The Use of Plant Extracts as Green Corrosion Inhibitors: A Review
Abstract
1. Introduction
2. Corrosion and Factors Affecting It
3. Corrosion Protection
4. Corrosion Inhibitors
5. GCIs
5.1. Background
5.2. Classification of GCIs
5.2.1. Amino Acids
5.2.2. Bio-Polymers
5.2.3. Drugs
5.2.4. Ionic Liquids
5.2.5. Plants
5.2.6. Surfactants
5.2.7. Vitamins and Food Supplements
5.2.8. Natural Gums
5.3. Using Plants as GCIs
6. Conclusions
Funding
Conflicts of Interest
References
- Wang, S.; He, Y.; Song, M. Global value chains, technological progress, and environmental pollution: Inequality towards developing countries. J. Environ. Manag. 2021, 277, 110999. [Google Scholar] [CrossRef]
- Rao, C.; Yan, B. Study on the interactive influence between economic growth and environmental pollution. Environ. Sci. Pollut. Res. 2020, 27, 39442–39465. [Google Scholar] [CrossRef] [PubMed]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.M.; Lamonier, J.F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Sheydaei, M. Investigation of Heavy Metals Pollution and Their Removal Methods: A Review. Geomicrobiol. J. 2024, 41, 213–230. [Google Scholar] [CrossRef]
- Sheydaei, M.; Pouraman, V.; Alinia-Ahandani, E.; Shahbazi-Ganjgah, S. PVCS/GO nanocomposites: Investigation of thermophysical, mechanical and antimicrobial properties. J. Sulfur Chem. 2022, 43, 376–390. [Google Scholar] [CrossRef]
- Sheydaei, M.; Pouraman, V.; Edraki, M.; Alinia-Ahandani, E.; Asadi-Sadeh, S.M. Targeted application of GO to improve mechanical and thermal properties of PVCS/RS composites. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 345–353. [Google Scholar] [CrossRef]
- Xia, D.H.; Deng, C.M.; Macdonald, D.; Jamali, S.; Mills, D.; Luo, J.L.; Strebl, M.G.; Amiri, M.; Jin, W.; Song, S.; et al. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Process. Technol. 2022, 112, 151–183. [Google Scholar] [CrossRef]
- Edraki, M.; Sheydaei, M. Investigation of the Dual Active/Barrier Corrosion Protection, Mechanical and Thermal Properties of a Vinyl Ester Coating Doped with Ginger Modified Clay Nanoparticles. Russ. J. Appl. Chem. 2022, 95, 1481–1488. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/Inorganic nanocomposite coatings with superior corrosion protection performance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Nazdracheva, T. Anti-corrosion coatings for protection of steel railway structures exposed to atmospheric environments: A review. Constr. Build. Mater. 2021, 288, 123115. [Google Scholar] [CrossRef]
- Soufeiani, L.; Foliente, G.; Nguyen, K.T.; San Nicolas, R. Corrosion protection of steel elements in façade systems—A review. J. Build. Eng. 2020, 32, 101759. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.Y.; Tardelli, J.D.; Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical Ti-6Al-4V and β-Ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Yang, T.; Cui, Y.; Li, Z.; Zeng, H.; Luo, S.; Li, W. Enhancement of the corrosion resistance of epoxy coating by highly stable 3, 4, 9, 10-perylene tetracarboxylic acid functionalized graphene. J. Hazard. Mater. 2018, 357, 475–482. [Google Scholar] [CrossRef]
- Shinato, K.W.; Zewde, A.A.; Jin, Y. Corrosion protection of copper and copper alloys in different corrosive medium using environmentally friendly corrosion inhibitors. Corros. Rev. 2020, 38, 101–109. [Google Scholar] [CrossRef]
- Baskar, P.; Annadurai, S.; Panneerselvam, S.; Prabakaran, M.; Kim, J. An Outline of Employing Metals and Alloys in Corrosive Settings with Ecologically Acceptable Corrosion Inhibitors. Surfaces 2023, 6, 380–409. [Google Scholar] [CrossRef]
- Pradhan, B. A study on effectiveness of inorganic and organic corrosion inhibitors on rebar corrosion in concrete: A review. Mater. Today Proc. 2022, 65, 1360–1366. [Google Scholar]
- Qiang, Y.; Zhang, S.; Zhao, H.; Tan, B.; Wang, L. Enhanced anticorrosion performance of copper by novel N-doped carbon dots. Corros. Sci. 2019, 161, 108193. [Google Scholar] [CrossRef]
- Edraki, M.; Sheydaei, M.; Zaarei, D.; Salmasifar, A.; Azizi, B. Protective nanocomposite coating based on ginger modified clay and polyurethane: Preparation, characterization and evaluation anti-corrosion and mechanical properties. Polym. Sci. Ser. B 2022, 64, 756–764. [Google Scholar] [CrossRef]
- Ollik, K.; Lieder, M. Review of the application of graphene-based coatings as anticorrosion layers. Coatings 2020, 10, 883. [Google Scholar] [CrossRef]
- Kanninen, P.; Eriksson, B.; Davodi, F.; Buan, M.E.; Sorsa, O.; Kallio, T.; Lindström, R.W. Carbon corrosion properties and performance of multi-walled carbon nanotube support with and without nitrogen-functionalization in fuel cell electrodes. Electrochim. Acta 2020, 332, 135384. [Google Scholar] [CrossRef]
- Thakur, A.; Ganjoo, R.; Kumar, A. Surface Modified Carbon Nanotubes in Corrosion Protection. In Surface Modified Carbon Nanotubes Volume 1: Fundamentals, Synthesis and Recent Trends; American Chemical Society: Washington, DC, USA, 2022; pp. 235–255. [Google Scholar]
- Sheydaei, M.; Edrki, M.; Javanbakht, S. Ganoderma lucidum-Modified Clay Epoxy Coating: Investigation of Thermal, Mechanical, Anticorrosion, and Antimicrobial Properties. Polym. Sci. Ser. B 2023, 65, 991–1000. [Google Scholar] [CrossRef]
- Edraki, M.; Sheydaei, M.; Vessally, E.; Salmasifar, A. Enhanced mechanical, anticorrosion and antimicrobial properties of epoxy coating via pine pollen modified clay incorporation. Iran. J. Chem. Chem. Eng. 2023, 42, 2775–2786. [Google Scholar]
- Ma, L.; Qiang, Y.; Zhao, W. Designing novel organic inhibitor loaded MgAl-LDHs nanocontainer for enhanced corrosion resistance. Chem. Eng. J. 2021, 408, 127367. [Google Scholar] [CrossRef]
- Sheydaei, M.; Edraki, M.; Abad, F.S. Matcha-modified clay polyurethane coating: Improving thermal, mechanical, antimicrobial, and anticorrosion performance. Iran. Polym. J. 2023, 32, 1643–1654. [Google Scholar] [CrossRef]
- Sheydaei, M.; Edraki, M.; Radeghi Mehrjou, S.M. Anticorrosion and Antimicrobial Evaluation of Sol-Gel Hybrid Coatings Containing Clitoria ternatea Modified Clay. Gels 2023, 9, 490. [Google Scholar] [CrossRef]
- Ji, G.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive effect of chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind. Eng. Chem. Res. 2013, 52, 10673–10681. [Google Scholar] [CrossRef]
- Ji, G.; Dwivedi, P.; Sundaram, S.; Prakash, R. Aqueous extract of Argemone mexicana roots for effective protection of mild steel in an HCl environment. Res. Chem. Intermed. 2016, 42, 439–459. [Google Scholar] [CrossRef]
- Tamalmani, K.; Husin, H. Review on corrosion inhibitors for oil and gas corrosion issues. Appl. Sci. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Khalaj, M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014, 4, 47313–47318. [Google Scholar] [CrossRef]
- Sharghi, H.; Khalifeh, R.; Doroodmand, M.M. Copper nanoparticles on charcoal for multicomponent catalytic synthesis of 1,2,3-Triazole derivatives from benzyl halides or alkyl halides, terminal alkynes and sodium azide in water as a “Green” solvent. Adv. Synth. Catal. 2009, 351, 207–218. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mater. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Seo, J.; Lee, S.; Elam, M.L.; Johnson, S.A.; Kang, J.; Arjmandi, B.H. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci. Nutr. 2014, 2, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, I.; Elfgren, L.; Bell, B.; Paulsson, B.; Niederleithinger, E.; Sandager Jensen, J.; Feltrin, G.; Täljsten, B.; Cremona, C.; Kiviluoma, R.; et al. Assessment of European railway bridges for future traffic demands and longer lives–EC project “Sustainable Bridges”. Struct. Infrastruct. Eng. 2005, 1, 93–100. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Atmospheric corrosion of different steels in marine, rural and industrial environments. Corros. Sci. 1999, 41, 1687–1702. [Google Scholar] [CrossRef]
- Wu, H.; Lei, H.; Chen, Y.F.; Qiao, J. Comparison on corrosion behaviour and mechanical properties of structural steel exposed between urban industrial atmosphere and laboratory simulated environment. Constr. Build. Mater. 2019, 211, 228–243. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine atmospheric corrosion of carbon steel: A review. Materials 2017, 10, 406. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Moricone, R.; Tugnoli, A.; Cozzani, V. Experimental investigation of the reactivity of sodium bicarbonate toward hydrogen chloride and sulfur dioxide at low temperatures. Ind. Eng. Chem. Res. 2019, 58, 6316–6324. [Google Scholar] [CrossRef]
- Corvo, F.; Minotas, J.; Delgado, J.; Arroyave, C. Changes in atmospheric corrosion rate caused by chloride ions depending on rain regime. Corros. Sci. 2005, 47, 883–892. [Google Scholar] [CrossRef]
- Freire, L.X.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wei, Y.; Wang, Z. Effect of different UV intensity on corrosion behavior of carbon steel exposed to simulated Nansha atmospheric environment. Mater. Chem. Phys. 2019, 237, 121855. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Pan, H.; Cao, F.; Wu, Z.; Lv, H.; Xu, Z. Synergisic effect of Mn, Cu, P with Cr content on the corrosion behavior of weathering steel as a train under the simulated industrial atmosphere. J. Alloys Compd. 2020, 834, 155095. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; De la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic concepts. Constr. Build. Mater. 2019, 213, 723–737. [Google Scholar] [CrossRef]
- Martínez, C.; Briones, F.; Villarroel, M.; Vera, R. Effect of atmospheric corrosion on the mechanical properties of SAE 1020 structural steel. Materials 2018, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, T.; Zhang, H.; Nie, B.; Wang, H.; Xu, S. Hysteretic behavior and cyclic constitutive model of corroded structural steel under general atmospheric environment. Constr. Build. Mater. 2021, 270, 121474. [Google Scholar] [CrossRef]
- Zeng, W.; Zhou, Q.; Zhang, H.; Qi, X. One-coat epoxy coating development for the improvement of UV stability by DPP pigments. Dye. Pigment. 2018, 151, 157–164. [Google Scholar] [CrossRef]
- Pflugfelder, J. Im Wandel: Korrosionsschutz für Eisenbahnbrücken aus Stahl. Stahlbau 2019, 88, 128–135. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.S.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Cen, H.; Gao, Y.; He, S.; Peng, Z.; Wu, C.; Chen, Z. Synergistic effect of surfactant and 1, 10-decanedithiol as corrosion inhibitor for zinc anode in alkaline electrolyte of zinc-air batteries. J. Colloid Interface Sci. 2024, 659, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Naddeo, C.; Guadagno, L. Enhancement of photooxidative and corrosion resistance of epoxy/graphene water-based coatings on metallic substrate. Prog. Org. Coat. 2019, 135, 7–18. [Google Scholar] [CrossRef]
- Bagherinia, M.A.; Sheydaei, M.; Giahi, M. Graphene oxide as a compatibilizer for polyvinyl chloride/rice straw composites. J. Polym. Eng. 2017, 37, 661–670. [Google Scholar] [CrossRef]
- Herrasti, P.; Recio, F.J.; Ocon, P.; Fatás, E. Effect of the polymer layers and bilayers on the corrosion behaviour of mild steel: Comparison with polymers containing Zn microparticles. Prog. Org. Coat. 2005, 54, 285–291. [Google Scholar] [CrossRef]
- Palanisamy, G. Corrosion Inhibitors; IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Rocca, E.; Faiz, H.; Dillmann, P.; Neff, D.; Mirambet, F. Electrochemical behavior of thick rust layers on steel artefact: Mechanism of corrosion inhibition. Electrochim. Acta 2019, 316, 219–227. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Unravelling the mechanisms of corrosion inhibition of iron by henna extract: A density functional theory study. Corros. Sci. 2018, 142, 102–109. [Google Scholar] [CrossRef]
- Yeganeh, M.; Khosravi-Bigdeli, I.; Eskandari, M.; Alavi Zaree, S.R. Corrosion inhibition of l-methionine amino acid as a Green corrosion inhibitor for stainless steel in the H2SO4 solution. J. Mater. Eng. Perform. 2020, 29, 3983–3994. [Google Scholar] [CrossRef]
- Abdallah, M.; Altass, H.M.; Al-Gorair, A.S.; Al-Fahemi, J.H.; Jahdaly, B.A.; Soliman, K.A. Natural nutmeg oil as a green corrosion inhibitor for carbon steel in 1.0 M HCl solution: Chemical, electrochemical, and computational methods. J. Mol. Liq. 2021, 323, 115036. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.A.; Kchaou, M. An overview of green corrosion inhibitors for sustainable and environment friendly industrial development. J. Adhes. Sci. Technol. 2021, 35, 673–690. [Google Scholar] [CrossRef]
- Hossain, S.M.; Razzak, S.A.; Hossain, M.M. Application of essential oils as green corrosion inhibitors. Arab. J. Sci. Eng. 2020, 45, 7137–7159. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Lgaz, H.; Al-Hadeethi, M.R.; Ali, I.H.; Masroor, S.; Chung, I.M. Green corrosion inhibition of mild steel by hydrazone derivatives in 1.0 M HCl. Coatings 2020, 10, 640. [Google Scholar] [CrossRef]
- Luo, Z.G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.F.; Liao, B.K.; Guo, X.P. Modified nano-lignin as a novel biomass-derived corrosion inhibitor for enhanced corrosion resistance of carbon steel. Corros. Sci. 2024, 227, 111705. [Google Scholar] [CrossRef]
- Proença, C.S.; Serrano, B.; Correia, J.; Araújo, M.E. Evaluation of tannins as potential green corrosion inhibitors of aluminium alloy used in aeronautical industry. Metals 2022, 3, 508. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations. Constr. Build. Mater. 2020, 245, 118464. [Google Scholar] [CrossRef]
- Harb, M.B.; Abubshait, S.; Etteyeb, N.; Kamoun, M.; Dhouib, A. Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arab. J. Chem. 2020, 13, 4846–4856. [Google Scholar] [CrossRef]
- Ghiasvandnia, P.; Sheydaei, M.; Edraki, M. Evaluation of toxic metals content and microbial contamination of parsley (Petroselinum crispum) prepared from local farms in Kushal and Layalestan regions (Lahijan city, north of Iran). Gıda Bilim. Ve Mühendisliği Araştırmaları 2023, 2, 23–27. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, C.; Ma, H.; Yang, Z.; Yang, K.; Liu, F.; Li, K.; Yang, Z.; Tang, S.; Guo, F.; et al. Heavy metal and Pb isotopic compositions of soil and maize from a major agricultural area in Northeast China: Contamination assessment and source apportionment. J. Geochem. Explor. 2020, 208, 106403. [Google Scholar] [CrossRef]
- Parajuli, D.; Sharma, S.; Oli, H.B.; Bohara, D.S.; Bhattarai, D.P.; Tiwari, A.P.; Yadav, A.P. Comparative study of corrosion inhibition efficacy of alkaloid extract of artemesia vulgaris and Solanum tuberosum in mild steel samples in 1 M sulphuric acid. Electrochem 2022, 3, 416–433. [Google Scholar] [CrossRef]
- Abdel-Karim, A.M.; El-Shamy, A.M. A review on green corrosion inhibitors for protection of archeological metal artifacts. J. Bio-Tribo-Corros. 2022, 8, 35. [Google Scholar] [CrossRef]
- Drakvik, E.; Altenburger, R.; Aoki, Y.; Backhaus, T.; Bahadori, T.; Barouki, R.; Brack, W.; Cronin, M.T.; Demeneix, B.; Bennekou, S.H.; et al. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ. Int. 2020, 134, 105267. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.S.J.; Ganesh, G.M. A comprehensive overview on corrosion in RCC and its prevention using various green corrosion inhibitors. Buildings 2022, 12, 1682. [Google Scholar] [CrossRef]
- Oli, H.B.; Thapa Magar, J.; Khadka, N.; Subedee, A.; Bhattarai, D.P.; Pant, B. Coriaria nepalensis Stem Alkaloid as a Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 713–727. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Zhang, Q.; Zhao, C.; Zhou, X.; Zheng, H.; Zhang, R.; Sun, Y.; Yan, Z. Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules 2023, 28, 2832. [Google Scholar] [CrossRef] [PubMed]
- Barbouchi, M.; Benzidia, B.; Aouidate, A.; Ghaleb, A.; El Idrissi, M. Theoretical modeling and experimental studies of Terebinth extracts as green corrosion inhibitor for iron in 3% NaCl medium. J. King Saud Univ. Sci. 2020, 32, 2995–3004. [Google Scholar] [CrossRef]
- Saraswat, V.; Yadav, M. Improved corrosion resistant performance of mild steel under acid environment by novel carbon dots as green corrosion inhibitor. Colloids Surf. A. 2021, 627, 127172. [Google Scholar] [CrossRef]
- Dehghani, A.; Ghahremani, P.; Mostafatabar, A.H.; Ramezanzadeh, B. Plant extracts: Probable alternatives for traditional inhibitors for controlling alloys corrosion against acidic media—A review. Biomass Convers. Biorefinery 2022, 14, 7467–7486. [Google Scholar] [CrossRef]
- Miralrio, A.; Espinoza Vázquez, A. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: A review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Sivakumar, P.R.; Srikanth, A.P. Green corrosion inhibitor: A comparative study. Sādhanā 2020, 45, 56. [Google Scholar] [CrossRef]
- Fuchs-Godec, R. A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution. Coatings 2021, 11, 971. [Google Scholar] [CrossRef]
- Sheydaei, M.; Shahbazi-Ganjgah, S.; Alinia-Ahandani, E.; Sheidaie, M.; Edraki, M. An overview of the use of plants, polymers and nanoparticles as antibacterial materials. Chem. Rev. Lett. 2022, 5, 207–216. [Google Scholar]
- Baymou, Y.; Bidi, H.; Ebn Touhami, M.; Allam, M.; Rkayae, M.; Belakhmima, R.A. Corrosion protection for cast iron in sulfamic acid solutions and studies of the cooperative effect between cationic surfactant and acid counterions. J. Bio- Tribo-Corros. 2018, 4, 11. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Nazdracheva, T.; Yavna, V. Comparative computational study of l-amino acids as green corrosion inhibitors for mild steel. Computation 2020, 9, 1. [Google Scholar] [CrossRef]
- Goni, L.K.; Mazumder, M.J.; Ali, S.A.; Nazal, M.K.; Al-Muallem, H.A. Biogenic amino acid methionine-based corrosion inhibitors of mild steel in acidic media. Int. J. Miner. Met. Mater. 2019, 26, 467–482. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Karishma, S.J. Review on biopolymers and composites–Evolving material as adsorbents in removal of environmental pollutants. Environ. Res. 2022, 212, 113114. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, J.; Cui, G.; Han, T.; Wu, Y. Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf. B 2020, 194, 111150. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Dalhatu, S.N.; Modu, K.A.; Mahmoud, A.A.; Zango, Z.U.; Umar, A.B.; Usman, F.; Dennis, J.O.; Alsadig, A.; Ibnaouf, K.H.; Aldaghri, O.A. L-arginine grafted chitosan as corrosion inhibitor for mild steel protection. Polymers 2023, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, N.; Alphonsa, J.; Cole, I.S.; Balasubramanian, K.; Bosco, I.G. Rapid investigation expiry drug green corrosion inhibitor on mild steel in NaCl medium. Mater. Sci. Eng. B 2019, 249, 114423. [Google Scholar] [CrossRef]
- Tanwer, S.; Shukla, S.K. Recent advances in the applicability of drugs as corrosion inhibitor on metal surface: A review. Curr. Res. Green Sustain. Chem. 2022, 5, 100227. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S. Drugs as environmentally sustainable corrosion inhibitors. In Sustainable Corrosion Inhibitors II: Synthesis, Design, and Practical Applications; American Chemical Society: Washington, DC, USA, 2021; pp. 1–17. [Google Scholar]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. Effective corrosion inhibition of mild steel by eco-friendly thiourea functionalized glucosamine derivatives in acidic solution. J. Colloid Interface Sci. 2021, 585, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Chafiq, M.; Chaouiki, A.; Albayati, M.R.; Lgaz, H.; Salghi, R.; AbdelRaheem, S.K.; Ali, I.H.; Mohamed, S.K.; Chung, I.M. Unveiled understanding on corrosion inhibition mechanisms of hydrazone derivatives based on naproxen for mild steel in HCl: A joint experimental/theoretical study. J. Mol. Liq. 2020, 320, 114442. [Google Scholar] [CrossRef]
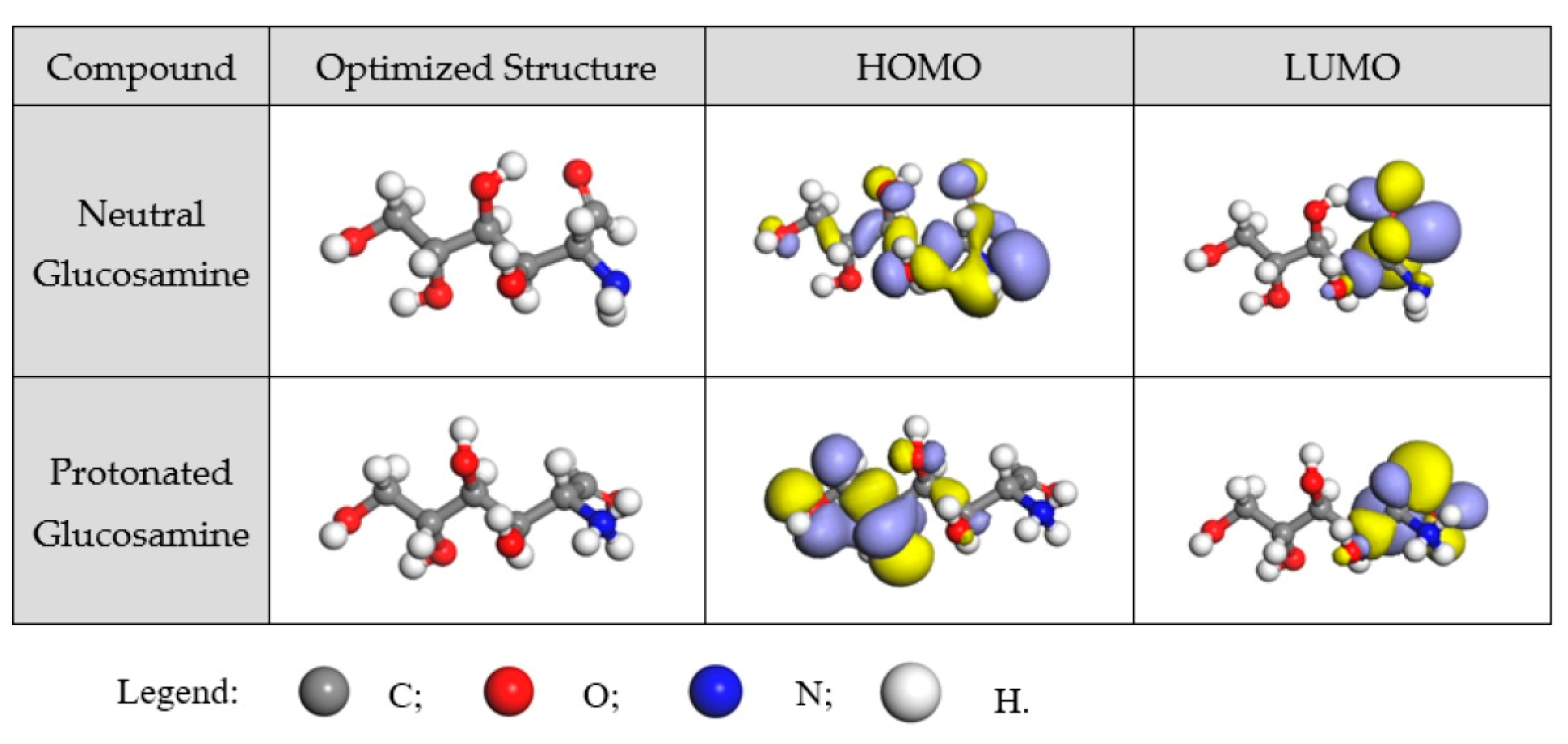
- Feng, L.; Zhang, S.; Zhou, Y.; Pan, R.; Du, H.; Liu, F.; Yang, Y. Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations. Crystals 2023, 13, 205. [Google Scholar] [CrossRef]
- Zunita, M.; Kevin, Y.J. Ionic liquids as corrosion inhibitor: From research and development to commercialization. Results Eng. 2022, 15, 100562. [Google Scholar] [CrossRef]
- Gurjar, S.; Sharma, S.K.; Sharma, A.; Ratnani, S. Performance of imidazolium based ionic liquids as corrosion inhibitors in acidic medium: A review. Appl. Surf. Sci. Adv. 2021, 6, 100170. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Fatyeyeva, K. Ionic liquids as green and sustainable steel corrosion inhibitors: Recent developments. Chem. Eng. J. 2021, 425, 131480. [Google Scholar] [CrossRef]
- Verma, C.; Alrefaee, S.H.; Quraishi, M.A.; Ebenso, E.E.; Hussain, C.M. Recent developments in sustainable corrosion inhibition using ionic liquids: A review. J. Mol. Liq. 2021, 321, 114484. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Olivares-Xometl, O.; Likhanova, N.V.; Lijanova, I.V.; Vargas-García, J.R.; Hernández-Ramírez, R.E. Adsorption and performance of ammonium-based ionic liquids as corrosion inhibitors of steel. J. Mol. Liq. 2018, 265, 151–163. [Google Scholar] [CrossRef]
- Ma, Y.; Han, F.; Li, Z.; Xia, C. Acidic-functionalized ionic liquid as corrosion inhibitor for 304 stainless steel in aqueous sulfuric acid. ACS Sustain. Chem. Eng. 2016, 4, 5046–5052. [Google Scholar] [CrossRef]
- Zouarhi, M. Bibliographical Synthesis on the Corrosion and Protection of Archaeological Iron by Green Inhibitors. Electrochem 2023, 4, 103–122. [Google Scholar] [CrossRef]
- Begum, A.A.; Vahith, R.M.; Kotra, V.; Shaik, M.R.; Abdelgawad, A.; Awwad, E.M.; Khan, M. Spilanthes acmella leaves extract for corrosion inhibition in acid medium. Coatings 2021, 11, 106. [Google Scholar] [CrossRef]
- Sliem, M.H.; Afifi, M.; Radwan, A.B.; Fayyad, E.M.; Shibl, M.F.; Heakal, F.E.; Abdullah, A.M. AEO7 surfactant as an eco-friendly corrosion inhibitor for carbon steel in HCl solution. Sci. Rep. 2019, 9, 2319. [Google Scholar] [CrossRef]
- Kousar, K.; Walczak, M.S.; Ljungdahl, T.; Wetzel, A.; Oskarsson, H.; Restuccia, P.; Ahmad, E.A.; Harrison, N.M.; Lindsay, R. Corrosion inhibition of carbon steel in hydrochloric acid: Elucidating the performance of an imidazoline-based surfactant. Corros. Sci. 2021, 180, 109195. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Verma, C.; Hussain, C.M.; Quraishi, M.A.; Alfantazi, A. Green surfactants for corrosion control: Design, performance and applications. Adv. Colloid Interface Sci. 2022, 311, 102822. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Elaraby, A.; El-samad, S.A.; Khamis, E.A.; Zaki, E.G. Theoretical and electrochemical evaluation of tetra-cationic surfactant as corrosion inhibitor for carbon steel in 1 M HCl. Sci. Rep. 2023, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, X. Asymmetric Gemini surfactants as corrosion inhibitors for carbon steel in acidic medium: Experimental and theoretical studies. Colloids Surf. A 2023, 660, 130850. [Google Scholar] [CrossRef]
- Yaagoob, I.Y.; Goni, L.K.; Mazumder, M.A.; Ali, S.A.; Quraishi, M.A.; Verma, C. Synthesis of polymeric surfactant containing bis-cationic motifs as a highly efficient acid corrosion inhibitor for C1018 carbon steel. New J. Chem. 2023, 47, 3445–3461. [Google Scholar] [CrossRef]
- Numin, M.S.; Jumbri, K.; Kee, K.E.; Hassan, A.; Borhan, N.; Matmin, J. DFT Calculation and MD Simulation Studies on Gemini Surfactant Corrosion Inhibitor in Acetic Acid Media. Polymers 2023, 15, 2155. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Gan, H.; Chen, Z.; Chen, M.; Fu, C. Eco-friendly approach to corrosion inhibition of AA5083 aluminum alloy in HCl solution by the expired Vitamin B1 drugs. J. Mol. Struct. 2021, 1244, 130881. [Google Scholar] [CrossRef]
- Argiz, C.; Arroyo, C.; Bravo, A.; Moragues, A.; Andrade, C.; Bolzoni, F. L-Ascorbic Acid as an Efficient Green Corrosion Inhibitor of Steel Rebars in Chloride Contaminated Cement Mortar. Materials 2022, 15, 8005. [Google Scholar] [CrossRef] [PubMed]
- Kesari, P.; Udayabhanu, G. Investigation of vitamin B12 as a corrosion inhibitor for mild steel in HCl solution through gravimetric and electrochemical studies. Ain Shams Eng. J. 2023, 14, 101920. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, D.; Zhang, H.; Wang, P.; Wang, M.; Wu, D.; Liu, C.; Ding, Z.; Zhang, M.; Xin, Z.; et al. Understanding the effect of vitamin B3, B6 and C as a corrosion inhibitor on the ordinary Portland cement hydration: Experiments and DFT study. Constr. Build. Mater. 2022, 331, 127294. [Google Scholar] [CrossRef]
- Manjunath, V.; Badhe, R.V.; McCoy, M.; Rynne, J.; Bhatti, A.; Segu, A.; Oral, E.; Jacobs, J.J.; Chastain, P., 2nd; Bijukumar, D.; et al. The role of Vitamin E in hip implant-related corrosion and toxicity: Initial outcome. J. Mech. Behav. Biomed. Mater. 2021, 123, 104769. [Google Scholar] [CrossRef]
- Maged, S.A.; Anaee, R.A.; Mathew, M.T. The Role of Uric Acid to Reduce the Corrosion of Co-Cr-Mo Alloy as Joint in Presence of Ca and Vitamin D3. J. Bio- Tribo-Corros. 2023, 9, 66. [Google Scholar] [CrossRef]
- Cai, L.; Guo, H.R.; Zhu, Y.Q.; Du, F.S.; Qi, J.T.; Cui, L.Y.; Liu, C.B.; Zeng, R.C. Biodegradation mechanisms of pure Mg in presence of glucose, vitamin C, and citric acid. Smart Mater. Manuf. 2023, 1, 100014. [Google Scholar] [CrossRef]
- Uzoma, I.E.; Solomon, M.M.; Loto, R.T.; Umoren, S.A. Aspartame as a Green and Effective Corrosion Inhibitor for T95 Carbon Steel in 15 wt.% HCl Solution. Sustainability 2022, 14, 6500. [Google Scholar] [CrossRef]
- Peter, A.; Obot, I.B.; Sharma, S.K. Use of natural gums as green corrosion inhibitors: An overview. Int. J. Ind. Chem. 2015, 6, 153–164. [Google Scholar] [CrossRef]
- Vaidya, N.R.; Aklujkar, P.; Rao, A.R. Modification of natural gums for application as corrosion inhibitor: A review. J. Coat. Technol. Res. 2022, 19, 223–239. [Google Scholar] [CrossRef]
- Korniy, S.A.; Zin, I.M.; Danyliak, M.-O.M.; Rizun, Y.Y. Eco-Friendly Metal Corrosion Inhibitors Based on Natural Polymers (A Review). Mater. Sci. 2023, 58, 567–578. [Google Scholar] [CrossRef]
- Palumbo, G.; Święch, D.; Górny, M. Guar Gum as an Eco-Friendly Corrosion Inhibitor for N80 Carbon Steel under Sweet Environment in Saline Solution: Electrochemical, Surface, and Spectroscopic Studies. Int. J. Mol. Sci. 2023, 24, 12269. [Google Scholar] [CrossRef] [PubMed]
- Timothy, U.J.; Ankah, N.K.; Umoren, P.S.; Solomon, M.M.; Igwe, I.O.; Umoren, S.A. Assessment of Berlinia grandiflora and cashew natural exudate gums as sustainable corrosion inhibitors for mild steel in an acidic environment. J. Environ. Chem. Eng. 2023, 11, 111578. [Google Scholar] [CrossRef]
- Núñez-Morales, J.; Jaramillo, L.I.; Espinoza-Montero, P.J.; Sánchez-Moreno, V.E. Evaluation of Adding Natural Gum to Pectin Extracted from Ecuadorian Citrus Peels as an Eco-Friendly Corrosion Inhibitor for Carbon Steel. Molecules 2022, 27, 2111. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Gum Arabic as an environmentally sustainable polymeric anticorrosive material: Recent progresses and future opportunities. Int. J. Biol. Macromol. 2021, 184, 118–134. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.; Seman, A.A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Sobri, S.A.; Ali, A.; et al. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Fazal, B.R.; Becker, T.; Kinsella, B.; Lepkova, K. A review of plant extracts as green corrosion inhibitors for CO2 corrosion of carbon steel. npj Mater. Degrad. 2022, 6, 5. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Reza, N.A.; Akhmal, N.H.; Fadil, N.A.; Taib, M.F. A review on plants and biomass wastes as organic green corrosion inhibitors for mild steel in acidic environment. Metals 2021, 11, 1062. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ibok, U.J.; Garg, R.; Garg, R.; Iqbal, A.; Amin, M.; Mustafa, F.; Egilmez, M.; Galal, A.M. A Brief review on Fruit and Vegetable Extracts as Corrosion Inhibitors in acidic environments. Molecules 2022, 27, 2991. [Google Scholar] [CrossRef]
- Ienașcu, I.M.; Căta, A.; Chis, A.A.; Ştefănuț, M.N.; Sfîrloagă, P.; Rusu, G.; Frum, A.; Arseniu, A.M.; Morgovan, C.; Rus, L.L.; et al. Some Brassicaceae Extracts as Potential Antioxidants and Green Corrosion Inhibitors. Materials 2023, 16, 2967. [Google Scholar] [CrossRef]
- Méndez, C.M.; Gervasi, C.A.; Pozzi, G.; Ares, A.E. Corrosion inhibition of aluminum in acidic solution by Ilex paraguariensis (Yerba Mate) extract as a green inhibitor. Coatings 2023, 13, 434. [Google Scholar] [CrossRef]
- Hernández, D.A.P.; Parra, E.R.; Arango, P.J.A.; Giraldo, B.S.; Medina, C.D.A. Innovative method for coating of natural corrosion inhibitor based on Artemisia vulgaris. Materials 2021, 14, 2234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Lai, J.; Qiang, S. Inhibition effect and mechanism explanation of perilla seed extract as a green corrosion inhibitor on Q235 carbon steel. Materials 2022, 15, 5394. [Google Scholar] [CrossRef] [PubMed]
- Kwolek, P.; Dychtoń, K.; Kościelniak, B.; Obłój, A.; Podborska, A.; Wojnicki, M. Gallic acid as a potential green corrosion inhibitor for aluminum in acidic solution. Metals 2022, 12, 250. [Google Scholar] [CrossRef]
- Vidhya, K.T.; Kakkassery, J.T.; Raphael, V.P.; Ragi, K.; Johnson, R. Ixora coccinea extract as an efficient eco-friendly corrosion inhibitor in acidic media: Experimental and theoretical approach. Curr. Chem. Lett. 2021, 10, 139–150. [Google Scholar]
- Iroha, N.B.; Maduelosi, N.J. Corrosion inhibitive action and adsorption behaviour of justicia secunda leaves extract as an eco-friendly inhibitor for aluminium in acidic media. Biointerface Res. Appl. Chem. 2021, 11, 13019–13030. [Google Scholar]
- Aralu, C.C.; Chukwuemeka-Okorie, H.O.; Akpomie, K.G. Inhibition and adsorption potentials of mild steel corrosion using methanol extract of Gongronema latifoliuim. Appl. Water Sci. 2021, 11, 22. [Google Scholar] [CrossRef]
- Fouda, A.S.; El-Gharkawy, E.S.; Ramadan, H.; El-Hossiany, A. Corrosion resistance of mild steel in hydrochloric acid solutions by clinopodium acinos as a green inhibitor. Biointerface Res. Appl. Chem. 2021, 11, 9786. [Google Scholar]
- Kaban, A.P.S.; Ridhova, A.; Priyotomo, G.; Elya, B.; Maksum, A.; Sadeli, Y.; Sutopo, S.; Aditiyawarman, T.; Riastuti, R.; Soedarsono, J.W. Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. East.-Eur. J. Enterp. Technol. 2021, 2, 110. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Kamboj, D.; Kaya, S.; Dagdag, O.; Guo, L. Corrosion inhibition, surface adsorption and computational studies of Momordica charantia extract: A sustainable and green approach. SN Appl. Sci. 2021, 3, 25. [Google Scholar] [CrossRef]
- Tehrani, M.E.; Ghahremani, P.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Theoretical and experimental assessment of a green corrosion inhibitor extracted from Malva sylvestris. J. Environ. Chem. Eng. 2021, 9, 105256. [Google Scholar] [CrossRef]
- Zuo, X.; Li, W.; Luo, W.; Zhang, X.; Qiang, Y.; Zhang, J.; Li, H.; Tan, B. Research of Lilium brownii leaves extract as a commendable and green inhibitor for X70 steel corrosion in hydrochloric acid. J. Mol. Liq. 2021, 321, 114914. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Jiang, H.; Gu, Y.; Li, X.; Xu, L.L. Pueraria lobata leaf extract as green corrosion inhibitor for low carbon steel in 1.0 M HCl solution. Res. Chem. Intermed. 2021, 47, 1051–1069. [Google Scholar] [CrossRef]
- Banu, A.M.; Farzana, B.A.; Ahamed, K.R. Evaluation of inhibitive performance of Allamanda cathartica leaves extract as a green corrosion inhibitor on mild steel in acid medium. Mater. Today Proc. 2021, 47, 2036–2047. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Qiang, Y. Corrosion retardation effect of a green cauliflower extract on copper in H2SO4 solution: Electrochemical and theoretical explorations. J. Mol. Liq. 2021, 321, 114450. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M. Peach pomace extract as novel cost-effective and high-performance green inhibitor for mild steel corrosion in NaCl solution: Experimental and theoretical research. Waste Biomass Valorization 2021, 12, 4623–4641. [Google Scholar] [CrossRef]
- Thomas, A.; Prajila, M.; Shainy, K.M.; Joseph, A. A green approach to corrosion inhibition of mild steel in hydrochloric acid using fruit rind extract of Garcinia indica (Binda). J. Mol. Liq. 2020, 312, 113369. [Google Scholar] [CrossRef]
- Tan, B.; He, J.; Zhang, S.; Xu, C.; Chen, S.; Liu, H.; Li, W. Insight into anti-corrosion nature of Betel leaves water extracts as the novel and eco-friendly inhibitors. J. Colloid Interface Sci. 2021, 585, 287–301. [Google Scholar] [CrossRef]
- Liu, Q.; Song, Z.; Han, H.; Donkor, S.; Jiang, L.; Wang, W.; Chu, H. A novel green reinforcement corrosion inhibitor extracted from waste Platanus acerifolia leaves. Constr. Build. Mater. 2020, 260, 119695. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.; Caballero-Briones, F.; Manickam, S.; Thomas, K.J.; Santos, D. Experimental and DFT studies on the ultrasonic energy-assisted extraction of the phytochemicals of Catharanthus roseus as green corrosion inhibitors for mild steel in NaCl medium. RSC Adv. 2020, 10, 5399–5411. [Google Scholar] [CrossRef] [PubMed]
- Ahanotu, C.C.; Onyeachu, I.B.; Solomon, M.M.; Chikwe, I.S.; Chikwe, O.B.; Eziukwu, C.A. Pterocarpus santalinoides leaves extract as a sustainable and potent inhibitor for low carbon steel in a simulated pickling medium. Sustain. Chem. Pharm. 2020, 15, 100196. [Google Scholar] [CrossRef]
- Fekkar, G.; Yousfi, F.; Elmsellem, H.; Aiboudi, M.; Ramdani, M.; Abdel-Rahman, I.; Hammouti, B.; Bouyazza, L. Eco-friendly Chamaerops humilis L. fruit extract corrosion inhibitor for mild steel in 1 M HCl. Int. J. Corros. Scale Inhib. 2020, 9, 446–459. [Google Scholar]
- Benahmed, M.; Selatnia, I.; Djeddi, N.; Akkal, S.; Laouer, H.J. Adsorption and corrosion inhibition properties of butanolic extract of Elaeoselinum thapsioides and its synergistic effect with Reutera lutea (Desf.) Maires (Apiaceae) on A283 carbon steel in hydrochloric acid solution. Chem. Afr. 2020, 3, 251–261. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Elhajjaji, F.; Taleb, M.; Salim, R.; Boukir, A. Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): Evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater. Today Proc. 2020, 27, 3193–3198. [Google Scholar] [CrossRef]
- Baran, E.; Cakir, A.; Yazici, B. Inhibitory effect of Gentiana olivieri extracts on the corrosion of mild steel in 0.5 M HCl: Electrochemical and phytochemical evaluation. Arab. J. Chem. 2019, 12, 4303–4319. [Google Scholar] [CrossRef]
- Divya, P.; Subhashini, S.; Prithiba, A.; Rajalakshmi, R. Tithonia diversifolia flower extract as green corrosion inhibitor for mild steel in acid medium. Mater. Today Proc. 2019, 18, 1581–1591. [Google Scholar] [CrossRef]
- Khan, M.; Abdullah, M.M.; Mahmood, A.; Al-Mayouf, A.M.; Alkhathlan, H.Z. Evaluation of Matricaria aurea extracts as effective anti-corrosive agent for mild steel in 1.0 M HCl and isolation of their active ingredients. Sustainability 2019, 11, 7174. [Google Scholar] [CrossRef]
- Jisha, M.; Hukuman, N.H.Z.; Leena, P.; Abdussalam, A.K. Electrochemical, computational and adsorption studies of leaf and floral extracts of Pogostemon quadrifolius (Benth.) as corrosion inhibitor for mild steel in hydrochloric acid. J. Mater. Environ. Sci. 2019, 10, 840–853. [Google Scholar]
- Hanini, K.; Merzoug, B.; Boudiba, S.; Selatnia, I.; Laouer, H.; Akkal, S. Influence of different polyphenol extracts of Taxus baccata on the corrosion process and their effect as additives in electrodeposition. Sustain. Chem. Pharm. 2019, 14, 100189. [Google Scholar] [CrossRef]
- Pal, S.; Lgaz, H.; Tiwari, P.; Chung, I.M.; Ji, G.; Prakash, R. Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peels as green corrosion inhibitors of mild steel in aggressive chloride media. J. Mol. Liq. 2019, 276, 347–361. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; Garcia-Anton, J. Green approach to corrosion inhibition of stainless steel in phosphoric acid of Artemesia herba albamedium using plant extract. J. Mater. Res. Technol. 2019, 8, 5763–5773. [Google Scholar] [CrossRef]
- Benabdellah, M.; Benkaddour, M.; Hammouti, B.; Bendahhou, M.; Aouniti, A. Inhibition of steel corrosion in 2 M H3PO4 by artemisia oil. Appl. Surf. Sci. 2006, 252, 6212–6217. [Google Scholar] [CrossRef]
- Victoria, S.N.; Prasad, R.; Manivannan, R. Psidium guajava leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int. J. Electrochem. Sci. 2015, 10, 2220–2238. [Google Scholar] [CrossRef]
- Messali, M.; Lgaz, H.; Dassanayake, R.; Salghi, R.; Jodeh, S.; Abidi, N.; Hamed, O. Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: Electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. 2017, 1145, 43–54. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- Karki, R.; Bajgai, A.K.; Khadka, N.; Thapa, O.; Mukhiya, T.; Oli, H.B.; Bhattarai, D.P. Acacia Catechu Bark Alkaloids as Novel Green Inhibitors for Mild Steel Corrosion in a One Molar Sulphuric Acid Solution. Electrochem 2022, 3, 668–687. [Google Scholar] [CrossRef]
- Thapa, O.; Magar, J.T.; Oli, H.B.; Rajaure, A.; Nepali, D.; Bhattarai, D.P.; Mukhiya, T. Alkaloids of Solanum Xanthocarpum Stem as Green Inhibitor for Mild Steel Corrosion in One Molar Sulphuric Acid Solution. Electrochem 2022, 3, 820–842. [Google Scholar] [CrossRef]
- Feng, Y.; He, J.; Zhan, Y.; An, J.; Tan, B. Insight into the anti-corrosion mechanism Veratrum root extract as a green corrosion inhibitor. J. Mol. Liq. 2021, 334, 116110. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. A green Brassica oleracea L extract as a novel corrosion inhibitor for Q235 steel in two typical acid media. Colloids Surf. A 2021, 616, 126077. [Google Scholar] [CrossRef]
- Simescu-Lazar, F.; Slaoui, S.; Essahli, M.; Bohr, F.; Lamiri, A.; Vanoye, L.; Chopart, J.P. Thymus satureoides oil as green corrosion Inhibitor for 316L stainless steel in 3% NaCl: Experimental and theoretical studies. Lubricants 2023, 11, 56. [Google Scholar] [CrossRef]
- Simović, A.R.; Grgur, B.N.; Novaković, J.; Janaćković, P.; Bajat, J. Black Pine (Pinus nigra) Essential Oil as a Green Corrosion Inhibitor for Carbon Steel. Metals 2023, 13, 508. [Google Scholar] [CrossRef]
- Mohammed, N.J.; Othman, N.K.; Taib, M.F.; Samat, M.H.; Yahya, S. Experimental and Theoretical Studies on Extract of Date Palm Seed as a Green Anti-Corrosion Agent in Hydrochloric Acid Solution. Molecules 2021, 26, 3535. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, S.; Hao, L.; Du, H.; Pan, R.; Huang, G.; Liu, H. Cucumber (Cucumis sativus L.) leaf extract as a green corrosion inhibitor for carbon steel in acidic solution: Electrochemical, functional and molecular analysis. Molecules 2022, 27, 3826. [Google Scholar] [CrossRef]
- Hammud, H.H.; Maache, S.A.; Al Otaibi, N.; Sheikh, N.S. An Integrated Experimental and Theoretical Studies on the Corrosion Inhibition of Carbon Steel by Harmal Extracts. Molecules 2022, 27, 7250. [Google Scholar] [CrossRef]
- Obot, I.B.; Solomon, M.M.; Onyeachu, I.B.; Umoren, S.A.; Meroufel, A.; Alenazi, A.; Sorour, A.A. Development of a green corrosion inhibitor for use in acid cleaning of MSF desalination plant. Desalination 2020, 495, 114675. [Google Scholar] [CrossRef]
- Hynes, N.R.; Vignesh, N.J.; Barile, C.; Velu, P.S.; Baskaran, T.; Jappes, J.T.; Al-Khashman, O.A.; Brykov, M.; Ene, A. Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract. Polymers 2022, 14, 1700. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.C.; Ribeiro, B.D.; Garrett, R.; Borges, R.M.; da Silva, T.U.; de Paula Machado, S.; de Araujo, J.R.; de Oliveira Massafra, S.; de Oliveira Junior, F.O.; D’Elia, E. Ziziphus joazeiro stem bark extract as a green corrosion inhibitor for mild steel in acid medium. Processes 2021, 9, 1323. [Google Scholar] [CrossRef]
- Baskar, P.; Rathinapriya, P.; Prabakaran, M. Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions. Processes 2022, 10, 1480. [Google Scholar] [CrossRef]
- Salmasifar, A.; Edraki, M.; Alibakhshi, E.; Ramezanzadeh, B.; Bahlakeh, G. Combined electrochemical/surface investigations and computer modeling of the aquatic Artichoke extract molecules corrosion inhibition properties on the mild steel surface immersed in the acidic medium. J. Mol. Liq. 2021, 327, 114856. [Google Scholar] [CrossRef]
- Salmasifar, A.; Edraki, M.; Alibakhshi, E.; Ramezanzadeh, B.; Bahlakeh, G. Theoretical design coupled with experimental study of the effectiveness of the inhibitive molecules based on Cynara scolymus L. extract toward chloride-induced corrosion of steel. J. Mol. Liq. 2021, 332, 115742. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Ranjbarghanei, M.; Javidparvar, A.A.; Guo, L.; Berdimurodov, E.; Ramezanzadeh, B. Theoretical and surface/electrochemical investigations of walnut fruit green husk extract as effective inhibitor for mild-steel corrosion in 1M HCl electrolyte. J. Mol. Liq. 2021, 338, 116550. [Google Scholar] [CrossRef]
- Elabbasy, H.M.; Elnagar, M.E.; Fouda, A.E. Chemical, electrochemical, and surface studies of Rosa damascene extract as a green corrosion inhibitor for carbon steel in sulfuric acid environment. J. Chin. Chem. Soc. 2024, 71, 385–396. [Google Scholar] [CrossRef]
- El Nemr, A.; Elhebshi, A.; Ashour, I.; El-Deab, M.S.; Barghout, N.A.; Eddy, N.O.; Ragab, S. Camphor tree bark extract as green corrosion inhibitor of LCS in 0.5 M H2SO4 with and without salt effect. J. Chin. Chem. Soc. 2024, 71, 174–196. [Google Scholar] [CrossRef]
- Eid, S.; Syam, S.M.; El-Etre, A.Y.; Sayed, N.H. Surface, electrochemical, and theoretical investigation on utilizing olive leaf extract as green inhibitor for copper corrosion in alkaline environment. Arab. J. Sci. Eng. 2024, 49, 147–164. [Google Scholar] [CrossRef]
- Barboza, G.K.; de Oliveira, M.C.; Neves, M.A.; Echevarria, A. Justicia brandegeeana as a green corrosion inhibitor for carbon steel in sulfuric acid. Green Chem. Lett. Rev. 2024, 17, 2320254. [Google Scholar] [CrossRef]
- Elhady, S.; Zaki, E.G.; El-Azabawy, O.E.; Fahim, I.S. Electrochemical evaluation of green corrosion inhibitor based on ground coffee waste in Petroleum fields. Results Eng. 2024, 21, 101880. [Google Scholar] [CrossRef]
- Eddahhaoui, F.Z.; Najem, A.; Elhawary, M.; Boudalia, M.; Campos, O.S.; Tabyaoui, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M. Experimental and computational aspects of green corrosion inhibition for low carbon steel in HCl environment using extract of Chamaerops humilis fruit waste. J. Alloys Compd. 2024, 977, 173307. [Google Scholar] [CrossRef]
- Batah, A.; Al-Moubaraki, A.H.; Noor, E.A.; Al-Ahmari, J.M.; Al-Ghamdi, A.A.; El Mouden, O.I.; Salghi, R.; Chafiq, M.; Chaouiki, A.; Ko, Y.G. Environmentally Benign Grape Seed Oil for Corrosion Inhibition: Cutting-Edge Computational Modeling Techniques Revealing the Intermolecular and Intramolecular Synergistic Inhibition Action. Coatings 2024, 14, 77. [Google Scholar] [CrossRef]
- El-Hashemy, M.A.; Almehmadi, A.M. Evaluation of Glebionis coronaria L. flower extract as a novel green inhibitor for mild steel corrosion in acidic environment. Biomass Convers. Biorefinery 2024, 14, 1–7. [Google Scholar] [CrossRef]
- Rahmouni, H.; Nigri, S.; Nacef, M.; Oumeddour, R.; Affoune, A.M. Analysis of Fig Leaf Extract as Steel Eco-friendly Corrosion Inhibitor in Acidic Medium: Electrochemical, Gravimetric, Spectroscopic, and Surface Studies. Anal. Bioanal. Electrochem. 2024, 16, 142–162. [Google Scholar]
- Yıldız, R.; Arslanhan, S.; Döner, A.; Baran, M.F. Corrosion behavior of mild steel in 1 M HCl with Cyclotrichium niveum as a green inhibitor. Mater. Chem. Phys. 2024, 312, 128654. [Google Scholar] [CrossRef]
- Ren, H.; Liu, Y.; Gong, Z.; Tan, B.; Deng, H.; Xiong, J.; Shao, P.; Dai, Q.; Cao, J.; Marzouki, R. Pumpkin Leaf Extract Crop Waste as a New Degradable and Environmentally Friendly Corrosion Inhibitor. Langmuir 2024, 40, 5738–5752. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.M.; Al-Sharif, M.S. Eco-Friendly Piper cubeba Official Extract Corrosion Inhibition of C-Steel in 1 M Sulfamic Acid. ACS Omega 2024, 9, 5024–5037. [Google Scholar] [CrossRef]
- Lebrini, M.; Suedile, F.; Salvin, P.; Roos, C.; Zarrouk, A.; Jama, C.; Bentiss, F. Bagassa guianensis ethanol extract used as sustainable eco-friendly inhibitor for zinc corrosion in 3% NaCl: Electrochemical and XPS studies. Surf. Interfaces 2020, 20, 100588. [Google Scholar] [CrossRef]
- Benzidia, B.; Hammouch, H.; Dermaj, A.; Benassaoui, H.; Abbout, S.; Hajjaji, N. Investigation of green corrosion inhibitor based on aloe vera (L.) burm. F. for the protection of bronze B66 in 3% NaCl. Anal. Bioanal. Electrochem. 2019, 11, 165–177. [Google Scholar]
- Devikala, S.; Kamaraj, P.; Arthanareeswari, M.; Pavithra, S. Green Corrosion inhibition of mild steel by Asafoetida extract extract in 3.5% NaCl. Mater. Today Proc. 2019, 14, 590–601. [Google Scholar] [CrossRef]
- Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous dried extract of skytanthus acutus meyen as corrosion inhibitor of carbon steel in neutral chloride solutions. Metals 2021, 11, 1992. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Jiang, Y.; Qian, Y.; Guo, X.; Wang, L.; Zhang, J. Orange peel extracts as biodegradable corrosion inhibitor for magnesium alloy in NaCl solution: Experimental and theoretical studies. J. Taiwan Inst. Chem. Eng. 2020, 115, 35–46. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Sanaei, Z.; Bahlakeh, G.; Ramezanzadeh, B. Highly effective inhibition of mild steel corrosion in 3.5% NaCl solution by green Nettle leaves extract and synergistic effect of eco-friendly cerium nitrate additive: Experimental, MD simulation and QM investigations. J. Mol. Liq. 2018, 256, 67–83. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Ghandchi, M.S. Santolina chamaecyparissus extract as a natural source inhibitor for 304 stainless steel corrosion in 3.5% NaCl. J. Ind. Eng. Chem. 2015, 31, 231–237. [Google Scholar] [CrossRef]
- Pradityana, A.; Shahab, A.; Noerochim, L.; Susanti, D. Inhibition of corrosion of carbon steel in 3.5% NaCl solution by Myrmecodia pendans extract. Int. J. Corros. 2016, 2016, 6058286. [Google Scholar] [CrossRef]
- Wamba-Tchio, O.R.; Pengou, M.; Teillout, A.L.; Baumier, C.; Mbomekallé, I.M.; De Oliveira, P.; Nanseu-Njiki, C.P.; Ngameni, E. Electrochemical study and experimental simulation of the synergistic effect of a formulation based on Ficus pumila Linn. Leaves extract and zinc sulfate on the XC38 steel corrosion inhibition in NaCl solution. J. Electroanal. Chem. 2022, 919, 116553. [Google Scholar] [CrossRef]
- Žbulj, K.; Hrnčević, L.; Bilić, G.; Simon, K. Dandelion-Root Extract as Green Corrosion Inhibitor for Carbon Steel in CO2-Saturated Brine Solution. Energies 2022, 15, 3074. [Google Scholar] [CrossRef]
- Loto, C.A.; Loto, R.T.; Popoola, A.P.I. Electrode potential monitoring of effect of plants extracts addition on the electrochemical corrosion behaviour of mild steel reinforcement in concrete. Int. J. Electrochem. Sci. 2011, 6, 3452–3465. [Google Scholar] [CrossRef]
- Asipita, S.A.; Ismail, M.; Abd Majid, M.Z.; Majid, Z.A.; Abdullah, C.; Mirza, J. Green Bambusa Arundinacea leaves extract as a sustainable corrosion inhibitor in steel reinforced concrete. J. Clean. Prod. 2014, 67, 139–146. [Google Scholar] [CrossRef]
- Valdez-Salas, B.; Vazquez-Delgado, R.; Salvador-Carlos, J.; Beltran-Partida, E.; Salinas-Martinez, R.; Cheng, N.; Curiel-Alvarez, M. Azadirachta indica leaf extract as green corrosion inhibitor for reinforced concrete structures: Corrosion effectiveness against commercial corrosion inhibitors and concrete integrity. Materials 2021, 14, 3326. [Google Scholar] [CrossRef] [PubMed]
- Naderi, R.; Bautista, A.; Velasco, F.; Soleimani, M.; Pourfath, M. Use of licorice plant extract for controlling corrosion of steel rebar in chloride-polluted concrete pore solution. J. Mol. Liq. 2022, 346, 117856. [Google Scholar] [CrossRef]

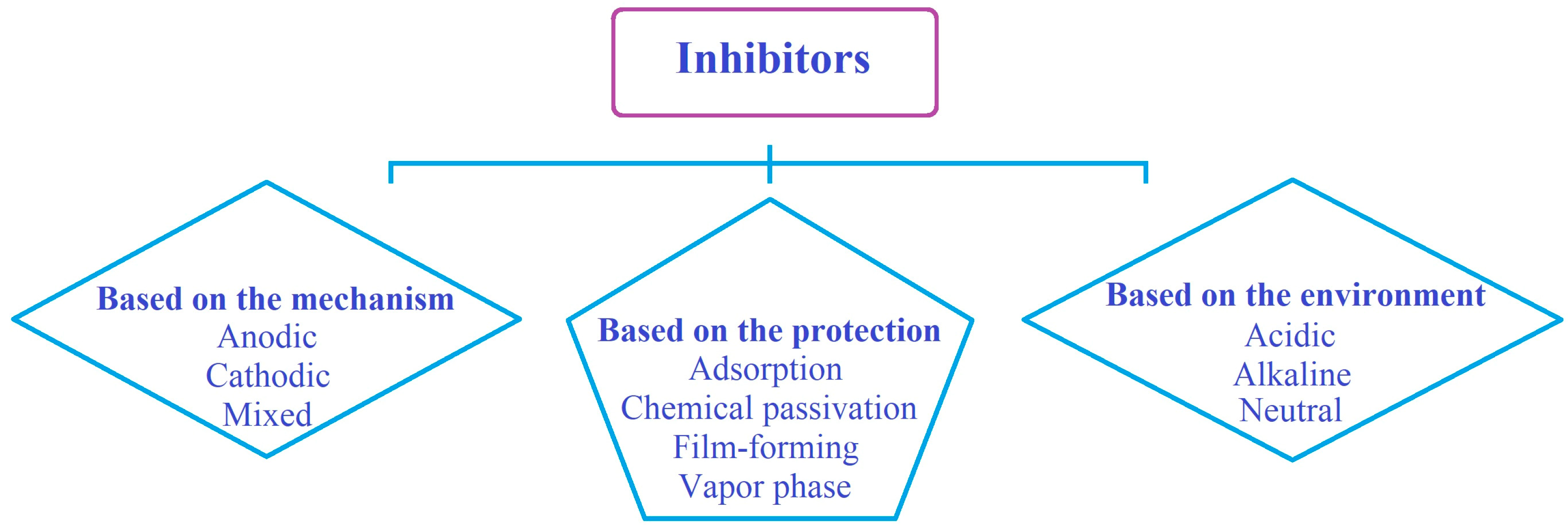
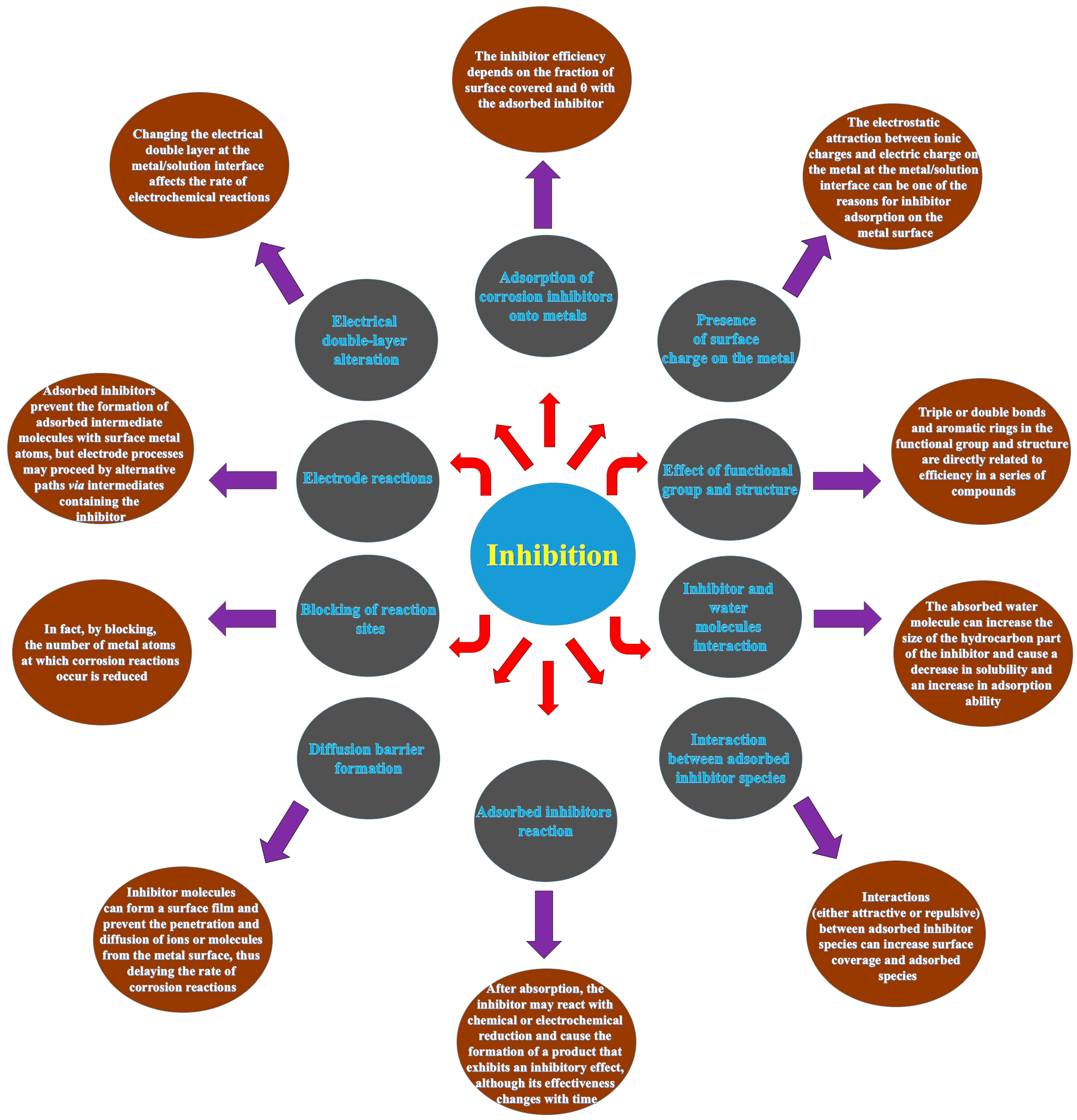
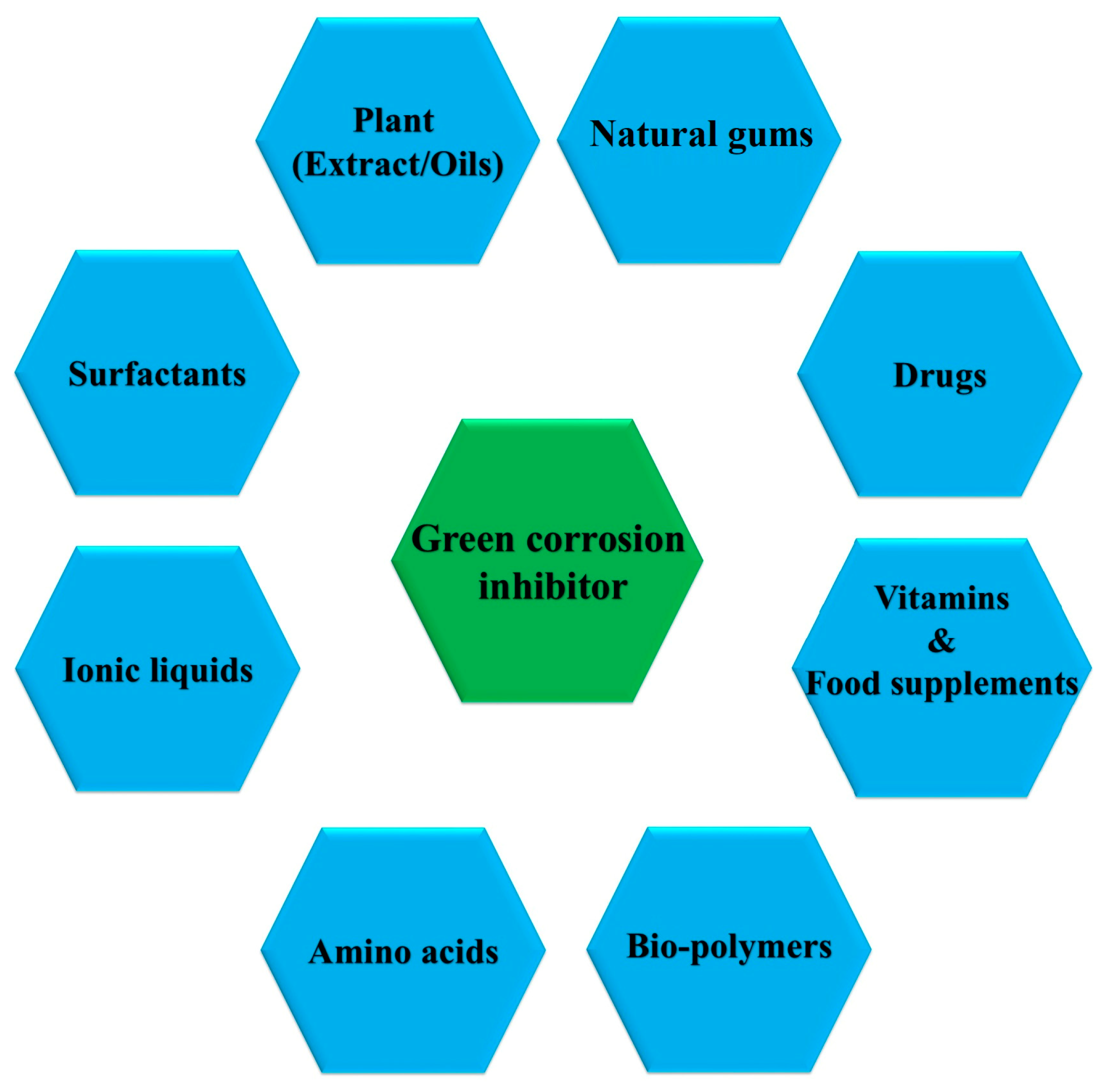

| Inhibitor | Corrosion Inhibition Method | The Most Common Inhibitor |
|---|---|---|
| Anodic | By forming a protective film along the anode, they increase their potential and reduce the corrosion reaction. | Chromates and tungstates |
| Cathodic | By blocking the cathode sites, they reduce the rate of the reduction reaction of the electrochemical corrosion cell, and as a result, the corrosion is reduced. | Elements arsenic and antimony |
| Mixed | By forming a film that causes deposits on the surface, they block the anodic and cathodic sites and delay corrosion. | Silicates and phosphates |
| Natural Gums | Brief Description |
|---|---|
| Gum arabic | Its other name is acacia gum, which is obtained from the Acacia Senegal tree and is used in drug delivery, adhesive, anti-corrosion agents, and food packaging. |
| Guar gum | It is obtained from the Cyamopsis tetragonoloba plant and is used in agriculture, drug delivery, biomedical, pharmaceuticals, coatings, and food industries. |
| Xanthan gum | It is secreted by the Xanthomonas campestris bacteria and is used in cosmetics, the oil industry, tissue engineering, and food industries. |
| Albizia gum | It is obtained from the Albizia tree and is used in the cosmetics, pharmaceutical, and food industries. |
| Dacroydes edulis gum | It is obtained from the Dacryodes edulis tree and is used in cosmetics, pharmaceutical, adhesive, and anti-corrosion agents. |
| Cashew tree gum | It is obtained from the Anacardium occidentale tree and is used in medicine, anti-corrosive agents, emulsifying agents, and coating. |
| Raphia hookeri gum | It is obtained from the Raphia hookeri tree and is used in pharmaceuticals, emulsifiers, and anti-corrosive agents. |
| Solvent | Acetone | Ethanol | Methanol | Water |
|---|---|---|---|---|
| Phytochemicals | Flavonols and Tannins | Alkaloids, Flavonols, Polyacetylenes, Polyphenols, Propolis, Sterols, Tannins, and Terpenoids | Anthocyanins, Flavonols, Lactones, Quassinoids, Phenones, Polyphenols, Saponins, Tannins, Terpenoids, Totarol, and Xanthoxyllines | Anthocyanins, Lectins, Polypeptides, Saponins, Starches, Tannins, and Terpenoids |
| Plant | Substrate | Corrosive Medium | Maximum Inhibition Efficiency (%) | Reference |
|---|---|---|---|---|
| Artemisia vulgaris—Solanum tuberosum | Mild steel | H2SO4 | 88.06–83.22 | [73] |
| Coriaria nepalensis | Mild steel | H2SO4 | 97.03 | [77] |
| Terebinth (Pistacia terebinthus L.) | Iron | Sodium chloride (NaCl) | 86.4 | [79] |
| Ixora coccinea | Mild steel | H2SO4-HCl | 77.96–89.38 | [144] |
| Justicia secunda | Aluminum | HCl | 94.30 | [145] |
| Gongronema latifolium | Mild steel | HCl | 81.69 | [146] |
| Clinopodium acinos | Mild steel | HCl | 89.90 | [147] |
| White tea | Mild steel | HCl | 96.00 | [148] |
| Momordica charantia | Carbon steel | H2SO4 | 93.51 | [149] |
| Malva sylvestris | Mild steel | NaCl | 91.00 | [150] |
| Lilium brownii | X70 steel | HCl | 85.00 | [151] |
| Pueraria lobata | 10# steel | HCl | 94.37 | [152] |
| Allamanda cathartica | Mild steel | H2SO4 | 72.75 | [153] |
| Cauliflower | Copper | H2SO4 | 99.00 | [154] |
| Peach pomace | Mild steel | NaCl | 88.00 | [155] |
| Binda rind | Mild steel | HCl | 97.33 | [156] |
| Betel | Carbon steel | HCl | 94.90 | [157] |
| Platanus acerifolia | Carbon steel | NaCl | 99.86 | [158] |
| Catharanthus roseus | Mild steel | NaCl | 84.00 | [159] |
| Mutiti | Low—carbon steel | H2SO4 | 86.23 | [160] |
| Chamaerops humilis | Mild steel | HCl | 88.00 | [161] |
| Elaeoselinum thapsioides | Carbon steel | HCl | 82.00 | [162] |
| Punica granatum L. | Mild steel | HCl | 87.30 | [163] |
| Gentiana olivieri | Mild steel | HCl | 89.70 | [164] |
| Tithonia diversifolia | Mild steel | HCl | 79.99 | [165] |
| Matricaria aurea | Mild steel | HCl | 93.56 | [166] |
| Pogostemon quadrifolius floral | Mild steel | HCl | 95.79 | [167] |
| Taxus baccata | Carbon steel | HCl | 74.26 | [168] |
| Prunus dulcis | Mild steel | HCl | 88.00 | [169] |
| Artemisia herba-alba | Stainless steel | Phosphoric acid (H3PO4) | 85.00 | [170] |
| Artemisia oil | Steel | H3PO4 | 74.00 | [171] |
| Psidium Guajava | Mild steel | H3PO4 | 82.00 | [172] |
| Guar gum | Carbon steel | H3PO4 | 95.00 | [173] |
| Rhynchostylis retusa | Mild steel | H2SO4 | 93.24 | [174] |
| Acacia catechu | Mild steel | H2SO4 | 98.54 | [175] |
| Solanum xanthocarpum | Mild steel | H2SO4 | 98.14 | [176] |
| Veratrum | Copper | H2SO4 | 97.00 | [177] |
| Brassica oleracea L. | Q235 steel | H2SO4-HCl | 92.30–93.80 | [178] |
| Thymus satureioides | 316L stainless steel | NaCl | 82.00 | [179] |
| Pinus nigra | Carbon steel | HCl | 97.00 | [180] |
| Palm seed | Carbon steel | HCl | 95.00 | [181] |
| Cucumis sativus L. | Carbon steel | H2SO4 | 92.80 | [182] |
| Harmal roots | Carbon steel | H2SO4 | 94.10 | [183] |
| Palm leaves | Carbon steel | HCl | 96.80 | [184] |
| Aerva lanata | Aluminum | NaCl | 88.00 | [185] |
| Ziziphus joazeiro | Mild steel | HCl | 94.70 | [186] |
| Trochodendron Aralioides | Mild steel | HCl | 96.42 | [187] |
| Aquatic Artichoke | Mild steel | HCl | 98.70 | [188] |
| Cynara scolymus L. | Mild steel | NaCl | 96.10 | [189] |
| Juglans regia L. | Mild steel | HCl | 95.00 | [190] |
| Rosa damascene | Carbon steel | H2SO4 | 75–96.1 | [191] |
| Cinnamomum camphora | Low carbon steel | H2SO4 | 89–97 | [192] |
| Olive leaf | Copper | KOH | 90.68 | [193] |
| Justicia brandegeeana | Carbon steel | H2SO4 | 82.41 | [194] |
| Coffee waste | Carbon steel | HCl | 76–96 | [195] |
| Chamaerops humilis | Carbon steel | HCl | 94 | [196] |
| Grape seed oil | Carbon steel | HCl | 79 | [197] |
| Glebionis coronaria L. flower | Mild steel | HCl | 95 | [198] |
| Fig leaf | Mild steel | HCl | 94 | [199] |
| Cyclotrichium niveum | Mild steel | HCl | 97.3 | [200] |
| Pumpkin leaf | Copper | H2SO4 | 89.98 | [201] |
| Pipper cubeba | Carbon steel | Sulfamic acid | 96 | [202] |
| Bagassa guianensis | Zinc | NaCl | 97 | [203] |
| Aloe vera (L.) Burm. F. | Bronze B66 | NaCl | 89 | [204] |
| Asafoetida | Mild steel | NaCl | 90 | [205] |
| Skytanthus acutus | Carbon Steel | NaCl | 90 | [206] |
| Orange peel | Magnesium alloy | NaCl | 85.7 | [207] |
| Nettle leaves | Mild steel | NaCl | 95 | [208] |
| Santolina chamaecyparissus | 304 stainless steel | NaCl | 86.9 | [209] |
| Myrmecodia Pendans | Carbon steel | NaCl | 91.41 | [210] |
| Ficus pumila Linn. | XC38 steel | NaCl | 91.3 | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheydaei, M. The Use of Plant Extracts as Green Corrosion Inhibitors: A Review. Surfaces 2024, 7, 380-403. https://doi.org/10.3390/surfaces7020024
Sheydaei M. The Use of Plant Extracts as Green Corrosion Inhibitors: A Review. Surfaces. 2024; 7(2):380-403. https://doi.org/10.3390/surfaces7020024
Chicago/Turabian StyleSheydaei, Milad. 2024. "The Use of Plant Extracts as Green Corrosion Inhibitors: A Review" Surfaces 7, no. 2: 380-403. https://doi.org/10.3390/surfaces7020024
APA StyleSheydaei, M. (2024). The Use of Plant Extracts as Green Corrosion Inhibitors: A Review. Surfaces, 7(2), 380-403. https://doi.org/10.3390/surfaces7020024







