Abstract
Microbial extracellular polymeric substances (EPSs) are emerging as sustainable alternatives to conventional corrosion inhibitors due to their eco-friendly nature, biodegradability, and functional versatility. Secreted by diverse microorganisms including bacteria, fungi, archaea, and algae, EPSs are composed mainly of polysaccharides, proteins, lipids, and nucleic acids. These biopolymers, chiefly polysaccharides and proteins, are accountable for surface corrosion prevention through biofilm formation, allowing microbial survival and promoting their environmental adaptation. Usually, EPS-mediated corrosion inhibitions can take place via different mechanisms: protective film formation, metal ions chelation, electrochemical property alteration, and synergy with inorganic inhibitors. Even though efficacious EPS corrosion prevention has been demonstrated in several former studies, the application of such microbial inhibitors remains, so far, a controversial topic due to the variability in their composition and compatibility toward diverse metal surfaces. Thus, this review outlines the microbial origins, biochemical properties, and inhibition mechanisms of EPSs, emphasizing their advantages and challenges in industrial applications. Advances in synthetic biology, nanotechnology, and machine learning are also highlighted and could provide new opportunities to enhance EPS production and functionality. Therefore, the adoption of EPS-based corrosion inhibitors represents a promising strategy for environmentally sustainable corrosion control.
1. Introduction
Metals play a crucial role in modern industries such as construction, aerospace, and energy due to their strength and adaptability [1]. They naturally emanate from stable mineral deposits, and their extraction demands significant energy consumption. Once purified, metals tend to revert to a more stable state through environmental interactions, a process called corrosion [2,3]. Corrosion involves electrochemical reactions where electrons are released from the anodes and absorbed on cathodes, influenced by factors such as pH and oxygen presence [4]. Microorganisms, such as bacteria and fungi, can accelerate corrosion by degrading materials through metabolic processes and biofilm formation [5,6]. These microbial biofilms can not only protect microorganisms from external risks but also promote corrosion by generating localized chemical imbalances and corrosive byproducts [7]. Corrosion can occur in various forms, including uniform degradation, localized damage, pitting, and crevice formation, all contributing to the gradual degradation of materials [8].
As a result, the degradation of materials compromises structural integrity in critical infrastructure, leading, hence, to significant safety risks [3]. Besides direct financial costs for repair or replacement, corrosion has broader economic impacts, involving reduced operational efficiency and environmental damage, such as ecosystem contamination due to leaks and spills [6]. In response, industries and governments are investing in advanced corrosion prevention strategies, such as using corrosion-resistant composites, surface treatments, and corrosion inhibitors [9]. Interestingly, the latter are widely studied and used to combat all forms of corrosion, including uniform and localized corrosion.
Corrosion inhibitors are typically added in low concentrations to reduce or prevent corrosion by interacting with material surfaces to create protective layers or altering environmental conditions [9]. They are commonly used in industries such as those involving oil and gas, cooling water systems, and metal surface preparation such as in reinforced concrete construction for coatings [10]. In fact, the ideal inhibitor should be non-toxic, effective at low concentrations, and have a potential anti-corrosion capability [9].
Within this framework, corrosion inhibitors can be classified based on their chemical composition (organic or inorganic), oxidizing characteristics, or application areas [10]. Inorganic inhibitors like phosphates, nitrites, and chromates are widely used for their durability and effectiveness at a wide range of temperatures [9]. Meanwhile, organic inhibitors, such as polyacrylates and phosphonates, are considered safer and more sustainable, functioning through adsorption onto metal surfaces [7,10]. However, both types of inhibitors face limitations, such as toxicity and poor sustainability, prompting the exploration of eco-friendly alternatives [11]. Hence, green inhibitors, derived from natural sources such as polysaccharides, amino acids, and biopolymers, are gaining popularity due to their sustainability, biodegradability, and safety [11]. Among these, biopolymers such as cellulose and chitosan are especially valuable for their adsorption capabilities and environmental compatibility [7]. Their complex structures improve their anticorrosive capability [9].
Furthermore, extracellular polymeric substances (EPSs), produced by microorganisms, are emerging as promising and eco-friendly corrosion inhibitors. Their unique properties viz their ability to form protective layers on metal surfaces present EPSs as a sustainable and ecological solution for corrosion prevention [10,12]. Thus, these green microbial inhibitors have gained great interest among researchers as suitable surrogates to reduce the environmental and toxicological impacts typically associated with traditional corrosion inhibitors [10,13]. Nonetheless, despite their proven anti-corrosion behavior across different environments, the usage of EPS-based inhibitors is still being considered a debatable issue in metal research due to the variety in their composition and lack of full understanding of their performance and mechanism of interaction with metals. Therefore, this review provides a deep insight into EPS-producing microorganisms, as well as their functional properties and main composition. Additionally, the anti-corrosion activity of EPSs on different metal surfaces under diverse environmental conditions is thoroughly investigated. Further, their inhibition mechanisms are described. Current challenges and future trends are also highlighted to boost secure, environmentally friendly, and cost-effective corrosion inhibition and management using EPS-based microbial inhibitors.
2. Extracellular Polymeric Substances (EPSs)
2.1. Definition of EPSs
Extracellular polymeric substances (EPSs) are high-molecular-weight natural polymers synthesized by microorganisms through various biochemical processes. These substances are categorized into two primary forms: soluble or loosely bound EPSs, which include dissolved macromolecules, slimes, and colloids, and tightly bound EPSs, encompassing capsular polymers, concentrated gels, and attached organic materials [14,15].
The production of EPSs is a fundamental survival strategy for microorganisms, providing protection against environmental stressors and facilitating biofilm formation [16,17].
2.2. Microorganisms Producing EPSs
EPSs are secreted by different microorganisms, including bacteria, archaea, fungi, and unicellular algae, as a survival mechanism in response to harsh environmental conditions [17,18]. The diversity of EPS-producing microorganisms underscores their ecological and biotechnological significance as well as their complex structure and versatile functionality. In fact, EPSs, composed of polysaccharides, proteins, lipids, nucleic acids, and other biomolecules, play a crucial role in microbial adaptation, biofilm formation, and ecological interactions [16,17].
Interestingly, the production of these biopolymers and their characteristics are influenced by the ecological origins of microorganisms, making them a subject of significant scientific and industrial interest [19,20,21]. EPS-producing microorganisms are, typically, found in diverse environments, from extreme habitats like geothermal springs and Antarctic ice to moderate habitats such as soil, freshwater ecosystems, and wastewater treatment plants. Most of these microorganisms grow in environments with a high carbon/nitrogen ratio and abundant organic substances. They belong to diverse groups (mesophiles, thermophiles, halophiles, etc.) adapted to specific environmental conditions [19,22,23].
Thermophilic microorganisms are among the most studied EPS producers. These organisms, including genera like Aeribacillus, Anoxybacillus, and Geobacillus, are isolated from geothermal springs and deep-sea hydrothermal vents. Thermophiles grow optimally between 55 and 80 °C, while hyperthermophiles, such as Sulfolobus and Thermococcus, grow above 80 °C [24,25]. These microorganisms utilize sugars like lactose, glucose, and sucrose as carbon sources, with EPS yield and composition varying based on the nutrient medium [26,27].
In contrast, Antarctic environments, characterized by extreme cold, high salinity, and intense radiation, host diverse microbial communities that produce EPSs as an adaptive strategy. These polymers play a pivotal role in protecting microorganisms from environmental stressors and contribute significantly to the organic carbon balance in aquatic ecosystems [16,28]. Interestingly, marine habitats, including seawater, sediments, and sea ice, are rich sources of EPS-producing bacteria. For example, Pseudoalteromonas species isolated from Antarctic seawater produce EPSs with high carbohydrate and uronic acid content, demonstrating cryoprotective and heavy metal-binding properties [29,30]. Similarly, Marinobacter sp. W1-16, isolated from surface seawater, produces EPSs with significant emulsifying and cryoprotective activities [19].
Sediments from Antarctic regions have also yielded EPS-producing bacteria like Pseudomonas sp. ID1, which produces EPSs with high emulsifying activity and cryoprotective properties [20]. Sea ice environments host unique EPS-producing microorganisms, such as Psychrobacter and Pseudoalteromonas species. The EPSs secreted by these microorganisms are composed of diverse monosaccharides, including glucose, mannose, and galactose, with variations in molecular weight and functional properties (immunomodulatory and cryoprotective), enabling them to grow in extreme cold conditions [21,28]. Moreover, terrestrial Antarctic environments have shown bacteria that produce EPSs with varying compositions depending on growth conditions, as reported for the strain Halomonas alkaliantarctica CRSS isolated from salt sediments [28]. Likewise, Parageobacillus thermantarcticus M1, isolated from geothermal soil, synthesizes two distinct EPSs [19,31]. Additionally, microalgae, such as Porphyridium cruentum and Chlorella vulgaris, are significant EPS producers, and the production is influenced by various environmental factors such as light intensity, temperature, and nutrient availability [23,32]. Nutrient availability, particularly that of nitrogen and phosphorus, significantly impacts EPS production in microalgae. Nitrogen limitation often induces higher EPS synthesis, as observed in Botryococcus braunii [32,33]. However, microalgae in wastewater environments, such as Chlorella sp., exhibit increased EPS production due to higher nutrient concentrations, resulting, hence, in an increase in EPS protein content [34]. Similar examples include sulfate-reducing bacteria like Desulfovibrionaceae and Desulfobacteriaceae, which produce EPSs rich in proteins and polysaccharides under specific conditions [35,36]. Also, thermophilic consortia, such as those isolated from corroded airplane engines, produce EPSs composed mainly of mannose and glucose residues, with surface (S)-layer proteins being the predominant protein component [27,37]. In addition, electroactive bacteria like Shewanella oneidensis produce EPSs containing redox-active components, such as c-type cytochromes, which facilitate extracellular electron transfer [36,38].
Finally, the production of EPSs is a fundamental survival strategy for microorganisms, enabling them to grow in extreme environments. These polymers not only protect producing microorganisms but also contribute to the organic carbon balance in microbial ecosystems [17,19].
2.3. EPS Functions, Composition, and Properties
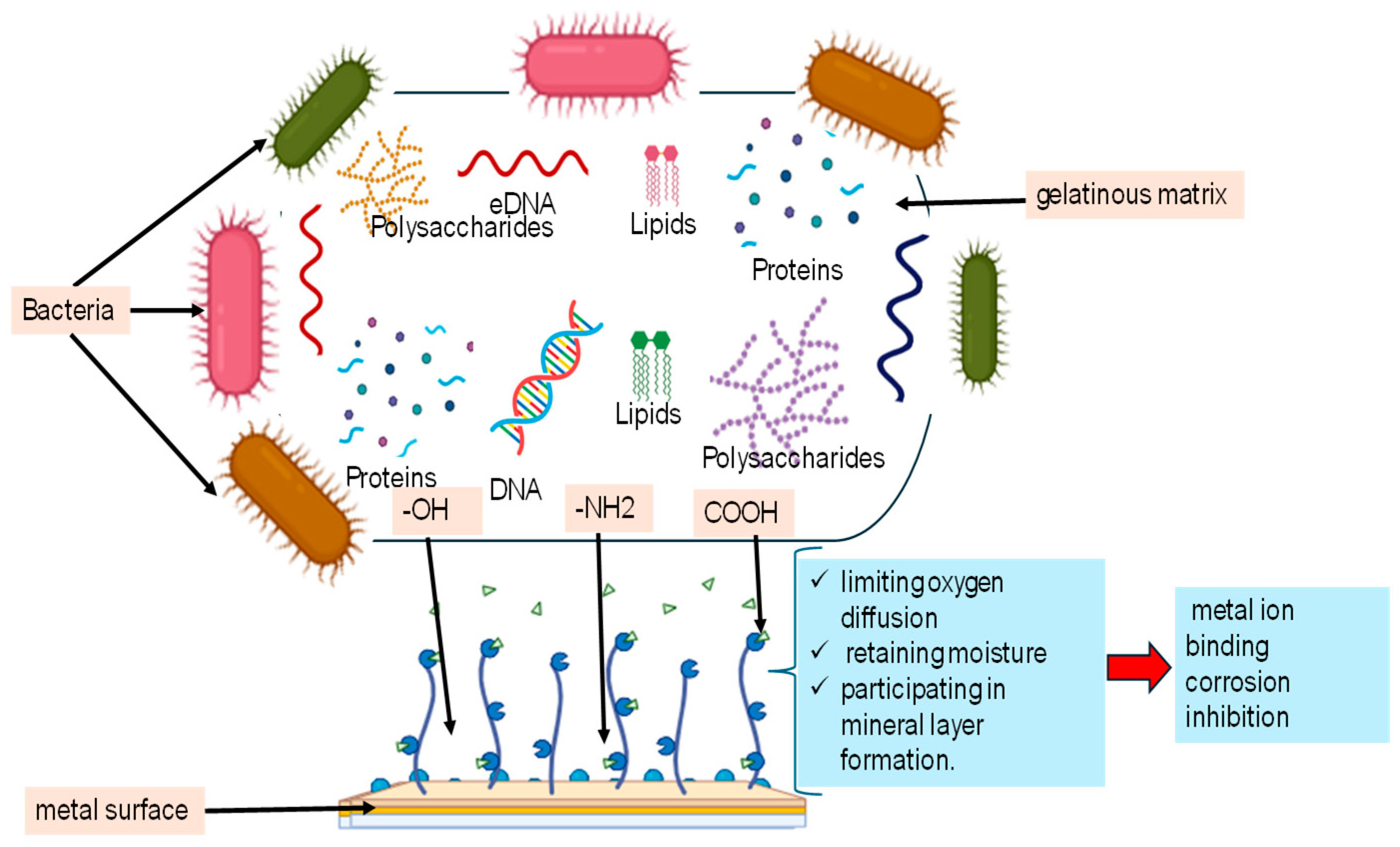
EPSs are vital biopolymers produced by microorganisms, serving a wide range of ecological and functional roles. The complex composition and multifunctional roles of EPSs are summarized in Figure 1, highlighting how different components contribute to corrosion inhibition.
Figure 1.
Composition and functional role of microbial extracellular polymeric substances (EPSs) in corrosion inhibition.
Table 1 summarizes the main functions of EPSs in microbial ecology. EPSs enable microorganisms to adhere to surfaces and form biofilms, creating a stable, gel-like matrix through interactions like hydrogen bonds and electrostatic forces [17,39]. Interestingly, they facilitate the transfer of genetic material, such as antibiotic resistance genes, within biofilms [40,41]. In symbiotic relationships, EPSs are essential for establishing connections between nitrogen-fixing bacteria and plants, as reported for Bradyrhizobium japonicum and Mesorhizobium loti [42,43]. Additionally, EPSs act as protective barriers for pathogens, protecting them from host immune responses and environmental toxins, as observed for Pseudomonas aeruginosa and Xanthomonas campestris [44,45]. Furthermore, EPSs work as carbon reserves during nutrient scarcity, allowing microorganisms like Azospirillum brasilense and Sinorhizobium meliloti to survive under limited resources [46,47]. They retain nutrients, including heavy metals which are useful for environmental cleanup, as demonstrated by Paenibacillus polymyxa and Anabaena variabilis [48,49]. Moreover, EPSs provide protection against environmental stresses (drought, high salinity, extreme temperatures, etc.), as reported for Azotobacter chroococcum and Nostoc calcicola [50,51]. On the other hand, they enhance resistance to antimicrobial agents and disinfectants, as observed in Staphylococcus epidermidis and Acinetobacter baumannii [52,53]. In agriculture, EPSs improve soil structure and water retention, promoting plant growth, as shown by Bacillus subtilis strain JCT1 [54].

Table 1.
Functions of EPSs in microbial ecology.
Overall, EPSs are multifunctional biopolymers with significant applications in ecology, industry, and biotechnology, as evidenced by their diverse roles and microbial sources.
EPSs are complex biopolymers, of which polysaccharides, such as glucose, galactose, and mannose, are the most abundant and form the primary structure of exopolysaccharides [54,63,64]. As visually summarized in Figure 1 and detailed in Table 2, the chemical composition of EPSs varies significantly depending on factors such as microbial species, environmental conditions, and extraction technique. For example, B. licheniformis produces EPSs composed of 89% polysaccharides (PSs) and 11% proteins (Ps) when grown on sucrose, with hydroxyl and carboxyl groups as the main functional groups [65]. Similarly, B. megaterium TF10 synthesizes EPSs with 76% PSs and 23% Ps from glucose, featuring hydroxyl, amide, carboxyl, and primary amine groups [66]. These functional groups play a prime role in adhesion, biofilm formation, and environmental adaptation [67].
The functional properties of EPSs, including biodegradability, hydrophilicity, and adsorption capacity, make them highly useful in various applications [54,63]. For instance, Halomonas sp. AAD6 produces EPSs with 90% PSs, 0.5% Ps, and 5.4% nucleic acids (NAs) from molasses, demonstrating strong hydrophilicity and adsorption capabilities [68]. Additionally, Chryseobacterium daeguense MBF-W6 synthesizes EPSs with 13.1% PSs, 32.4% Ps, and 6.8% NAs from glucose and tryptone, featuring carboxyl, hydroxyl, and methoxyl groups [69]. These EPSs facilitate extracellular electron transfer, with components like cytochromes and humic acids acting as electron mediators [70,71]. This electron transfer is essential for microbial respiration and can also influence the metal corrosion processes [70,72].
Industrially, EPSs are used as stabilizers, thickeners, and emulsifiers in different sectors such as food and cosmetics [73]. For example, X. campestris produces EPSs with 95% PSs and 5% Ps from glucose, which are widely used as xanthan gum owing to their high viscosity and solubility [74]. Further, Leuconostoc mesenteroides synthesizes EPSs with 90% PSs and 10% Ps from sucrose, which are used as edible coatings due to their antimicrobial properties [75]. In agriculture, EPSs enhance soil structure and fertility, promoting improved crop yields [76]. For instance, the B. subtilis strain JCT1 produces EPSs that promote soil aggregation and water retention [54].
Other microorganisms, such as Proteus mirabilis TJ-1, produce EPSs with 63.1% PSs and 30.9% Ps from glucose, featuring hydroxyl and carboxyl groups [76]. A. indicus ATCC9540 synthesizes EPSs with 97.7% PSs and 2.3% Ps from latifolia flower extract, containing O-acetyl, orcinol, carboxyl, and hydroxyl groups [77]. Additionally, Serratia marcescens produces EPSs with 75% PSs, 20% Ps, and 5% NAs from glucose, featuring hydroxyl, carboxyl, and amide groups [78]. In addition, Serratia sp. synthesizes EPSs with 80% PSs, 15% Ps, and 5% NAs from sucrose containing hydroxyl, carboxyl, and pyruvate groups [79]. Similarly, Lactobacillus rhamnosus produces EPSs with 85% PSs and 15% Ps from glucose, featuring hydroxyl, carboxyl, and amide groups [80]. Pseudomonas aeruginosa synthesizes EPSs with 70% PSs, 25% Ps, and 5% NAs from glycerol, containing carboxyl, hydroxyl, and pyruvate groups [81]. Streptococcus thermophilus produces EPSs with 80% PSs and 20%Ps from lactose featuring hydroxyl, carboxyl, and phosphate groups [82]. Enterobacter cloacae synthesizes EPSs with 70% PSs, 25% Ps, and 5% NAs from glucose, containing hydroxyl, carboxyl, and amide groups [83]. Klebsiella pneumoniae produces EPSs with 85% PSs, 10% Ps, and 5% NAs from sucrose, featuring hydroxyl, carboxyl, and pyruvate groups [84]. Finally, Rhizobium radiobacter synthesizes EPSs with 80% PSs, 15% Ps, and 5% NAs from glucose, containing hydroxyl, carboxyl, and amide groups [85].
Considering all these multiple synthesis methods, EPSs’ properties are largely determined by their specific components (polysaccharides, proteins, lipids, nucleic acids, and humic acids). Polysaccharides, which constitute 40–95% of the EPS matrix, have essential functions including cell adhesion, aggregation, water retention, the adsorption of organic and inorganic compounds, enzyme binding, nutrient provision, and cellular protection. Moreover, their concentration and structural features are responsible for the surface properties and biodegradability of EPSs [67,86,87].
In addition, proteins, making up 1–50% of EPSs, significantly contribute to the structural and functional integrity of EPSs and perform similar roles, including adhesion, aggregation, water retention, compound sorption, enzyme binding, electron transfer, and cell protection [67]. Like polysaccharides, proteins significantly affect flocculation, biosorption, and the surface characteristics of EPSs [67,86,87]. Additionally, nucleic acids, present at 1–10%, contribute to adhesion, aggregation, nutrient provision, genetic exchange, and cellular export processes. Their influence on flocculation and biosorption depends on their quantity and composition [67,86,87]. Further, lipids, also at 1–10%, primarily facilitate the export of cellular components and have a minor role in flocculation and biosorption [67,86,87]. Lastly, humic substances, although their concentration is not well-defined, contribute to adhesion and electron transfer. However, their impact on flocculation and biosorption is relatively minor and relies on their concentration and nature [67,86,87].
Hence, the chemical diversity of EPSs, determined by the ecological habitat of their producers, offers significant biotechnological potential. Furthermore, it is worth noting that this comprehensive investigation of EPS-producing microorganisms from varied environments reveals their survival strategies and supports innovation towards their promising applications in medicine, environmental science, and industry.

Table 2.
EPS composition.
Table 2.
EPS composition.
| Microorganism | Substrate | EPS Composition (%) | Main Functional Groups | Extraction Techniques | References |
|---|---|---|---|---|---|
| B. licheniformis | Sucrose | PS: 89; P: 11 | Hydroxyl, Carboxyl | Ethanol precipitation | [65] |
| B. megaterium TF10 | Glucose | PS: 76; P: 23 | Hydroxyl, Amide, Carboxyl, Primary Amine | Ethanol precipitation | [66] |
| P. mirabilis TJ-1 | Glucose | PS: 63.1; P: 30.9 | Hydroxyl, Carboxyl | Ethanol precipitation | [77] |
| Halomonas sp. AAD6 | Molasses | PS: 90; P: 0.5; NA: 5.4 | Hydroxyl, Carboxyl | Ethanol precipitation | [68] |
| Chryseobacterium daeguense MBF-W6 | Glucose, tryptone | PS: 13.1; P: 32.4; NA: 6.8 | Carboxyl, Hydroxyl, Methoxyl | Ethanol precipitation | [69] |
| A. indicus ATCC 9540 | Latifolia flower extract | PS: 97.7; P: 2.3 | O-acetyl, Orcinol, Carboxyl, Hydroxyl | Ethanol precipitation | [77] |
| S. marcescens | Glucose | PS: 75; P: 20; NA: 5 | Hydroxyl, Carboxyl, Amide | Ethanol precipitation | [78] |
| Serratia sp. | Sucrose | PS: 80; P:15; NA: 5 | Hydroxyl, Carboxyl, Pyruvate | Ultracentrifugation | [79] |
| L. rhamnosus | Glucose | PS: 85; P: 15 | Hydroxyl, Carboxyl, Amide | Ethanol precipitation | [80] |
| P. aeruginosa | Glycerol | PS: 70; P: 25; NA: 5 | Carboxyl, Hydroxyl, Pyruvate | Ultracentrifugation | [81] |
| X. campestris | Glucose | PS: 95; P: 5 | Acetyl, Carboxyl, Hydroxyl | Ethanol precipitation | [73] |
| L. mesenteroides | Sucrose | PS: 90; P: 10 | Hydroxyl, Carboxyl | Ethanol precipitation | [74] |
| S. thermophilus | Lactose | PS: 80; P: 20 | Hydroxyl, Carboxyl, Phosphate | Dialysis and ethanol precipitation | [82] |
| E. cloacae | Glucose | PS: 70; P: 25; NA: 5 | Hydroxyl, Carboxyl, Amide | Ethanol precipitation | [83] |
| K. pneumoniae | Sucrose | PS: 85; P: 10; NA: 5 | Hydroxyl, Carboxyl, Pyruvate | Ethanol precipitation | [84] |
| R. radiobacter | Glucose | PS: 80; P: 15; NA: 5 | Hydroxyl, Carboxyl, Amide | Ethanol precipitation | [85] |
PS: polysaccharide, P: protein, NA: nucleic acid.
3. Corrosion Inhibition by Microbial Extracellular Polymeric Substances
3.1. Effectiveness of EPSs in Corrosion Inhibition
EPSs produced by microorganisms have emerged as promising green corrosion inhibitors [10,13]. These polymers are biodegradable, non-toxic, and renewable, making them ideal candidates for sustainable corrosion protection [63,88]. EPSs from different microorganisms exhibit varying levels of corrosion inhibition, as reported in Table 3 [89,90].

Table 3.
Example of microbial EPSs with corrosion inhibition activity.
For instance, P. mosselii F01 produces a glycolipid-based EPS that achieves 87% corrosion inhibition for carbon steel in HCl [91]. Similarly, Marinobacter salsuginis produces proteins and carbohydrates that provide 91.16% inhibition on X80 carbon steel in NaCl [92]. Additionally, the γ-polyglutamate EPS from B. licheniformis has demonstrated 90% corrosion inhibition on aluminum 2024 [93]. Additionally, S. mutans produces a polysaccharide EPS that, when combined with sodium molybdate, enhances corrosion inhibition on X70 steel in NaCl up to 82% [90].
Lactic acid bacteria produce various exopolysaccharides that protect carbon steel in NaCl, with corrosion rates ranging from 0.315 to 0.992 m/year [89]. Likewise, Vibrio sp. EF187016 secretes an EPS that reduces corrosion by 68% on X80 carbon steel in NaCl [94]. EPSs derived from wastewater sludge also show important corrosion inhibition of 78.89% on carbon steel in NaCl [63]. In addition, microorganisms from wastewater treatment plants produce protein- and polysaccharide-rich EPSs that provide 66.40% inhibition for cast iron [95].
On the other hand, P. putida and B. megaterium exhibit lower corrosion inhibition efficiencies, with 59.63% and 35.04%, respectively, for A3 carbon steel [96,97]. Iron-oxidizing bacteria from sludge form protective biofilms with proteins and polysaccharides on Q235 mild carbon steel in NaCl [98].
Marine isolates of P. stutzeri and Shewanella putrefaciens exhibit corrosion inhibition mechanisms through biomineralization, with efficiencies of 72% and 75%, respectively, on steel surfaces [99,100]. Furthermore, a Halomonas sp. EPS has been shown to achieve 80% inhibition on mild steel in NaCl environments [101], and K. pneumoniae produces a biofilm-forming EPS that results in 85% inhibition on stainless steel [102]. Additionally, an A. baumannii EPS has achieved 83% inhibition on carbon steel [103].
3.2. Mechanisms of Corrosion Inhibition by EPSs
As reported above, several studies have demonstrated that microbial cultures and/or the produced EPSs possess the ability to inhibit metal corrosion [3,89,104]. EPSs consisting of complex mixtures of various compounds (polysaccharides, proteins, nucleic acids, and lipids, etc.) are secreted by microorganisms and form biofilms on metal surfaces [105,106]. In fact, biofilms act as protective barriers, inhibiting the corrosion process through various mechanisms. These mechanisms are mainly the formation of protective films, complexation with metal ions, and the role of microbial activity in corrosion inhibition as well as synergistic effects with inorganic corrosion inhibitors [107,108]. EPSs inhibit corrosion through multiple interconnected mechanisms, primarily involving surface adsorption, electrochemical modification, and chemical interactions. These are explored below.
Table 4 presents the possible mechanisms involved in EPS-mediated corrosion inhibition and the main influencing factors.

Table 4.
Pathways of EPS-mediated corrosion inhibition mechanism and main influencing factors.
3.2.1. Formation of Protective Layer on Metal Surface
The well-described mechanism by which EPSs inhibit corrosion is the formation of a compact adsorbed film on the metal surface. The adsorption of EPS molecules onto the metal surface occurs due to the interaction between the functional groups (carboxyl, amino, hydroxyl, etc.) in EPSs and metal, allowing, hence, the formation of a protective layer [90,94,117]. This layer acts as a physical barrier, preventing corrosive species such as oxygen and chloride ions from reaching the metal surface [95,109,119]. Regarding the corrosion inhibition mechanism, EPSs can promote the formation of a protective mineralization layer, thereby suppressing metal corrosion [119,120]
In this context, Dong et al. [121] investigated the impact of extracted EPSs on the corrosion behavior of carbon steel. Their findings revealed that low concentrations of EPSs reduced corrosion by limiting the diffusion of oxygen. Conversely, at elevated EPS levels, the inhibitory effect diminished as a result of increased binding between EPSs and iron ions [121].
3.2.2. Complexation with Metal Ions
The complexation of EPSs with metal ions is another critical mechanism of corrosion inhibition. The functional groups of EPSs can react with positively charged metal ions to form stable complexes that prevent corrosion [108,111,117]. The formed complexes can either adsorb onto the metal surface, creating a protective layer [90,110], or remain in solution, reducing the availability of free metal ions for corrosion reactions [112,122]. For instance, EPSs can chelate with Fe2+ ions, preventing their oxidation to Fe3+ and reducing the formation of corrosive iron oxides [110]. This complexation decreases, as well, the rate of metal dissolution [112]. Additionally, the complexation between EPSs and metal ions modifies the electrochemical behavior of the metal surface, signaling a decrease in the corrosion rate [89,117]. The chelation process is particularly effective in slightly acidic environments, where the ionization of carboxyl groups in EPSs is enhanced, facilitating stronger binding with metal ions [90]. The formed complexes can further interact with metal ions to create biomineralized layers (through oxidation and precipitation) on the metal surface, effectively controlling corrosion caused by environmental factors [116,123]. Additionally, EPSs have the ability to regulate the kinetic pathway of CaCO3 biomineralization, impacting both nucleation and crystal growth processes, as reported in circulating cooling water systems [116,122]. Interestingly, EPSs enhance the stability of corrosion product layers (iron oxide, iron phosphate, etc.) by improving their adhesion to the metal surface [107]. As an example, Pseudomonas cichorii produces an EPS that forms a protective iron–EPS complex on mild steel, boosting corrosion resistance [107].
3.2.3. Microbial-Mediated Corrosion Inhibition Mechanisms
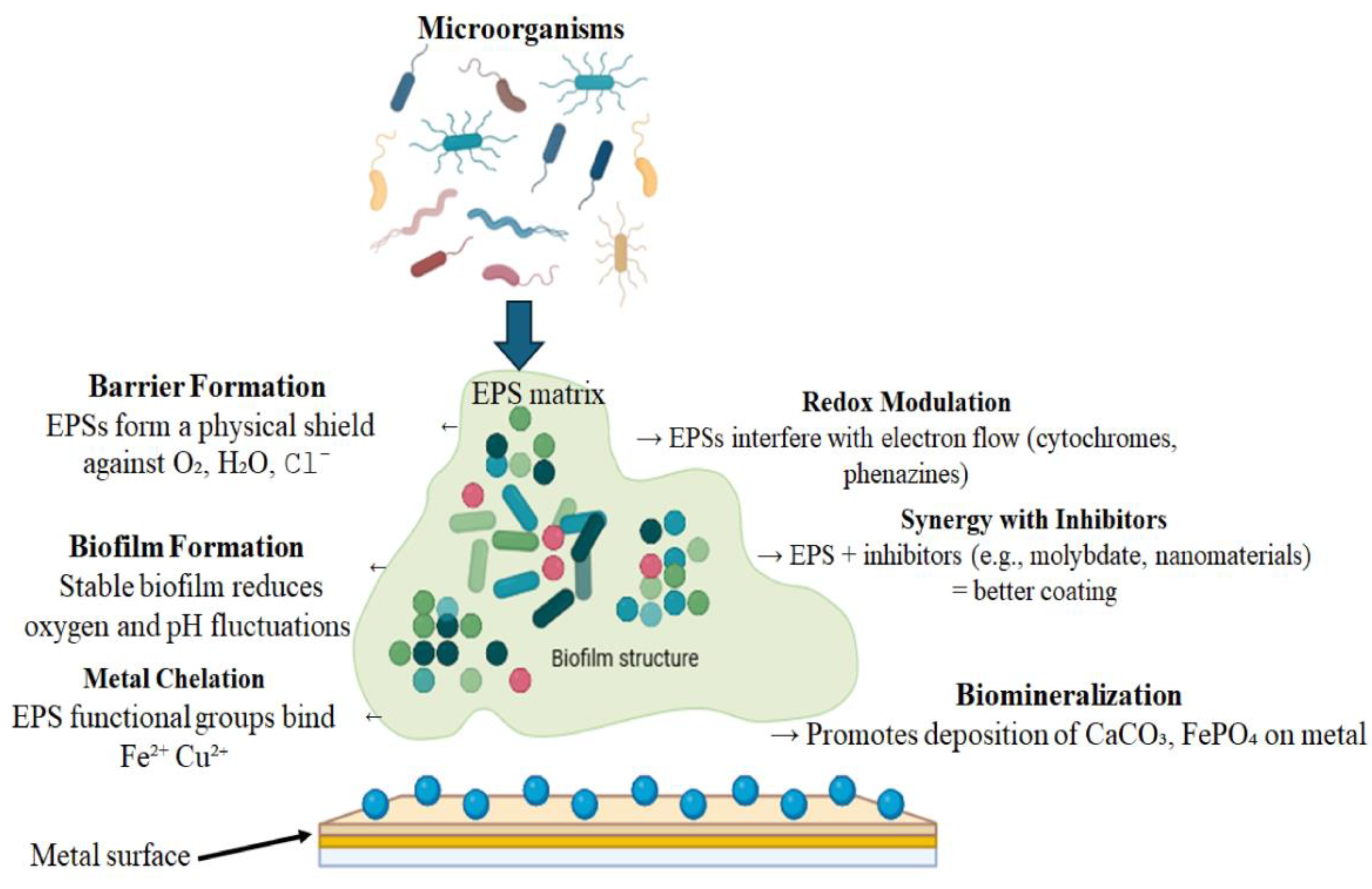
Microorganisms actively inhibit corrosion through three synergistic mechanisms (Figure 2) mediated by biofilm formation [104]. The structured microbial communities within their self-produced EPS matrices [105] create protective microenvironments that significantly alter metal corrosion behavior [124]. First, aerobic respiration depletes dissolved oxygen, a critical cathodic reactant, as demonstrated by Vibrio spp. biofilms that achieve 68% corrosion inhibition on X80 carbon steel through combined oxygen consumption and physical EPS barriers [94,112]. Second, microbial metabolites including organic acids chemically neutralize aggressive species like chlorides, particularly in marine environments where chloride-induced corrosion predominates [114,124,125]. Third, the EPS matrix stabilizes corrosion products such as iron sulfides into adherent, protective surface films that provide durable protection [107,122]. These active biological processes work in concert with passive EPS adsorption effects to deliver comprehensive corrosion inhibition across diverse environments.
Figure 2.
Schematic overview of EPS-mediated metal corrosion inhibition mechanisms.
3.2.4. Synergistic Effects of EPSs and Corrosion Inhibitors
The corrosion inhibition efficiency of EPSs can be significantly enhanced through synergistic combinations with other inhibitors. These hybrid systems exhibit superior performance compared to individual components, with EPS–nanomaterial composites showing particular promise for next-generation protective coating applications [118,125,126].
Research conducted by Xu et al. (2023) [126] demonstrated a synergistic effect between EPSs and a carbon-based layer, improving electron utilization and protective film formation. Similarly, in metallic systems, the combination of EPSs and inorganic compounds such as sodium molybdate has been shown to enhance corrosion resistance by forming complex multilayered barriers [118]. This synergistic effect arises from the ability of EPSs to create an acidic microenvironment rich in organic acids, which react with molybdate ions to form adsorbed protective complexes. The reduction of Mo(VI) to Mo(V) on the metal surface enhances the formation of a durable film, while the co-adsorption of EPSs and molybdate-derived compounds contributes to a denser, more uniform barrier that blocks corrosive agents [125]. Generally, synergistic corrosion inhibition mechanisms involving organic and inorganic inhibitors rely on complementary actions [118,127]. Organic inhibitors typically form a primary barrier on the metal surface, while inorganic inhibitors, such as cerium or molybdate ions, fill defects or enhance film stability [127,128]. Moreover, the microbial activity in EPS-producing biofilms can play a dual role by modifying electrochemical conditions and forming bio-corrosion layers, as observed by Jia et al. (2017) [129] in carbon steel under organic carbon starvation.
3.3. Main Factors Influencing EPS-Mediated Corrosion Inhibition
The properties of EPSs vary significantly across microbial strains, with direct implications for industrial application [130], particularly in corrosion inhibition where strain-specific functional groups determine interfacial adhesion [131]. The effectiveness of EPSs in corrosion inhibition is controlled by various factors, including temperature, pH, EPS concentration, biofilm thickness, microbial strain specificity, growth conditions, extraction methods, and EPS composition [90,121,130]. Understanding these factors is crucial for optimizing EPS-based corrosion inhibition strategies.
Temperature plays a significant role in the performance of EPSs as corrosion inhibitors [90]. Studies indicate that higher temperatures reduce the inhibition efficiency of EPSs, likely due to thermal desorption [90]. For instance, Chen et al. [89] reported a decline in corrosion inhibition efficiency from 97.13% at 15 °C to 62.46% at 45 °C. Similarly, Dong et al. [121] found that an EPS effectively prevented steel corrosion in a 3% NaCl solution, but its protective effect reduced at higher temperatures because of thermal desorption. Moreover, the decrease in EPS anti-corrosion performance can be assigned to the deactivation of enzymatic electron shuttles under high temperature [112]. Furthermore, pH significantly influences EPS adhesion to metal surfaces [130]. Under acidic conditions, the protonation of functional groups reduces EPS binding capacity, thereby decreasing metal ion adsorption [130]. Additional factors governing protection efficiency include the inhibitor concentration and exposure duration [132]. At low concentrations, EPSs may promote corrosion by acting as electron shuttles [112]. However, at higher concentrations, EPSs form compact protective films that inhibit corrosion [112]. Moradi et al. [133] demonstrated that the corrosion prevention effect increased with both exposure duration and EPS concentration. In the same context, biofilm thickness plays a critical role in corrosion inhibition efficiency [94].
The properties of EPSs vary significantly across microbial species and growth conditions [130]. As reported in the literature, EPSs exhibit strain-specific characteristics [130]. Moreover, additional variables including microbial growth media, growth time, and experimental protocols further influence EPS performance [111]. Interestingly, the corrosion inhibiting properties of EPSs are governed by their macromolecular composition and the used extraction protocol [105,106]. For example, heat-based extraction methods preferentially yield EPSs with higher relative protein contents [106]. However, the variability of functional groups in EPSs enhances corrosion inhibition through synergistic mechanisms [134,135]. Key functional groups such as carboxyl, hydroxyl, and amino groups chelate metal ions and promote the formation of stable protective films on metal surfaces [134,135]. Generally, extraction processing methodologies significantly influence both purity and interfacial adhesion characteristics of EPSs [136]. Notably, EPSs harvested during the stationary phase demonstrate superior inhibition efficiency [95]. Moreover, the synergistic effects of the produced compounds have an impact on corrosion inhibition [91]. Specifically, rhamnolipid biosurfactants from Pseudomonas spp. form protective surface films through oxygen lone–pair interactions with metal substrates [137].
Taking into consideration all these multiple interdependent factors affecting EPS-mediated corrosion inhibition such as the environmental conditions, biofilm properties, strain-specific EPS characteristics, and production parameters, the optimization of these parameters can enhance the performance of EPSs as sustainable corrosion inhibitors [90,94,121]. Thus, future research should focus on standardizing synthetic EPS formulations and improving extraction techniques [106].
4. Challenges, Limitations, and Future Prospective of EPSs as Corrosion Inhibitors
EPSs have emerged as eco-friendly corrosion inhibitors, offering important advantages over conventional synthetic inhibitors due to their biodegradability, safety, and capacity to form protective biofilms on metal surfaces [113,138]. However, several challenges currently limit their widespread industrial application [63,138]. One major issue is the variability in EPS composition, which is highly dependent on microbial strains, growth conditions, and extraction methods, leading to irregular corrosion inhibition performance. The extraction methodology employed significantly influences EPS composition, as residual impurities or inactive constituents may reduce their inhibitory efficacy. Furthermore, EPS performance can be substantially diminished under aggressive operational conditions, including elevated temperatures, chemical media, or pH variability [113,139,140]. The mechanisms governing EPS adsorption and protective action [141,142] remain incompletely characterized, limiting optimization efforts. Otherwise, certain EPSs may accelerate corrosion during initial exposure prior to the formation of the protective film, raising reliability concerns for practical application [113]. Moreover, large-scale EPS production is limited by various factors, mainly low yields, expensive culture media requirements, costly purification processing, and difficulty in matching the economic viability of established inhibitors such as chromates [143,144].
Despite these limitations, significant research efforts are advancing innovative strategies to improve the efficacy and practical deployment of EPS-based corrosion inhibitors. One promising approach involves developing synergistic formulation by combining EPSs with inorganic inhibitors such as molybdate. This formulation shows enhanced corrosion inhibition efficiency through complementary protection mechanisms. For instance, experimental studies reveal that synergistic combinations of sodium molybdate with S. mutans EPSs can achieve exceptional corrosion inhibition efficiencies exceeding 90% on carbon steel substrates [90]. An advanced research direction using genetic engineering can be employed to modify microbial strains and optimize EPS production, with enhanced specific functional groups that improve metal binding and protective film stability. Recently, genetic engineering techniques have enabled the precise control of genes for polysaccharide production, leading to more robust and consistent EPS materials [127,145,146].
Furthermore, advances in synthetic biology offer promising opportunities to design microbial cell factories that secrete EPSs with tailored functionalities, by incorporating specific functional groups or enhancing structural stability under industrial conditions. These engineered strains could enable the production of next-generation, smart, and sustainable corrosion inhibitors.
Interestingly, researchers are developing smart multifunctional EPSs with stimuli-responsive properties that can adapt their protective behavior in response to environmental changes, such as pH fluctuations or mechanical stress. These intelligent systems have the potential to transform corrosion protection in dynamic industrial environments, where operating conditions vary significantly [10,137]. Computational approaches, including artificial intelligence and molecular modeling, are able to transform EPS research by predicting molecular interactions and designing high-performance inhibitors. EPS datasets can be analyzed by machine learning to identify protective structural features [10,147]. Moreover, molecular dynamics simulations may elucidate atomic-scale adsorption behavior, offering potential insights into interfacial interactions. These tools simplify inhibitor discovery and minimize experimental screening requirements [147]. Moreover, the modification of EPSs and/or their use in combination with other materials could enhance the corrosion inhibition. For example, oxidized and acetylated EPS modifications in styrene–acrylic coatings can provide effective marine corrosion protection for over a year [118]. Therefore, researchers should explore integrating EPSs with nanotechnology to develop innovative hybrid coatings with superior corrosion inhibition properties.
On the production side, utilizing waste materials as growth media for microbial growth significantly reduces EPS production costs while enhancing sustainability. This approach aligns with circular economy principles by transforming waste into valuable resources and minimizing environmental impact [63,88,143,148].
Although EPS production by microbial strains is a viable option as microbial growth, EPS extraction, and purification processes are well established, production costs remain a limiting factor for large-scale implementation. To reduce costs, key considerations must include various factors including strain selection (high-yield native strains, genetically modified strains, strains with efficient biosynthesis pathways, etc.), growth media (considering composition, low-cost alternatives, culture operating conditions, etc.), harvesting methods (extraction, purification, and storage techniques), and corrosion inhibition mechanisms [143,144,148]. Future research directions should focus on optimizing microbial growth conditions [148], enhancing EPS performance through targeted molecular design [149], and validating laboratory findings in real conditions [150]. As reported by Jia et al. [141], understanding how different microorganisms interact in biofilms is essential for applications in real conditions. To substitute conventional agents with EPS inhibitors, is important to develop both standardized testing protocols and production methods at a large scale [63,139]. Moreover, there should be close collaboration between interdisciplinary researchers including microbiologists, materials scientists, and corrosion engineers to ensure the EPS-based inhibitor transition from the laboratory scale to industrial applications [104,141], allowing the implementation of sustainable corrosion protection strategies [10].
5. Conclusions
Microbial extracellular polymeric substances (EPSs) present a compelling and sustainable alternative to conventional corrosion inhibitors. Their unique biochemical composition, rich in polysaccharides, proteins, and functional groups, enables them to form protective biofilms, chelate metal ions, and alter the local electrochemical environment, thereby mitigating corrosion effectively. Numerous studies have demonstrated the high efficiency of EPSs in protecting various metal surfaces, particularly in saline and acidic environments. Despite their significant promise, several challenges remain, including variability in EPS composition, sensitivity to environmental conditions, and production costs. However, ongoing advancements in genetic engineering, synthetic biology, artificial intelligence, and sustainable bioprocessing offer new avenues to overcome these limitations. Future research should prioritize standardizing production methods, optimizing microbial strains, and developing smart EPS-based formulations tailored to industrial needs. Overall, EPS-based corrosion inhibitors align with global sustainability goals and offer a biotechnological path forward for green and efficient corrosion management in diverse sectors such as construction, marine infrastructure, and energy systems.
Author Contributions
Conceptualization, F.B.R.; investigation, N.S., W.M., B.O., M.K., and F.B.R.; data curation, N.S., W.M., B.O., M.K., and F.B.R.; writing—original draft preparation, N.S., W.M., B.O., Z.A., and F.B.R.; writing—review and editing, W.M. and M.K.; supervision, W.M., M.K., Z.A., and F.B.R.; project administration, F.B.R.; funding acquisition, Z.A. and W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Deanship of Scientific Research at University of Bisha-Saudi Arabia, through the Fast-Track Research Support Program.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vafadar, A.; Guzzomi, F.; Rassau, A.; Hayward, K. Advances in Metal Additive Manufacturing: A Review of Common Processes, Industrial Applications, and Current Challenges. Appl. Sci. 2021, 11, 1213. [Google Scholar] [CrossRef]
- Schweitzer, P.E.P.A. Fundamentals of Metallic Corrosion: Atmospheric and Media Corrosion of Metals; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zarasvand, K.A.; Rai, V.R. Microorganisms: Induction and Inhibition of Corrosion in Metals. Int. Biodeterior. Biodegrad. 2014, 87, 66–74. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of Iron by Sulfate-Reducing Bacteria: New Views of an Old Problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, J.; Mulder, J.; Wang, Q.; Liu, C.; He, N. High Environmental Costs behind Rapid Economic Development: Evidence from Economic Loss Caused by Atmospheric Acid Deposition. J. Environ. Manag. 2023, 334, 117511. [Google Scholar] [CrossRef]
- Singh, A.K. Microbially Induced Corrosion and Its Mitigation; Springer Briefs in Materials; Springer: Singapore, 2020; ISBN 978-981-15-8017-8. [Google Scholar]
- Ibrahim, A.; Hawboldt, K.; Bottaro, C.; Khan, F. Review and Analysis of Microbiologically Influenced Corrosion: The Chemical Environment in Oil and Gas Facilities. Corros. Eng. Sci. Technol. 2018, 53, 549–563. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Isahak, W.N.R.W.; Al-Azzawi, W.K. Corrosion Inhibitors: Natural and Synthetic Organic Inhibitors. Lubricants 2023, 11, 174. [Google Scholar] [CrossRef]
- Kamachi Mudali, U.; Jayaraj, J.; Raman, R.K.S.; Raj, B. Corrosion: An Overview of Types, Mechanism, and Requisites of Evaluation. In Non-Destructive Evaluation of Corrosion and Corrosion-Assisted Cracking; Singh, R., Raj, B., KamachiMudali, U., Singh, P., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 75–91. ISBN 978-1-118-35005-8. [Google Scholar]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Current and Emerging Trends of Inorganic, Organic and Eco-Friendly Corrosion Inhibitors. RSC Adv. 2024, 14, 31877–31920. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Asaduzzaman Chowdhury, M.; Kchaou, M. An Overview of Green Corrosion Inhibitors for Sustainable and Environment Friendly Industrial Development. J. Adhes. Sci. Technol. 2021, 35, 673–690. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Zhang, Q.; Zhao, C.; Zhou, X.; Zheng, H.; Zhang, R.; Sun, Y.; Yan, Z. Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules 2023, 28, 2832. [Google Scholar] [CrossRef]
- Sapkota, S.C.; Dahal, D.; Yadav, A.; Dhakal, D.; Sharma, R.K.; Saini, G. A Paradigm Shift in Corrosion Inhibition Using Botanical Extracts: From Conventional Methods to Advanced Methods for Reinforcing Steel. Innov. Infrastruct. Solut. 2025, 10, 104. [Google Scholar] [CrossRef]
- Fu, M.; Cheng, X.; Li, J.; Chen, S.; Dou, W.; Liu, G. Influence of Soluble, Loosely Bound and Tightly Bound Extracellular Polymeric Substances (EPS) Produced by Desulfovibrio Vulgaris on EH40 Steel Corrosion. Corros. Sci. 2023, 221, 111342. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting Extracellular Polymeric Substances (EPS) Controlling Strategies for Performance Enhancement of Biological Wastewater Treatments: An Overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Krembs, C.; Eicken, H.; Junge, K.; Deming, J.W. High Concentrations of Exopolymeric Substances in Arctic Winter Sea Ice: Implications for the Polar Ocean Carbon Cycle and Cryoprotection of Diatoms. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 2163–2181. [Google Scholar] [CrossRef]
- Al-Sayegh, A.; Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A. Draft Genome Sequence of Bacillus Subtilis AS2, a Heavy Crude Oil-Degrading and Biosurfactant-Producing Bacterium Isolated from a Soil Sample. Genome Announc. 2017, 5, e00969-17. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Finore, I.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Production and Biotechnological Potential of Extracellular Polymeric Substances from Sponge-Associated Antarctic Bacteria. Appl. Environ. Microbiol. 2018, 84, e01624-17. [Google Scholar] [CrossRef]
- Carrión, O.; Delgado, L.; Mercade, E. New Emulsifying and Cryoprotective Exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Singha, T.K. Microbial Extracellular Polymeric Substances: Production, Isolation and Applications. IOSR J. Pharm. 2012, 2, 271–281. [Google Scholar]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Gomes, E.; De Souza, A.R.; Orjuela, G.L.; Da Silva, R.; De Oliveira, T.B.; Rodrigues, A. Applications and Benefits of Thermophilic Microorganisms and Their Enzymes for Industrial Biotechnology. In Gene Expression Systems in Fungi: Advancements and Applications; Schmoll, M., Dattenböck, C., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 459–492. ISBN 978-3-319-27949-7. [Google Scholar]
- Dick, G.J. The Microbiomes of Deep-Sea Hydrothermal Vents: Distributed Globally, Shaped Locally. Nat. Rev. Microbiol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Sardari, R.R.; Kulcinskaja, E.; Ron, E.Y.; Björnsdóttir, S.; Friðjónsson, Ó.H.; Hreggviðsson, G.Ó.; Karlsson, E.N. Evaluation of the Production of Exopolysaccharides by Two Strains of the Thermophilic Bacterium Rhodothermus Marinus. Carbohydr. Polym. 2017, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fuso, A.; Bancalari, E.; Castellone, V.; Caligiani, A.; Gatti, M.; Bottari, B. Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods 2023, 12, 215. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Extracellular Polymeric Substances with Metal Adsorption Capacity Produced by Pseudoalteromonas sp. MER144 from Antarctic Seawater. Environ. Sci. Pollut. Res. 2018, 25, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Mancuso Nichols, C.A.; Garon, S.; Bowman, J.P.; Raguénès, G.; Guezennec, J. Production of Exopolysaccharides by Antarctic Marine Bacterial Isolates. J. Appl. Microbiol. 2004, 96, 1057–1066. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Finore, I.; Di Marco, G.; Michaud, L.; Giudice, A.L. Isolation, Characterization and Optimization of EPSs Produced by a Cold-Adapted Marinobacter Isolate from Antarctic Seawater. Antarct. Sci. 2019, 31, 69–79. [Google Scholar] [CrossRef]
- Finore, I.; Lama, L.; Di Donato, P.; Romano, I.; Tramice, A.; Leone, L.; Nicolaus, B.; Poli, A. Parageobacillus Thermantarcticus, an Antarctic Cell Factory: From Crop Residue Valorization by Green Chemistry to Astrobiology Studies. Diversity 2019, 11, 128. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- García-Cubero, R.; Cabanelas, I.T.D.; Sijtsma, L.; Kleinegris, D.M.M.; Barbosa, M.J. Production of Exopolysaccharide by Botryococcus braunii CCALA 778 under Laboratory Simulated Mediterranean Climate Conditions. Algal Res. 2018, 29, 330–336. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Liu, N.; Ge, F.; Xiao, H.; Yang, Y. Role of Extracellular Polymeric Substances from Chlorella Vulgaris in the Removal of Ammonium and Orthophosphate under the Stress of Cadmium. Bioresour. Technol. 2015, 190, 299–306. [Google Scholar] [CrossRef]
- Dhanya, B.E.; Athmika; Rekha, P.D. Characterization of an Exopolysaccharide Produced by Enterobacter sp. YU16-RN5 and Its Potential to Alleviate Cadmium Induced Cytotoxicity In Vitro. 3 Biotech 2021, 11, 491. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic Iron-Oxidizing Bacteria Produce Organic Stalks to Control Mineral Growth: Implications for Biosignature Formation. ISME J. 2011, 5, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the Applications of Their Enzymes as New Biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular Polymeric Substances from Shewanella sp. HRCR-1 Biofilms: Characterization by Infrared Spectroscopy and Proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. In Microbial Biofilms; Ghannoum, M., Parsek, M., Whiteley, M., Mukherjee, P.K., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 163–199. ISBN 978-1-68367-091-9. [Google Scholar]
- Hendrickx, L.; Hausner, M.; Wuertz, S. Natural Genetic Transformation in Monoculture Acinetobacter sp. Strain BD413 Biofilms. Appl. Environ. Microbiol. 2003, 69, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Oh, E.; Jeon, B. Enhanced Transmission of Antibiotic Resistance in Campylobacter Jejuni Biofilms by Natural Transformation. Antimicrob. Agents Chemother. 2014, 58, 7573–7575. [Google Scholar] [CrossRef]
- Kawaharada, Y.; Kelly, S.; Nielsen, M.W.; Hjuler, C.T.; Gysel, K.; Muszyński, A.; Carlson, R.W.; Thygesen, M.B.; Sandal, N.; Asmussen, M.H. Receptor-Mediated Exopolysaccharide Perception Controls Bacterial Infection. Nature 2015, 523, 308–312. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Walker, G.C. Succinoglycan Is Required for Initiation and Elongation of Infection Threads during Nodulation of Alfalfa by Rhizobium meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar] [CrossRef]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of Polysaccharides in Pseudomonas aeruginosa Biofilm Development. Curr. Opin. Microbiol. 2007, 10, 644–648. [Google Scholar] [CrossRef]
- Koczan, J.M.; McGrath, M.J.; Zhao, Y.; Sundin, G.W. Contribution of Erwinia amylovora Exopolysaccharides Amylovoran and Levan to Biofilm Formation: Implications in Pathogenicity. Phytopathology® 2009, 99, 1237–1244. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Ptacek, D.; Vanderleyden, J.; Dutto, P.; Labandera-Gonzalez, C.; Caballero-Mellado, J.; Aguirre, J.F.; Kapulnik, Y. Responses of Agronomically Important Crops to Inoculation with Azospirillum. Funct. Plant Biol. 2001, 28, 871–879. [Google Scholar] [CrossRef]
- Sengupta, S.; Dey, S. Microbial Exo-Polysaccharides (EPS): Role in Agriculture and Environment. Agric. Food 2019, 1, 4–8. [Google Scholar]
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Viscardi, S.; Ventorino, V.; Duran, P.; Maggio, A.; De Pascale, S.; Mora, M.L.; Pepe, O. Assessment of Plant Growth Promoting Activities and Abiotic Stress Tolerance of Azotobacter Chroococcum Strains for a Potential Use in Sustainable Agriculture. J. Soil Sci. Plant Nutr. 2016, 16, 848–863. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Hashem, A.; Abd_Allah, E.F.; Santoyo, G.; Kumar, A.; Gupta, R.K. Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils. Microorganisms 2023, 11, 2507. [Google Scholar] [CrossRef]
- Davenport, E.K.; Call, D.R.; Beyenal, H. Differential Protection from Tobramycin by Extracellular Polymeric Substances from Acinetobacter baumannii and Staphylococcus aureus Biofilms. Antimicrob. Agents Chemother. 2014, 58, 4755–4761. [Google Scholar] [CrossRef] [PubMed]
- Farber, B.F.; Kaplan, M.H.; Clogston, A.G. Staphylococcus Epidermidis Extracted Slime Inhibits the Antimicrobial Action of Glycopeptide Antibiotics. J. Infect. Dis. 1990, 161, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Lloret, J.; Wulff, B.B.H.; Rubio, J.M.; Downie, J.A.; Bonilla, I.; Rivilla, R. Exopolysaccharide II Production Is Regulated by Salt in the Halotolerant Strain Rhizobium meliloti EFB1. Appl. Environ. Microbiol. 1998, 64, 1024–1028. [Google Scholar] [CrossRef]
- Kumar, S.C.; Kumar, M.; Singh, R.; Saxena, A.K. Population and Genetic Diversity of Rhizobia Nodulating Chickpea in Indo-Gangetic Plains of India. Braz. J. Microbiol. 2024, 55, 4057–4075. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from Extremophiles: From Fundamentals to Biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef]
- Miguel, P.S.B.; de Oliveira, M.N.V.; Delvaux, J.C.; de Jesus, G.L.; Borges, A.C.; Tótola, M.R.; Neves, J.C.L.; Costa, M.D. Diversity and Distribution of the Endophytic Bacterial Community at Different Stages of Eucalyptus Growth. Antonie Van Leeuwenhoek 2016, 109, 755–771. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical Transport along Bacterial Nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. USA 2010, 107, 18127–18131. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides 2024, 5, 241–287. [Google Scholar] [CrossRef]
- Lehman, A.P.; Long, S.R. Exopolysaccharides from Sinorhizobium meliloti Can Protect against H2O2-Dependent Damage. J. Bacteriol. 2013, 195, 5362–5369. [Google Scholar] [CrossRef]
- Grobe, S.; Wingender, J.; Flemming, H.-C. Capability of Mucoid Pseudomonas aeruginosa to Survive in Chlorinated Water. Int. J. Hyg. Environ. Health 2001, 204, 139–142. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An Overview of the Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Go, L.C.; Holmes, W.; Depan, D.; Hernandez, R. Evaluation of Extracellular Polymeric Substances Extracted from Waste Activated Sludge as a Renewable Corrosion Inhibitor. PeerJ 2019, 7, e7193. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Yu, Y.; Li, Q.; Wang, H.; Chen, R.; He, N. Production and Characterization of a Novel Bioflocculant from Bacillus licheniformis. Appl. Environ. Microbiol. 2010, 76, 2778–2782. [Google Scholar] [CrossRef]
- Yuan, S.-J.; Sun, M.; Sheng, G.-P.; Li, Y.; Li, W.-W.; Yao, R.-S.; Yu, H.-Q. Identification of Key Constituents and Structure of the Extracellular Polymeric Substances Excreted by Bacillus megaterium TF10 for Their Flocculation Capacity. Environ. Sci. Technol. 2011, 45, 1152–1157. [Google Scholar] [CrossRef]
- Poli, A.; Nicolaus, B.; Denizci, A.A.; Yavuzturk, B.; Kazan, D. Halomonas smyrnensis sp. Nov., a Moderately Halophilic, Exopolysaccharide-Producing Bacterium. Int. J. Syst. Evol. Microbiol. 2013, 63, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and Characterization of an Intracellular Bioflocculant by Chryseobacterium daeguense W6 Cultured in Low Nutrition Medium. Bioresour. Technol. 2010, 101, 1044–1048. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular Electron Transfer Mechanisms between Microorganisms and Minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Wang, D.; Lekbach, Y.; Xu, D. Extracellular Electron Transfer in Microbial Biocorrosion. Curr. Opin. Electrochem. 2021, 29, 100763. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Nielsen, S.; Joseph, S.D.; Huang, D.; Thomas, T. A Combination of Biochar–Mineral Complexes and Compost Improves Soil Bacterial Processes, Soil Quality, and Plant Properties. Front. Microbiol. 2016, 7, 372. [Google Scholar] [CrossRef]
- Oleksy, M.; Klewicka, E. Exopolysaccharides Produced by Lactobacillus sp.: Biosynthesis and Applications. Crit. Rev. Food Sci. Nutr. 2016, 58, 450–462. [Google Scholar] [CrossRef]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L. Xanthan Gum Biosynthesis and Application: A Biochemical/Genetic Perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Aman, A.; Siddiqui, N.N.; Qader, S.A.U. Characterization and Potential Applications of High Molecular Weight Dextran Produced by Leuconostoc mesenteroides AA1. Carbohydr. Polym. 2012, 87, 910–915. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Z.; Wang, X.; Yang, A.; Chen, L.; Zhao, J.; Leonard, D.; Jaffrezic-Renault, N. Production and Characterization of a Bioflocculant by Proteus Mirabilis TJ-1. Bioresour. Technol. 2008, 99, 6520–6527. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.V.; Salunkhe, R.B.; Patil, C.D.; Patil, D.M.; Salunke, B.K. Bioflocculant Exopolysaccharide Production by Azotobacter Indicus Using Flower Extract of Madhuca latifolia L. Appl. Biochem. Biotechnol. 2010, 162, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.A.; Martin, S.M. Extracellular Polysaccharides of Serratia marcescens. Can. J. Biochem. 1964, 42, 1403–1413. [Google Scholar] [CrossRef]
- Bezawada, J.; Hoang, N.V.; More, T.T.; Yan, S.; Tyagi, N.; Tyagi, R.D.; Surampalli, R.Y. Production of Extracellular Polymeric Substances (EPS) by Serratia sp. 1 Using Wastewater Sludge as Raw Material and Flocculation Activity of the EPS Produced. J. Environ. Manag. 2013, 128, 83–91. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Jin, M.; Haobin, Z.; Li, Q.; Shao, D.; Jiang, C.; Huang, Q.; Yang, H.; Shi, J.; Hussain, N. Functional Characterization and Biotechnological Potential of Exopolysaccharide Produced by Lactobacillus rhamnosus Strains Isolated from Human Breast Milk. LWT 2018, 89, 638–647. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef]
- Laws, A.; Gu, Y.; Marshall, V. Biosynthesis, Characterisation, and Design of Bacterial Exopolysaccharides from Lactic Acid Bacteria. Biotechnol. Adv. 2001, 19, 597–625. [Google Scholar] [CrossRef]
- Naik, M.M.; Pandey, A.; Dubey, S.K. Biological Characterization of Lead-Enhanced Exopolysaccharide Produced by a Lead Resistant Enterobacter Cloacae Strain P2B. Biodegradation 2012, 23, 775–783. [Google Scholar] [CrossRef]
- Benincasa, M.; Lagatolla, C.; Dolzani, L.; Milan, A.; Pacor, S.; Liut, G.; Tossi, A.; Cescutti, P.; Rizzo, R. Biofilms from Klebsiella Pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides. Microorganisms 2016, 4, 26. [Google Scholar] [CrossRef]
- Kavitake, D.; Marchawala, F.Z.; Delattre, C.; Shetty, P.H.; Pathak, H.; Andhare, P. Biotechnological Potential of Exopolysaccharide as a Bioemulsifier Produced by Rhizobium Radiobacter CAS Isolated from Curd. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100202. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in Bacterial Exopolysaccharides: From Production to Biotechnological Applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The Biofilm Matrix: Multitasking in a Shared Space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Su, W.; Tang, B.; Fu, F.; Huang, S.; Zhao, S.; Bin, L.; Ding, J.; Chen, C. A New Insight into Resource Recovery of Excess Sewage Sludge: Feasibility of Extracting Mixed Amino Acids as an Environment-Friendly Corrosion Inhibitor for Industrial Pickling. J. Hazard. Mater. 2014, 279, 38–45. [Google Scholar] [CrossRef]
- Chen, S.; Wu, T.; Zeng, F.; Chen, K.; Li, L.; Qu, Q. Streptococcus Mutans Soluble Extracellular Polymeric Substances and Sodium Molybdate as Mixed Corrosion Inhibitor for X70 Steel. J. Mater. Sci. 2023, 58, 2915–2934. [Google Scholar] [CrossRef]
- Finkenstadt, V.L.; Bucur, C.B.; Côté, G.L.; Evans, K.O. Bacterial Exopolysaccharides for Corrosion Resistance on Low Carbon Steel. J. Appl. Polym. Sci. 2017, 134, 45032. [Google Scholar] [CrossRef]
- Parthipan, P.; Sabarinathan, D.; Angaiah, S.; Rajasekar, A. Glycolipid Biosurfactant as an Eco-Friendly Microbial Inhibitor for the Corrosion of Carbon Steel in Vulnerable Corrosive Bacterial Strains. J. Mol. Liq. 2018, 261, 473–479. [Google Scholar] [CrossRef]
- Khan, M.S.; Yang, C.; Zhao, Y.; Pan, H.; Zhao, J.; Shahzad, M.B.; Kolawole, S.K.; Ullah, I.; Yang, K. An Induced Corrosion Inhibition of X80 Steel by Using Marine Bacterium Marinobacter salsuginis. Colloids Surf. B Biointerfaces 2020, 189, 110858. [Google Scholar] [CrossRef]
- Örnek, D.; Jayaraman, A.; Syrett, B.; Hsu, C.-H.; Mansfeld, F.; Wood, T. Pitting Corrosion Inhibition of Aluminum 2024 by Bacillus Biofilms Secreting Polyaspartate or γ-Polyglutamate. Appl. Microbiol. Biotechnol. 2002, 58, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, M.; Fan, Y.; Li, Z.; Cristiani, P.; Chen, X.; Xu, D.; Wang, F.; Gu, T. Marine Vibrio Spp. Protect Carbon Steel against Corrosion through Secreting Extracellular Polymeric Substances. NPJ Mater. Degrad. 2022, 6, 6. [Google Scholar] [CrossRef]
- Jin, J.; Wu, G.; Zhang, Z.; Guan, Y. Effect of Extracellular Polymeric Substances on Corrosion of Cast Iron in the Reclaimed Wastewater. Bioresour. Technol. 2014, 165, 162–165. [Google Scholar] [CrossRef]
- Pusparizkita, Y.M.; Schmahl, W.W.; Setiadi, T.; Ilsemann, B.; Reich, M.; Devianto, H.; Harimawan, A. Evaluation of Bio-Corrosion on Carbon Steel by Bacillus Megaterium in Biodiesel and Diesel Oil Mixture. J. Eng. Technol. Sci. 2020, 52, 370–384. [Google Scholar] [CrossRef]
- Suma, M.S.; Basheer, R.; Sreelekshmy, B.R.; Vipinlal, V.; Sha, M.A.; Jineesh, P.; Krishnan, A.; Archana, S.R.; Saji, V.S.; Shibli, S.M.A. Pseudomonas Putida RSS Biopassivation of Mild Steel for Long Term Corrosion Inhibition. Int. Biodeterior. Biodegrad. 2019, 137, 59–67. [Google Scholar] [CrossRef]
- Ruan, X.; Yang, L.; Wang, Y.; Dong, Y.; Xu, D.; Zhang, M. Biofilm-Induced Corrosion Inhibition of Q235 Carbon Steel by Tenacibaculum mesophilum D-6 and Bacillus sp. Y-6. Metals 2023, 13, 649. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Qian, H.; Huang, L.; Ma, L.; Hao, X.; Zhang, D. Microbiologically Influenced Corrosion Inhibition of Carbon Steel via Biomineralization Induced by Shewanella putrefaciens. NPJ Mater. Degrad. 2021, 5, 59. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Tan, Y.; Meng, G.; Liu, H.; Cheng, Y.; Liu, H. Characterizations of the Biomineralization Film Caused by Marine Pseudomonas stutzeri and Its Mechanistic Effects on X80 Pipeline Steel Corrosion. J. Mater. Sci. Technol. 2022, 125, 15–28. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Dou, W.; Wang, Y.; Sun, J.; Liu, G. Microbiologically Influenced Corrosion Inhibition of Two Marine Structural Steels Caused by Halomonas titanicae in Aerobic Environments. Eng. Fail. Anal. 2023, 154, 107668. [Google Scholar] [CrossRef]
- Ejileugha, C.; Ezealisiji, K.M.; Ezejiofor, A.N.; Orisakwe, O.E. Microbiologically Influenced Corrosion: Uncovering Mechanisms and Discovering Inhibitor—Metal and Metal Oxide Nanoparticles as Promising Biocorrosion Inhibitors. J. Bio-Tribo-Corros. 2021, 7, 109. [Google Scholar] [CrossRef]
- Muzammil, S.; Khurshid, M.; Nawaz, I.; Siddique, M.H.; Zubair, M.; Nisar, M.A.; Imran, M.; Hayat, S. Aluminium Oxide Nanoparticles Inhibit EPS Production, Adhesion and Biofilm Formation by Multidrug Resistant Acinetobacter baumannii. Biofouling 2020, 36, 492–504. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Understanding Microbial Inhibition of Corrosion. A Comprehensive Overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Relations between Extraction Protocols for Activated Sludge Extracellular Polymeric Substances (EPS) and EPS Complexation Properties: Part I. Comparison of the Efficiency of Eight EPS Extraction Methods. Enzym. Microb. Technol. 2006, 38, 237–245. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H.P. Extraction of Extracellular Polymeric Substances (EPS) of Sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Ghafari, M.D.; Bahrami, A.; Rasooli, I.; Arabian, D.; Ghafari, F. Bacterial Exopolymeric Inhibition of Carbon Steel Corrosion. Int. Biodeterior. Biodegrad. 2013, 80, 29–33. [Google Scholar] [CrossRef]
- Chongdar, S.; Gunasekaran, G.; Kumar, P. Corrosion Inhibition of Mild Steel by Aerobic Biofilm. Electrochim. Acta 2005, 50, 4655–4665. [Google Scholar] [CrossRef]
- Ignatova-Ivanova, T.; Ivanov, R.; Iliev, I.; Ivanova, I. Study of the Anticorrosion Effect of Exopolysaccharides Produced by Lactobacillus Delbrueckii B5 Cultivated on Different Carbohydrates. Biotechnol. Biotechnol. Equip. 2012, 26, 224–227. [Google Scholar]
- Finkenstadt, V.L.; Côté, G.L.; Willett, J.L. Corrosion Protection of Low-Carbon Steel Using Exopolysaccharide Coatings from Leuconostoc mesenteroides. Biotechnol. Lett. 2011, 33, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, T.; Zhang, G.; Wang, W.; Dong, S.; Cheng, Y.; Liu, H. Corrosion Inhibition of Carbon Steel in CO2-Containing Oilfield Produced Water in the Presence of Iron-Oxidizing Bacteria and Inhibitors. Corros. Sci. 2016, 105, 149–160. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Asif, M.; Zhang, G.; Liu, H. The Corrosion Behavior and Mechanism of Carbon Steel Induced by Extracellular Polymeric Substances of Iron-Oxidizing Bacteria. Corros. Sci. 2017, 114, 102–111. [Google Scholar] [CrossRef]
- Ismail, K.M.; Gehrig, T.; Jayaraman, A.; Wood, T.K.; Trandem, K.; Arps, P.J.; Earthman, J.C. Corrosion Control of Mild Steel by Aerobic Bacteria under Continuous Flow Conditions. Corrosion 2002, 58, 417–423. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically Influenced Corrosion: Looking to the Future. Int. Microbiol. 2005, 8, 169. [Google Scholar]
- Zuo, R.; Wood, T.K. Inhibiting Mild Steel Corrosion from Sulfate-Reducing and Iron-Oxidizing Bacteria Using Gramicidin-S-Producing Biofilms. Appl. Microbiol. Biotechnol. 2004, 65, 747–753. [Google Scholar] [CrossRef]
- Li, S.; Qu, Q.; Li, L.; Xia, K.; Li, Y.; Zhu, T. Bacillus Cereus S-EPS as a Dual Bio-Functional Corrosion and Scale Inhibitor in Artificial Seawater. Water Res. 2019, 166, 115094. [Google Scholar] [CrossRef] [PubMed]
- Arthur, D.E.; Jonathan, A.; Ameh, P.O.; Anya, C. A Review on the Assessment of Polymeric Materials Used as Corrosion Inhibitor of Metals and Alloys. Int. J. Ind. Chem. 2013, 4, 2. [Google Scholar] [CrossRef]
- Scheerder, J.; Breur, R.; Slaghek, T.; Holtman, W.; Vennik, M.; Ferrari, G. Exopolysaccharides (EPS) as Anti-Corrosive Additives for Coatings. Prog. Org. Coat. 2012, 75, 224–230. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Wang, J.; Wang, Y.; Li, W.; Xu, J. Electroactive Polymeric Coating with Enhanced Anticorrosion Performance Based on Polyaniline and Graphene Oxide. ACS Appl. Mater. Interfaces 2018, 10, 40317–40327. [Google Scholar]
- Zhang, J.; Li, Z.; Sun, W.; Li, X.; Cui, M.; Zhou, E.; Wang, F.; Xu, D. Extracellular Polysaccharides of Tenacibaculum mesophilum D-6 Play a Major Role During Its Corrosion Protection for X80 Carbon Steel in Seawater. Corros. Sci. 2025, 249, 112811. [Google Scholar] [CrossRef]
- Dong, Z.H.; Liu, T.; Liu, H.F. Influence of EPS Isolated from Thermophilic Sulphate-Reducing Bacteria on Carbon Steel Corrosion. Biofouling 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, S.; Liu, T.; Zhao, Q.; Wang, Y.; Guo, N.; Chang, X.; Liu, T.; Dong, Y.; Yin, Y. Bacillus Subtilis Inhibits Vibrio Natriegens-Induced Corrosion via Biomineralization in Seawater. Front. Microbiol. 2019, 10, 1111. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Corrosión de Origen Microbiológico: Mirando al Futuro. Int. Microbiol. 2005, 8, 169–180. [Google Scholar]
- Ford, T.; Mitchell, R. The Ecology of Microbial Corrosion. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1990; Volume 11, pp. 231–262. ISBN 978-1-4684-7614-9. [Google Scholar]
- Xu, H.; Wang, Y.; Li, X.; Zhang, M.; Chen, Q.; Zhou, L.; Wu, Z.; Liu, J.; Liu, X.; Gao, M. Synergistic effect of extracellular polymeric substances and carbon layer on electron utilization of Fe@C during anaerobic treatment of refractory wastewater. Water Res. 2023, 231, 119609. [Google Scholar] [CrossRef]
- Hao, J.; Li, P.; Zhang, X.; Wang, L.; Chen, Y.; Zhou, D.; Liu, Q.; Feng, S. Overview of Recent Developments in Composite Epoxy Resin in Organic Coating on Steel (2020–2024). Materials 2025, 18, 1531. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Nguyen, T.T.; Tran, H.Q.; Le, V.T.; Dang, N.T.; Pham, V.C.; Doan, T.H.; Bui, X.H. Synthesis and Corrosion Inhibition Potential of Cerium/Tetraethylenepentamine Dithiocarbamate Complex on AA2024-T3 in 3.5% NaCl. Materials 2022, 15, 6631. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Wang, X.; Zhou, Y.; Li, H.; Zhou, X.; Hu, L.; Zhang, T. Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Gong, S.; Song, C.; Tian, H.; Yan, Z.; Wang, S. Roles of Microbial Extracellular Polymeric Substances in Corrosion and Scale Inhibition of Circulating Cooling Water. ACS EST Water 2023, 3, 743–755. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Duan, J.; Shi, X.; Zhang, Y.; Guan, F.; Sand, W.; Hou, B. Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 5566. [Google Scholar] [CrossRef]
- Lavanya, M. A Brief Insight into Microbial Corrosion and Its Mitigation with Eco-Friendly Inhibitors. J. Bio Tribo Corros. 2021, 7, 125. [Google Scholar] [CrossRef]
- Moradi, M.; Song, Z.; Xiao, T. Exopolysaccharide Produced by Vibrio neocaledonicus sp. as a Green Corrosion Inhibitor: Production and Structural Characterization. J. Mater. Sci. Technol. 2018, 34, 2447–2457. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. A Theoretical Study on the Inhibition Efficiencies of Some Amino Acids as Corrosion Inhibitors of Nickel. Corros. Sci. 2010, 52, 3435–3443. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Cai, Q.-R.; He, X.-M.; Gao, L.-X.; Zhou, G.-D. Inhibition Effect of Some Amino Acids on Copper Corrosion in HCl Solution. Mater. Chem. Phys. 2008, 112, 353–358. [Google Scholar] [CrossRef]
- Stadler, R.; Wei, L.; Fürbeth, W.; Grooters, M.; Kuklinski, A. Influence of Bacterial Exopolymers on Cell Adhesion of Desulfovibrio vulgaris on High Alloyed Steel: Corrosion Inhibition by Extracellular Polymeric Substances (EPS). Mater. Corros. 2010, 61, 1008–1016. [Google Scholar] [CrossRef]
- Soares da Silva, R.d.C.F.; Selva Filho, A.A.P.; Faccioli, Y.E.S.; Silva, Y.K.; Oliveira, K.W.; Araujo, G.P.; Rocha e Silva, N.M.P.; Converti, A.; Sarubbo, L.A. Application of Pseudomonas Cepacia CCT 6659 Biosurfactant as a Metal Corrosion Inhibitor in a Constructed Accelerated Corrosion Chamber (ACC). Fermentation 2024, 10, 602. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Moradi, M.; Xiao, T.; Song, Z. Investigation of Corrosion Inhibitory Process of Marine Vibrio neocaledonicus sp. Bacterium for Carbon Steel. Corros. Sci. 2015, 100, 186–193. [Google Scholar] [CrossRef]
- Go, L.C.; Depan, D.; Holmes, W.E.; Gallo, A.; Knierim, K.; Bertrand, T.; Hernandez, R. Kinetic and Thermodynamic Analyses of the Corrosion Inhibition of Synthetic Extracellular Polymeric Substances. PeerJ Mater. Sci. 2020, 2, e4. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically Influenced Corrosion and Current Mitigation Strategies: A State of the Art Review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Zuo, R. Biofilms: Strategies for Metal Corrosion Inhibition Employing Microorganisms. Appl. Microbiol. Biotechnol. 2007, 76, 1245–1253. [Google Scholar] [CrossRef]
- Yadav, M.K.; Song, J.H.; Vasquez, R.; Lee, J.S.; Kim, I.H.; Kang, D.-K. Methods for Detection, Extraction, Purification, and Characterization of Exopolysaccharides of Lactic Acid Bacteria—A Systematic Review. Foods 2024, 13, 3687. [Google Scholar] [CrossRef]
- Sellami, M.; Oszako, T.; Miled, N.; Ben Rebah, F. Industrial Wastewater as Raw Material for Exopolysaccharide Production by Rhizobium leguminosarum. Braz. J. Microbiol. 2015, 46, 407–413. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Zhou, X.; Gu, Y.; Zhou, H.; Shen, L.; Zeng, W. The Roles of Extracellular Polymeric Substances of Pandoraea sp. XY-2 in the Removal of Tetracycline. Bioprocess Biosyst. Eng. 2020, 43, 1951–1960. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Z.; Zeng, Z.; Guo, N.; Lei, Y.; Liu, T.; Sun, S.; Chang, X.; Yin, Y.; Wang, X. Marine Bacteria Provide Lasting Anticorrosion Activity for Steel via Biofilm-Induced Mineralization. ACS Appl. Mater. Interfaces 2018, 10, 40317–40327. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent Developments in Sustainable Corrosion Inhibitors: Design, Performance and Industrial Scale Applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Sellami, M.; Frikha, F.; Aribi, A.; Taktak, I.; Miled, N.; Rebah, F.B. Optimization of Wastewater Based Media for Biopolymers Production by Rhizobium leguminosarum. Environ. Eng. Manag. J. (EEMJ) 2018, 17, 1329–1335. [Google Scholar]
- Korniy, S.A.; Zin, I.M.; Danyliak, M.-O.M.; Rizun, Y.Y. Eco-Friendly Metal Corrosion Inhibitors Based on Natural Polymers (A Review). Mater. Sci. 2023, 58, 567–578. [Google Scholar] [CrossRef]
- Seviour, R.J.; McNeil, B.; Fazenda, M.L.; Harvey, L.M. Operating Bioreactors for Microbial Exopolysaccharide Production. Crit. Rev. Biotechnol. 2011, 31, 170–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).