Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity
Abstract
1. Introduction
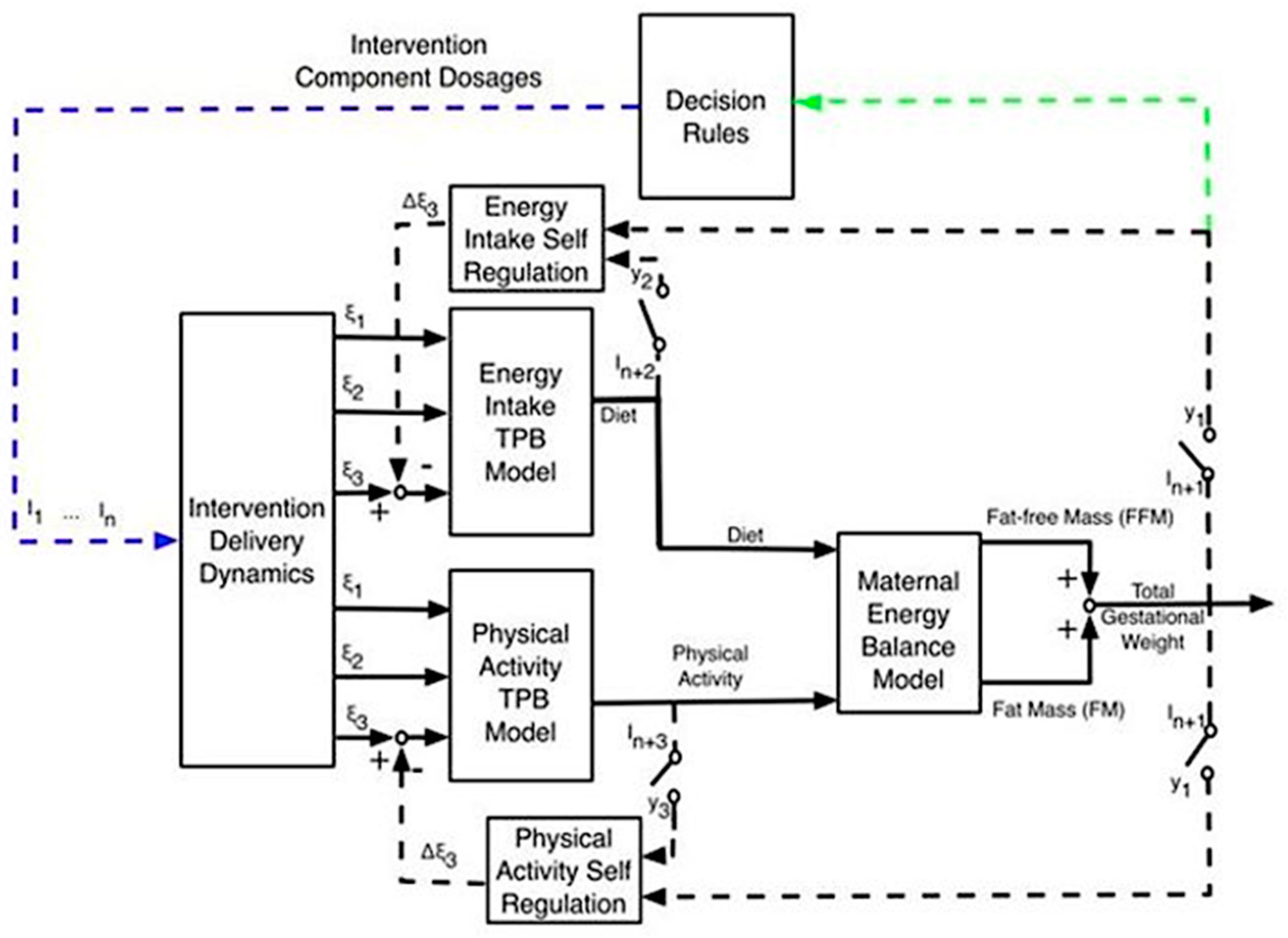
2. Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.4. Data Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, K.A. Alterations in sleep during pregnancy and postpartum: A review of 30 years of research. Sleep Med. Rev. 1998, 2, 231–242. [Google Scholar] [CrossRef]
- Lee, K.A.; Zaffke, M.E.; McEnany, G. Parity and sleep patterns during and after pregnancy. Obstet. Gynecol. 2000, 95, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Signal, T.L.; Gander, P.H.; Sangalli, M.R.; Travier, N.; Firestone, R.T.; Tuohy, J.F. Sleep duration and quality in healthy nulliparous and multiparous women across pregnancy and post-partum. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.L.; Richoux, S.E.; Beebe, K.R.; Lee, K.A. Sleep disruption and duration in late pregnancy is associated with excess gestational weight gain among overweight and obese women. Birth 2017, 44, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.H.; Au Yeung, E.; Law, B.M.H. Effectiveness of physical activity interventions on pregnancy related outcomes among pregnant women: A systematic review. Int. J. Environ. Res. Pub. Health 2019, 16, 1840. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Micheli, K.; Komninos, I.; Bagkeris, E.; Roumeliotaki, T.; Koutis, A.; Kogevinas, M.; Chatzi, L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology 2011, 22, 738–744. [Google Scholar] [CrossRef]
- Nunnery, D.; Ammerman, A.; Dharod, J. Predictors and outcomes of excess gestational weight gain among low-income pregnant women. Health Care Women Int. 2018, 39, 19–33. [Google Scholar] [CrossRef]
- Okun, M.L.; Luther, J.F.; Wisniewski, S.R.; Sit, D.; Prairie, B.A.; Wisner, K.L. Disturbed sleep, a novel risk factor for preterm birth? J. Womens Health 2012, 21, 54–60. [Google Scholar] [CrossRef]
- Polley, B.A.; Wing, R.R.; Sims, C.J. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1494–1502. [Google Scholar] [CrossRef]
- Phelan, S.; Phipps, M.G.; Abrams, B.; Darroch, F.; Schaffner, A.; Wing, R.R. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for Delivery Study. Am. J. Clin. Nutr. 2011, 93, 772–779. [Google Scholar] [CrossRef]
- Savage, J.S.; Marini, M.; Birch, L.L. Dietary energy density predicts women’s weight change over 6 y. Am. J. Clin. Nutr. 2008, 88, 677–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebire, N.J.; Jolly, M.; Harris, J.P.; Wadsworth, J.; Joffe, M.; Beard, R.W.; Regan, L.; Robinson, S. Maternal obesity and pregnancy outcome: A study of 287,213 pregnancies in London. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.; E Navarro-Barrientos, J.; Rivera, D.E.; Heymsfield, S.B.; Bredlau, C.; Redman, L.M.; Martin, C.K.; A Lederman, S.; Collins, L.M.; Butte, N.F. Dynamic energy-balance model predicting gestational weight gain. Am. J. Clin. Nutr. 2012, 85, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Ellis, K.J.; Wong, W.W.; Hopkinson, J.M.; Smith, E. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am. J. Obstet. Gynecol. 2003, 189, 423–432. [Google Scholar] [CrossRef]
- Butte, N.F.; Wong, W.W.; Treuth, M.S.; Ellis, K.J.; Smith, E.O. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr. 2004, 79, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Deshpande, S.; Rivera, D.E.; Danielle, S.D.; Jennifer, S.S. Hybrid model predictive control for sequential decision policies in adaptive behavioral interventions. In Proceedings of the 2014 American Control Conference, Portland, OR, USA, 4–6 June 2014. [Google Scholar]
- Dong, Y.; Rivera, D.E.; Downs, D.S.; Savage, J.S.; Thomas, D.M.; Collins, L.M. Hybrid model predictive control for optimizing gestational weight gain behavioral interventions. In Proceedings of the 2013 American Control Conference, Washington, DC, USA, 17–19 June 2013. [Google Scholar]
- Dong, Y.; Rivera, D.E.; Thomas, D.M.; Navarro-Barrientos, J.E.; Downs, D.S.; Savage, J.S.; Collins, L.M. A dynamical systems model for improving gestational weight gain behavioral interventions. In Proceedings of the 2012 American Control Conference (ACC), Montreal, QC, Canada, 27–29 June 2012. [Google Scholar]
- Downs, D.S.; Savage, J.S.; Rivera, D.E.; Smyth, J.M.; Rolls, B.J.; Hohman, E.E.; McNitt, K.M.; Kunselman, A.R.; Stetter, C.; Pauley, A.M.; et al. Individually-Tailored, adaptive intervention to manage gestational weight gain: Protocol for a randomized controlled trial in overweight and obese women. JMIR Res. Protoc. 2018, 7, e150. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary Reference Intakes: The Essential Guide to Nutrient Requirement; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- McGowan, C.A.; McAuliffe, F.M. Maternal nutrient intakes and levels of under-reporting during early pregnancy. Eur. J. Clin. Nutr. 2012, 66, 906–913. [Google Scholar] [CrossRef]
- Wennberg, A.L.; Isaksson, U.; Sandström, H.; Lundqvist, A.; Hörnell, A.; Hamberg, K. Swedish women’s food habits during pregnancy up to six months post-partum: A longitudinal study. Sex. Reprod. Healthc. 2016, 8, 31–36. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice. Committee Opinion No. 650 Summary. physical activity and exercise during pregnancy and the postpartum period. Obstet. Gynecol. 2015, 126, 1326–1327. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; US Dept of Health and Human Services: Washington, DC, USA, 2018.
- Hui, A.L.; Back, L.; Ludwig, S.; Gardiner, P.F.; Sevenhuysen, G.; Dean, H.; Sellers, E.A.; McGavock, J.; Morris, M.; Jiang, D.; et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy body mass index in a randomized control trial. BMC Preg. Childbirth 2014, 14, 331. [Google Scholar] [CrossRef]
- Redman, L.M.; Gilmore, L.A.; Breaux, J.; Thomas, D.J.; Elkind-Hirsch, K.E.; Stewart, T.; Hsia, D.S.; Burton, J.H.; Apolzan, J.W.; E Cain, L.; et al. Effectiveness of SmartMoms, a novel eHealth intervention for management of gestational weight gain: Randomized controlled pilot trial. JMIR Mhealth Uhealth 2017, 5, e133. [Google Scholar] [CrossRef] [PubMed]
- Pauley, A.M.; Moore, G.; Mama, S.; Molenaar, P.; Symons Downs, D. Systematic review of the associations between prenatal sleep behaviors and components of energy balance for regulating weight gain. Sleep Health. Under Review.
- Chang, M.-W.; Brown, R.; Nitzke, S.; Smith, B.; Eghtedary, K. Stress, sleep, depression and dietary intakes among low-income overweight and obese pregnant women. Matern. Child. Health J. 2015, 19, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Van Lee, L.; Chia, A.-R.; Loy, S.L.; Colega, M.T.; Tham, E.K.H.; Cai, S.; Yap, F.; Godfrey, K.M.; Teoh, O.H.; Goh, D.Y.T.; et al. Sleep and dietary patterns in pregnancy: Findings from the GUSTO Cohort. Int. J. Environ. Res. Publich Health 2017, 14, 1409. [Google Scholar] [CrossRef]
- Duke, C.H.; Williamson, J.A.; Snook, K.R.; Finch, K.C.; Sullivan, K. Association between fruit and vegetable consumption and sleep quantity in pregnant women. Matern. Child. Health J. 2017, 21, 966–973. [Google Scholar] [CrossRef]
- Merkx, A.; Ausems, M.; Budé, L.; De Vries, R.; Nieuwenhuijze, M.J. Weight gain in healthy pregnant women in relation to pre-pregnancy BMI, diet and physical activity. Midwifery 2015, 31, 693–701. [Google Scholar] [CrossRef]
- Restall, A.; Taylor, R.S.; Thompson, J.M.D.; Deralie, F.; Gustaaf, A.D.; Louise, C.K.; Lucilla, P.; Lesley, M.E.M. Risk factors for excessive gestational weight gain in a health, nulliparous cohort. J. Obes. 2014, 2014, 148391. [Google Scholar] [CrossRef]
- Pauley, A.M.; Figueiro, M.G.; Symons Downs, D. Correspondence between self-report and actigraphy measures of sleep behaviors in pregnant women with overweight/obesity. Sleep Med. Under Review.
- Jarman, M.; Yuan, Y.; Pakseresht, M.; Shi, Q.; Robson, P.J.; Bell, R.C.; the Alberta Pregnancy Outcomes and Nutrition study team the ENRICH team; Enrich, T.; Team, E. Patterns and trajectories of gestational weight gain: A prospective cohort study. CMAJ Open 2016, 4, E338–E345. [Google Scholar] [CrossRef]
- Santos, S.; Eekhout, I.; Voerman, E.; Gaillard, R.; Barros, H.; Charles, M.-A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; Corpeleijn, E.; et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. 2018, 16. [Google Scholar] [CrossRef]
- Symons Downs, D.; Savage, J.S.; Rivera, D.E.; Pauley, A.M.; Leonard, K.S.; Hohman, E.E.; Guo, P.; Stetter, C.; Kunselman, A. Adaptive, behavioral intervention impacts weight gain, physical activity, energy intake, and motivational determinants: Results of a feasibility trial in pregnant women with overweight/obesity. J. Beh. Med. Under Review.
- Symons Downs, D.; Rivera, D.E.; Savage, J.S.; Stetter, C.; Kunselman, A.; Pauley, A.M.; Leonard, K.S.; Guo, P.; Hohman, E.E. Optimizing an individually-tailored, adaptive intervention to manage weight in overweight and obese pregnant women. Annals Beh. Med. 2019, 53, S149–S150. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- De Zambotti, M.; Claudatos, S.; Inkelis, S.; Colrain, I.M.; Baker, F.C. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol. Int. 2015, 32, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Rivera, D.E.; Downs, D.S.; Savage, J.S. Semi-physical identification and state estimation of energy intake for interventions to manage gestational weight gain. In Proceedings of the 2016 American Control. Conference, Boston, MA, USA, 6–8 July 2016. [Google Scholar]
- Guo, P.; Rivera, D.E.; Pauley, A.M.; Leonard, K.S.; Savage, J.S.; Downs, D.S. A “Model-on-Demand” methodology for energy intake estimation to improve gestational weight control interventions. In Proceedings of the 18th IFAC Symposium on System Identification (SYSID 2018), Stockholm, Sweden, 9–11 July 2018; pp. 144–149. [Google Scholar]
- Guo, P.; Rivera, D.E.; Savage, J.S.; Hohman, E.E.; Pauley, A.M.; Leonard, K.S.; Downs, D.S. System identification approaches for energy intake estimation: Enhancing interventions for managing gestational weight gain. IEEE Trans. Control. Syst. Technol. 2018, 1–16. [Google Scholar] [CrossRef]
- Kubala, A.G.; Santos, E.C.; Barone Gibbs, B.; Buysee, D.J.; Patel, S.R.; Hall, M.H.; Kline, C.E. Field-based sleep measurement: Concordance between commercial activity monitors and an actigraph. Sleep 2018, 41, A123–A124. [Google Scholar] [CrossRef]
- Lichtman, S.W.; Pisarska, K.; Berman, E.R.; Pestone, M.; Dowling, H.; Offenbacher, E.; Weisel, H.; Heshka, S.; Matthews, D.E.; Heymsfield, S.B. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N. Engl. J. Med. 1992, 327, 1893–1898. [Google Scholar] [CrossRef]
- Ferguson, T.; Rowlands, A.V.; Olds, T.; Maher, C. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 42. [Google Scholar] [CrossRef]
- Storm, F.A.; Heller, B.W.; Mazzà, C. Step detection and activity recognition accuracy of seven physical activity monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar] [CrossRef]
- Hood, K.M.; Marr, C.; Kirk-Sorrow, J.; Farmer, J.; Lee, C.M.; Kern, M.; Bagley, J.R. Validity and reliability of a Wi-Fi smart scale to estimate body composition. Health Technol. 2019, 9, 839–846. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Inc.: New Jersey, NJ, USA, 1988. [Google Scholar]
- Quené, H.; Van den Bergh, H. On multi-level modeling of data from repeated measures designs: A tutorial. Speech Commun. 2004, 43, 103–121. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesely: Boston, MA, USA, 1977. [Google Scholar]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Downs, D.S.; DiNallo, J.M.; Birch, L.L.; Paul, I.M.; Ulbrecht, J.S. Randomized face-to-face vs. home exercise interventions in pregnant women with gestational diabetes. Psychol. Sport Exerc. 2017, 30, 73–81. [Google Scholar] [CrossRef]
- Downs, D.S.; Feinberg, M.; Hillemeier, M.M.; Weisman, C.S.; Chase, G.A.; Chuang, C.H.; Parrott, R.; Francis, L.A. Design of the Central Pennsylvania Women’s Health Study (CePAWHS) strong healthy women intervention: Improving preconceptional health. Mat. Child. Health J. 2009, 13, 18–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dodeen, H.M. Effectiveness of valid mean substitution in treating missing data in attitude assessment. Assess. Eval. Higher Ed. 2003, 28, 505–513. [Google Scholar] [CrossRef]
- Zuraikat, F.; Makarem, N.; Liao, M.; St-Onge, M.-P.; Aggarwal, B. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2020, 9, e014587. [Google Scholar] [CrossRef]
- Gay, C.L.; Lee, K.A.; Lee, S.Y. Sleep patterns and fatigue in new mothers and fathers. Biol. Res. Nurs. 2004, 5, 311–318. [Google Scholar] [CrossRef]
- Lee, K.A. Sleep and fatigue. Ann. Rev. Nurs. Res. 2001, 19, 249–273. [Google Scholar] [CrossRef]
| Mean | SD | N (%) | |
|---|---|---|---|
| Age | 30.6 | 3.2 | |
| Gestational Week at Study Entry | 10.1 | 1.6 | |
| Pre-pregnancy BMI | 31.8 | 3.2 | |
| OW | 14 (58.3) | ||
| OB | 10 (41.7) | ||
| Race | |||
| White | 24 (100) | ||
| Employment | |||
| Full-Time | 21 (87.5) | ||
| Other | 3 (12.5) | ||
| Education | |||
| High School | 1 (4.2) | ||
| College | 11 (45.8) | ||
| Graduate/Professional | 12 (50.0) | ||
| Family Income | |||
| $10–20,000 | 1 (4.2) | ||
| $20–40,000 | 5 (20.8) | ||
| $40–100,000 | 10 (41.7) | ||
| >$100,000 | 8 (33.3) | ||
| Marital Status | |||
| Married | 22 (91.6) | ||
| Single | 1 (4.2) | ||
| Divorced | 1 (4.2) | ||
| Parity | |||
| Nulliparous | 18 (75) | ||
| Primiparous | 6 (25) |
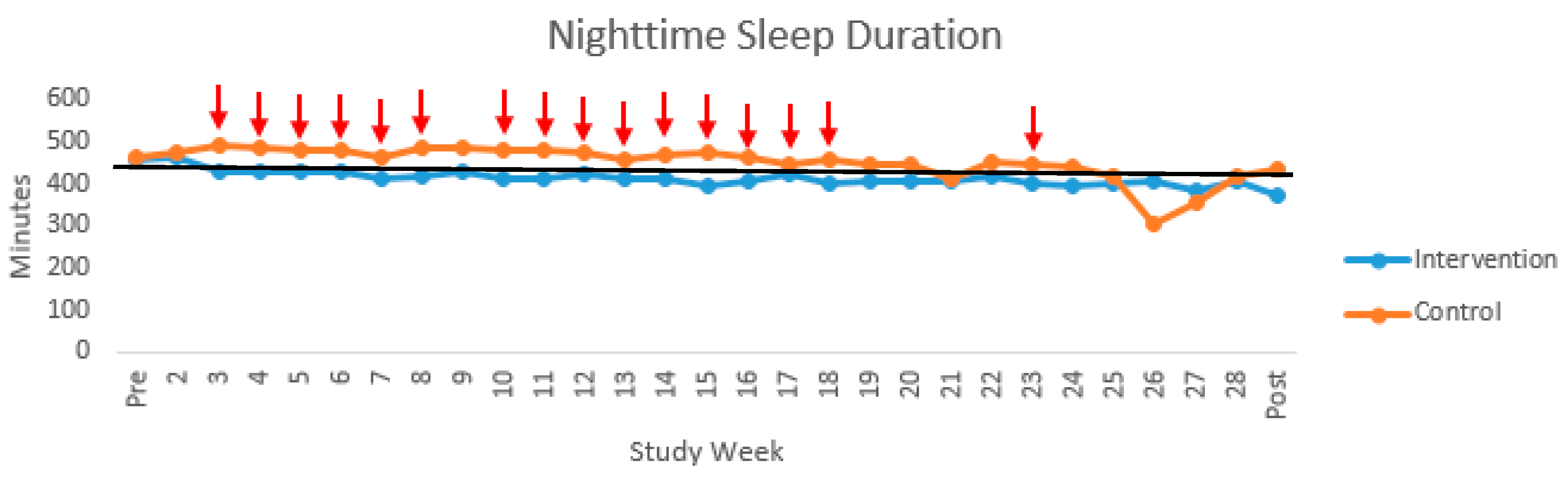
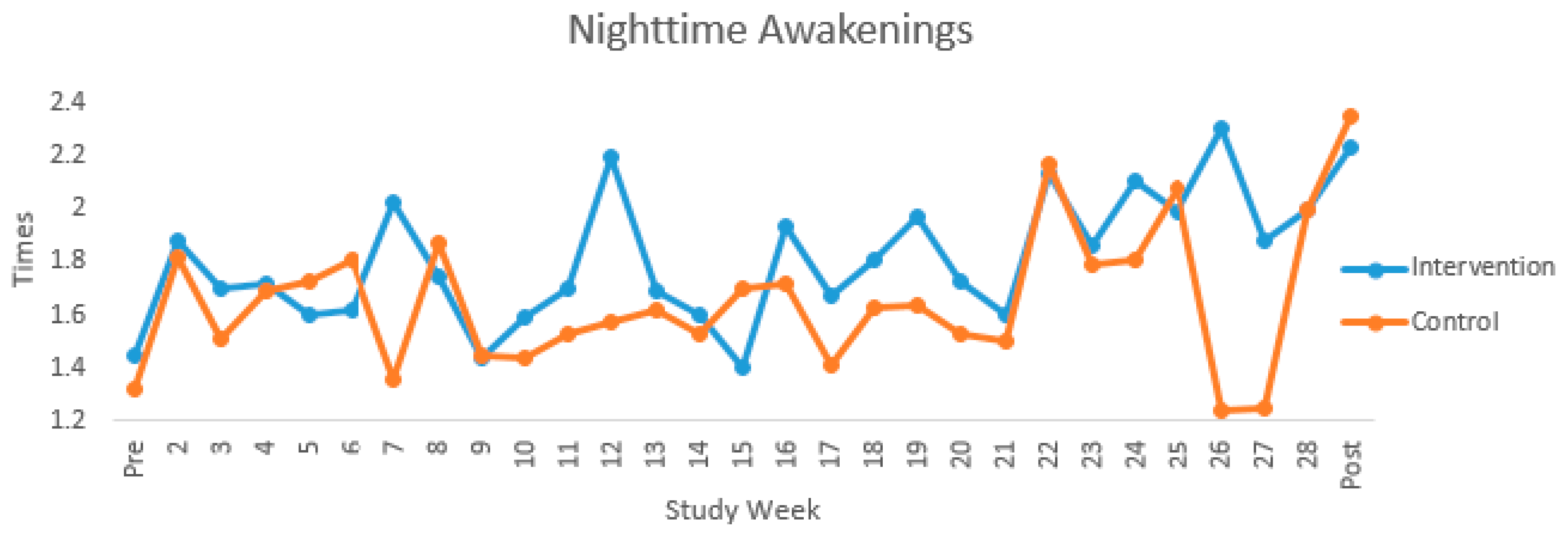
| Nighttime Sleep Duration (min) | Nighttime Awakenings | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Intervention | Control | Overall | Intervention | Control | |||||||||||||
| Study Week | N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD | N | M | SD |
| Pre | 15 | 460.97 | 43.32 | 5 | 459.33 | 20.68 | 10 | 461.78 | 52.22 | 16 | 1.37 | 1.04 | 6 | 1.45 | 1.20 | 10 | 1.32 | 1.00 |
| 2 | 19 | 471.91 | 49.04 | 8 | 465.50 | 36.23 | 11 | 476.57 | 57.91 | 19 | 1.85 | 0.79 | 8 | 1.88 | 0.42 | 11 | 1.82 | 0.99 |
| 3 | 20 | 460.58 | 75.03 | 10 | 428.90 | 43.32 | 10 | 492.27 | 88.18 | 20 | 1.60 | 0.79 | 10 | 1.70 | 0.82 | 10 | 1.51 | 0.79 |
| 4 | 21 | 456.64 | 53.07 | 11 | 429.46 | 27.80 | 10 | 486.54 | 59.19 | 21 | 1.70 | 0.71 | 11 | 1.72 | 0.89 | 10 | 1.69 | 0.49 |
| 5 | 21 | 454.84 | 52.90 | 11 | 432.12 | 38.18 | 10 | 479.84 | 57.22 | 21 | 1.66 | 0.73 | 11 | 1.60 | 0.75 | 10 | 1.73 | 0.75 |
| 6 | 20 | 454.33 | 59.63 | 11 | 432.30 | 28.11 | 9 | 481.25 | 77.30 | 20 | 1.70 | 0.80 | 11 | 1.62 | 0.90 | 9 | 1.81 | 0.69 |
| 7 | 21 | 436.63 | 47.70 | 11 | 411.83 | 33.59 | 10 | 463.90 | 47.16 | 21 | 1.71 | 0.74 | 11 | 2.02 | 0.78 | 10 | 1.36 | 0.55 |
| 8 | 21 | 450.06 | 67.54 | 11 | 419.63 | 49.46 | 10 | 483.54 | 71.01 | 21 | 1.80 | 0.76 | 11 | 1.74 | 0.79 | 10 | 1.87 | 0.76 |
| 9 | 22 | 457.11 | 96.72 | 11 | 428.07 | 33.05 | 11 | 486.15 | 129.22 | 22 | 1.45 | 0.67 | 11 | 1.44 | 0.55 | 11 | 1.45 | 0.80 |
| 10 | 23 | 450.91 | 58.74 | 10 | 413.85 | 47.00 | 13 | 479.41 | 51.43 | 23 | 1.51 | 0.71 | 10 | 1.59 | 0.56 | 13 | 1.44 | 0.81 |
| 11 | 22 | 451.95 | 59.06 | 9 | 412.46 | 37.74 | 13 | 479.29 | 56.35 | 22 | 1.60 | 0.72 | 9 | 1.70 | 0.54 | 13 | 1.53 | 0.84 |
| 12 | 21 | 450.69 | 71.13 | 10 | 421.49 | 45.27 | 11 | 477.24 | 81.52 | 21 | 1.86 | 0.94 | 10 | 2.19 | 0.90 | 11 | 1.57 | 0.92 |
| 13 | 22 | 437.97 | 54.41 | 9 | 411.80 | 38.68 | 13 | 456.08 | 57.58 | 22 | 1.65 | 0.70 | 9 | 1.69 | 0.73 | 13 | 1.62 | 0.71 |
| 14 | 23 | 445.00 | 54.82 | 10 | 413.67 | 52.31 | 13 | 469.10 | 44.83 | 23 | 1.56 | 0.56 | 10 | 1.60 | 0.48 | 13 | 1.53 | 0.63 |
| 15 | 22 | 438.36 | 85.34 | 10 | 396.35 | 78.49 | 12 | 473.37 | 76.95 | 22 | 1.56 | 0.76 | 10 | 1.40 | 0.76 | 12 | 1.70 | 0.76 |
| 16 | 21 | 438.96 | 64.96 | 9 | 405.50 | 42.31 | 12 | 464.05 | 69.05 | 21 | 1.81 | 0.76 | 9 | 1.93 | 0.63 | 12 | 1.72 | 0.86 |
| 17 | 20 | 437.27 | 60.04 | 9 | 422.61 | 31.35 | 11 | 449.26 | 75.58 | 20 | 1.53 | 0.81 | 9 | 1.67 | 0.83 | 11 | 1.41 | 0.82 |
| 18 | 21 | 432.44 | 66.65 | 9 | 400.49 | 44.59 | 12 | 456.40 | 71.89 | 21 | 1.71 | 0.77 | 9 | 1.81 | 0.89 | 12 | 1.63 | 0.70 |
| 19 | 22 | 427.57 | 62.62 | 10 | 407.43 | 62.49 | 12 | 444.35 | 60.12 | 22 | 1.79 | 0.69 | 10 | 1.97 | 0.43 | 12 | 1.64 | 0.84 |
| 20 | 22 | 428.58 | 58.48 | 10 | 408.48 | 41.07 | 12 | 445.32 | 66.91 | 22 | 1.62 | 0.48 | 10 | 1.73 | 0.51 | 12 | 1.53 | 0.47 |
| 21 | 19 | 410.76 | 90.25 | 10 | 408.57 | 64.10 | 9 | 413.19 | 117.00 | 20 | 1.55 | 0.74 | 10 | 1.60 | 0.57 | 10 | 1.50 | 0.91 |
| 22 | 18 | 431.91 | 65.83 | 10 | 417.19 | 61.32 | 8 | 450.32 | 70.66 | 18 | 2.15 | 0.92 | 10 | 2.13 | 0.82 | 8 | 2.17 | 1.10 |
| 23 | 18 | 422.56 | 54.33 | 9 | 399.93 | 51.12 | 9 | 445.18 | 50.07 | 18 | 1.82 | 0.75 | 9 | 1.86 | 0.51 | 9 | 1.79 | 0.96 |
| 24 | 16 | 414.96 | 72.02 | 9 | 396.55 | 56.95 | 7 | 438.62 | 86.50 | 16 | 1.97 | 0.61 | 9 | 2.10 | 0.67 | 7 | 1.81 | 0.53 |
| 25 | 13 | 409.86 | 98.60 | 7 | 401.24 | 63.77 | 6 | 419.93 | 135.00 | 13 | 2.03 | 0.79 | 7 | 1.99 | 0.50 | 6 | 2.08 | 1.10 |
| 26 | 8 | 370.36 | 92.01 | 5 | 408.32 | 31.71 | 3 | 307.09 | 134.21 | 8 | 1.90 | 0.90 | 5 | 2.30 | 0.26 | 3 | 1.24 | 1.29 |
| 27 | 4 | 369.52 | 50.54 | 2 | 381.78 | 65.54 | 2 | 357.25 | 52.58 | 4 | 1.56 | 0.83 | 2 | 1.88 | 1.24 | 2 | 1.25 | 0.35 |
| 28 | 3 | 412.02 | 21.61 | 2 | 409.17 | 29.76 | 1 | 417.73 | - | 2 | 2.00 | 0.00 | 1 | 2.00 | - | 1 | 2.00 | - |
| Post | 17 | 405.65 | 72.10 | 8 | 375.44 | 53.57 | 9 | 432.50 | 78.51 | 17 | 2.29 | 0.80 | 8 | 2.23 | 0.71 | 9 | 2.35 | 0.92 |
| Parameters | Estimate | Standard Error | p-Value | Effect Sizes | |
|---|---|---|---|---|---|
| Nighttime Sleep Duration | Intercept | 4572.56 | 1260.33 | <0.01 | |
| Within-Person NSD | −0.36 | 0.22 | 0.13 | 0.33 | |
| Between-Person NSD | −3.87 | 2.85 | 0.19 | 0.28 | |
| Week | 13.57 | 2.25 | <0.0001 * | ||
| Study Group | 346.47 | 280.56 | 0.23 | ||
| Week by Study Group | −2.74 | 4.41 | 0.53 | ||
| Within-Person NSD by Week | 0.01 | 0.02 | 0.61 | 0.10 | |
| Within-Person NSD by Study Group | 0.33 | 0.53 | 0.53 | 0.13 | |
| Within-Person NSD by Week by Study Group | 0.03 | 0.06 | 0.67 | 0.09 | |
| Nighttime Awakenings | Intercept | 3417.36 | 489.71 | <0.0001 | |
| Within-Person Awakenings | 41.93 | 27.57 | 0.13 | 0.31 | |
| Between-Person Awakenings | −318.52 | 277.90 | 0.26 | 0.23 | |
| Week | 13.30 | 2.26 | <0.0001 * | ||
| Study Group | 59.08 | 257.97 | 0.82 | ||
| Week by Study Group | −3.14 | 4.43 | 0.48 | ||
| Within-Person Awakenings by Week | −4.05 | 10.88 | 0.71 | ||
| Within-Person Awakenings by Study Group | −63.46 | 99.03 | 0.52 | ||
| Within-Person Awakenings by Week by Study Group | 4.34 | 6.53 | 0.51 | 0.14 |
| Parameters | Estimate | Standard Error | p-Value | Effect Sizes | |
|---|---|---|---|---|---|
| Nighttime Sleep Duration | Intercept | 883.38 | 409.57 | 0.04 | |
| Within-Person NSD | −0.07 | 0.17 | 0.69 | 0.08 | |
| Between-Person NSD | −0.97 | 0.93 | 0.31 | 0.21 | |
| Week | 0.73 | 0.77 | 0.35 | ||
| Study Group | −78.94 | 92.66 | 0.40 | ||
| Week by Study Group | −2.22 | 1.51 | 0.14 | ||
| Within-Person NSD by Week | −0.03 | 0.01 | 0.03 * | 0.61 | |
| Within-Person NSD by Study Group | 0.11 | 0.34 | 0.74 | 0.07 | |
| Within-Person NSD by Week by Study Group | 0.09 | 0.03 | <0.001 * | 0.70 | |
| Nighttime Awakenings | Intercept | 741.49 | 148.07 | <0.0001 | |
| Within-Person Awakenings | 2.72 | 9.37 | 0.77 | 0.06 | |
| Between-Person Awakenings | −167.56 | 84.01 | 0.06 # | 0.41 | |
| Week | 1.12 | 0.75 | 0.13 | ||
| Study Group | −128.85 | 73.53 | 0.09# | ||
| Week by Study Group | −1.75 | 1.47 | 0.24 | ||
| Within-Person Awakenings by Week | −2.44 | 1.12 | 0.03 * | 0.45 | |
| Within-Person Awakenings by Study Group | 5.72 | 18.75 | 0.76 | 0.06 | |
| Within-Person Awakenings by Week by Study Group | 0.62 | 2.2 | 0.78 | 0.06 |
| Parameters | Estimate | Standard Error | p-Value | Effect Sizes | |
|---|---|---|---|---|---|
| Nighttime Sleep Duration | Intercept | 1.73 | 1.65 | 0.31 | |
| Within-Person NSD | −9.9 × 10−5 | 0.002 | 0.96 | 0.10 | |
| Between-Person NSD | −0.001 | 0.004 | 0.71 | 0.05 | |
| Week | 0.14 | 0.02 | <0.0001 * | ||
| Study Group | 0.09 | 0.41 | 0.83 | ||
| Week by Study Group | 0.07 | 0.04 | 0.09 | ||
| Within-Person NSD by Week | 2.4 × 10−4 | 2.2 × 10−4 | 0.29 | 0.22 | |
| Within-Person NSD by Study Group | 0.002 | 0.005 | 0.61 | 0.08 | |
| Within-Person NSD by Week by Study Group | 0.002 | 0.001 | 0.005 * | 0.74 | |
| Nighttime Awakenings | Intercept | 1.80 | 0.66 | 0.01 | |
| Within-Person Awakenings | 0.76 | 0.42 | 0.07 # | 0.37 | |
| Between-Person Awakenings | −0.39 | 0.37 | 0.31 | 0.22 | |
| Week | 0.12 | 0.02 | <0.0001 * | ||
| Study Group | −0.05 | 0.35 | 0.88 | ||
| Week by Study Group | 0.05 | 0.04 | 0.19 | ||
| Within-Person Awakenings by Week | 0.21 | 0.03 | <0.0001 * | 1.4 | |
| Within-Person Awakenings by Study Group | −0.03 | 0.83 | 0.97 | 0.01 | |
| Within-Person Awakenings by Week by Study Group | 0.2042 | 0.06182 | 0.001 * | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauley, A.M.; Hohman, E.E.; Leonard, K.S.; Guo, P.; McNitt, K.M.; Rivera, D.E.; Savage, J.S.; Downs, D.S. Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity. Clocks & Sleep 2020, 2, 487-501. https://doi.org/10.3390/clockssleep2040036
Pauley AM, Hohman EE, Leonard KS, Guo P, McNitt KM, Rivera DE, Savage JS, Downs DS. Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity. Clocks & Sleep. 2020; 2(4):487-501. https://doi.org/10.3390/clockssleep2040036
Chicago/Turabian StylePauley, Abigail M., Emily E. Hohman, Krista S. Leonard, Penghong Guo, Katherine M. McNitt, Daniel E. Rivera, Jennifer S. Savage, and Danielle Symons Downs. 2020. "Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity" Clocks & Sleep 2, no. 4: 487-501. https://doi.org/10.3390/clockssleep2040036
APA StylePauley, A. M., Hohman, E. E., Leonard, K. S., Guo, P., McNitt, K. M., Rivera, D. E., Savage, J. S., & Downs, D. S. (2020). Short Nighttime Sleep Duration and High Number of Nighttime Awakenings Explain Increases in Gestational Weight Gain and Decreases in Physical Activity but Not Energy Intake among Pregnant Women with Overweight/Obesity. Clocks & Sleep, 2(4), 487-501. https://doi.org/10.3390/clockssleep2040036





