Abstract
It is widely recognized that fine surface structures found in nature contribute to surface functionality, and studies on the design of functional materials based on biomimetics have been actively conducted. In this study, polymer thin films with hierarchically ordered surface structure were prepared by combining both nanoimprinting using anodically oxidized porous alumina (AAO) as a template and surface-initiated atom transfer radical polymerization (SI-ATRP). To prepare such polymer films, we designed a new copolymer (poly{[2-(4-methyl-2-oxo-2H-chromen-7-yloxy)ethyl methacrylate]-co-[2-(2-bromo-2-methylpropionyloxy)ethyl methacrylate]}; poly(MCMA-co-HEMABr)) with coumarin moieties and α-haloester moieties in the pendants. The MCMA repeating units function to fix the pillar structure by photodimerization, and the HEMABr ones act as the polymerization initiation sites for SI-ATRP on the pillar surfaces. Surface structures consisting of vertically oriented multiple pillars were fabricated on the spin-coated poly(MCMA-co-HEMABr) thin films by nanoimprinting using an AAO template. Then, the coumarin moieties inside each pillar were crosslinked by UV light irradiation to fix the pillar structure. SEM observation confirmed that the internally crosslinked pillar structures were maintained even when immersed in organic solvents such as 1,2-dichloroethane and anisole, which are employed as solvents under SI-ATRP conditions. Finally, poly(2,2,2-trifluoroethyl methacrylate) and poly(N-isopropylacrylamide) chains were grafted onto the thin film by SI-ATRP, respectively, to prepare the hierarchically ordered surface structure. Furthermore, in this study, the surface properties as well as the thermoresponsive hydrophilic/hydrophobic switching of the obtained polymer films were investigated. The surface morphology and chemistry of the films with and without pillar structures were compared, especially the interfacial properties expressed as wettability. Grafting poly(TFEMA) increased the static contact angle for both flat and pillar films, and the con-tact angle of the pillar film surface increased from 104° for the flat film sample to 112°, suggesting the contribution of the pillar structure. Meanwhile, the pillar film surface grafted with poly(NIPAM) brought about a significant change in wettability when changing the temperature between 22 °C and 38 °C.
1. Introduction
The concept of biomimetics has great significance in creating excellent functional materials. In particular, various surface functional materials have been designed, inspired by the unique shapes, geometric patterns, and hierarchical structures of living organisms and plants [1,2,3,4]. Some of them have realized unique surface functions based on the synergistic effect between surface morphology and chemical properties. For example, lotus leaves with fractal surface structures containing hydrophobic waxes exhibit superhydrophobic surfaces, which is the so-called lotus effect [5,6,7,8]. The shell of a snail is made of a composite of minerals, mainly calcium carbonate and proteins, and has a superhydrophilic surface due to a thin layer of water trapped in the submicron-sized micro-grooves that cover its surface, and it exhibits oil-repellent and antifouling properties [9,10]. Biomimetic materials with surface microstructures are being developed based on nanotechnology. Essentially, nanotechnology manufacturing techniques can be divided into two broad categories: “top–down” and “bottom–up” approaches [11,12]. The “top–down” approach includes microfabrication techniques such as photolithography, dip-pen nanolithography, electron beam patterning, nanoimprint lithography [13,14,15,16,17,18,19,20]. On the other hand, the “bottom–up” approaches involve self-assembly methods that allow for fine patterning at the nanoscale level, providing well-defined nanoscale structures via molecular self-assembly [21,22]. Recently, the combination of nanoimprinting (a “top–down” approach) and SI-ATRP (a “bottom–up” approach) has been developed to prepare hierarchical surface structures [23]; however, there are few reports of surface functional materials produced using this method, and they have a lower surface roughness that can be more appropriately described as a disk-like morphology rather than a surface pillar morphology. The objective of this study was to develop new surface functional materials by combining the features of these two techniques. The fabrication process is outlined as follows (Figure 1): The first step in the fabrication process was to prepare a polymer thin film with vertically aligned pillar structures as the primary structure, which was achieved by using a hot-press nanoimprinting technique. A through-hole anodic alumina oxide (AAO) mold was used as the template for this process. In order to carry out graft modification on the pillar surfaces in organic solvents in the subsequent process, the pillar structure needed to be fixed. This was achieved by crosslinking formation inside each pillar by photo-irradiating the reactive groups previously introduced in the base polymer material. As a result, the pillar thin film was structurally fixed and insoluble in organic solvents. Next, we performed surface-initiated graft polymerization using the controlled radical polymerization (ATRP) initiation site (α-haloester site) on the pillar surface that was incorporated into the base polymer along with the crosslinking sites, to form graft chains on the pillar surface as secondary structures. We hoped that by grafting functional monomers, the surface properties based on the vertically aligned multiple pillar structure (primary structure) and the chemical properties of the grafted polymer chains (secondary structure, corresponding to the sheath of each pillar) on the pillar surfaces would work cooperatively and synergistically to realize tunable and unique surface functional properties. In this study, we attempted to improve the hydrophobicity of the pillar film surfaces by grafting fluorine-containing polymer chains (poly(2,2,2-trifluoroethyl methacrylate), poly(TFEMA)) and also attempted to realize hydrophilic/hydrophobic switching by grafting a temperature-responsive poly(N-isopropylacrylamide) (poly(NIPAM)); this enabled us to explore the possibility of imparting hydrophilic/hydrophobic functions by designing the chemical structure of the grafted polymer chains (secondary structure) that covered each pillar.
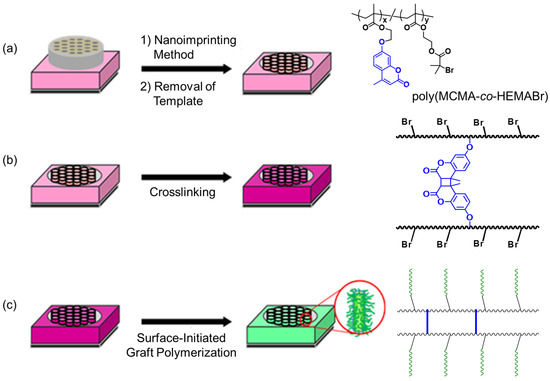
Figure 1.
Preparation of a polymer thin film with designed hierarchically ordered surface structure by a combination of nanoimprinting and controlled polymerization. (a) Preparation of poly(MCMA-co-HEMABr) films with pillar structures on their surfaces. (b) Fixation of the pillar structure by photocrosslinking. (c) Surface-initiated graft polymerization from the crosslinked pillar films.
2. Materials and Methods
2.1. Preparation of Poly (MCMA-co-HEMABr) Films with Nano-Pillar Structure Surfaces (CL-Poly (MCMA-co-HEMABr))
The Si wafers underwent a 6 h treatment with heated 35% H2O2 aqueous solution, and Si wafers with amino groups on their surfaces were produced by subjecting them to 3 wt% 3-Aminopropyltrimethoxysilane aqueous solution for 30 min. Thin films were prepared over the Si wafers by spin-coating (1200 rpm, 20 s) using 10 wt% poly(MCMA-co-HEMABr) toluene solution. Films with nano-pillar structure surfaces were prepared by thermal nanoimprinting using AAO with a pore diameter of 0.2 μm as a template. The nanoimprinting processes were accomplished by using an Eitre-3 Nano Imprint Lithography system. The thermal imprinting conditions were 130 °C for 3 min under 20 bar. After the imprinting process, the temperature was decreased down to 60 °C under applied pressure. The AAO template (Al2O3) was dissolved in 1 molL−1 NaOH aqueous solution for 10 h. Thereafter, the resulting polymer film was irradiated with a 150 W high-pressure mercury lamp emitting at 365 nm for 5 h at room temperature (distance to sample = 5 cm).
2.2. SI-ATRP of TFEMA from Nano-Pillar Films of CL-Poly(MCMA-co-HEMABr) [24]
A Si wafer coated with CL-poly(MCMA-co-HEMABr) was added to a Schlenk flask containing CuCl (11 mg, 0.11 mmol) and CuCl2 (1.0 mg, 7.4 μmol). TFEMA (1.84 g, 10.9 mmol), TPMA (32 mg, 0.11 mmol), EBI (21 mg, 0.11 mmol), and anisole (1.77 g) were added via a syringe. The flask was purged via three cycles of freezing, pumping, thawing, and backfilling with N2. The reaction was sealed and heated at 85 °C for 5 h and then cooled. The reaction mixture was analyzed by SEC and 1H NMR spectroscopy. The wafer was removed from the flask and rinsed liberally with anisole and MeOH.
2.3. SI-ATRP of NIPAM from Nano-Pillar Films of Poly(MCMA-co-HEMABr) [25]
A Si wafer coated with CL-poly(MCMA-co-HEMABr) was added to a Schlenk flask containing CuCl (6 mg, 0.06 mmol). NIPAM (0.67 g, 6.2 mmol), Me6TREN (28 mg, 0.12 mmol), EBI (12 mg, 0.06 mmol), ascorbic acid (11 mg, 0.06 mmol), and 2-propanol/water (4/1, v/v) (0.67 g) were added via a syringe. The flask was purged via three cycles of freezing, pumping, thawing, and backfilling with N2. The reaction was sealed and heated at 25 °C for 5 h and then cooled. The reaction mixture was analyzed by SEC and 1H NMR spectroscopy. The wafer was removed from the flask and rinsed liberally with 2-propanol and water.
2.4. Contact Angle Measurements
Water contact angles on various films were measured using a static contact angle meter, CA-S150 (Kyowa Interface Science Co., Ltd., Niiza, Japan), by placing a drop of distilled water (10 μL) on a substrate equipped with a water circulation system at 20 to 45 °C. Samples for contact angle measurements (2 cm × 2 cm) were dried in a vacuum oven at 60 °C for 12 h before measurement. Water contact angles were measured in the temperature range of 20 to 45 °C using a plate temperature controller. Five measurements were taken at different positions on each substrate, and the average value was calculated.
3. Results and Discussion
3.1. Synthesis of Poly(MCMA-co-HEMABr)
For the preparation of polymer thin films with hierarchically ordered surface structure, we designed a new copolymer (poly{[2-(4-methyl-2-oxo-2H-chromen-7-yloxy)ethyl methacrylate]-co-[2-(2-bromo-2-methylpropionyloxy)ethyl methacrylate]}; poly(MCMA-co-HEMABr)) with coumarin moieties and α-haloester moieties. The synthesis of methacrylate monomers with coumarin moieties (MCMA) was carried out by the condensation of methacryloyl chloride with a coumarin derivative according to a previous report [26]. And the synthesis of methacrylate monomers with α-haloester moieties (HEMABr) was carried out by the condensation of 2-hydroxyethyl methacrylate with 2-bromoisobutyryl bromide according to a previous report [27]. MCMA and HEMABr were copolymerized using AIBN in 1,2-dichloroethane at 60 °C for 4 h ([MCMA]0/[HEMABr]0/[AIBN]0 = 45/55/1, [MCMA]0 + [HEMABr]0 = 25 wt%). The obtained 1,2-dichloroethane solution of the reaction mixture was poured into a large amount of MeOH to remove the unreacted monomers and precipitate the polymer. The obtained polymer was characterized by 1H NMR and size exclusion chromatography (SEC). Figure 2 shows the 1H NMR spectrum of the isolated copolymer (Mn = 150,000; Mw/Mn = 2.89). 1H NMR analysis of poly(MCMA-co-HEMABr) showed a series of characteristic resonances, including those of the MCMA moieties (peaks b at δ = 6.5–7.0 ppm and δ = 7.4–7.7 ppm, peak c at δ = 6.1 ppm, and peak d at δ = 2.4 ppm), the HEMABr moieties (peak e at δ = 1.9 ppm), and the MMA main chain protons (peaks f at δ = 0.8–1.4 ppm). The integration ratio of the characteristic signals (peaks b, c, d, and e in Figure 2) supported the formation of a copolymer of MCMA and HEMABr with a composition ratio of 40:60. The synthesized poly(MCMA-co-HEMABr) had both crosslinkable sites (coumarin moieties) and polymerization initiation sites (α-haloester moieties) and was used to produce a thin film with vertically aligned pillars on its surface.
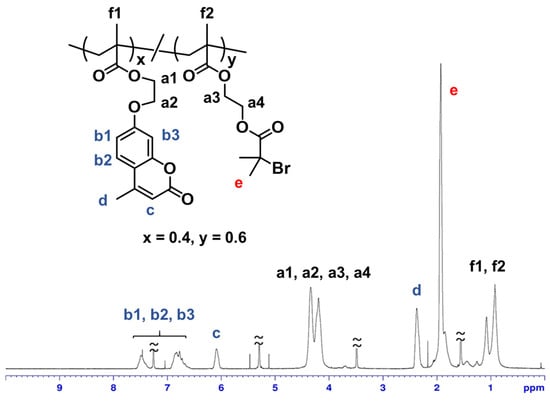
Figure 2.
1H NMR spectrum of poly(MCMA-co-HEMABr) in CDCl3.
3.2. Preparation of Poly(MCMA-co-HEMABr) Films With Pillar Structures on Their Surfaces
Since temperature setting is important in hot-press nanoimprinting, the glass transition temperature of the obtained poly(MCMA-co-HEMABr) was examined by differential scanning calorimetry (DSC) before nanoimprinting and found to be 75 °C (heating rate; 10 °C/min). (Figure S1). Therefore, the set temperature for hot-press nanoimprinting was 130 °C, which is sufficiently higher than the glass transition temperature of the same bath copolymer. A 1,2-dichloroethane solution of the copolymer was then spin-cast (1200 rpm, 20 s) onto a Si wafer pretreated with 3-aminopropyltrimethoxysilane to produce a thin film. This pretreatment was performed to improve the interaction between the copolymer and the Si wafer, thereby preventing the copolymer thin film from peeling off under SI-ATRP conditions. The spin-coated film showed uniform coloring due to structural coloring, suggesting a constant film thickness. Furthermore, the thickness of the resulting film was measured at five points using contact probe analysis, which also indicated a uniform film thickness at approximately 1400 nm. The obtained polymer film was nanoimprinted using an AAO as a template at 130 °C for 3 min under 20 bar. The formation of pillar structures is determined by the combination of temperature and pressure. In this experiment, the pressure was fixed at 20 bar based on preliminary considerations, and the formation behavior of pillar structures was examined by changing the temperature. When the temperature was increased to 140 °C, which is above 130 °C, it was observed that the length of the formed pillars increased, but some defects existed in the pillar structure. On the other hand, when the temperature was reduced to 120 °C and 110 °C, the length of the formed pillars decreased. Furthermore, at 100 °C, the template was not transferred to the polymer film. The AAO template was removed by etching with 1 molL−1 NaOH aqueous solution. After washing with water and drying, the surface morphology of the polymer film was observed by SEM (Figure 3a). Vertically aligned pillar structures with a diameter of 260 ± 30 nm and a length of 1000 ± 200 nm were observed, confirming that the fine pore structure of the AAO used as a template had been transferred to the polymer film surface. As shown in Figure 4a, no Al peaks were observed in the XPS analysis of the pillar film surface after the AAO was removed by dissolving, confirming the complete removal of the AAO. Furthermore, no traces of undissolved AAO were observed in the SEM observation of the resulting pillar film.
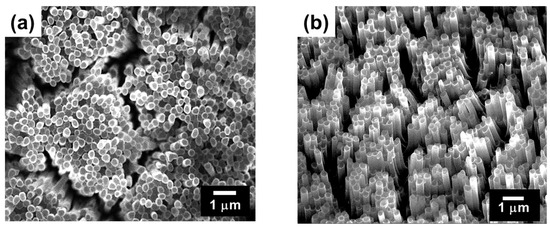
Figure 3.
SEM images of the surface of the (a) poly(MCMA-co-HEMABr) pillar film and (b) CL-poly(MCMA-co-HEMABr) pillar film.
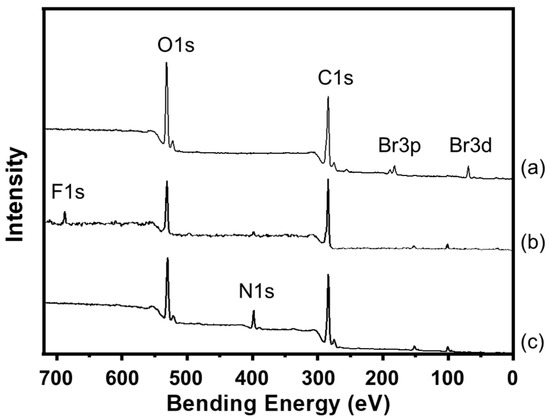
Figure 4.
XPS spectra of the surfaces of the (a) CL-poly(MCMA-co-HEMA-Br) pillar film, (b) CL-poly((MCMA-co-HEMABr)-g-poly(TFEMA)) pillar film and (c) CL-poly((MCMA-co-HEMABr)-g-poly(NIPAM)) pillar film.
Next, the pillar structure was fixed by photocrosslinking inside each pillar. As shown in Figure 5, UV absorption spectroscopy confirmed that the photochemical dimerization of the pendant coumarin moieties in the copolymer film proceeded in the solid state. Upon irradiation with UV light (150 W high-pressure mercury lamp; λ = 365 nm), the intensity of the absorption band originating from the coumarin groups at 280–350 nm gradually decreased [24,28]. The reaction rate of the coumarin moiety was calculated from the decrease in the peak intensity (absorbance at 320 nm) of the UV-vis spectra in Figure 4. Consequently, the reaction rate of the coumarin moiety was estimated to be approximately 80%. The photodimerization of the coumarin moieties occurred both intramolecularly and intermolecularly, the latter contributing to the fixation of the pillar structure. When the surface morphology of a film sample (crosslinked poly(MCMA-co-HEMABr); CL-poly(MCMA-co-HEMABr)) obtained by the photodimerization of the coumarin pendants was observed by SEM, it was confirmed that the aligned individual pillar structures were maintained and their size was almost unchanged (Figure 3b) compared to those in the SEM image before photoirradiation (Figure 3a). When the light irradiation time was 1 to 3 h, the polymer pillar structure could not be maintained and partially dissolved. Furthermore, the crosslinked pillar film was insoluble even when immersed in 1,2-dichloroethane, a good solvent for the starting copolymer, suggesting that the fixation of the pillar structure was achieved by the formation of crosslinks inside the pillars.
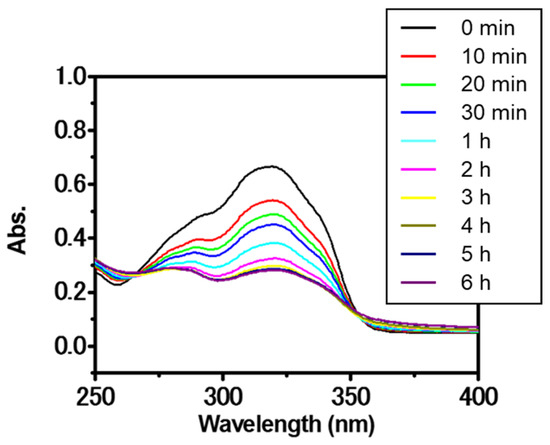
Figure 5.
UV spectral change in poly(MCMA-co-HEMA-Br) film upon irradiation with UV light (λ = 365 nm).
3.3. SI-ATRP from the Pillar Films of CL-Poly(MCMA-co-HEMABr)
For smooth, flat surfaces, the hydrophilicity or hydrophobicity is derived from the chemical properties of the material. On the other hand, for uneven surfaces, the hydrophilicity or hydrophobicity of the surface is determined not only by the size and hierarchical structure of the fine uneven morphology but also by the contribution of the chemical properties of the material covering the surface. The polymer film used in this study had a vertically aligned pillar structure fabricated by nanoimprinting as primary structure, which resulted in an uneven surface structure. Furthermore, surface-initiated graft polymerization was performed on the surface of this pillar film to form polymer graft chains as secondary structures. In other words, the obtained polymer film had a unique hierarchical structure in which, in addition to the pillar structure with submicron-sized unevenness, a nanoscale surface structure based on the grafted polymer chains was formed. Therefore, we envisaged that by selecting the type of polymer chain to be grafted, it would be possible to widely tune the properties of the pillar film surface through the synergistic effect of the surface morphology of the pillar structure and the chemical properties of the grafted polymer chain. Specifically, we expected that the hydrophobicity based on the uneven structure would be further improved by grafting a fluorine-containing monomer, and furthermore, we expected that the hydrophobic/hydrophilic switching ability of the surface due to an external stimulus (temperature change) would be amplified by grafting polymer chains whose hydrophilic/hydrophilic properties could be changed depending on the temperature.
To perform functionalization with grafted polymer chains, the surface-initiated ATRP of 2,2,2-trifluoroethyl methacrylate (TFEMA) and N-isopropylacrylamide (NIPAM) was carried out using the polymerization initiation sites on the pillar surfaces. For the graft polymerization of TFEMA, the polymer thin films were heated at 85 °C for 5 h in a Schlenk flask with the monomer anisole as a solvent, Cu catalysts, a ligand, and the initiator after degassing by three freeze–pump–thaw cycles ([TFEMA]0/[CuCl]0/[CuCl2]0/[TPMA]0/[EBI]0 = 100/1/0.1/1/1, TPMA: tris(2-pyridylmethyl) amine, EBI; ethyl 2-bromoisobutyrate) [26]. To ensure surface-initiated ATRP, EBI was added to the polymerization system as a free initiator. After graft polymerization, the polymer film (CL-poly(MCMA-co-HEMABr)-g-poly(TFEMA)) was thoroughly rinsed and carefully dried to remove the physisorbed polymers and solvents from the film sample. Since it was practically difficult to directly analyze the molecular characteristics of the polymer grafted onto the pillar film, the polymer derived from the free initiator (free polymer) generated in the solution was analyzed as an indicator of the grafted polymer. When the polymerization behavior in solution is controlled, the molecular weight and molecular weight distribution of surface grafted chains are expected to be very close to those of the polymers derived from the free initiators [29]. The molecular weight (Mn) and molecular weight distribution (Mw/Mn) of the free poly(TFEMA) were 5600 and 1.26, respectively, according to SEC analysis.
Next, we investigated the SI-ATRP of NIPAM from the polymer thin films. Graft polymerization was conducted at 25 °C in a Schlenk flask with the monomer 2-propanol/water (4/1, v/v) as a mixed solvent, a Cu catalyst, a ligand, a reducing agent, and the initiator after degassing by three freeze–pump–thaw cycles ([NIPAM]0/[CuCl]0/[Me6TREN]0/[ascorbic acid]0/[EBI]0 = 100/1/2/1/1, Me6TREN: tris [2-(dimethylamino)ethyl]amine) [27]. After graft polymerization, the polymer films (CL-poly(MCMA-co-HEMABr)-g-poly(NIPAM)) were thoroughly rinsed with 2-propanol to remove the physisorbed polymers from the film sample. The Mn and Mw/Mn of poly(NIPAM) were 7200 and 1.55, respectively, which were determined by SEC based on the free poly(NIPAM). SEM observation of the film surfaces after graft modification confirmed that the pillar structures were maintained even after the graft polymerization for both TFEMA and NIPAM (Figure 6). The growth of graft polymers on the pillar surfaces was confirmed by X-ray photoelectron spectroscopy (XPS) (Figure 4). All the pillar films exhibited C1s (285 eV) and O1s (531 eV) peaks corresponding to the methacrylate polymer chains. The poly(TFEMA)-grafted sample showed an F1s (685 eV) peak corresponding to the TFEMA component, while the poly(NIPAM)-grafted sample showed an N1s (398 eV) peak corresponding to the NIPAM component. To further confirm the progress of graft polymerization, Fourier transform infrared (FTIR) spectroscopy was used to investigate the structural changes in the polymer film before and after the graft modification (Figure 7). The FTIR spectrum of the crosslinked pillar film measured in transmission mode showed a group of peaks originating from the methacrylate polymer, with the main peaks assignable to C=O stretching (1730 cm−1) and C-H stretching (2950 cm−1). In the grafted pillar thin films, new peaks due to the chemical structure of the polymer brush were observed. For example, when TFEMA was polymerized, the absorption due to C=O stretching vibration (1730 cm−1) increased relatively compared to the C=C stretching (1620 cm−1), and when poly(NIPAM) was grafted, a new absorption due to the amide N-H bending vibration (1550 cm−1) was observed. These results confirmed that the grafted polymers were successfully introduced onto the pillar film surfaces by SI-ATRP and the expected secondary structure was constructed.
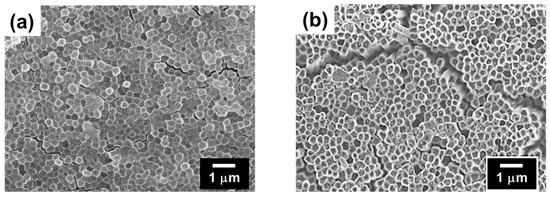
Figure 6.
SEM images of the surface of the (a) CL-poly((MCMA-co-HEMABr)-g-poly(TFEMA)) pillar film and (b) CL-poly((MCMA-co-HEMABr)-g-poly(NIPAM)) pillar film.
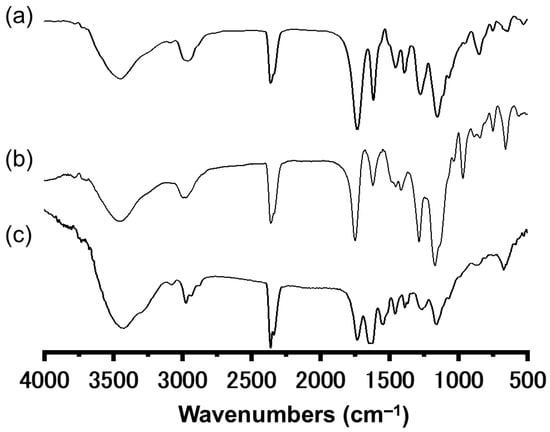
Figure 7.
IR spectra obtained for the (a) CL-poly(MCMA-co-HEMA-Br) pillar film, (b) CL-poly((MCMA-co-HEMABr)-g-poly(TFEMA)) pillar film, and (c) CL-poly((MCMA-co-HEMABr)-g-poly(NIPAM)) pillar film.
3.4. Wattability of Polymer Film Surfaces
In order to ascertain the effect of the pillar structure on the interfacial properties of the film surface (especially the gas-phase interface), a flat film prepared by spin-coating was used as a control sample, and a comparative study was conducted focusing on wettability, which is one of the evaluation indices for interfacial activity. First, to clarify the change in surface properties caused by the formation of the pillar structure, a comparative analysis of the static water contact angles of the flat film and the pillar film was conducted. The contact angles were measured to be 80° for the flat film and 16° for the columnar film (Figure 8a,b), which was probably due to the capillary action induced by the pillared structure of the surface, as shown in Figure 3b, rather than an increase in hydrophilicity due to increased surface roughness based on the pillar structure (Wenzel’s theory). Since poly(TFEMA) is hydrophobic [30], the wettability of the surface was reduced by grafting a fluoropolymer onto the film surface. The static contact angle of a water droplet on the flat film surface grafted with poly(TFEMA) was 104° (Figure 8c), whereas the value was 112° on the pillar film surface grafted with poly(TFEMA) (Figure 8d), both of which were significantly more hydrophobic than the sample before grafting. It is noteworthy here that the difference in wettability of the pillar film before and after grafting with poly(TFEMA) was significantly large, and the sample grafted with poly(TFEMA) retained its pillared structure (Figure 6b) but no longer showed the capillary effect and became highly water-repellent.
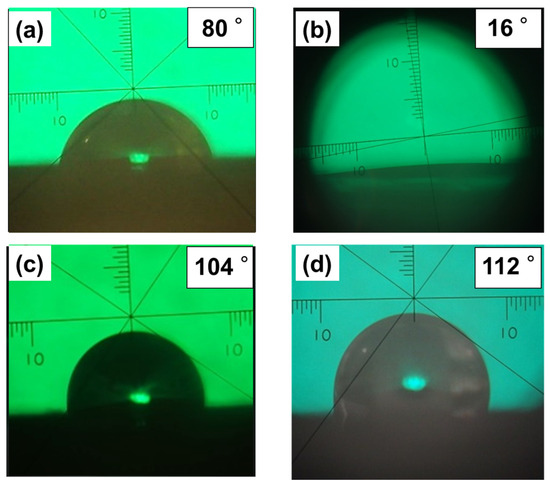
Figure 8.
Images of water droplets in static contact angle measurements on surfaces of several specimens: (a) CL-poly(MCMA-co-HEMABr) flat film; (b) CL-poly(MCMA-co-HEMABr) pillar film; (c) CL-poly(MCMA-co-HEMABr) flat film grafted with poly(TFEMA); (d) CL-poly(MCMA-co-HEMABr) pillar film grafted with poly(TFEMA).
Poly(NIPAM), a representative thermoresponsive polymer, changes its conformation and hydrophilicity/hydrophobicity in response to temperature changes in an aqueous environment. That is, below the phase transition temperature (Tpt) of about 32 °C, it forms an extended conformation due to hydration and becomes hydrophilic, and when the temperature rises above Tpt, it dehydrates and assumes a collapsed conformation and becomes hydrophobic. Furthermore, this hydrophilic/hydrophobic switching due to the phase transition is reversible with temperature changes [31,32]. When poly(NIPAM) is fixed to a material surface, the change in the properties of the polymer chain determines the surface properties, and the interfacial properties (hydrophilicity/hydrophobicity) of the material surface can be controlled by changing the temperature [33]. A limited number of reports have demonstrated that the introduction of nanoscale roughness onto a surface by grafting can have a significant effect on the wettability of the surface [23,34]. Therefore, it is interesting to investigate the effect of poly(NIPAM)-grafted pillar surfaces on interfacial properties, such as wettability. Figure 9a shows the static contact angle of a water droplet on the surface of the poly(NIPAM)-grafted flat film and pillar film as a function of temperature. For both sample surfaces, an increase in the water contact angle was observed between 31 °C and 35 °C during the heating process, and it was found that this temperature-responsive change in wettability was significantly amplified by the presence of the pillar structure. As shown in Figure 9b, when the temperature was increased from 22 °C to 38 °C, the water contact angle of the poly(NIPAM)-grafted flat film increased by only 13°, whereas the water contact angle of the poly(NIPAM)-grafted pillar film increased significantly to 68°. This improvement in wettability due to the temperature response of poly(NIPAM) chains is consistent with another report [23,34]. Furthermore, when the temperature was repeatedly increased and decreased in the range from 22 °C to 38 °C, the water contact angles of both sample surfaces changed reversibly, with the amplitude of change being significantly larger for the grafted pillar film. In addition, the amplitude of the water contact angle was maintained within the range of the number of trials in this experiment, indicating that the hydrophilic/hydrophobic switching of the sample surface could be reversibly controlled without attenuation (Figure 9c). These results indicate that the functionality of the film surface was amplified by the synergistic effect between the surface morphology of the pillar structures (primary structure) and the chemical properties of the grafted polymer chains (secondary structure) introduced onto the surface.
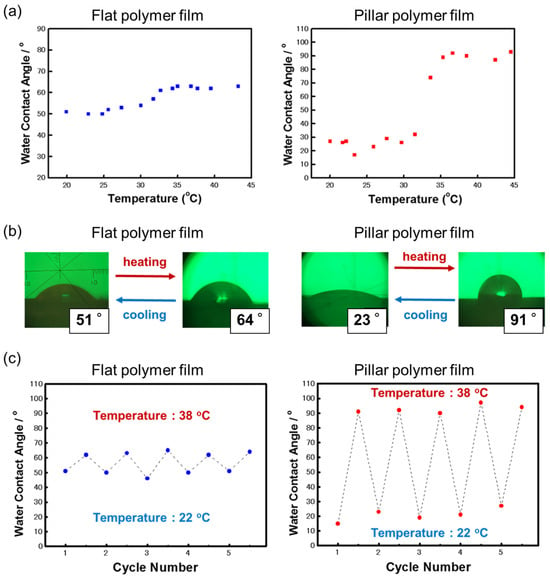
Figure 9.
(a) Change in water contact angle upon the heating of the poly(NIPAM)-grafted flat and pillar films. (b) Images of water droplets in static contact angle measurements on these sample surfaces at 22 °C and 38 °C. (c) Changes in water contact angle on the sample surfaces after five heating and cooling cycles in the temperature range of 22 °C and 38 °C.
4. Conclusions
A new method to fabricate polymer thin films with hierarchically ordered surface structures was achieved by combining nanoimprinting using an AAO template with the SI-ATRP of TFEMA and NIPAM. In this study, the surface morphology and chemistry of the films with and without pillar structures were compared, especially the interfacial properties expressed as wettability. Grafting poly(TFEMA) increased the static contact angle for both flat and pillar films, and the contact angle of the pillar film surface increased from 104° for the flat film sample to 112°, suggesting the contribution of the pillar structure. Meanwhile, the pillar film surface grafted with poly(NIPAM) brought about a significant change in wettability by changing the temperature between 22 °C and 38 °C. Furthermore, it was shown that the interfacial properties of the film surface (hydrophilic/hydrophobic switching) could be reversibly and reproducibly controlled by temperature. This experiment also revealed that the presence of pillar structures significantly affected the interfacial properties of the film surfaces. These findings indicate that the functionality of the film surfaces is expressed through a synergistic effect between the surface morphology of the pillar structure (primary structure) and the chemical properties of the polymer chains introduced to the pillar surface by graft modification (secondary structure). The results presented in this study are expected to be applied to new nanomaterials research, such as the creation of hierarchical surface functional materials by combining top–down and bottom–up techniques.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/surfaces8030048/s1. Methods, Chemical and Reagents, poly(MCMA-co-HEMABr), and Figure S1: DCS trace of poly(MCMA-co-HEMABr).
Author Contributions
Conceptualization, M.M.; methodology, J.M. and M.M.; validation, J.M.; formal analysis, D.S. and T.N.; investigation, J.M.; resources, J.M. and M.M.; data curation, D.S., T.N., and J.M.; writing—original draft preparation, J.M.; writing—review and editing, M.M. visualization, J.M.; supervision, M.M.; project administration, M.M.; funding acquisition, J.M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the JSPS KAKENHI, Grant-in-Aid for Scientific Research (C), grant numbers 20K05605 and 22K05216.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Y.; Zheng, G.; Jiang, N.; Ying, G.; Li, Y.; Cai, X.; Meng, J.; Mai, L.; Guo, M.; Zhang, Y.S.; et al. Nature-inspired micropatterns. Nat. Rev. Methods Primers 2023, 3, 68. [Google Scholar] [CrossRef]
- Himel, M.H.; Sikder, B.; Ahmed, T.; Muhaimin, S. Biomimicry in nanotechnology: A comprehensive review. Nanoscale Adv. 2023, 5, 596–614. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Koh, J.J.; Wang, J.; Lyu, Z. 3D-printed biomimetic structures for energy and environmental applications. DeCarbon 2024, 3, 10026. [Google Scholar] [CrossRef]
- Yang, M.; Kotov, N.A. Quantitative biomimetics of high-performance materials. Nat. Rev. Mater. 2025, 10, 382–395. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical explanation of the lotus effect: Superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, S.; Wang, L.; Zheng, Y. Lotus effect in wetting and self-cleaning. Biotribology 2016, 5, 31–43. [Google Scholar] [CrossRef]
- Nine, M.J.; Tung, T.T.; Alotaibi, F.; Tran, D.N.H.; Losic, D. Facile Adhesion-Tuning of Superhydrophobic Surfaces between “Lotus” and “Petal” Effect and their Influence on Icing and Deicing Properties. ACS Appl. Mater. Interfaces 2017, 9, 8393–8402. [Google Scholar] [CrossRef]
- Wang, L. A critical review on robust self-cleaning properties of lotus leaf. Soft Matter 2023, 19, 1058–1075. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Xue, Z.; Gao, J.; Meng, J.; Wang, S.; Jiang, L. Clam’s Shell Inspired High-Energy Inorganic Coatings with Underwater Low Adhesive Superoleophobicity. Adv. Mater. 2012, 24, 3401–3405. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Cook, A.B.; Clemons, T.D. Bottom-Up versus Top-Down Strategies for Morphology Control in Polymer-Based Biomedical Materials. Adv. Nanobiomed. Res. 2022, 2, 2100087. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, C.; Zhang, H. Bio-Inspired Subnanofluidics: Advanced Fabrication and Functionalization. Small Methods 2023, 8, 2300278. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Shao, J.; Ding, Y. Fabrication of Well-Defined Mushroom-Shaped Structures for Biomimetic Dry Adhesive by Conventional Photolithography and Molding. ACS Appl. Mater. Interfaces 2014, 6, 2213–2218. [Google Scholar] [CrossRef]
- Alameda, M.T.; Alameda, T.; Osorio, M.R.; Herna, J.J. Multilevel Hierarchical Topographies by Combined Photolithography and Nanoimprinting Processes To Create Surfaces with Controlled Wetting. ACS Appl. Nano Mater. 2019, 2, 4727–4733. [Google Scholar] [CrossRef]
- Hirtz, M.; Oikonomou, A.; Georgiou, T.; Fuchs, H.; Vijayaraghavan, A. Multiplexed biomimetic lipid membranes on graphene by dip-pen nanolithography. Nat. Commun. 2013, 4, 2591. [Google Scholar] [CrossRef]
- Liu, G.; Petrosko, S.H.; Zheng, Z.; Mirkin, C. Evolution of Dip-Pen Nanolithography (DPN): From Molecular Patterning to Materials Discovery. Chem. Rev. 2020, 120, 6009–6047. [Google Scholar] [CrossRef]
- Kim, T.; Pang, C.; Suh, K.Y. Shape-Tunable Polymer Nanofibrillar Structures by Oblique Electron Beam Irradiation. Langmuir 2009, 25, 8879–8882. [Google Scholar] [CrossRef]
- Qin, N.; Qian, Z.G.; Zhou, C.; Xia, X.X.; Tao, T.H. 3D electron-beam writing at sub-15 nm resolution using spider silk as a resist. Nat. Commun. 2021, 12, 5133. [Google Scholar] [CrossRef]
- Yang, Y.; He, H.; Li, Y.; Qiu, J. Using Nanoimprint Lithography to Create Robust, Buoyant, Superhydrophobic PVB/SiO2 Coatings on wood Surfaces Inspired by Red roses petal. Sci. Rep. 2019, 9, 9961. [Google Scholar] [CrossRef]
- Nowduri, B.; Schulte, S.; Decker, D.; Schäfer, K.-H.; Saumer, M. Biomimetic Nanostructures Fabricated by Nanoimprint Lithography for Improved Cell-Coupling. Adv. Funct. Mater. 2020, 30, 2004227. [Google Scholar] [CrossRef]
- Tu, R.S.; Tirrell, M. Bottom-up design of biomimetic assemblies. Adv. Drug Deliv. Rev. 2004, 56, 1537–1563. [Google Scholar] [CrossRef]
- Song, J.; Malathong, V.; Bertozzi, C.R. Mineralization of synthetic polymer scaffolds: A bottom-up approach for the development of artificial bone. J. Am. Chem. Soc. 2005, 127, 3366–3372. [Google Scholar] [CrossRef]
- Nagase, K.; Onuma, T.; Yamato, M.; Takeda, N.; Okano, T. Enhanced Wettability Changes by Synergistic Effect of Micro/Nanoimprinted Substrates and Grafted Thermoresponsive Polymer Brushes. Macromol. Rapid Commun. 2015, 36, 1965–1970. [Google Scholar] [CrossRef]
- Flejszar, M.; Ślusarczyk, K.; Hochół, A.; Chmielarz, P.; Wytrwal, M.; Wolski, K.; Spilarewicz, K.; Awsiuk, K.; Raczkowska, J. Sequential SI-ATRP in μL-scale for surface nanoengineering: A new concept for designing polyelectrolyte nanolayers formed by complex architecture polymers. Eur. Polym. J. 2023, 194, 112142. [Google Scholar] [CrossRef]
- Gury, L.; Kamble, S.; Parisi, D.; Zhang, J.; Lee, J.; Abdullah, A.; Matyjaszewski, K.; Bockstaller, M.R.; Vlassopoulos, D.; Fytas, G. Internal Microstructure Dictates Interactions of Polymer-Grafted Nanoparticles in Solution. Macromolecules 2021, 54, 7234–7243. [Google Scholar] [CrossRef]
- Onbulak, S.; Rzayev, J. Cylindrical Nanocapsules from Photo-Cross-Linkable Core-Shell Bottlebrush Copolymers. Polym. Chem. 2015, 6, 764–771. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Gaynor, S.G.; Kulfan, A.; Podwika, M. Preparation of Hyperbranched Polyacrylates by Atom Transfer Radical Polymerization. 1. Acrylic AB* Monomers in “Living” Radical Polymerizations. Macromolecules 1997, 30, 5192–5194. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Nishimura, I.; Minoda, M. Living Cationic Polymerization of a Coumarin-Substituted Vinyl Ether and Reversible Photoinduced Crosslinking of the Resulting Homopolymers and Amphiphilic Block Copolymers. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4701–4707. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef]
- Xia, Z.; Feng, Z.; Wu, R.; Niu, Z.; He, J.; Bai, C. Tough, hydrophobic, pressure-resistant, and self-cleaning underwater engineering materials based on copolymerization of butadiene and trifluoroethyl methacrylate. ACS Appl. Polym. Mater. 2023, 5, 8241–8249. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Osváth, Z.; Iván, B. The Dependence of the Cloud Point, Clearing Point, and Hysteresis of Poly(N-isopropylacrylamide) on Experimental Conditions: The Need for Standardization of Thermoresponsive Transition Determinations. Macromol. Chem. Phys. 2017, 218, 1600470. [Google Scholar] [CrossRef]
- Thiele, S.; Andersson, J.; Dahlin, A.; Hailes, R.L.N. Tuning the Thermoresponsive Behavior of Surface-Attached PNIPAM Networks: Varying the Crosslinker Content in SI-ATRP. Langmuir 2021, 37, 3391–3398. [Google Scholar] [CrossRef]
- Yu, Q.; Li, X.; Zhang, Y.; Yuan, L.; Zhao, T.; Chen, H. The synergistic effects of stimuli-responsive polymers with nano- structured surfaces: Wettability and protein adsorption. RSC Adv. 2011, 1, 262–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
