Cell Adhesion Strength Indicates the Antithrombogenicity of Poly(2-methoxyethyl acrylate) (PMEA): Potential Candidate for Artificial Small-Diameter Blood Vessel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fabrication of Polymer-Coated Substrates
2.3. Contact Angle (CA)
2.4. Cell Culture
2.5. Cell Attachment and Proliferation Assay
2.6. Immunocytochemical Analysis
2.7. HUVECs–Polymer Interaction by SCFS
2.8. Human Platelet Adhesion Test
2.9. Platelet–Polymer Substrate Interaction by SCFS
2.10. FM-AFM of Single HUVEC Surface
2.11. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Properties of PMEA-Analogue-Coated Surface
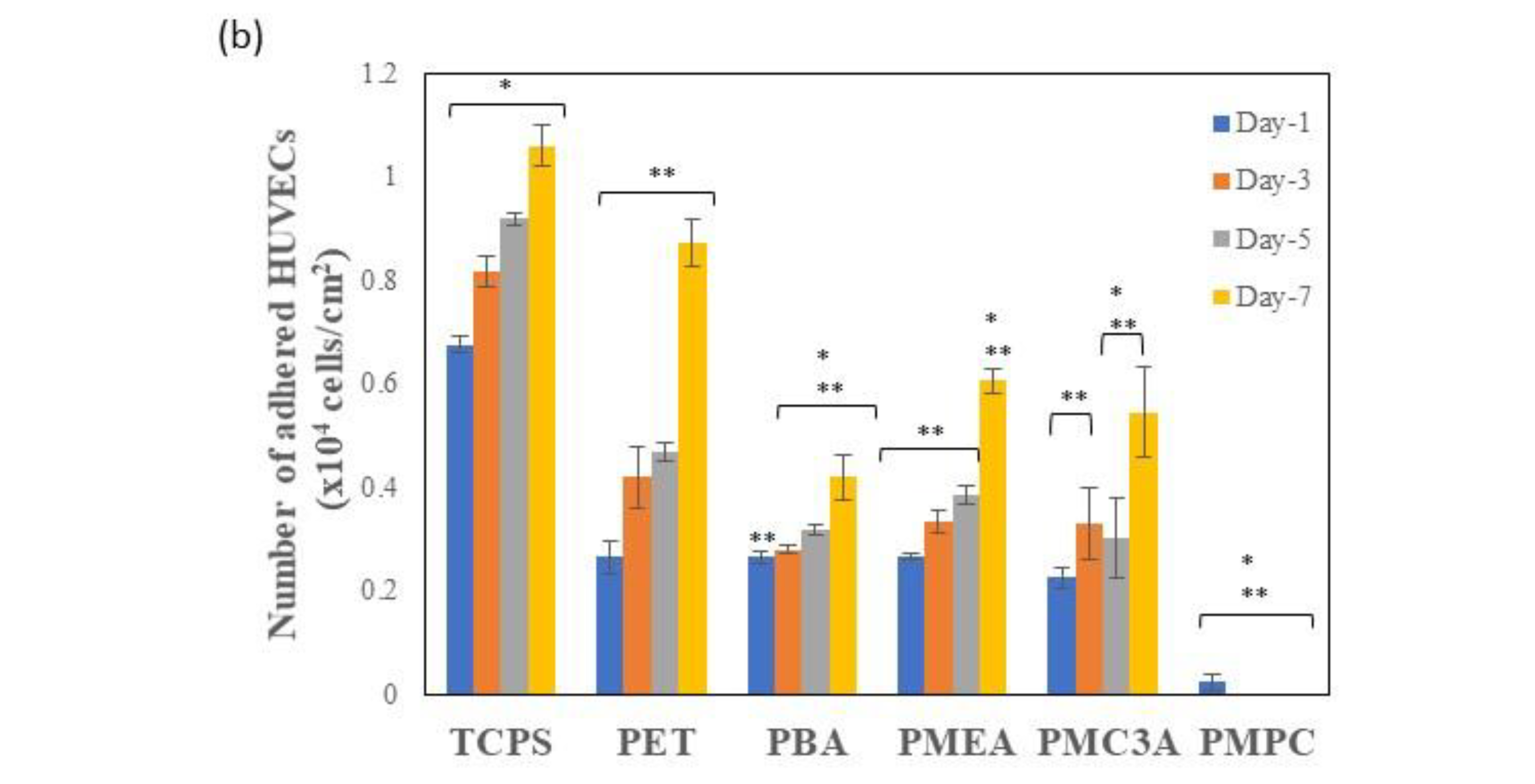
3.2. Cell Attachment and Proliferation Assay
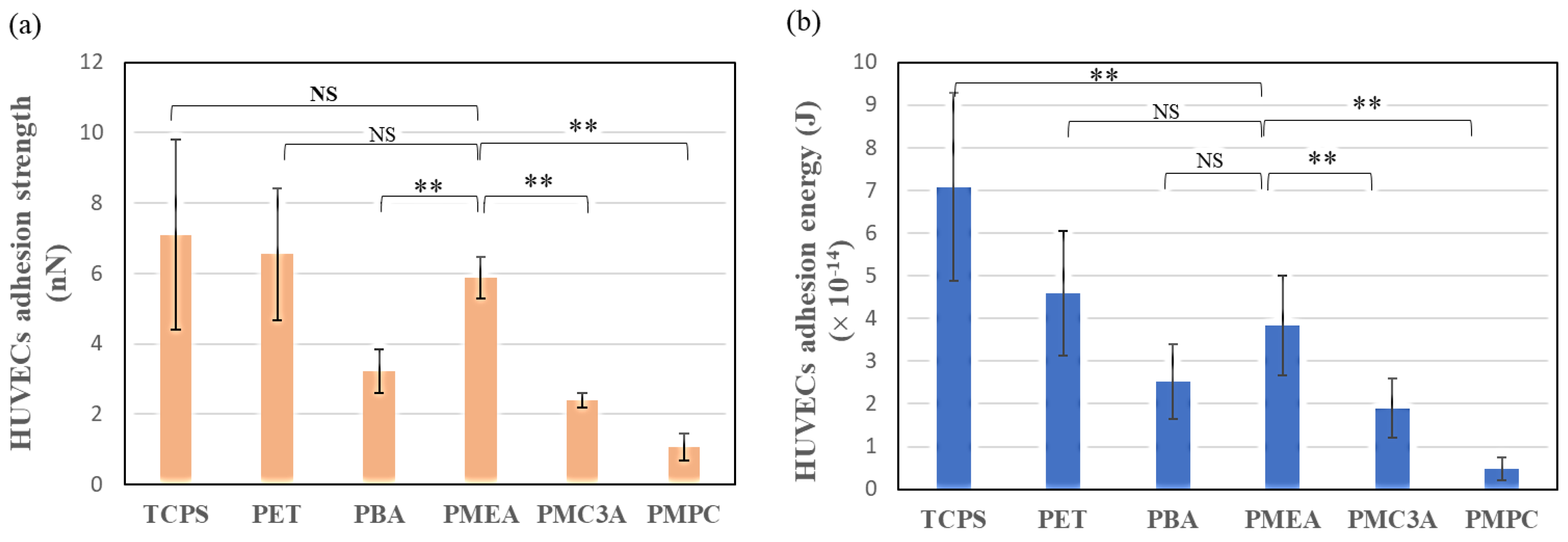
3.3. Measurement of Cell–Substrate Interaction Behavior by SCFS
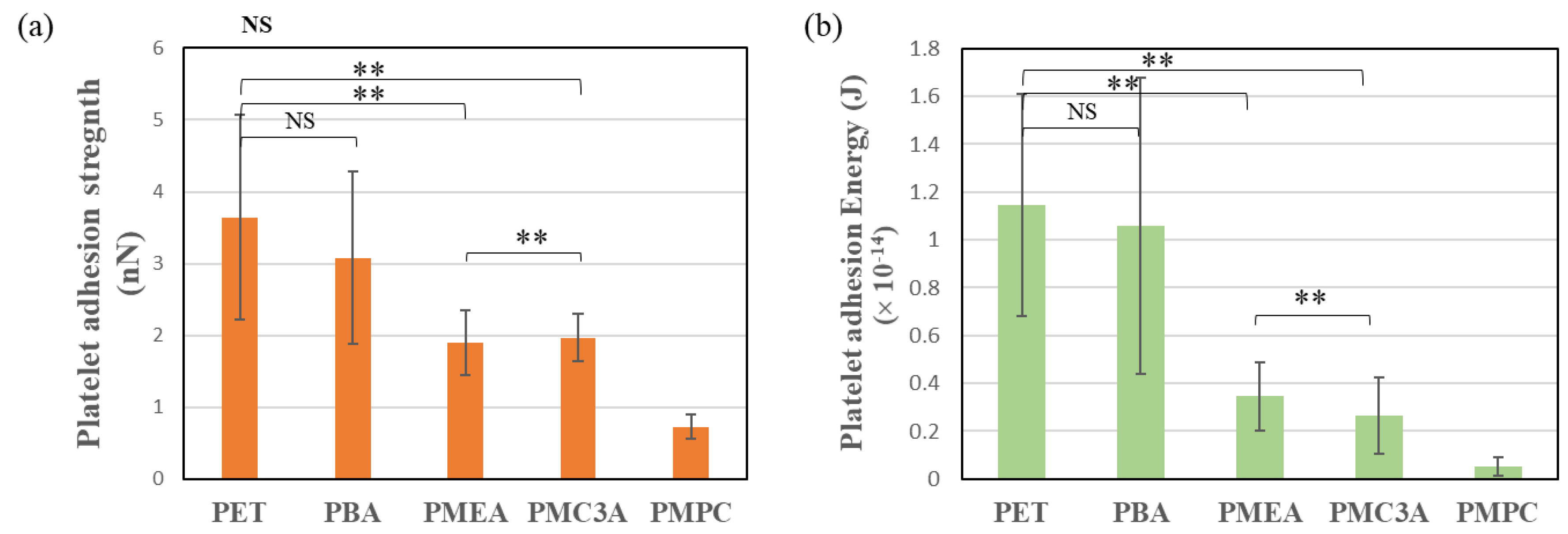
3.4. Human Platelet Adhesion Test
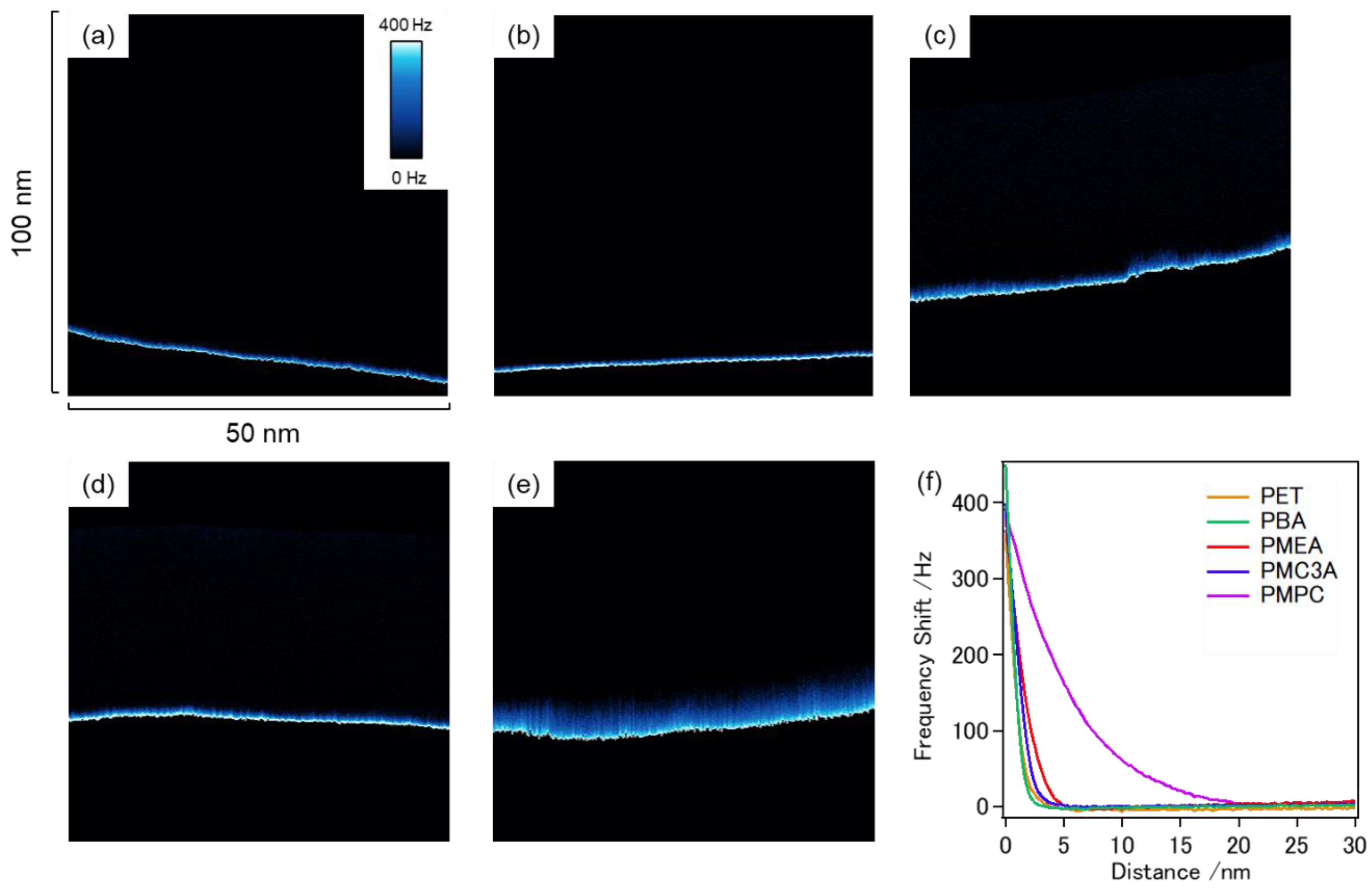
3.5. FM-AFM Observation of Coated Polymer Surfaces
3.6. Relationship between IW and Cell Adhesion Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials science: An introduction to materials in medicine. MRS Bull. 2004, 31, 162–164. [Google Scholar]
- Tsuruta, T. Contemporary Topics in Polymeric Materials for Biomedical Applications. Adv. Polym. Sci. 1996, 126, 1–55. [Google Scholar] [CrossRef]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell. 2009, 5, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. Modelling approach in cell/material interactions studies. Biomaterials 2006, 27, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Motomura, T.; Kawada, M.; Anzai, T.; Kasori, Y.; Shiroya, T.; Shimura, K.; Onishi, M.; Mochizuki, A. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)-relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials 2000, 21, 1471–1481. [Google Scholar] [CrossRef]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Healthc. Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxidative Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future perspectives in small-diameter vascular graft engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Li, S. Advances in vascular tissue engineering. J. Med. Biomech. 2016, 31, E333–E339. [Google Scholar] [CrossRef]
- Tara, S.; Rocco, K.A.; Hibino, N.; Sugiura, T.; Kurobe, H.; Breuer, C.K.; Shinoka, T. Vessel Bioengineering. Circ. J. 2014, 78, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, A.; Hang, R.; Li, W.; Zhang, W.; Li, P.; Wang, G.; Bai, L.; Yu, X.F.; Wang, H.; Tong, L.; et al. Linker-free covalent immobilization of heparin, SDF-1α, and CD47 on PTFE surface for antithrombogenicity, endothelialization and anti-inflammation. Biomaterials 2017, 140, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Weidenbacher, L.; Müller, E.; Guex, A.G.; Zündel, M.; Schweizer, P.; Marina, V.; Adlhart, C.; Vejsadová, L.; Pauer, R.; Spiecker, E.; et al. In Vitro Endothelialization of Surface-Integrated Nanofiber Networks for Stretchable Blood Interfaces. ACS Appl. Mater. Interfaces 2019, 11, 5740–5751. [Google Scholar] [CrossRef]
- Noy, J.-M.; Chen, F.; Akhter, D.T.; Houston, Z.H.; Fletcher, N.L.; Thurecht, K.J.; Stenzel, M.H. Direct Comparison of Poly(ethylene glycol) and Phosphorylcholine Drug-Loaded Nanoparticles In Vitro and In Vivo. Biomacromolecules 2020, 21, 2320–2333. [Google Scholar] [CrossRef]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modifcation of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. II. Graft-polymerization onto fabric through 2-methacryloyloxyethyl isocyanate and interaction between fabric and platelets. Biomaterials 2000, 21, 327–333. [Google Scholar] [CrossRef]
- Park, H.H.; Sun, K.; Seong, M.; Kang, M.; Park, S.; Hong, S.; Jung, H.; Jang, J.; Kim, J.; Jeong, H.E. Lipid-Hydrogel-Nanostructure Hybrids as Robust Biofilm-Resistant Polymeric Materials. ACS Macro Lett. 2019, 8, 64–69. [Google Scholar] [CrossRef]
- Suhara, H.; Sawa, Y.; Nishimura, M.; Oshiyama, H.; Yokoyama, K.; Saito, N.; Matsuda, H. Efficacy of a new coating material, PMEA, for cardiopulmonary bypass circuits in a porcine model. Ann. Thorac. Surg. 2001, 71, 1603–1608. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Interaction between water and hydrophilic polymers. Thermochim. Acta 1998, 308, 3–22. [Google Scholar] [CrossRef]
- Morita, S.; Tanaka, M.; Ozaki, Y. Time-resolved in situ ATR-IR observations of the process of sorption of water into a poly(2-methoxyethyl acrylate) film. Langmuir 2007, 23, 3750–3761. [Google Scholar] [CrossRef]
- Miwa, Y.; Ishida, H.; Saitô, H.; Tanaka, M.; Mochizuki, A. Network structures and dynamics of dry and swollen poly(acrylate)s. Characterization of high- and low-frequency motions as revealed by suppressed or recovered intensities (SRI) analysis of 13C NMR. Polymer 2009, 50, 6091–6099. [Google Scholar] [CrossRef]
- Tanaka, M.; Motomura, T.; Ishii, N.; Shimura, K.; Onishi, M.; Mochizuki, A.; Hatakeyama, T. Cold crystallization of water in hydrated poly(2-methoxyethyl acrylate) (PMEA). Polym. Int. 2000, 49, 1709–1713. [Google Scholar] [CrossRef]
- Hoshiba, T.; Nikaido, M.; Tanaka, M. Characterization of the attachment mechanisms of tissue-derived cell lines to blood-compatible polymers. Adv. Healthc. Mater. 2014, 3, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Yoshihiro, A.; Tanaka, M. Evaluation of initial cell adhesion on poly (2-methoxyethyl acrylate) (PMEA) analogous polymers. J. Biomater. Sci. Polym. Ed. 2017, 28, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of human umbilical vein endothelial cells (HUVEC) as a model to study cardiovascular disease: A review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef]
- Onat, D.; Brillon, D.; Colombo, P.C.; Schmidt, A.M. Human vascular endothelial cells: A model system for studying vascular inflammation in diabetes and atherosclerosis. Curr. Diabetes Rep. 2011, 11, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Schleger, C.; Platz, S.J.; Deschl, U. Development of an in vitro model for vascular injury with human endothelial cells. ALTEX Altern. Anim. Exp. 2004, 21, 12–19. [Google Scholar]
- Vion, A.C.; Ramkhelawon, B.; Loyer, X.; Chironi, G.; Devue, C.; Loirand, G.; Tedgui, A.; Lehoux, S.; Boulanger, C.M. Shear stress regulates endothelial microparticle release. Circ. Res. 2013, 112, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Fearon, I.M.; Gaça, M.D.; Nordskog, B.K. In vitro models for assessing the potential cardiovascular disease risk associated with cigarette smoking. Toxicol. In Vitro 2013, 27, 513–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, C.; Aoki, M.; Tanaka, M. Blood-compatible poly(2-methoxyethyl acrylate) for the adhesion and proliferation of endothelial and smooth muscle cells. Colloids Surf. B Biointerfaces 2016, 145, 586–596. [Google Scholar] [CrossRef]
- Asai, F.; Seki, T.; Hoshino, T.; Liang, X.; Nakajima, K.; Takeoka, Y. Silica Nanoparticle Reinforced Composites as Transparent Elastomeric Damping Materials. ACS Appl. Nano Mater. 2021, 4, 4140–4152. [Google Scholar] [CrossRef]
- Asai, F.; Seki, T.; Sugawara-Narutaki, A.; Sato, K.; Odent, J.; Coulembier, O.; Raquez, J.M.; Takeoka, Y. Tough and Three-Dimensional-Printable Poly(2-methoxyethyl acrylate)-Silica Composite Elastomer with Antiplatelet Adhesion Property. ACS Appl. Mater. Interfaces 2020, 12, 46621–46628. [Google Scholar] [CrossRef]
- Watanabe, K.; Miwa, E.; Asai, F.; Seki, T.; Urayama, K.; Nakatani, T.; Fujinami, S.; Hoshino, T.; Takata, M.; Liu, C.; et al. Highly Transparent and Tough Filler Composite Elastomer Inspired by the Cornea. ACS Mater. Lett. 2020, 2, 325–330. [Google Scholar] [CrossRef]
- Kobayashi, S.; Wakui, M.; Iwata, Y.; Tanaka, M. Poly(ω-methoxyalkyl acrylate)s: Nonthrombogenic Polymer Family with Tunable Protein Adsorption. Biomacromolecules 2017, 18, 4214–4223. [Google Scholar] [CrossRef]
- Nishida, K.; Anada, T.; Kobayashi, S.; Ueda, T.; Tanaka, M. Effect of bound water content on cell adhesion strength to water-insoluble polymers. Acta Biomater. 2021, 134, 313–324. [Google Scholar] [CrossRef]
- Friedrichs, J.; Legate, K.R.; Schubert, R.; Bharadwaj, M.; Werner, C.; Müller, D.J.; Benoit, M. A practical guide to quantify cell adhesion using single-cell force spectroscopy. Methods 2013, 60, 169–178. [Google Scholar] [CrossRef]
- Sato, K.; Kobayashi, S.; Kusakari, M.; Watahiki, S.; Oikawa, M.; Hoshiba, T.; Tanaka, M. The Relationship between Water Structure and Blood Compatibility in Poly(2-methoxyethyl Acrylate) (PMEA) Analogues. Macromol. Biosci. 2015, 15, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kobayashi, S.; Murakami, D.; Aratsu, F.; Kashiwazaki, A.; Hoshiba, T.; Fukushima, K. Design of Polymeric Biomaterials: The “Intermediate Water Concept”. Bull. Chem. Soc. Jpn. 2019, 92, 2043–2057. [Google Scholar] [CrossRef]
- Morita, S.; Tanaka, M.; Kitagawa, K.; Ozaki, Y. Hydration structure of poly(2-methoxyethyl acrylate): Comparison with a 2-methoxyethyl acetate model monomer. J. Biomater. Sci. Polym. Ed. 2010, 21, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.T.; Sonoda, T.; Urata, S.; Koguchi, R.; Kobayashi, S.; Tanaka, M. Elucidating the Feature of Intermediate Water in Hydrated Poly(ω-methoxyalkyl acrylate)s by Molecular Dynamics Simulation and Differential Scanning Calorimetry Measurement. ACS Biomater. Sci. Eng 2020, 6, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Zografi, G. The Relationship Between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef]
- Hoshiba, T.; Orui, T.; Endo, C.; Sato, K.; Yoshihiro, A.; Minagawa, Y.; Tanaka, M. Adhesion-based simple capture and recovery of circulating tumor cells using a blood-compatible and thermo-responsive polymer-coated substrate. RSC Adv. 2016, 6, 89103–89112. [Google Scholar] [CrossRef]
- Murakami, D.; Kobayashi, S.; Tanaka, M. Interfacial Structures and Fibrinogen Adsorption at Blood-Compatible Polymer/Water Interfaces. ACS Biomater. Sci. Eng. 2016, 2, 2122–2126. [Google Scholar] [CrossRef]
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nishida, K.; Baba, K.; Murakami, D.; Tanaka, M. Nanoscopic analyses of cell-adhesive protein adsorption on poly(2-methoxyethyl acrylate) surfaces. Biomater. Sci. 2022, 10, 2953–2963. [Google Scholar] [CrossRef]
- Mescola, A.; Canale, C.; Prato, M.; Diaspro, A.; Berdondini, L.; Maccione, A.; Dante, S. Specific Neuron Placement on Gold and Silicon Nitride-Patterned Substrates through a Two-Step Functionalization Method. Langmuir 2016, 32, 6319–6327. [Google Scholar] [CrossRef]
- Oropesa-Nuñez, R.; Mescola, A.; Vassalli, M.; Canale, C. Impact of experimental parameters on cell–cell force spectroscopy signature. Sensors 2021, 21, 1069. [Google Scholar] [CrossRef]
- Hozumi, K.; Otagiri, D.; Yamada, Y.; Sasaki, A.; Fujimori, C.; Wakai, Y.; Uchida, T.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Cell surface receptor-specific scaffold requirements for adhesion to laminin-derived peptide-chitosan membranes. Biomaterials 2010, 31, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Tersteeg, C.; Roest, M.; Mak-Nienhuis, E.M.; Ligtenberg, E.; Hoefer, I.E.; de Groot, P.G.; Pasterkamp, G. A fibronectin-fibrinogen-tropoelastin coating reduces smooth muscle cell growth but improves endothelial cell function. J. Cell. Mol. Med. 2012, 16, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Colella, S.; Languino, L.R.; Balconi, G.; Corbascio, G.C.; Marchisio, P.C. Fibrinogen induces adhesion, spreading, and microfilament organization of human endothelial cells in vitro. J. Cell Biol. 1987, 104, 1403–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.B.; Grunkemeier, J.M.; Horbett, T.A. Human plasma fibrinogen adsorption and platelet adhesion to polystyrene. J. Biomed. Mater. Res. 1999, 44, 130–139. [Google Scholar] [CrossRef]
- Murakami, D.; Segami, Y.; Ueda, T.; Tanaka, M. Control of interfacial structures and anti-platelet adhesion property of blood-compatible random copolymers. J. Biomater. Sci. Polym. Ed. 2020, 31, 207–218. [Google Scholar] [CrossRef]
- Tanaka, M.; Mochizuki, A.; Ishii, N.; Motomura, T.; Hatakeyama, T. Study of blood compatibility with poly(2-methoxyethyl acrylate). Relationship between water structure and platelet compatibility in poly(2-methoxyethylacrylate-co-2-hydroxyethylmethacrylate). Biomacromolecules 2002, 3, 36–41. [Google Scholar] [CrossRef]
- Murakami, D.; Kitahara, Y.; Kobayashi, S.; Tanaka, M. Thermosensitive Polymer Biocompatibility Based on Interfacial Structure at Biointerface. ACS Biomater. Sci. Eng. 2018, 4, 1591–1597. [Google Scholar] [CrossRef]
- Ueda, T.; Murakami, D.; Tanaka, M. Analysis of interaction between interfacial structure and fibrinogen at blood-compatible polymer/water interface. Front. Chem. 2018, 6, 542. [Google Scholar] [CrossRef]
- Murakami, D.; Mawatari, N.; Sonoda, T.; Kashiwazaki, A.; Tanaka, M. Effect of the Molecular Weight of Poly(2-methoxyethyl acrylate) on Interfacial Structure and Blood Compatibility. Langmuir 2019, 35, 2808–2813. [Google Scholar] [CrossRef]
- Murakami, D.; Nishimura, S.n.; Tanaka, Y.; Tanaka, M. Observing the repulsion layers on blood-compatible polymer-grafted interfaces by frequency modulation atomic force microscopy. Mater. Sci. Eng. C 2021, 133, 112596. [Google Scholar] [CrossRef] [PubMed]
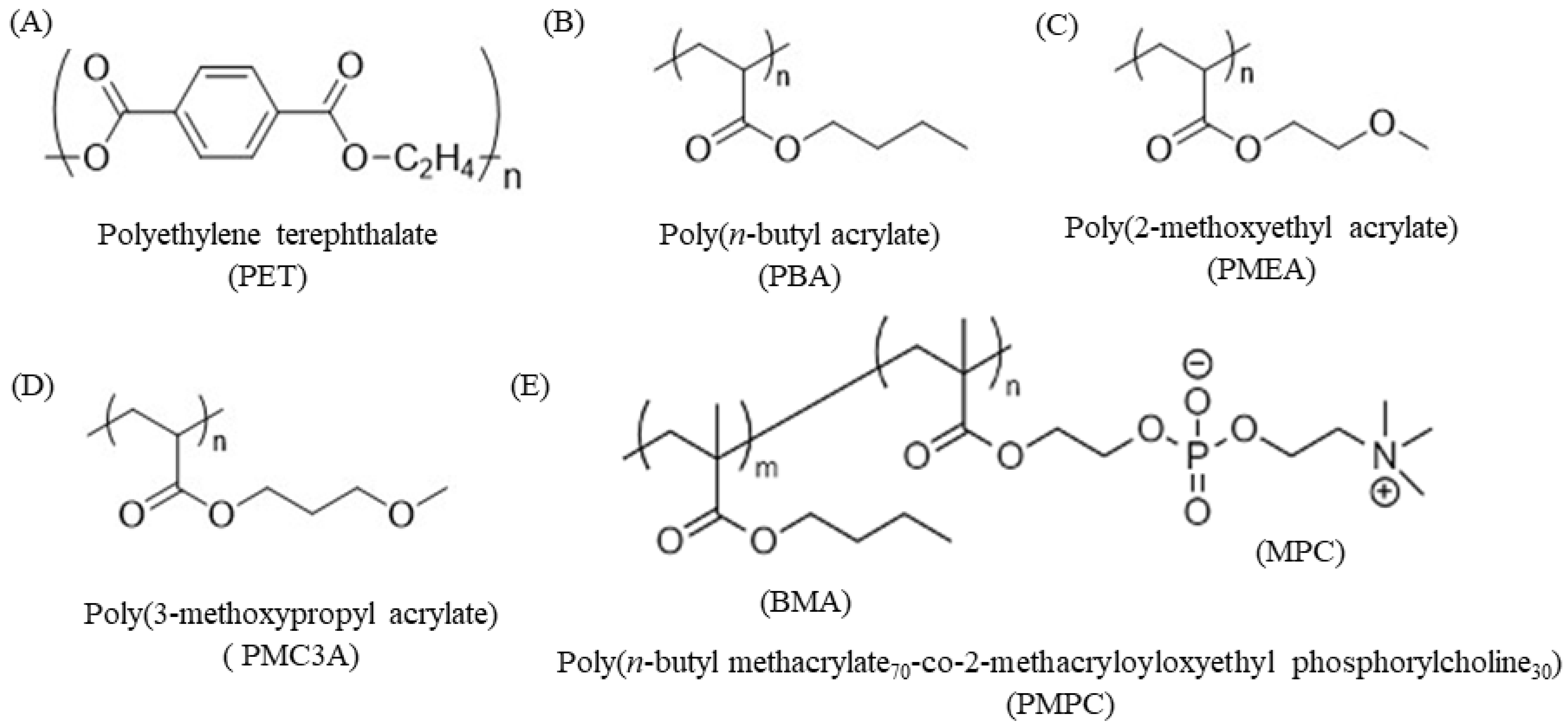




| Polymers | Mn | Mw/Mn | Tg Dry a | Tg Wet a | IW a,b | NFW a,b | FW c | EWC d |
|---|---|---|---|---|---|---|---|---|
| (kg/mol) | (°C) | (°C) | (wt%) | (wt%) | (wt%) | (wt%) | ||
| PBA | 62.8 | 1.41 | −47 | −48 | 0.31 | 0.45 | 0.54 | 1.3 |
| PMEA | 26.9 | 2.73 | −35 | −51 | 3.7 | 2.5 | 2.5 | 8.7 |
| PMC3A | 20.8 | 3.83 | −48 | −58 | 2.8 | 3.1 | 1.7 | 7.6 |
| PMPC | - | - | - | - | 11.11 | 33.33 | - | - |
| Polymers | CA [deg] | ||
|---|---|---|---|
| Sessile Water Drops | Captive Air Bubble | ||
| (30 s) | (30 s) | 24 h | |
| PET | 73.3 (±0.9) | 125.5 (±2.2) | 125.4 (±0.5) |
| PBA | 83.8 (±1.9) | 126.7 (±2.8) | 125.0 (±1.7) |
| PMEA | 44.3 (±2.1) | 134.0 (±0.9) | 132.9 (±1.8) |
| PMC3A | 52.1 (±0.5) | 126.9 (±1.0) | 127.8 (±0.7) |
| PMPC | 108.9 (±0.5) | 152.4 (±2.9) | 150.0 (±3.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.A.; Murakami, D.; Tanaka, M. Cell Adhesion Strength Indicates the Antithrombogenicity of Poly(2-methoxyethyl acrylate) (PMEA): Potential Candidate for Artificial Small-Diameter Blood Vessel. Surfaces 2022, 5, 365-382. https://doi.org/10.3390/surfaces5030027
Haque MA, Murakami D, Tanaka M. Cell Adhesion Strength Indicates the Antithrombogenicity of Poly(2-methoxyethyl acrylate) (PMEA): Potential Candidate for Artificial Small-Diameter Blood Vessel. Surfaces. 2022; 5(3):365-382. https://doi.org/10.3390/surfaces5030027
Chicago/Turabian StyleHaque, Md Azizul, Daiki Murakami, and Masaru Tanaka. 2022. "Cell Adhesion Strength Indicates the Antithrombogenicity of Poly(2-methoxyethyl acrylate) (PMEA): Potential Candidate for Artificial Small-Diameter Blood Vessel" Surfaces 5, no. 3: 365-382. https://doi.org/10.3390/surfaces5030027
APA StyleHaque, M. A., Murakami, D., & Tanaka, M. (2022). Cell Adhesion Strength Indicates the Antithrombogenicity of Poly(2-methoxyethyl acrylate) (PMEA): Potential Candidate for Artificial Small-Diameter Blood Vessel. Surfaces, 5(3), 365-382. https://doi.org/10.3390/surfaces5030027







