastewater components. J. Hazard. Mater. 2015, 285, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Prasse, C.; Stalter, D.; Schulte-Oehlmann, U.; Oehlmann, J.; Ternes, T.A. Spoilt for choice: A critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Res. 2015, 87, 237–270. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Rodríguez, L.; Oller, I.; Klamerth, N.; Agüera, A.; Rodríguez, E.M.; Malato, S. Application of solar AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res. 2013, 47, 1521–1528. [Google Scholar] [CrossRef]
- Keen, O.S.; McKay, G.; Mezyk, S.P.; Linden, K.G.; Rosario-Ortiz, F.L. Identifying the factors that influence the reactivity of effluent organic matter with hydroxyl radicals. Water Res. 2014, 50, 408–419. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Prihod’ko, R.V.; Soboleva, N.M. Photocatalysis: Oxidative processes in water treatment. J. Chem. 2013, 2013, 168701. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review–part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Zareei Pour, F.; Sabzehmeidani, M.M.; Karimi, H.; Madadi Avargani, V.; Ghaedi, M. Superhydrophobic–superoleophilic electrospun nanofibrous membrane modified by the chemical vapor deposition of dimethyl dichlorosilane for efficient oil-water separation. J. Appl. Polym. Sci. 2019, 136, 47621. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. A potential natural solar light active photocatalyst using magnetic ZnFe2O4@TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution. J. Clean. Prod. 2020, 268, 122023. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. Photocatalytic degradation of model pharmaceutical pollutant by novel magnetic TiO2@ZnFe2O4/Pd nanocomposite with enhanced photocatalytic activity and stability under solar light irradiation. J. Environ. Manag. 2020, 271, 110964. [Google Scholar] [CrossRef]
- Naushad, M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ahmadpour, N.; Homaeigohar, S. Photocatalytic and antibacterial properties of Ag-CuFe2O4@WO3 magnetic nanocomposite. Nanomaterials 2021, 11, 298. [Google Scholar] [CrossRef]
- Liu, F.; Liang, J.; Chen, L.; Tong, M.; Liu, W. Photocatalytic removal of diclofenac by Ti doped BiOI microspheres under visible light irradiation: Kinetics, mechanism, and pathways. J. Mol. Liq. 2019, 275, 807–814. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Sörme, P.; Ellor, B. The removal of pharmaceuticals during wastewater treatment: Can it be predicted accurately? Sci. Total Environ. 2019, 676, 222–230. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Mudgal, S.; De Toni, A.; Lockwood, S.; Sales, K.; Backhaus, T.; Sorensen, B.H. Study on the environmental risks of medicinal products. In BIO Intelligence Service; European Commission: Brussels, Belgium, 2013; Available online: https://hal.science/hal-03859880 (accessed on 12 January 2024).
- Sheng, C.; Agwu Nnanna, A.G.; Liu, Y.; Vargo, J.D. Removal of trace pharmaceuticals from water using coagulation and powdered activated carbon as pretreatment to ultrafiltration membrane system. Sci. Total Environ. 2016, 550, 1075–1083. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Removal of trimethoprim, sulfamethoxazole, and triclosan by the green alga Nannochloris sp. J. Hazard. Mater. 2016, 315, 70–75. [Google Scholar] [CrossRef]
- Williams, R.T.; Cook, J.C. Exposure to pharmaceuticals present in the environment. Ther. Innov. Regul. Sci. 2007, 41, 133–141. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Steger-Hartmann, T.; Meyer, M. Biodegradability of the anti-tumour agent ifosfamide and its occurrence in hospital effluents and communal sewage. Water Res. 1997, 31, 2705–2710. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Cataluña Veses, R.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Layton, A.; Gregory, B.; Seward, J.; Schultz, T.; Sayler, G. Mineralization of steroidal hormones by biosolids in wastewater treatment systems in Tennessee USA. Environ. Sci. Technol. 2000, 34, 3925–3931. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced oxidation processes for water and wastewater viral disinfection. a systematic review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Chen, T.; Wang, H.; Lu, W. Graphene TiO2 nanocomposites with high photocatalytic activity for the degradation of sodium pentachlorophenol. J. Environ. Sci. 2014, 26, 2114–2122. [Google Scholar] [CrossRef]
- Sharma, G.; Alothman, Z.A.; Kumar, A.; Sharma, S.; Kumar Ponnusamy, S.; Naushad, M. Fabrication and characterization of a nanocomposite hydrogel for combined photocatalytic degradation of a mixture of malachite green and fast green dye. Nanotechnol. Environ. Eng. 2017, 2, 4. [Google Scholar] [CrossRef]
- Akter, S.; Islam, S.; Kabir, H.; Shaikh, A.A.; Gafur, A. UV/TiO2 photodegradation of metronidazole, ciprofloxacin and sulfamethoxazole in aqueous solution: An optimization and kinetic study. Arab. J. Chem. 2022, 15, 103900. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, A.; Mandal, M.K.; Dubey, K.K. TiO2 based photocatalysis: A valuable approach for the removal of pharmaceuticals from aquatic environment. Int. J. Environ. Sci. Technol. 2023, 20, 4569–4584. [Google Scholar] [CrossRef]
- Manasa, M.; Chandewar, P.R.; Mahalingam, H. Photocatalytic degradation of ciprofloxacin & norfloxacin and disinfection studies under solar light using boron & cerium doped TiO2 catalysts synthesized by green EDTA-citrate method. Catal. Today 2020, 375, 522–536. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Martins, P.; Kappert, S.; Nga Le, H.; Sebastian, V.; Kühn, K.; Alves, M.; Pereira, L.; Cuniberti, G.; Melle-Franco, M.; Lanceros-Méndez, S. Enhanced photocatalytic activity of Au/TiO2 nanoparticles against ciprofloxacin. Catalysts 2020, 10, 234. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Martínez-Piernas, A.B.; Bertakis, Y.; Xekoukoulotakis, N.P.; Agüera, A.; Sánchez-Pérez, J.A. Ti photocatalysis under natural solar radiation for the degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions at pilot plant scale. Water Res. 2019, 166, 115037. [Google Scholar] [CrossRef] [PubMed]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV-vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B Environ. 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Rodriguez, J.J.; Belver, C. C-modified TiO2 using lignin as carbon precursor for the solar photocatalytic degradation of acetaminophen. Chem. Eng. J. 2019, 358, 1574–1582. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. Chem. Eng. J. 2020, 392, 124867. [Google Scholar] [CrossRef]
- Murgolo, S.; Moreira, I.S.; Piccirillo, C.; Castro, P.M.L.; Ventrella, G.; Cocozza, C.; Mascolo, G. Photocatalytic degradation of diclofenac by hydroxyapatite–TiO2 composite material: Identification of transformation products and assessment of toxicity. Materials 2018, 11, 1779. [Google Scholar] [CrossRef]
- Czech, B.; Tyszczuk-Rotko, K. Visible-light-driven photocatalytic removal of acetaminophen from water using a novel MWCNT-TiO2-SiO2 photocatalysts. Separ. Purif. Technol. 2018, 206, 343–355. [Google Scholar] [CrossRef]
- Payan, A.; Akbar Isari, A.; Gholizade, N. Catalytic decomposition of sulfamethazine antibiotic and pharmaceutical wastewater using Cu-TiO2@functionalized SWCNT ternary porous nanocomposite: Influential factors, mechanism, and pathway studies. Chem. Eng. J. 2019, 361, 1121–1141. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.M.; Nadagouda, M.N.; Dionysiou, D.D. Solar light-assisted remediation of domestic wastewater by N,B-TiO2 nanoparticles for potable reuse. Appl. Catal. B Environ. 2020, 269, 118807. [Google Scholar] [CrossRef]
- Carbuloni, C.F.; Savoia, J.E.; Santos, J.S.P.; Pereira, C.A.A.; Marques, R.G.; Ribeiro, V.A.S.; Ferrari, A.M. Degradation of metformin in water by TiO2–ZrO2 photocatalysis. J. Environ. Manag. 2020, 262, 110347. [Google Scholar] [CrossRef]
- Escudeiro de Oliveira, M.; Barroso, B.L.; de Almeida, J.; Moraes, M.L.L.; de Arruda Rodrigues, C. Photoelectrocatalytic degradation of 17a-ethinylestradiol and estrone under UV and visible light using nanotubular oxide arrays grown on Ti-0.5wt%W. Environ. Res. 2020, 191, 110044. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Ncibi, M.C.; Thangaraj, S.K.; Jänis, J.; Seyedsalehi, M.; Sillanpää, M. Removal of pharmaceutically active compounds (PhACs) from real membrane bioreactor (MBR) effluents by photocatalytic degradation using composite Ag2O/P-25 photocatalyst. Separ. Purif. Technol. 2019, 215, 317–328. [Google Scholar] [CrossRef]
- Gomathi Devi, L.; Kavitha, R. A review on plasmonic metal TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogenerated electrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Yin, X.; Que, W.; Liao, Y.; Xie, H.; Fei, D. Ag-TiO2 nanocomposites with improved photocatalytic properties prepared by a low temperature process in polyethylene glycol. Colloids Surf. A 2012, 410, 153–158. [Google Scholar] [CrossRef]
- Fei, J.; Li, J. Controlled preparation of porous TiO2-Ag nanostructures through supramolecular assembly for plasmon-enhanced photocatalysis. Adv. Mater. 2015, 27, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.-J.; Lin, J.-S.; Yang, J.-L.; Zhang, F.-L.; Lin, X.-M.; Zhang, Y.-J.; Jin, S.; Li, J.-F. Plasmonic photocatalysis: Mechanism, applications and perspectives. Chin. J. Struct. Chem. 2023, 42, 100066. [Google Scholar] [CrossRef]
- Kaur, R.; Pal, B. Co-catalysis effect of different morphological facets of as prepared Ag nanostructures for the photocatalytic oxidation reaction by Ag-TiO2 aqueous slurry. Mater. Chem. Phys. 2013, 143, 393–399. [Google Scholar] [CrossRef]
- Gang, R.; Xia, Y.; Xu, L.; Zhang, L.; Ju, S.; Wang, Z.; Koppala, S. Size controlled Ag decorated TiO2 plasmonic photocatalysts for tetracycline degradation under visible light. Surf. Interfaces 2022, 31, 102018. [Google Scholar] [CrossRef]
- Litter, M.I. Mechanisms of removal of heavy metals and arsenic from water by TiO2-heterogeneous photocatalysis. Pure Appl. Chem. 2015, 87, 557–567. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.L.; Zhou, P. Preparation of porous nano-calcium titanate microspheres and its adsorption behavior for heavy metal ion in water. J. Hazard. Mater. 2011, 186, 971–977. [Google Scholar] [CrossRef]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Kyung, H.; Lee, J.; Choi, W. Simultaneous and synergistic conversion of dyes and heavy metal ions in aqueous TiO2 suspensions under visible-light illumination. Environ. Sci. Technol. 2005, 39, 2376–2382. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jalali, M.; Afkhami, A. Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chem. Eng. Commun. 2013, 200, 448–470. [Google Scholar] [CrossRef]
- Poursani, A.S.; Nilchi, A.; Hassani, A.; Shariat, S.M.; Nouri, J. The synthesis of nano TiO2 and its use for removal of lead ions from aqueous solution. J. Water Resour. Protect. 2016, 8, 438–448. [Google Scholar] [CrossRef]
- Luo, T.; Cui, J.; Hu, S.; Huang, Y.; Jing, C. Arsenic removal and recovery from copper smelting wastewater using TiO2. Environ. Sci. Technol. 2010, 44, 9094–9098. [Google Scholar] [CrossRef]
- Yan, M.; Zeng, G.; Li, X.; Zhao, C.; Yang, G.; Gong, J.; Chen, G.; Tang, L.; Huang, D. Titanium dioxide nanotube arrays with silane coupling agent modification for heavy metal reduction and persistent organic pollutant degradation. New J. Chem. 2017, 41, 4377–4389. [Google Scholar] [CrossRef]
- Skubal, L.R.; Meshkov, N.K. Reduction and removal of mercury from water using arginine-modified TiO2. J. Photochem. Photobiol. A Chem. 2002, 148, 211–214. [Google Scholar] [CrossRef]
- Zhang, F.-S.; Nriagu, J.O.; Itoh, H. Photocatalytic removal and recovery of mercury from water using TiO2-modified sewage sludge carbon. J. Photochem. Photobiol. A Chem. 2004, 167, 223–228. [Google Scholar] [CrossRef]
- Dou, B.; Chen, H. Removal of toxic mercury(II) from aquatic solutions by synthesized TiO2 nanoparticles. Desalination 2011, 269, 260–265. [Google Scholar] [CrossRef]
- Xu, S.; Du, A.J.; Liu, J.; Ng, J.; Sun, D.D. Highly efficient CuO incorporated TiO2 nanotube photocatalyst for hydrogen production from water. Int. J. Hydrogen Energy 2011, 36, 6538–6545. [Google Scholar] [CrossRef]
- Xu, S.C.; Pan, S.S.; Xu, Y.; Luo, Y.Y.; Zhang, Y.X.; Li, G.H. Efficient removal of Cr (VI) from wastewater under sunlight by Fe(II)-doped TiO2 spherical shell. J. Hazard. Mater. 2014, 283, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Guo, M.; Xu, F.; Wang, P.; Du, Y.; Na, P. One-pot synthesis of Mn-doped TiO2 grown on graphene and the mechanism for removal of Cr(VI) and Cr (III). J. Hazard. Mater. 2016, 310, 188–198. [Google Scholar] [CrossRef]
- Gullipilli, S.; Rupakula, R.B. Adsorption studies of Cr(VI) and Cu(II) metal ions from aqueous solutions by synthesized Ag and Mg co-doped TiO2 nanoparticles. Separ. Sci. Technol. 2018, 54, 2983–2992. [Google Scholar] [CrossRef]
- Eddy, D.R.; Rahayu, I.; Wyantuti, S.; Hartati, Y.W.; Firdaus, M.L.; Bahti, H.H. Photocatalytic activity of gadolinium doped TiO2 particles for decreasing heavy metal chromium (VI) concentration. J. Phys. Conf. Ser. 2018, 1080, 012013. [Google Scholar] [CrossRef]
- Luo, Z.; Qu, L.; Jia, J.; Wang, J.; Jiang, S.; Wu, Z.; Wu, X. TiO2/EDTA-rich carbon composites: Synthesis, characterization and visible-light-driven photocatalytic reduction of Cr(VI). Chin. Chem. Lett. 2018, 29, 547–550. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kanasaki, R.; Nozaki, T.; Shoji, R.; Sato, K. Improving effect of MnO2 addition on TiO2-photocatalytic removal of lead ion from water. J. Water Environ. Technol. 2017, 15, 35–42. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Hu, H.; Chang, M.; Chen, D.; Zhang, M.; Wu, L.; Li, X. Nonuniform doping outperforms uniform doping for enhancing the photocatalytic efficiency of Au-doped TiO2 nanotubes in organic dye degradation. Ceram. Int. 2017, 43, 9053–9059. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Cui, X.; Yao, W.; Duan, T.; Zhu, W. Capture of Cs+ and Sr2+ from aqueous solutions by using Cr doped TiO2 nanotubes. J. Nanosci. Nanotechnol. 2017, 17, 3943–3950. [Google Scholar] [CrossRef]
- Garza-Arévalo, J.I.; García-Montes, I.; Reyes, M.H.; Guzmán-Mar, J.L.; Rodríguez-González, V.; Reyes, L.H. Fe doped TiO2 photocatalyst for the removal of As (III) under visible radiation and its potential application on the treatment of As-contaminated groundwater. Mater. Res. Bull. 2016, 73, 145–152. [Google Scholar] [CrossRef]
- Seema, K.M.; Mamba, B.B.; Njuguna, J.; Bakhtizin, R.Z.; Mishra, A.K. Removal of lead (II) from aqeouos waste using (CD-PCL-TiO2) bio-nanocomposites. Int. J. Biol. Macromol. 2018, 109, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chang, W.; Huang, Z.; Feng, X.; Ma, L.; Qi, X.; Li, Z. Enhanced removal of toxic Cr(VI) in tannery wastewater by photoelectrocatalysis with synthetic TiO2 hollow spheres. Appl. Surf. Sci. 2017, 405, 102–110. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, Q.; Jin, R.; Gao, S.; Yuan, Q.; Rong, W.; Wang, R. Photoelectrocatalytic removal of organic dyes and Cr(VI) ions using Ag3PO4 nanoparticles sensitized TiO2 nanotube arrays. Mater. Chem. Phys. 2017, 199, 209–215. [Google Scholar] [CrossRef]
- Hang, Y.; Yin, H.; Ji, Y.; Liu, Y.; Lu, Z.; Wang, A.; Shen, L.; Yin, H. Adsorption performances of naked and 3-aminopropyl triethoxysilane-modified mesoporous TiO2 hollow nanospheres for Cu2+, Cd2+, Pb2+, Cr(VI) ions. J. Nanosci. Nanotechnol. 2017, 17, 5539–5549. [Google Scholar] [CrossRef]
- Abbas, K.K.; Al-Ghaban, A.M.H.A. Enhanced solar light photoreduction of innovative TiO2 nanospherical shell by reduced graphene oxide for removal silver ions from aqueous media. J. Environ. Chem. Eng. 2019, 7, 103168. [Google Scholar] [CrossRef]
- Gan, W.; Shang, X.; Li, X.H.; Zhang, J.; Fu, X. Achieving high adsorption capacity and ultrafast removal of methylene blue and Pb2+ by graphene-like TiO2@C. Colloids Surf. A 2019, 561, 218–225. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2-based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Shen, T.; Hidaka, H.; Pelizzetti, E.; Serpone, N. TiO2-assisted photodegradation of dye pollutants II. Adsorption and degradation kinetics of eosin in TiO2 dispersions under visible light irradiation. Appl. Catal. B Environ. 1998, 15, 147–156. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Ma, W.; Zhao, J.; Hidaka, H.; Serpone, N. Effect of transition metal ions on the TiO2-assisted photodegradation of dyes under visible irradiation: A probe for the interfacial electron transfer process and reaction mechanism. J. Phys. Chem. B 2002, 106, 318–324. [Google Scholar] [CrossRef]
- Gola, D.; Malik, A.; Namburath, M.; Ahammad, S.Z. Removal of industrial dyes and heavy metals by Beauveria bassiana: FTIR, SEM, TEM and AFM investigations with Pb(II). Environ. Sci. Pollut. Res. 2018, 25, 20486–20496. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Mukhopadhyay, S.; Nath, M. TiO2@ZIF-8: A novel approach of modifying micro-environment for enhanced photo-catalytic dye degradation and high usability of TiO2 nanoparticles. Mater. Lett. 2016, 164, 571–574. [Google Scholar] [CrossRef]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Singh, R.; Kumari, P.; Chavan, P.D.; Datta, S.; Dutta, S. Synthesis of solvothermal derived TiO2 nanocrystals supported on ground nano egg shell waste and its utilization for the photocatalytic dye degradation. Opt. Mater. 2017, 73, 377–383. [Google Scholar] [CrossRef]
- Ljubas, D.; Smoljanić, G.; Juretić, H. Degradation of Methyl Orange and Congo Red dyes by using TiO2 nanoparticles activated by the solar and the solar-like radiation. J. Environ. Manag. 2015, 161, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, M.; Chowdhury, S.; Sufian, S.; Omar, A.A. Enhanced photocatalytic activity of Orange II in aqueous solution using solvent-based TiO2 nanotubes: Kinetic, equilibrium and thermodynamic studies. J. Clean. Prod. 2018, 203, 848–859. [Google Scholar] [CrossRef]
- Pol, R.; Guerrero, M.; García-Lecina, E.; Altube, A.; Rossinyol, E.; Garroni, S.; Baró, M.D.; Pons, J.; Sort, J.; Pellicer, E. Ni-, Pt- and (Ni/Pt)-doped TiO2 nanophotocatalysts: A smart approach for sustainable degradation of Rhodamine B dye. Appl. Catal. B Environ. 2016, 181, 270–278. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Saravanan, R.; Ravichandran, K.; Gracia, F.; Gupta, V.K. Intermediate state created by dopant ions (Mn, Co and Zr) into TiO2 nanoparticles for degradation of dyes under visible light. J. Mol. Liq. 2016, 223, 652–659. [Google Scholar] [CrossRef]
- Kerkez-Kuyumcu, Ö.; Kibar, E.; Dayioʇlu, K.; Gedik, F.; Akin, A.N.; Özkara Aydinoǧlu, S. A comparative study for removal of different dyes over M/TiO2(M ¼ Cu, Ni, Co, Fe, Mn and Cr) photocatalysts under visible light irradiation. J. Photochem. Photobiol. A Chem. 2015, 311, 176–185. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, S.; Singh, V. Preparation, characterization and photocatalytic degradation kinetics of Reactive Red dye 198 using N, Fe codoped TiO2 nanoparticles under visible light. Desalin. Water Treat. 2016, 57, 9237–9246. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Kumar Mehta, S.; Sinha, A.S.K.; Kansal, S.K. Efficient photocatalytic degradation of brilliant green using Sr-doped TiO2 nanoparticles. Ceram. Int. 2015, 41, 3533–3540. [Google Scholar] [CrossRef]
- Brindha, A.; Sivakumar, T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A Chem. 2017, 340, 146–156. [Google Scholar] [CrossRef]
- McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J.D.; Morris, M.A. A facile route to synthesis of S-doped TiO2 nanoparticles for photocatalytic activity. J. Mol. Catal. A Chem. 2015, 406, 51–57. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, Y.; Wu, Y.; Zhang, Y.N.; Zuo, T. Low-cost Y-doped TiO2 nanosheets film with highly reactive {001} facets from CRT waste and enhanced photocatalytic removal of Cr(VI) and methyl orange. ACS Sustain. Chem. Eng. 2016, 4, 1794–1803. [Google Scholar] [CrossRef]
- Li, Q.; Zong, L.; Li, C.; Yang, J. Reprint of photocatalytic reduction of CO2 on MgO/TiO2 nanotube films. Appl. Surf. Sci. 2014, 319, 16–20. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Anwar, D.I.; Mulyadi, D. Synthesis of Fe-TiO2 composite as a photocatalyst for degradation of methylene blue. Procedia Chem 2015, 17, 49–54. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef]
- Talebi, S.; Chaibakhsh, N.; Moradi-Shoeili, Z. Application of nanoscale ZnS/TiO2 composite for optimized photocatalytic decolorization of a textile dye. J. Appl. Res. Technol. 2017, 15, 378–385. [Google Scholar] [CrossRef]
- Hui, C.; Lei, Z.; Xitang, W.; Shujing, L.; Zhongxing, L. Preparation of nanoporous TiO2/SiO2 composite with rice husk as template and its photocatalytic property. Rare Met. Mater. Eng. 2015, 44, 1607–1611. [Google Scholar] [CrossRef]
- Li, J.; Zhen, D.; Sui, G.; Zhang, C.; Deng, Q.; Jia, L. Nanocomposite of Cu-TiO2-SiO2 with high photoactive performance for degradation of rhodamine B dye in aqueous wastewater. J. Nanosci. Nanotechnol. 2012, 12, 6265–6270. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Shi, F.; Hu, S.; Jiang, S.; Liu, S.; Liu, D.; Tian, X. F/W codoped TiO2-SiO composite aerogels with improved visible light-driven photocatalytic activity. J. Solid State Chem. 2019, 275, 8–15. [Google Scholar] [CrossRef]
- Shao, L.; Liu, H.; Zeng, W.; Zhou, C.; Li, D.; Wang, L.; Lan, Y.; Xu, F.; Liu, G. Immobilized and photocatalytic performances of PDMS-SiO2-chitosan@TiO2 composites on pumice under simulated sunlight irradiation. Appl. Surf. Sci. 2019, 478, 1017–1026. [Google Scholar] [CrossRef]
- Tang, Y.; Di, W.; Zhai, X.; Yang, R.; Qin, W. NIR-responsive photocatalytic activity and mechanism of NaYF4:Yb,Tm@TiO2 core-shell nanoparticles. ACS Catal. 2013, 3, 405–412. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, M.F.; Cerrato, G.; Morandi, S.; Gatto, S.; Oldani, V.; Di Michele, A.; Bianchi, C.L. Photoactive TiO2-montmorillonite composite for degradation of organic dyes in water. J. Photochem. Photobiol. A Chem. 2015, 295, 57–63. [Google Scholar] [CrossRef]
- Alagarasi, A.; Rajalakshmi, P.U.; Shanthi, K.; Selvam, P. Solar light photocatalytic activity of mesoporous nanocrystalline TiO2, SnO2, and TiO2-SnO2 composites. Mater. Today Sustain. 2019, 5, 100016. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kanimozhi, K.; Arularasu, M.V.; Ramalingam, G.; Kaviyarasu, K. Self-cleaning mechanism of synthesized SnO2/TiO2 nanostructure for photocatalytic activity application for waste water treatment. Surf. Interfaces 2019, 17, 100346. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Li, L.; Yi, C.; Lv, H.; Song, Q. Litchi-like CdS/CdTiO3-TiO2 composite: Synthesis and enhanced photocatalytic performance for crystal violet degradation and hydrogen production. RSC Adv. 2016, 6, 51374–51386. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Saputera, W.H.; Groenen, E.J.; Mul, G. A novel TiO2 composite for photocatalytic wastewater treatment. J. Catal. 2014, 310, 75–83. [Google Scholar] [CrossRef]
- Rahimi, B.; Jafari, N.; Abdolahnejad, A.; Farrokhzadeh, H.; Ebrahimi, A. Application of efficient photocatalytic process using a novel BiVO/TiO2-NaY zeolite composite for removal of acid orange 10 dye in aqueous solutions: Modeling by response surface methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 103253. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A.; Prakoso, S.P. Fabrication of Ag2O/TiO2 composites on nanographene platelets for the removal of organic pollutants: Influence of oxidants and inorganic anions. Appl. Surf. Sci. 2019, 480, 697–708. [Google Scholar] [CrossRef]
- Visa, M.; Isac, L.; Duta, A. New fly ash TiO2 composite for the sustainable treatment of wastewater with complex pollutants load. Appl. Surf. Sci. 2015, 339, 62–68. [Google Scholar] [CrossRef]
- Jaseela, P.K.; Garvasis, J.; Joseph, A. Selective adsorption of methylene blue (MB) dye from aqueous mixture of MB and methyl orange (MO) using mesoporous titania (TiO2)-poly vinyl alcohol (PVA) nanocomposite. J. Mol. Liq. 2019, 286, 110908. [Google Scholar] [CrossRef]
- Yun, J.; Jin, D.; Lee, Y.S.; Kim, H. Photocatalytic treatment of acidic waste water by electrospun composite nanofibers of pH-sensitive hydrogel and TiO2. Mater. Lett. 2010, 64, 2431–2434. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, M.; Zhai, Q.; Li, S.; Jiang, Y. Polydopamine tethered CPO/HRPTiO2 nano-composites with high bio-catalytic activity, stability and reusability: Enzyme-photo bifunctional synergistic catalysis in water treatment. Chem. Eng. J. 2018, 347, 703–710. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J. Colloid Interface Sci. 2012, 371, 101–106. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an azo dye Sunset Yellow under UV-A light using TiO2/CAC composite catalysts. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 100–108. [Google Scholar] [CrossRef]
- Hassan, M.E.; Liu, G.; Omer, E.O.M.; Goja, A.M.; Acharya, S. Silver embedded C-TiO2 exhibits improved photocatalytic properties with potential application in waste water treatment. Arab. J. Chem. 2018, 12, 1134–1140. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide-TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ahamad, T.; Ahmad, N.; Khan, M.Z. Removal enhancement of acid navy blue dye by GO-TiO2 nanocomposites synthesized using sonication method. Mater. Chem. Phys. 2019, 238, 121906. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Alsanea, A.A. TiO2/carbon dots decorated reduced graphene oxide composites from waste car bumper and TiO2 nanoparticles for photocatalytic applications. Arab. J. Chem. 2018, 13, 3082–3091. [Google Scholar] [CrossRef]
- Ranjith, R.; Renganathan, V.; Chen, S.M.; Selvan, N.S.; Rajam, P.S. Green synthesis of reduced graphene oxide supported TiO2/Co3O4 nanocomposite for photocatalytic degradation of methylene blue and crystal violet. Ceram. Int. 2019, 45, 12926–12933. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2. Adv. Powder Technol. 2019, 30, 1089–1098. [Google Scholar] [CrossRef]
- Zhang, J.J.; Fang, S.S.; Mei, J.Y.; Zheng, G.P.; Zheng, X.C.; Guan, X.X. High-efficiency removal of rhodamine B dye in water using g-C3N4 and TiO2 co-hybridized 3D graphene aerogel composites. Separ. Purif. Technol. 2018, 194, 96–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Chi, L.; Qian, Y.; Guo, J.; Wang, X.; Arandiyan, H.; Jiang, Z. Novel g-C3N4/TiO2/PAA/PTFE ultrafiltration membrane enabling enhanced antifouling and exceptional visible-light photocatalytic self-cleaning. Catal. Today 2019, 335, 527–537. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Niaei, A.; Rasoulifard, M.H.; Khataee, A.R. Immobilization of TiO2 nanopowder on glass beads for the photocatalytic decolorization of an azo dye C.I. Direct Red 23. J. Environ. Sci. Health 2005, 40, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; Machado, A.E.d.H.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Farrokhnia, A.; García-López, E.I.; Zargar Shoushtari, M. Codeposition of Fe3O4 nanoparticles sandwiched between g-C3N4 and TiO2 nanosheets: Structure, characterization and high photocatalytic activity for efficiently degradation of dye pollutants. Phys. Chem. Res. 2019, 7, 65–80. [Google Scholar] [CrossRef]
- Gu, W.; Lu, F.; Wang, C.; Kuga, S.; Wu, L.; Huang, Y.; Wu, M. Face-to-Face interfacial assembly of ultrathin g-C3N4 and anatase TiO2 nanosheets for enhanced solar photocatalytic activity. ACS Appl. Mater. Interfaces 2017, 9, 28674–28684. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Liu, F.; Yue, C.; Ling, C.; Li, A. A recyclable nanosheet of Mo/N-doped TiO2 nanorods decorated on carbon nanofibers for organic pollutants degradation under simulated sunlight irradiation. Chemosphere 2019, 215, 280–293. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Wang, F.; Lei, W.; Xia, M. Sensitization of TiO2 nanosheets with Cu-biphenylamine framework to enhance photocatalytic degradation performance of toxic organic contaminants: Synthesis, mechanism and kinetic studies. Nanotechnology 2018, 29, 375605. [Google Scholar] [CrossRef]
- Nair, A.K.; Jagadeesh Babu, P.E. Ag-TiO2 nanosheet embedded photocatalytic membrane for solar water treatment. J. Environ. Chem. Eng. 2017, 5, 4128–4133. [Google Scholar] [CrossRef]
- Du, F.; Zuo, X.; Yang, Q.; Yang, B.; Li, G.; Ding, Z.; Wu, M.; Ma, Y.; Jin, S.; Zhu, K. Facile assembly of TiO2 nanospheres/SnO2 quantum dots composites with excellent photocatalyst activity for the degradation of methyl orange. Ceram. Int. 2016, 42, 12778–12782. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, L.; Wang, C.; Lin, B.; Lyu, S.; Chu, X.; Chi, Y.; Yang, X.; Wang, X. Pd/TiO2 nanospheres with three-dimensional hyperstructure for enhanced photodegradation of organic dye. Chem. Res. 2019, 35, 667–673. [Google Scholar] [CrossRef]
- Ghaforyan, H.; Ghaffary, T.; Pincak, R.; Ebrahimzadeh, M. Effects of ultrasound waves intensity on the removal of Congo Red color from the textile industry wastewater by Fe3O4@TiO2 core-shell nanospheres. arXiv 2017, arXiv:1708.04683. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of synthesized magnetic TiO2-based membranes for wastewater treatment: A review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef]
- Cao, J.; Song, X.Z.; Kang, X.; Dai, Z.; Tan, Z. One-pot synthesis of oleic acid modified monodispersed mesoporous TiO2 nanospheres with enhanced visible light photocatalytic performance. Adv. Powder Technol. 2018, 29, 1925–1932. [Google Scholar] [CrossRef]
- Liu, D.; Tian, R.; Wang, J.; Nie, E.; Piao, X.; Li, X.; Sun, Z. Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation. Chemosphere 2017, 185, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, M.; Azis, T.; Maulidiyah, M.; Aladin, A.; Hafid, N.A.; Salim, L.O.A.; Wibowo, D. Photocurrent responses of metanil yellow and remazol red B organic dyes by using TiO2/Ti electrode. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 12048. [Google Scholar] [CrossRef]
- Yilmaz, P.; Lacerda, A.M.; Larrosa, I.; Dunn, S. Photoelectrocatalysis of rhodamine B and solar hydrogen production by TiO2 and Pd/TiO2 catalyst systems. Electrochim. Acta 2017, 231, 641–649. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.; Chen, S.; Quan, X.; Zhao, H. Integrating plasmonic nanoparticles with TiO2 photonic crystal for enhancement of visible-light-driven photocatalysis. Environ. Sci. Technol. 2012, 46, 1724–1730. [Google Scholar] [CrossRef]
- Liao, G.; Chen, S.; Quan, X.; Chen, H.; Zhang, Y. Photonic crystal coupled TiO2/polymer hybrid for efficient photocatalysis under visible light irradiation. Environ. Sci. Technol. 2010, 44, 3481–3485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Chen, S.L.; Wang, A.J. Dual-bandgap effect of photonic crystals on TiO2 photocatalytic activity in ultraviolet and visible light regions. Catal. Surv. 2019, 23, 23–32. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Yuan, X.; Huo, M.; Zhao, Y.; Lu, Y.; Qiu, Y. Incorporation of Cu2O nanocrystals into TiO2 photonic crystal for enhanced UV-visible light driven photocatalysis. J. Alloys Compd. 2015, 644, 734–741. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Quan, X.; Zhang, Y. Structuring a TiO2-based photonic crystal photocatalyst with Schottky junction for efficient photocatalysis. Environ. Sci. Technol. 2010, 44, 451–455. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Liu, F.T.; Zhao, Y.; Wang, J.; Wang, C.W. Photonic crystal structural-induced Cu3SnS4/Ti3+-TiO2 p-n coaxial heterojunction arrays for light-driven H2 production and pollutant degradation. Mater. Des. 2017, 133, 426–434. [Google Scholar] [CrossRef]
- Vela, N.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Recent overview on the abatement of pesticide residues in water by photocatalytic treatment using TiO2. In Application of Titanium Dioxide; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Pan, X.; Li, J. Protecting the environment and public health from pesticides. Environ. Sci. Technol. 2012, 46, 5658–5659. [Google Scholar] [CrossRef]
- Shuman-Goodier, M.E.; Propper, C.R. A meta-analysis synthesizing the effects of pesticides on swim speed and activity of aquatic vertebrates. Sci. Total Environ. 2016, 565, 758–766. [Google Scholar] [CrossRef]
- Blair, A.; Ritz, B.; Wesseling, C.; Beane Freeman, L. Pesticides and human health. Occup. Environ. Med. 2015, 72, 81–82. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Bonner, M.R. Occupational pesticide exposures and cancer risk: A review. J. Toxicol. Environ. Health Part B 2012, 15, 238–263. [Google Scholar] [CrossRef]
- Vanraes, P.; Ghodbane, H.; Davister, D.; Wardenier, N.; Nikiforov, A.; Verheust, Y.P.; Van Hulle, S.W.H.; Hamdaoui, O.; Vandamme, J.; Van Durme, J.; et al. Removal of several pesticides in a falling water film DBD reactor with activated carbon textile: Energy efficiency. Water Res. 2017, 116, 1–12. [Google Scholar] [CrossRef]
- Farner Budarz, J.; Cooper, E.M.; Gardner, C.; Hodzic, E.; Ferguson, P.L.; Gunsch, C.K.; Wiesner, M.R. Chlorpyrifos degradation via photoreactive TiO2 nanoparticles: Assessing the impact of a multi-component degradation scenario. J. Hazard. Mater. 2019, 372, 61–68. [Google Scholar] [CrossRef]
- Amalraj, A.; Thirumalaisamy, S.; Rajeswari, A.; Pius, A. Photocatalytic degradation of quinalphos and profenofos pesticides using UV irradiated TiO2 nanoparticles-a kinetic study. Mater. Focus 2016, 5, 377–384. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, C.; Zhang, G.; Dionysiou, D.D.; Nadagouda, M.N. PEG-assisted synthesis of crystal TiO2 nanowires with high specific surface area for enhanced photocatalytic degradation of atrazine. Chem. Eng. J. 2015, 268, 170–179. [Google Scholar] [CrossRef]
- Ahmari, H.; Heris, S.Z.; Khayyat, M.H. The effect of titanium dioxide nanoparticles and UV irradiation on photocatalytic degradation of Imidaclopride. Environ. Technol. 2018, 39, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Dadban Shahamat, Y.; Farzadkia, M.; Esrafili, A.; Asgharnia, H. Photocatalytic degradation and mineralization of diazinon in aqueous solution using nano-TiO2 (Degussa, P25): Kinetic and statistical analysis. Desalin. Water Treat. 2015, 55, 555–563. [Google Scholar] [CrossRef]
- Suhaimy, S.H.M.; Lai, C.W.; Tajuddin, H.A.; Samsudin, E.M.; Johan, M.R. Impact of TiO2 nanotubes’ morphology on the photocatalytic degradation of simazine pollutant. Materials 2018, 11, 66. [Google Scholar] [CrossRef]
- Tabasideh, S.; Maleki, A.; Shahmoradi, B.; Ghahremani, E.; McKay, G. Sonophotocatalytic degradation of diazinon in aqueous solution using iron-doped TiO2 nanoparticles. Separ. Purif. Technol. 2017, 189, 186–192. [Google Scholar] [CrossRef]
- Mermana, J.; Sutthivaiyakit, P.; Blaise, C.; Gagné, F.; Charnsethikul, S.; Kidkhunthod, P.; Sutthivaiyakit, S. Photocatalysis of S-metolachlor in aqueous suspension of magnetic cerium-doped mTiO2 core-shell under simulated solar light. Environ. Sci. Pollut. Res. 2017, 24, 4077–4092. [Google Scholar] [CrossRef] [PubMed]
- Umar, K.; Aris, A.; Ahmad, H.; Parveen, T.; Jaafar, J.; Majid, Z.A.; Reddy, A.V.B.; Talib, J. Synthesis of visible light active doped TiO2 for the degradation of organic pollutants-methylene blue and glyphosate. J. Anal. Sci. Technol. 2016, 7, 29. [Google Scholar] [CrossRef]
- Maddila, S.; Oseghe, E.O.; Jonnalagadda, S.B. Photocatalyzed ozonation by Ce doped TiO2 catalyst degradation of pesticide Dicamba in water. J. Chem. Technol. Biotechnol. 2016, 91, 385–393. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Rey, A.; Álvarez, P.M.; Beltrán, F.J.; Li Puma, G. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B Environ. 2014, 178, 74–81. [Google Scholar] [CrossRef]
- Joseph, A.I.J.; Thiripuranthagan, S. Non-metal doped titania photocatalysts for the degradation of neonicotinoid insecticides under visible light irradiation. J. Nanosci. Nanotechnol. 2018, 18, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Komtchou, S.; Dirany, A.; Drogui, P.; Delegan, N.; El Khakani, M.A.; Robert, D.; Lafrance, P. Degradation of atrazine in aqueous solution with electrophotocatalytic process using TiO2-x photoanode. Chemosphere 2016, 157, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.B.; Sari Erkan, H.; Çaglak, A.; Bakırdere, S.; Engin, G.O. Optimization of atrazine removal from synthetic groundwater by electrooxidation process using titanium dioxide and graphite electrodes. Separ. Sci. Technol. 2019, 55, 3036–3045. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. Available online: https://www.sciencedirect.com/science/article/pii/0378109785903003 (accessed on 12 January 2024). [CrossRef]
- Maness, P.-C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef]
- Cai, R.; Hashimoto, K.; Itoh, K.; Kubota, Y.; Fujishima, A. Photokilling of malignant cells with ultrafine TiO2 powder. Bull. Chem. Soc. Jpn. 1991, 64, 1268–1273. [Google Scholar] [CrossRef]
- Karunakaran, C. Solar Photocatalytic Disinfection of Bacteria. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Prasad, G.K.; Ramacharyulu, P.V.R.K.; Merwyn, S.; Agarwal, G.S.; Srivastava, A.R.; Beer Singh, G.P.R.; Vijayaraghavan, R. Photocatalytic inactivation of spores of Bacillus anthracis using titania nanomaterials. J. Hazard. Mater. 2011, 185, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.M.; Paulman, H.; Sela, O.; Ktaitzer, T.; Cikurel, H.; Zuckerman, I.; Meir, A.; Aharoni, A.; Adin, A. UV disinfection of wastewater effluents for unrestricted irrigation. Water Sci. Technol. 2006, 54, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Egli, T.; Köster, W.; Meile, L. Pathogenic microbes in water and food: Changes and challenges. FEMS Microbiol. Rev. 2002, 26, 111–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.A.; Baruah, S.; Anal, A.K.; Dutta, J. Heterogeneous photocatalysis for removal of microbes from water. Environ. Chem. Lett. 2012, 10, 145–151. [Google Scholar] [CrossRef]
- Leung, T.L.F.; Bates, A.E. More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J. Appl. Ecol. 2013, 50, 215–222. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B Environ. 2015, 176–177, 70–75. [Google Scholar] [CrossRef]
- Long, M.; Wang, J.; Zhuang, H.; Zhang, Y.; Wu, H.; Zhang, J. Performance and mechanism of standard nano-TiO2 (P-25) in photocatalytic disinfection of foodborne microorganisms-Salmonella typhimurium and Listeria monocytogenes. Food Control 2014, 39, 68–74. [Google Scholar] [CrossRef]
- Makropoulou, T.; Panagiotopoulou, P.; Venieri, D. N-doped TiO2 photocatalysts for bacterial inactivation in water. J. Chem. Technol. Biotechnol. 2018, 93, 2518–2526. [Google Scholar] [CrossRef]
- Milosevic, I.; Jayaprakash, A.; Greenwood, B.; van Driel, B.; Rtimi, S.; Bowen, P. Synergistic effect of fluorinated and N doped TiO2 nanoparticles leading to different microstructure and enhanced photocatalytic bacterial inactivation. Nanomaterials 2017, 7, 391. [Google Scholar] [CrossRef]
- Choi, S.Y.; Cho, B. Extermination of influenza virus H1N1 by a new visible-light induced photocatalyst under fluorescent light. Virus Res. 2018, 248, 71–73. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Rao, G.; Brastad, K.S.; Zhang, Q.; Robinson, R.; He, Z.; Li, Y. Enhanced disinfection of Escherichia coli and bacteriophage MS2 in water using a copper and silver loaded titanium dioxide nanowire membrane. Front. Environ. Sci. Eng. 2016, 10, 11. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Inactivation of MS2 coliphage in sewage by solar photocatalysis using metal-doped TiO2. Appl. Catal. B Environ. 2015, 178, 54–64. [Google Scholar] [CrossRef]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-based nanostructured materials for microbial inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A review of pharmaceuticals and endocrinedisrupting compounds: Sources, effects, removal, and detections. Water. Air. Soil Pollut. 2013, 224, 1770. [Google Scholar] [CrossRef]
- Burkhardt-Holm, P. Endocrine disruptors and water quality: A state-of-the-art review. Int. J. Water Resour. Dev. 2010, 26, 477–493. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Tetreault, G.R.; Bennett, C.J.; Shires, K.; Knight, B.; Servos, M.R.; McMaster, M.E. Intersex and reproductive impairment of wild fish exposed to multiple municipal wastewater discharges. Aquat. Toxicol. 2011, 104, 278–290. [Google Scholar] [CrossRef]
- Tabata, A.; Kashiwada, S.; Ohnishi, Y.; Ishikawa, H.; Miyamoto, N.; Itoh, M.; Magara, Y. Estrogenic influences of estradiol-17 beta, p-nonylphenol and bis-phenol-A on Japanese medaka (Oryzias latipes) at detected environmental concentrations. Water Sci. Technol. 2001, 43, 109–116. [Google Scholar] [CrossRef]
- Villeneuve, S.; Cyr, D.; Lynge, E.; Orsi, L.; Sabroe, S.; Merletti, F.; Gorini, G.; Morales Suarez Varela, M.; Ahrens, W.; Baumgardt-Elms, C.; et al. Occupation and occupational exposure to endocrine disrupting chemicals in male breast cancer: A case-control study in Europe. Occup. Environ. Med. 2010, 67, 837–844. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Z.; Qing, D.; He, Y.; Wu, T.; Miao, M.; Wang, J.; Weng, X.; Ferber, J.R.; Herrinton, L.J.; et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum. Reprod. 2009, 25, 519–527. [Google Scholar] [CrossRef]
- Tong, A.Y.C.; Braund, R.; Warren, D.S.; Peake, B.M. TiO2-assisted photodegradation of pharmaceuticals—A review. Cent. Eur. J. Chem. 2012, 10, 989–1027. [Google Scholar] [CrossRef]
- AlAani, H.; Hashem, S.; Karabet, F. Photocatalytic (UV-A/TiO2) and photolytic (UV-A) degradation of steroid hormones: Ethinyl estradiol, levonorgestrel, and progesterone. Int. J. Chem. Res. 2017, 10, 1061–1070. [Google Scholar]
- Arlos, M.J.; Liang, R.; Hatat-Fraile, M.M.; Bragg, L.M.; Zhou, N.Y.; Servos, M.R.; Andrews, S.A. Photocatalytic decomposition of selected estrogens and their estrogenic activity by UV-LED irradiated TiO2 immobilized on porous titanium sheets via thermal-chemical oxidation. J. Hazard. Mater. 2016, 318, 541–550. [Google Scholar] [CrossRef]
- Sornalingam, K.; McDonagh, A.; Zhou, J.L.; Johir, M.A.H.; Ahmed, M.B. Photocatalysis of estrone in water and wastewater: Comparison between Au-TiO2 nanocomposite and TiO2, and degradation by-products. Sci. Total Environ. 2018, 610–611, 521–530. [Google Scholar] [CrossRef]
- Zatloukalová, K.; Obalová, L.; Koči, K.; Čapek, L.; Matěj, Z.; Šnajdhaufová, H.; Ryczkowski, J.; Słowik, G. Photocatalytic degradation of endocrine disruptor compounds in water over immobilized TiO2 photocatalysts. Iran. J. Chem. Chem. Eng. IJCCE 2017, 36, 29–38. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.M.; Tuberty, S.; Hambourger, M.; Bandala, E.R. Resource efficiency analysis for photocatalytic degradation and mineralization of estriol using TiO2 nanoparticles. Chemosphere 2017, 184, 1270–1285. [Google Scholar] [CrossRef]
- Solcova, O.; Spacilova, L.; Maleterova, Y.; Morozova, M.; Ezechias, M.; Kresinova, Z. Photocatalytic water treatment on TiO2 thin layers. Desalin. Water Treat. 2016, 57, 11631–11638. [Google Scholar] [CrossRef]
- Ruokolainen, M.; Gul, T.; Permentier, H.; Sikanen, T.; Kostiainen, R.; Kotiaho, T. Comparison of TiO2 photocatalysis, electrochemically assisted Fenton reaction and direct electrochemistry for simulation of phase i metabolism reactions of drugs. Eur. J. Pharmaceut. Sci. 2016, 83, 36–44. [Google Scholar] [CrossRef]
- Pazoki, M.; Parsa, M.; Farhadpour, R. Removal of the hormones dexamethasone (DXM) by Ag doped on TiO2 photocatalysis. J. Environ. Chem. Eng. 2016, 4, 4426–4434. [Google Scholar] [CrossRef]
- Chow, K.L.; Man, Y.B.; Tam, N.F.Y.; Liang, Y.; Wong, M.H. Removal of decabromodiphenyl ether (BDE-209) using a combined system involving TiO2 photocatalysis and wetland plants. J. Hazard. Mater. 2017, 322, 263–269. [Google Scholar] [CrossRef]
- Vasapollo, G.; Mele, G.; Del Sole, R.; Pio, I.; Li, J.; Mazzetto, S.E. Use of novel cardanol-porphyrin hybrids and their TiO2-based composites for the photodegradation of 4-Nitrophenol in water. Molecules 2011, 16, 5769–5784. [Google Scholar] [CrossRef]
- Sraw, A.; Kaur, T.; Pandey, Y.; Sobti, A.; Wanchoo, R.K.; Toor, A.P. Fixed bed recirculation type photocatalytic reactor with TiO2 immobilized clay beads for the degradation of pesticide polluted water. J. Environ. Chem. Eng. 2018, 6, 7035–7043. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Highly ordered TiO2 nanotube arrays wrapped with g-C3N4 nanoparticles for efficient charge separation and increased photoelectrocatalytic degradation of phenol. J. Hazard. Mater. 2018, 344, 369–380. [Google Scholar] [CrossRef]
- Martins, A.S.; Nuñez, L.; Lanza, M.R.d.V. Enhanced photoelectrocatalytic performance of TiO2 nanotube array modified with WO3 applied to the degradation of the endocrine disruptor propyl paraben. J. Electroanal. Chem. 2017, 802, 33–39. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of titanium dioxide nanoparticles exposure on human health-a review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Heilgeist, S.; Sekine, R.; Sahin, O.; Stewart, R.A. Finding Nano: Challenges involved in monitoring the presence and fate of engineered titanium dioxide nanoparticles in aquatic environments. Water 2021, 13, 734. [Google Scholar] [CrossRef]
- Jeon, S.-K.; Kim, E.-J.; Lee, J.; Lee, S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard. Mater. 2016, 317, 312–318. [Google Scholar] [CrossRef]
- Choi, S.; Johnston, M.; Wang, G.-S.; Huang, C.P. A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO2 and ZnO. Sci. Total Environ. 2018, 625, 1321–1329. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lessons learned: Are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016, 568, 470–479. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Ramasubbu, S. Fate and behaviour of TiO2 nanoparticles in the soil: Their impact on staple food crops. Water Air Soil Pollut. 2021, 232, 274. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Pu, Y.; Yin, L.; Li, Y.; Zhang, X.; Liang, G.; Li, X.; Zhang, J. Small-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivo. J. Nanosci. Nanotechnol. 2010, 10, 5161–5169. [Google Scholar] [CrossRef]
- Acar, M.; Bulut, Z.; Ateş, A.; Nami, B.; Koçak, N.; Yıldız, B. Titanium dioxide nanoparticles induce cytotoxicity and reduce mitotic index in human amniotic fluid-derived cells. Hum. Exp. Toxicol. 2015, 34, 74–82. [Google Scholar] [CrossRef]
- Biola-Clier, M.; Gaillard, J.-C.; Rabilloud, T.; Armengaud, J.; Carriere, M. Titanium dioxide nanoparticles alter the cellular phosphoproteome in A549 Cells. Nanomaterials 2020, 10, 185. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Du, X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 2009, 34, 559–567. [Google Scholar] [CrossRef]
- Lu, N.; Zhu, Z.; Zhao, X.; Tao, R.; Yang, X.; Gao, Z. Nano titanium dioxide photocatalytic protein tyrosine nitration: A potential hazard of TiO2 on skin. Biochem. Biophys. Res. Commun. 2008, 370, 675–680. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.; Wang, L.; Kisin, E.; Murray, A.R.; Schwegler-Berry, D.; Shvedova, A.A.; Castranova, V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L87–L97. [Google Scholar] [CrossRef]
- Hamilton, R.F., Jr.; Buford, M.C.; Wood, M.B.; Arnone, B.; Morandi, M.; Holian, A. Engineered carbon nanoparticles alter macrophage immune function and initiate airway hyper-responsiveness in the BALB/c mouse model. Nanotoxicology 2007, 1, 104–117. [Google Scholar] [CrossRef]
- Boffetta, P.; Gaborieau, V.; Nadon, L.; Parent, M.F.; Weiderpass, E.; Siemiatycki, J. Exposure to titanium dioxide and risk of lung cancer in a population-based study from Montreal. Scand. J. Work Environ. Health 2001, 27, 227–232. [Google Scholar] [CrossRef]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Dehghanian, A.R. The application of titanium dioxide (TiO2) nanoparticles in the photo-thermal therapy of melanoma cancer model. Iran J. Basic Med. Sci. 2018, 21, 1133–1139. [Google Scholar] [CrossRef]
- Fujiwara, R.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Cancer therapeutic effects of titanium dioxide nanoparticles are associated with oxidative stress and cytokine induction. Pathobiology 2015, 82, 243–251. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.; Liu, J.; Feng, X.; Zhou, T.; Shao, L. Is neurotoxicity of metallic nanoparticles the cascades of oxidative stress? Nanoscale Res. Lett. 2016, 11, 291. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H.; Demokritou, P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv. Colloid Interface Sci. 2017, 246, 165–180. [Google Scholar] [CrossRef]
- Patri, A.; Umbreit, T.; Zheng, J.; Nagashima, K.; Goering, P.; Francke-Carroll, S.; Gordon, E.; Weaver, J.; Miller, T.; Sadrieh, N.; et al. Energy dispersive X-ray analysis of titanium dioxide nanoparticle distribution after intravenous and subcutaneous injection in mice. J. Appl. Toxicol. 2009, 29, 662–672. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environ. Sci. Pollut. Res. Int. 2015, 22, 5519–5530. [Google Scholar] [CrossRef]
- Sager, T.M.; Kommineni, C.; Castranova, V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: Role of particle surface area. Part. Fibre Toxicol. 2008, 5, 17. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar] [CrossRef]
- Nemmar, A.; Melghit, K.; Al-Salam, S.; Zia, S.; Dhanasekaran, S.; Attoub, S.; Al-Amri, I.; Ali, B.H. Acute respiratory and systemic toxicity of pulmonary exposure to rutile Fe-doped TiO2 nanorods. Toxicology 2011, 279, 167–175. [Google Scholar] [CrossRef]
- Li, N.; Duan, Y.; Hong, M.; Zheng, L.; Fei, M.; Zhao, X.; Wang, J.; Cui, Y.; Liu, H.; Cai, J.; et al. Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticules. Toxicol. Lett. 2010, 195, 161–168. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Haberl, N.; Kozempel, J.; Hirn, S.; Wenk, A.; Schleh, C.; Schäffler, M.; Lipka, J.; Semmler-Behnke, M.; et al. Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: Part 1. Nanotoxicology 2017, 11, 434–442. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Schleh, C.; Kozempel, J.; Wenk, A.; Haberl, N.; Hirn, S.; Schäffler, M.; Lipka, J.; Semmler-Behnke, M.; et al. Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: Part 2. Nanotoxicology 2017, 11, 443–453. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.; Li, B.; Ge, C.; Zhou, G.; et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol. Lett. 2008, 183, 72–80. [Google Scholar] [CrossRef]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol. Vitr. 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- Grissa, I.; Guezguez, S.; Ezzi, L.; Chakroun, S.; Sallem, A.; Kerkeni, E.; Elghoul, J.; El Mir, L.; Mehdi, M.; Cheikh, H.B.; et al. The effect of titanium dioxide nanoparticles on neuroinflammation response in rat brain. Environ. Sci. Pollut. Res. Int. 2016, 23, 20205–20213. [Google Scholar] [CrossRef]
- Valentini, X.; Deneufbourg, P.; Paci, P.; Rugira, P.; Laurent, S.; Frau, A.; Stanicki, D.; Ris, L.; Nonclercq, D. Morphological alterations induced by the exposure to TiO2 nanoparticles in primary cortical neuron cultures and in the brain of rats. Toxicol. Rep. 2018, 5, 878–889. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Zhang, Z.; Schluesener, H.J. Apoptosis induced by titanium dioxide nanoparticles in cultured murine microglia N9 cells. Chin. Sci. Bull. 2009, 54, 3830–3836. [Google Scholar] [CrossRef]
- Ze, Y.; Hu, R.; Wang, X.; Sang, X.; Ze, X.; Li, B.; Su, J.; Wang, Y.; Guan, N.; Zhao, X.; et al. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 470–478. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Gurr, J.R.; Wang, A.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Huerta-García, E.; Pérez-Arizti, J.A.; Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Reeves, J.F.; Davies, S.J.; Dodd, N.J.; Jha, A.N. Hydroxyl radicals (*OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat. Res. 2008, 640, 113–122. [Google Scholar] [CrossRef]
- Rajapakse, K.; Drobne, D.; Valant, J.; Vodovnik, M.; Levart, A.; Marinsek-Logar, R. Acclimation of Tetrahymena thermophila to bulk and nano-TiO2 particles by changes in membrane fatty acids saturation. J. Hazard. Mater. 2012, 221–222, 199–205. [Google Scholar] [CrossRef]
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative determination of OH radical generation and its cytotoxicity induced by TiO2-UVA treatment. Toxicol. In Vitro 2002, 16, 629–635. [Google Scholar] [CrossRef]
- Petković, J.; Küzma, T.; Rade, K.; Novak, S.; Filipič, M. Pre-irradiation of anatase TiO2 particles with UV enhances their cytotoxic and genotoxic potential in human hepatoma HepG2 cells. J. Hazard. Mater. 2011, 196, 145–152. [Google Scholar] [CrossRef]
- Barthel, A.; Klotz, L.O. Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biol. Chem. 2005, 386, 207–216. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Geiser, M.; Casaulta, M.; Kupferschmid, B.; Schulz, H.; Semmler-Behnke, M.; Kreyling, W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am. J. Respir. Cell Mol. Biol. 2008, 38, 371–376. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Liu, X.; Sui, B.; Sun, J. Size-and shape-dependent effects of titanium dioxide nanoparticles on the permeabilization of the blood-brain barrier. J. Mater. Chem. B 2017, 5, 9558–9570. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.M.; Schürch, S.; Haenni, B.; Kapp, N.; Gehr, P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ. Sci. Technol. 2006, 40, 4353–4359. [Google Scholar] [CrossRef]
- Stone, V.; Nowack, B.; Baun, A.; van den Brink, N.; von der Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellöv, M.; Joner, E.; et al. Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physico-chemical characterization. Sci. Total Environ. 2010, 408, 1745–1754. [Google Scholar] [CrossRef]
- Li, L.; Sillanpää, M.; Risto, M. Influences of water properties on the aggregation and deposition of engineered titanium dioxide nanoparticles in natural waters. Environ. Pollut. 2016, 219, 132–138. [Google Scholar] [CrossRef]
- Skrabal, S.A.; Terry, C.M. Distributions of dissolved titanium in porewaters of estuarine and coastal marine sediments. Mar. Chem. 2002, 77, 109–122. [Google Scholar] [CrossRef]
- Shi, X.H.; Jiang, P.; Wang, H.Y.; Zhang, S.H.; Ji, T.; Liu, W.J.; Zhou, H.Y. The redshifted hydrogen Balmer and metastable He i absorption line system in mini-FeLoBAL quasar SDSS J112526. 12+ 002901.3: A parsec-scale accretion inflow? Astrophys. J. 2016, 829, 96. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.H.; Zhang, H.; Zhao, L. UV irradiation induced transformation of TiO2 nanoparticles in water: Aggregation and photoreactivity. Environ. Sci. Technol. 2014, 48, 11962–11968. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Shon, H.K.; Bolan, N.S.; El Saliby, I.; Vigneswaran, S. Sources, Distribution, Environmental Fate, and Ecological Effects of Nanomaterials in Wastewater Streams. Crit. Rev. Environ. Sci. Technol. 2015, 45, 277–318. [Google Scholar] [CrossRef]
- Valério, A.; Sárria, M.P.; Rodriguez-Lorenzo, L.; Hotza, D.; Espiña, B.; Gómez González, S.Y. Are TiO2 nanoparticles safe for photocatalysis in aqueous media? Nanoscale Adv. 2020, 2, 4951–4960. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Maldonado, M.I.; Malato, S.; Sánchez, B. Regeneration approaches for TiO2 immobilized photocatalyst used in the elimination of emerging contaminants in water. Catal. Today 2014, 230, 27–34. [Google Scholar] [CrossRef]
- Laisney, J.; Rosset, A.; Bartolomei, V.; Predoi, D.; Truffier-Boutry, D.; Artous, S.; Bergé, V.; Brochardd, G.; Michaud-Soret, I. TiO2 nanoparticles coated with bio-inspired ligands for the safer-by-design development of photocatalytic paints. Environ. Sci. Nano 2021, 8, 297–310. [Google Scholar] [CrossRef]
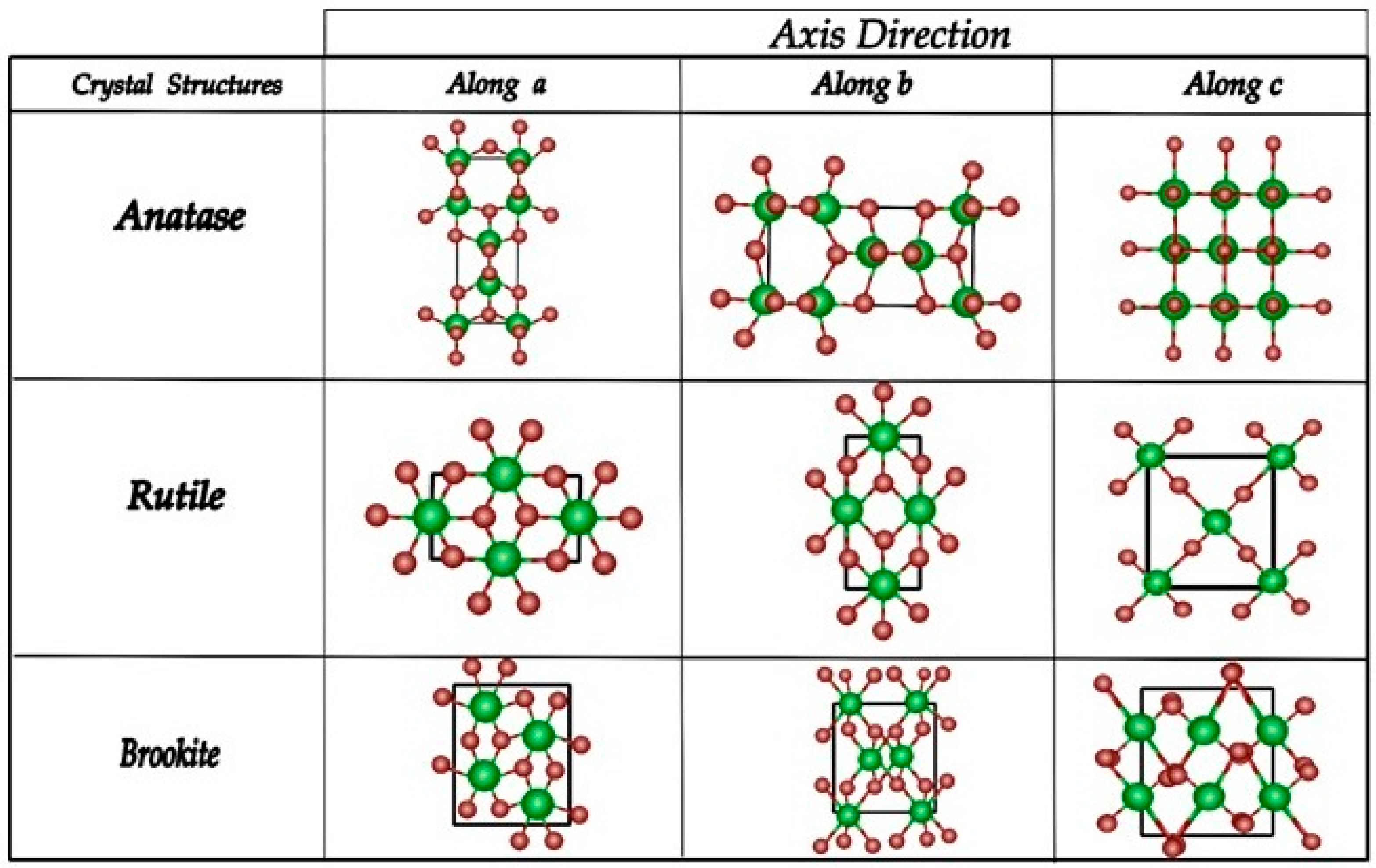
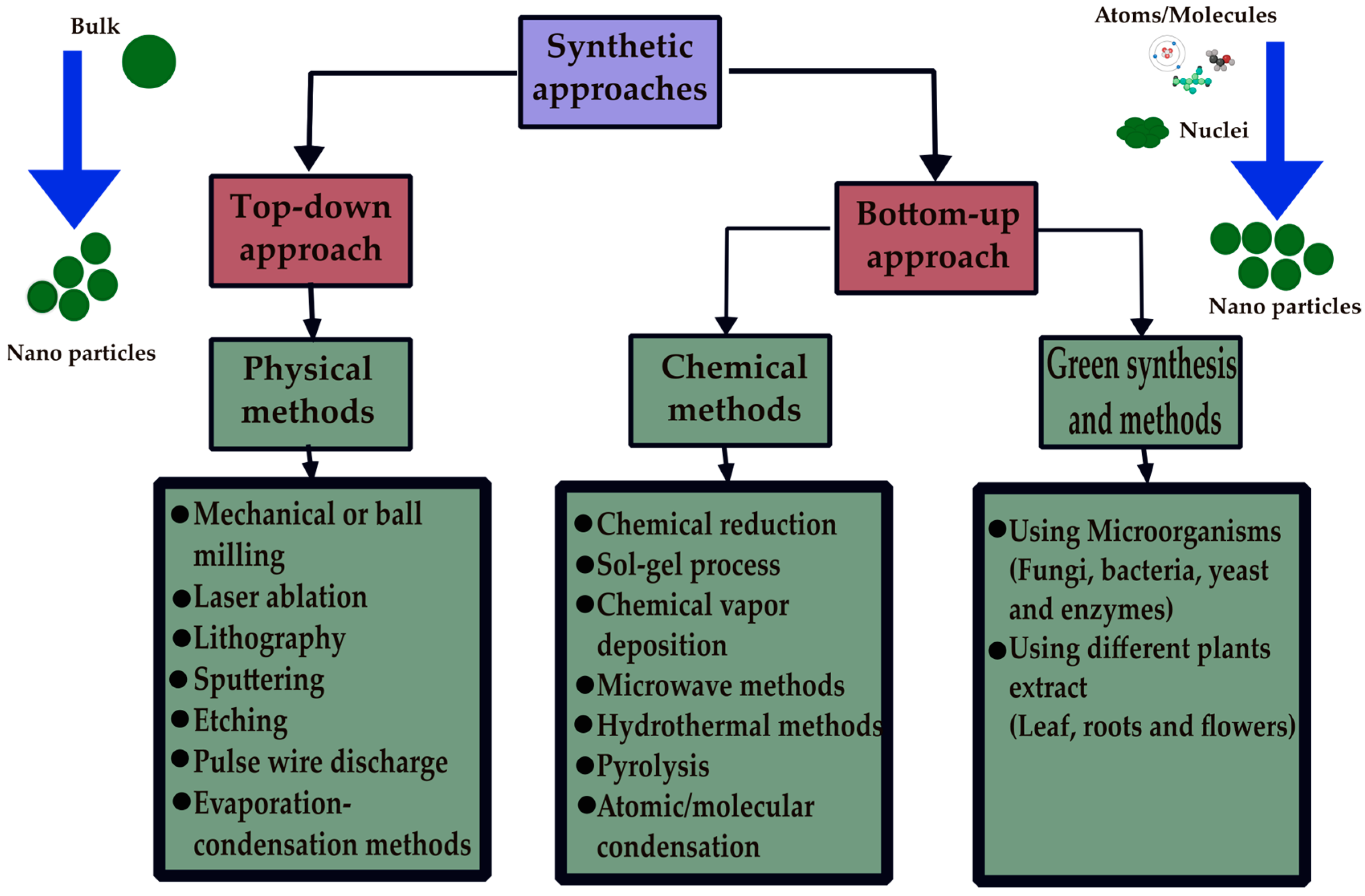
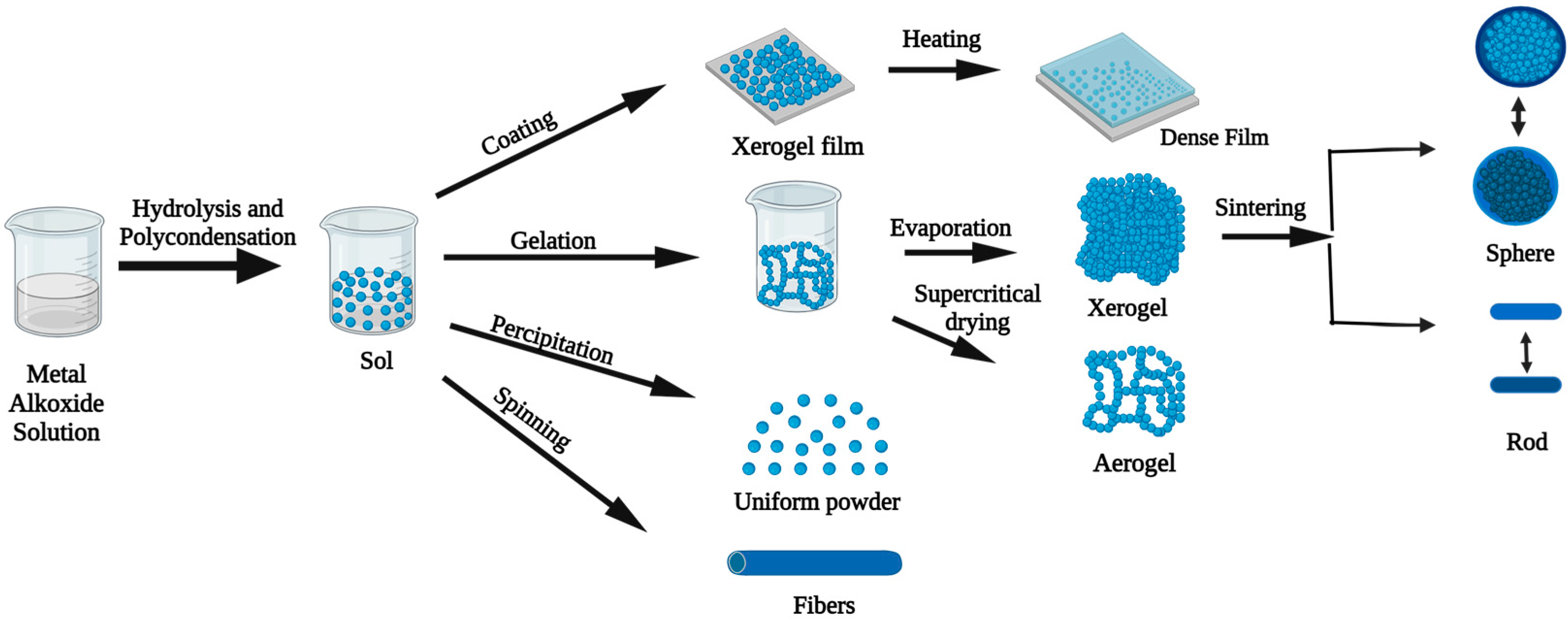
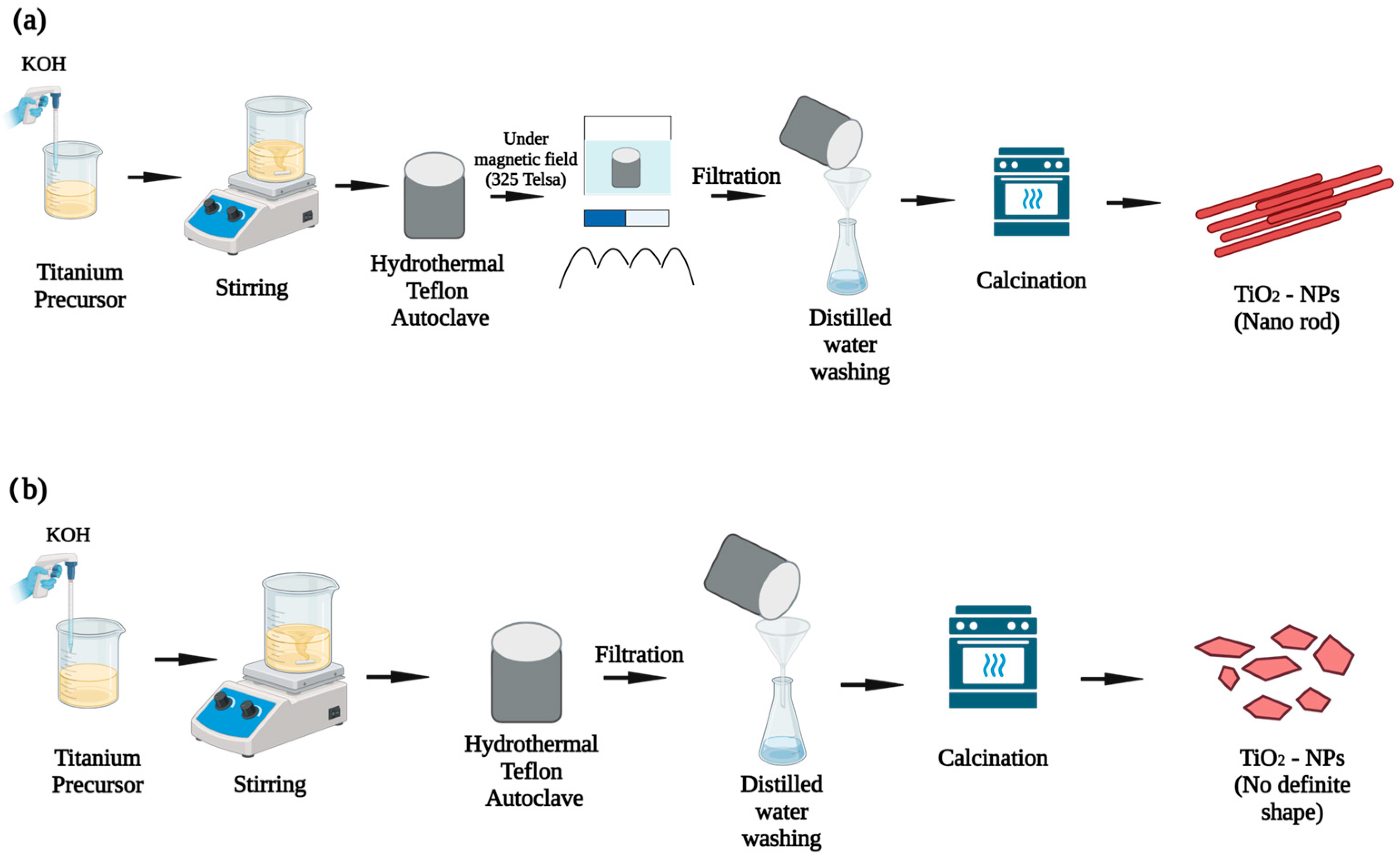
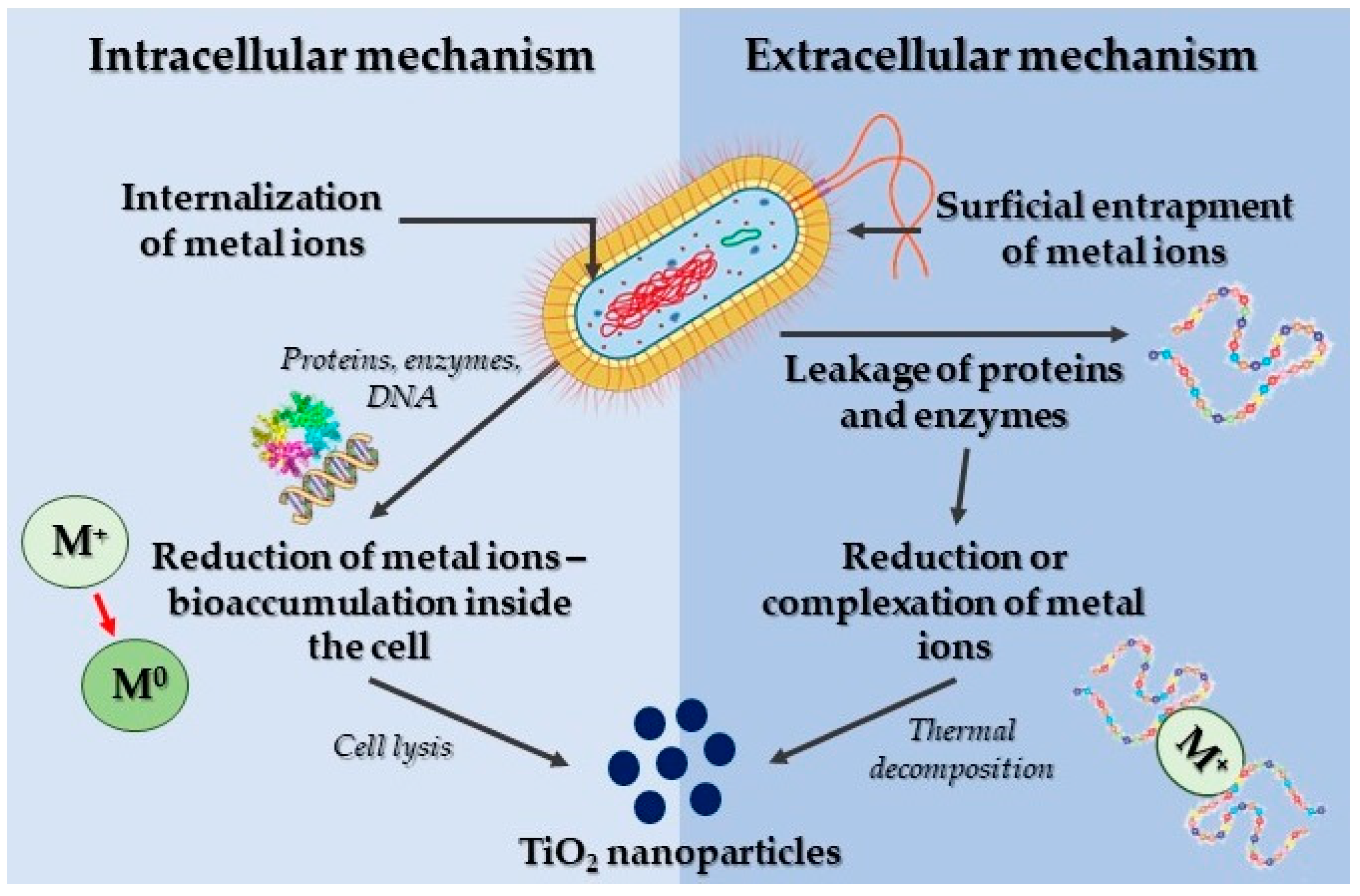
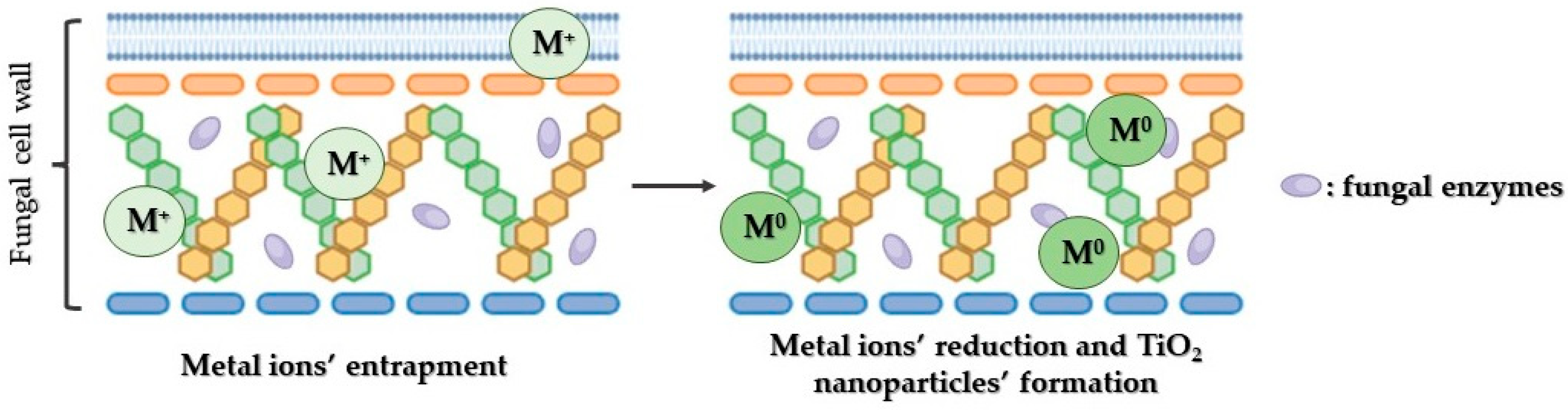
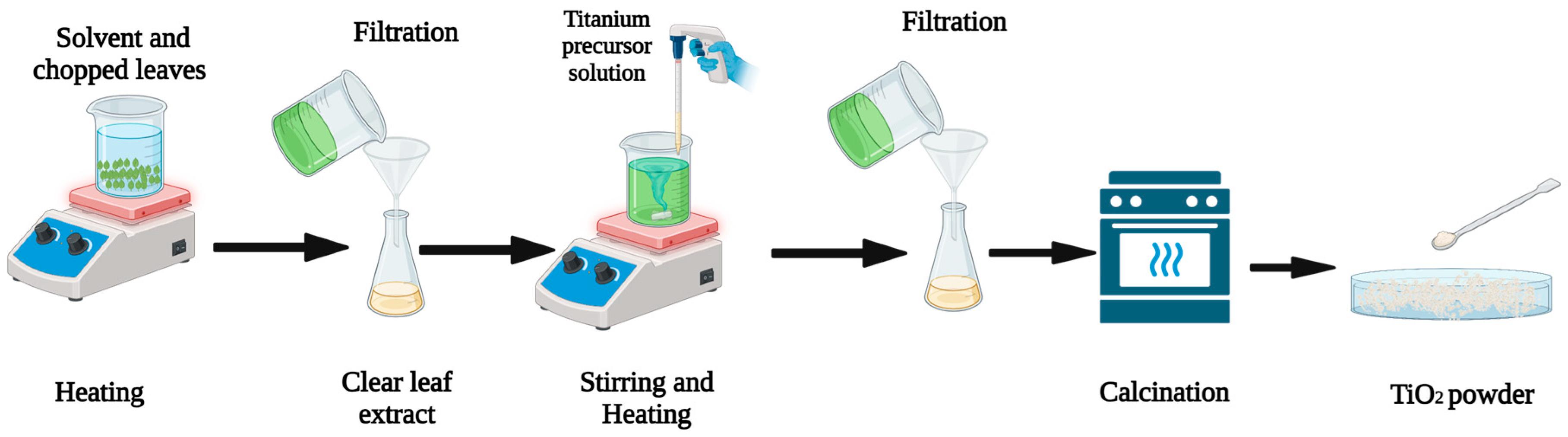
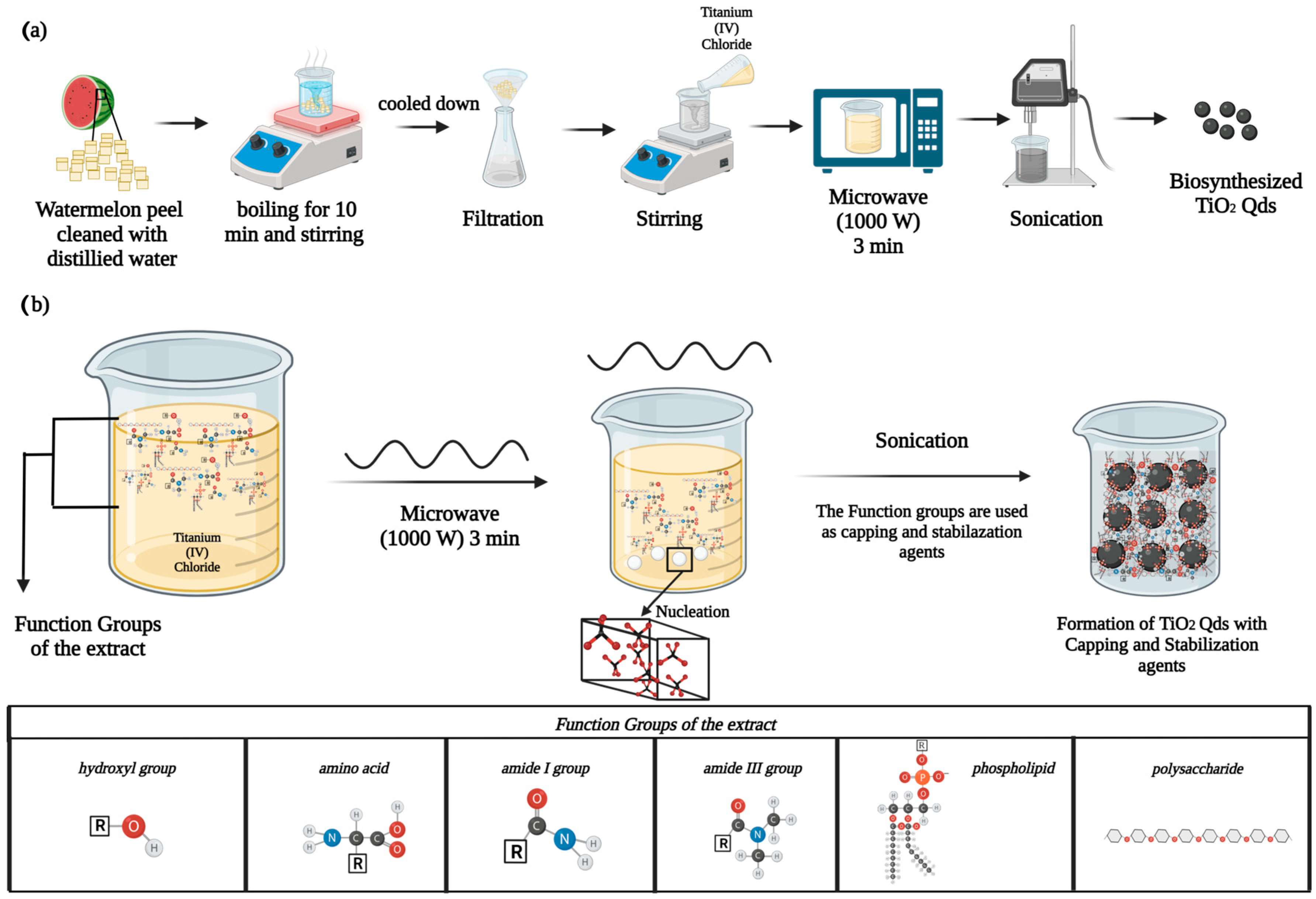
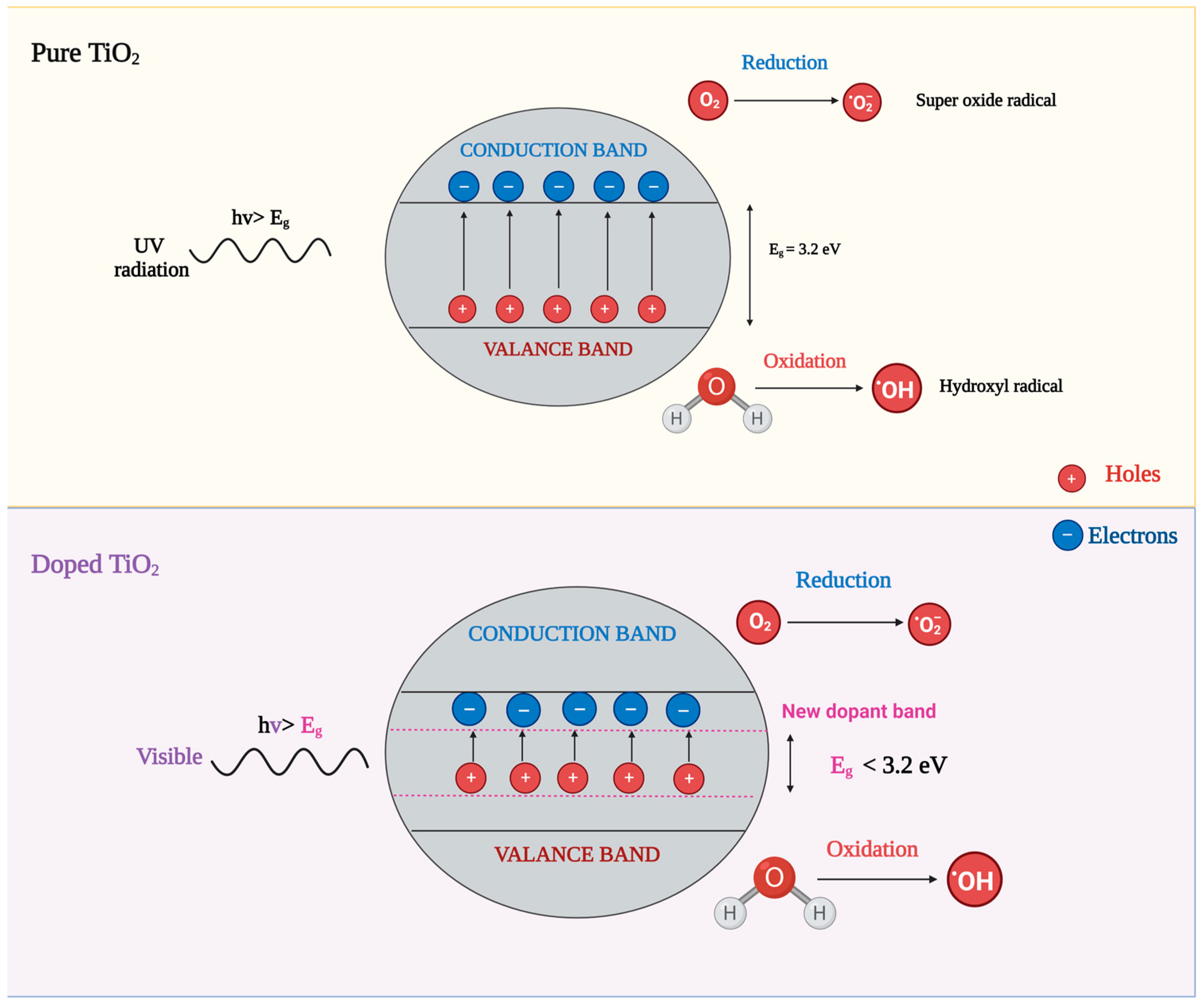

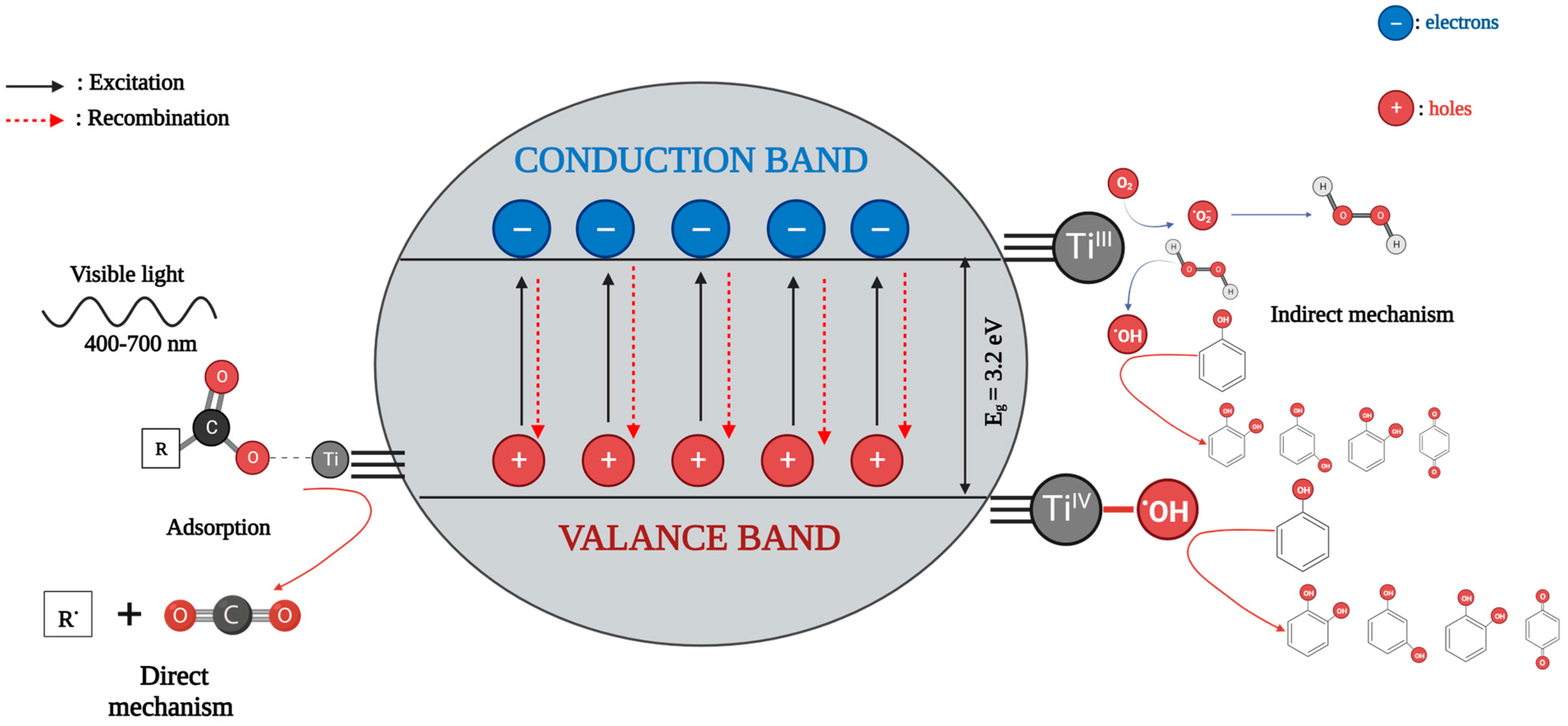

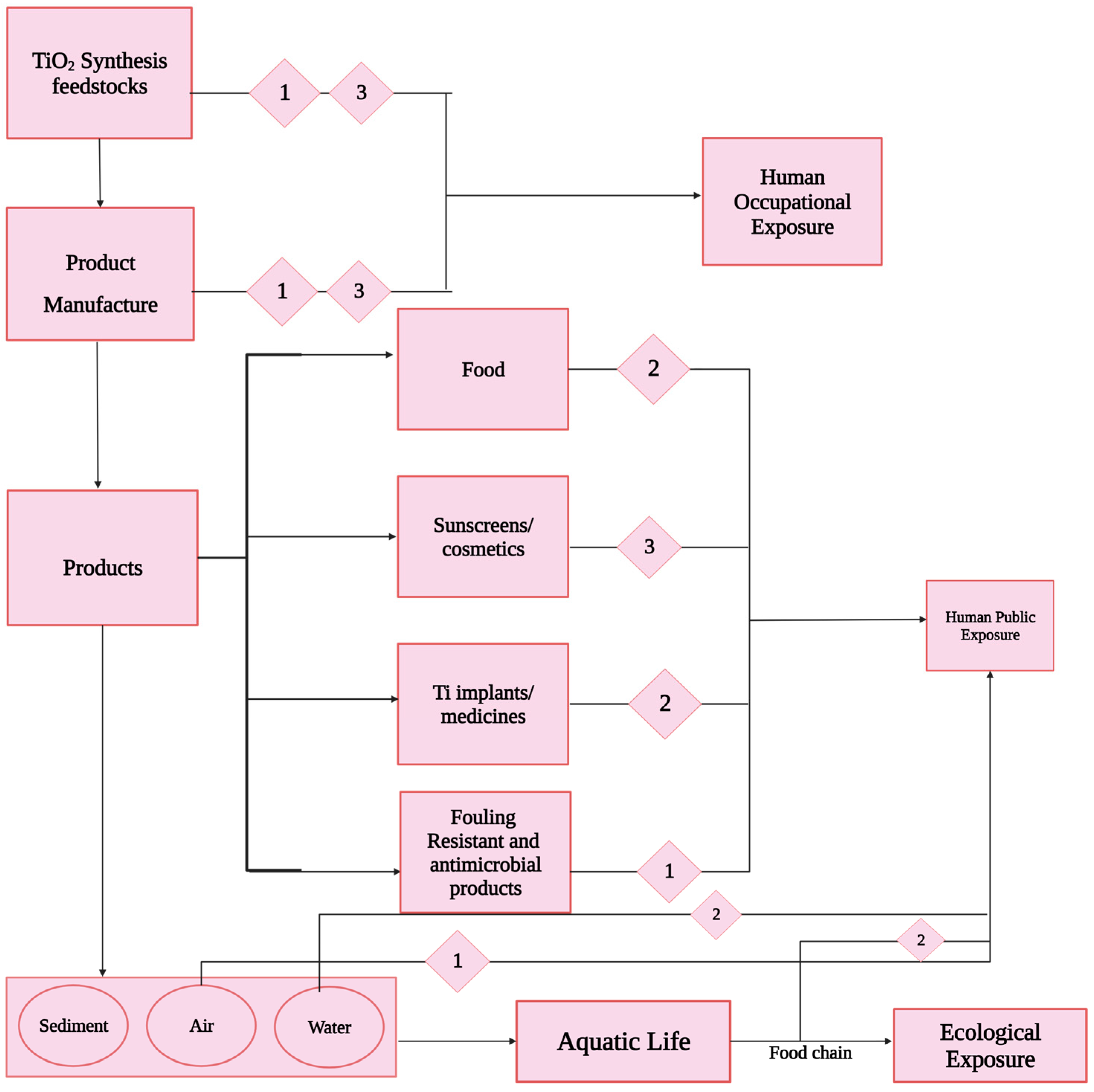
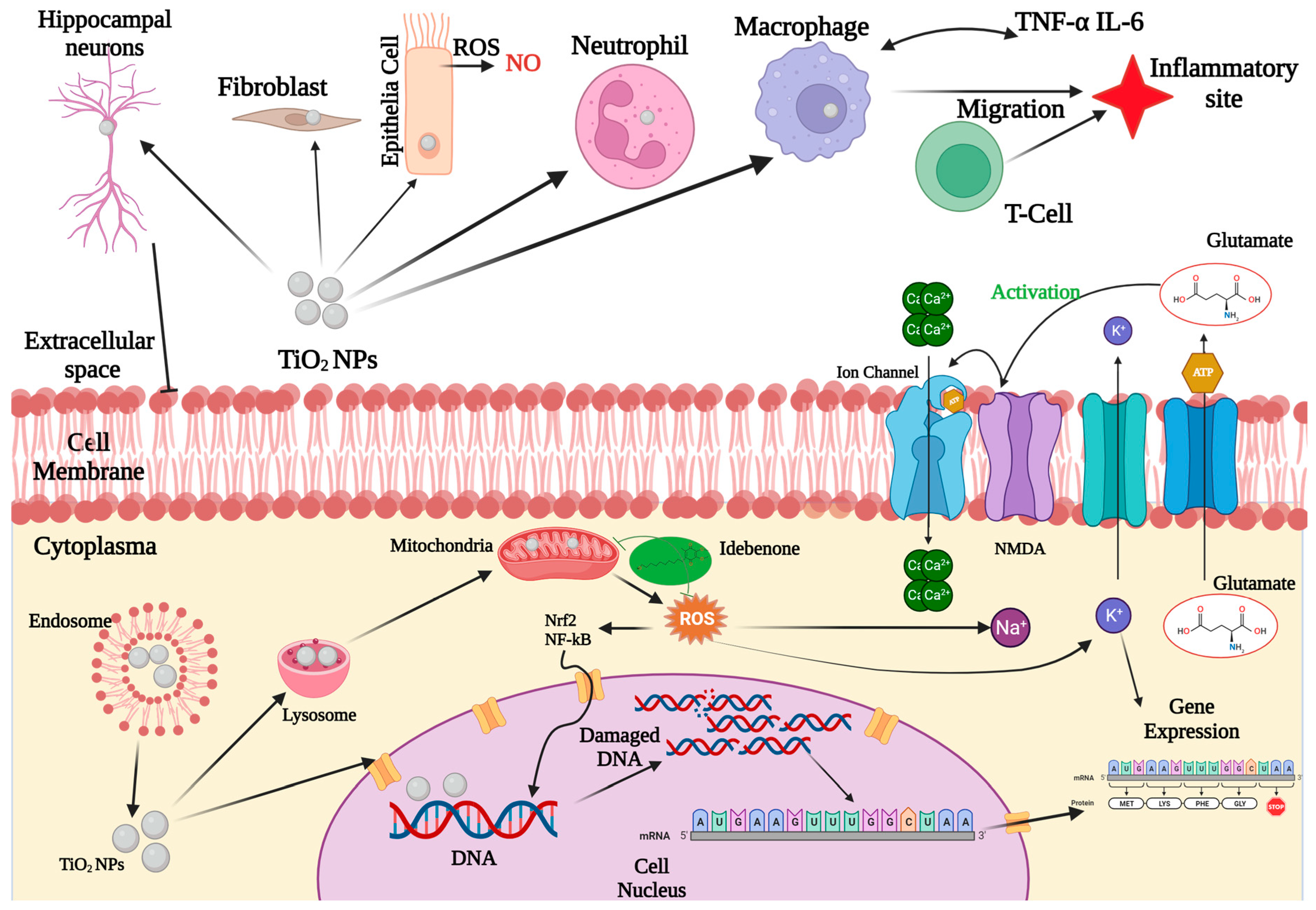
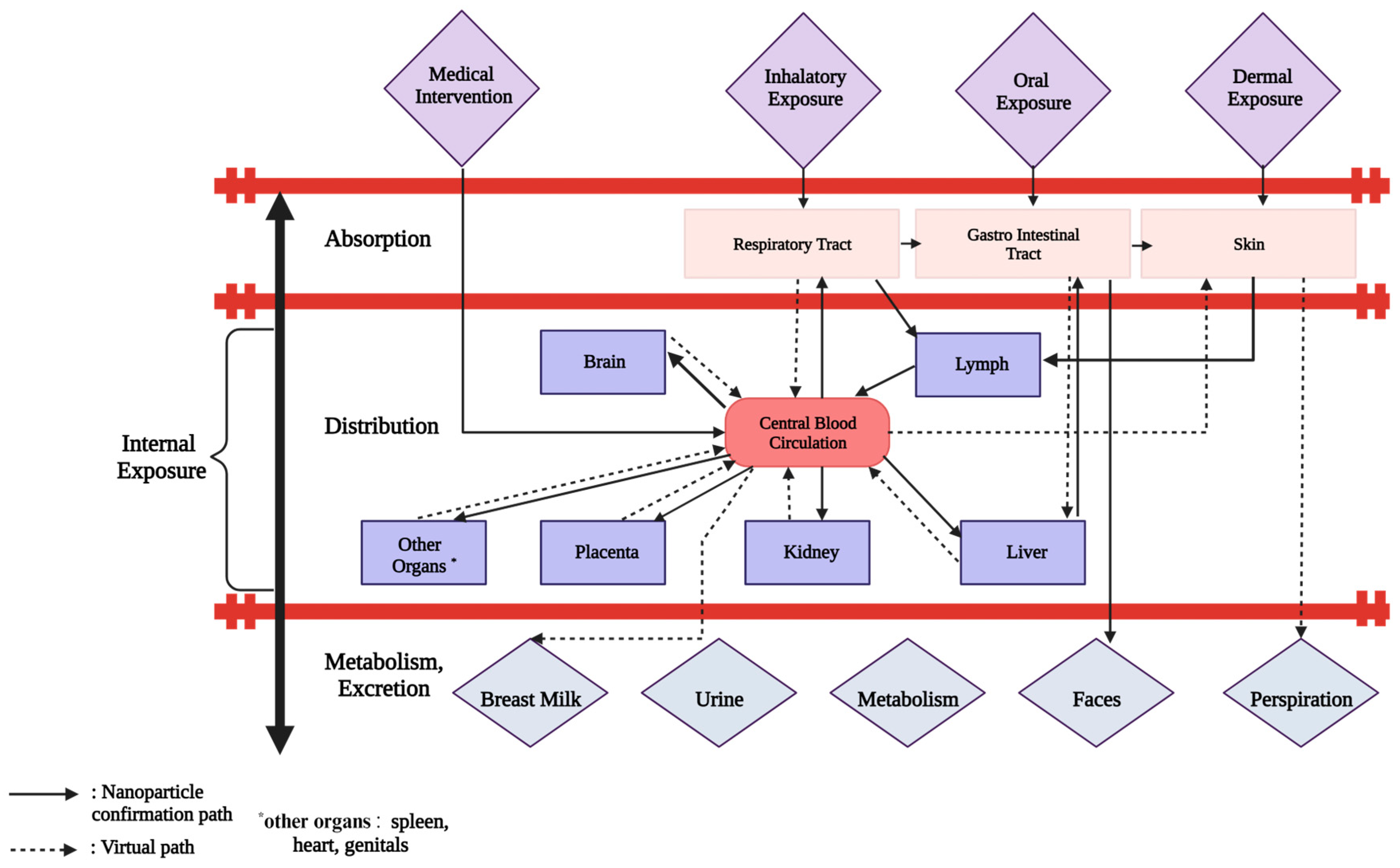
| Feature | Anatase | Rutile | Brookite |
|---|---|---|---|
| Crystal structure | Tetragonal I41/Amd (141) | Tetragonal P42/mnm (136) | Orthorhombic Pbc21 (29) |
| Lattice parameters a, b, c (Å) | a = b = 3.785 c = 9.514 | a = b = 4.593 c = 2.958 | a = 5.502 b = 8.942 c = 5.144 |
| Lattice volume (Å3) | 136.25 | 62.07 | 257.38 |
| Specific Gravity (g/cm3) | 3.8–3.9 | ≥4.2 | 3.9–4.1 |
| Hardness (Mohs scale) | 5.5–6.0 | 5.5–6.0 | 5.5–6.0 |
| Density (kg/m3) | 3830 | 4240 | 4170 |
| Refractive index | ng = 2.5688, np = 2.6584 | ng = 2.9467, np = 2.6506 | ng = 2.809, np = 2.677 |
| Band-gap (eV) | 3.2–3.26 | 2.8–3.0 | 2.9 |
| Absorption band-gap (eV) | 2.04 | 1.78 | 2.20 |
| Ti-O bond length (Å) | 1.92–1.95 | 1.91–1.94 | 1.87–2.04 |
| O-O bond length (Å) | 2.43 | 2.43 | 2.49 |
| Synthetic Method | Benefits | Drawbacks |
|---|---|---|
| Physical |
|
|
| Chemical |
|
|
| Green/biosynthesis |
|
|
| Synthetic Method | Benefits | Drawbacks |
|---|---|---|
| Sol–gel |
|
|
| Hydrothermal |
|
|
| Chemical Vapor Deposition (CVD) |
|
|
| Physical Vapor Deposition (PVD) |
|
|
| Green/biosynthesis |
|
|
| Green Synthetic Method | Benefits | Drawbacks |
|---|---|---|
| Microorganism-assisted method |
|
|
| Plant-assisted method |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatou, M.-A.; Syrrakou, A.; Lagopati, N.; Pavlatou, E.A. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions 2024, 5, 135-194. https://doi.org/10.3390/reactions5010007
Gatou M-A, Syrrakou A, Lagopati N, Pavlatou EA. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions. 2024; 5(1):135-194. https://doi.org/10.3390/reactions5010007
Chicago/Turabian StyleGatou, Maria-Anna, Athanasia Syrrakou, Nefeli Lagopati, and Evangelia A. Pavlatou. 2024. "Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review" Reactions 5, no. 1: 135-194. https://doi.org/10.3390/reactions5010007
APA StyleGatou, M.-A., Syrrakou, A., Lagopati, N., & Pavlatou, E. A. (2024). Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions, 5(1), 135-194. https://doi.org/10.3390/reactions5010007










