1. Introduction
Since prehistoric times, artists have sought ways to harness naturally occurring materials to either improve colorant adhesion to substrates or to fine tune the shade of the final artistic product. Milk appears to have been used as an artist’s material for a long time as shown by some recent archaeological evidence. Residue amounts of bovid milk peptides were found co-present with ochre on a stone tool dated to 49,000 years ago in a cave at Sibudu in South Africa [
1]. Additionally, peptides from both cow and goat milk were also detected along with colorants on the fragments of the destroyed Buddha statues at Bāmiyān, Afghanistan created between the 5th and 7th century C.E. [
2]. Because of its adhesive properties, casein has been used along with egg, animal glue, polysaccharide gums, plant resins, fats or oils, and beeswax as an organic binder for pigments on sculptures and paintings [
3].
In the textile field, dyers have long experimented with mordants and other materials with adhesive properties [
4] as dye binders to fabric. In doing so, they would have undoubtedly noticed any subtle effects of mordants on hue modification. Furthermore, it has recently been shown from studies of papyral documents from the early centuries C.E. in the Near East that dyers in antiquity had a keen need to subtly vary the hue of their products in order to satisfy the demands of their clients [
5]. This might have been a motivating factor for dyers to broaden the type of additives to their dyebaths to include material such as milk. However, it is unknown how commonplace the use of milk was in dyeing practice among textile artisans throughout history.
The Leyden and Stockholm papyri [
6] are collections of documents recovered in the 19th century from burial sites near Thebes in central Egypt, a portion of which are now preserved by the Museum of Antiquities at the University of Leyden and the remaining by the Swedish Royal Academy of Antiquities. Written in Greek and thought to be compiled between the 3rd and 4th century C.E., the documents are short recipes providing generalized instructions to artisans to perform a variety of crafts ranging from coloring of gemstones, cleaning and purifying pearls, creating imitation precious metals, and mordanting and dyeing wool. An English translation was published by Earle Radcliffe Caley in 1932 [
6]. Among the 110 entries, Stockholm recipe 109 (S109) specifies a particular method that includes the use of a mordant in wool yarn preparation along with goat milk with orchil dyeing [
6]:
“109. Dyeing in Phoenician Color with Archil
Roll up the wool and sift ashes over it. Separate the rolls in a
convenient manner and again shift ashes over them until the wool
becomes clean and branny. Shake it out on the following day and
rinse it out. After the washing, boil it with 6 chus of salt water for
each mina of wool, mix in half a mina of alum and mordant the
wool therein in the way mentioned. Rinse it out. Then cook, in
rainwater, until it boils, three times as much archil as the weight of
the wool. Pour in goat’s milk and stir up. Put the wool in and stir
again until the color is thoroughly soaked in. Then take the wool
out, rinse it and dry it, but in doing so protect it from smoke.”
The requirements are unusual for orchil, which is a direct dye without the need for mordants [
4]. Goat milk is thought to improve dye fastness [
4], and the use of mordants and milk could be hypothesized to improve the color, speed of the dyeing process, or to conserve labor. Several years ago, Mamie’s Schoolhouse, a studio for natural dyeing at Cape Breton Island, Nova Scotia, Canada, initiated a project to implement the dyeing protocols specified in recipes of the Leyden and Stockholm papyri with a group of contemporary natural dyers [
7]. One of the present authors (I.W.) who participated in the project embarked on an effort aiming to explore the effects of milk in orchil dyeing as described in S109.
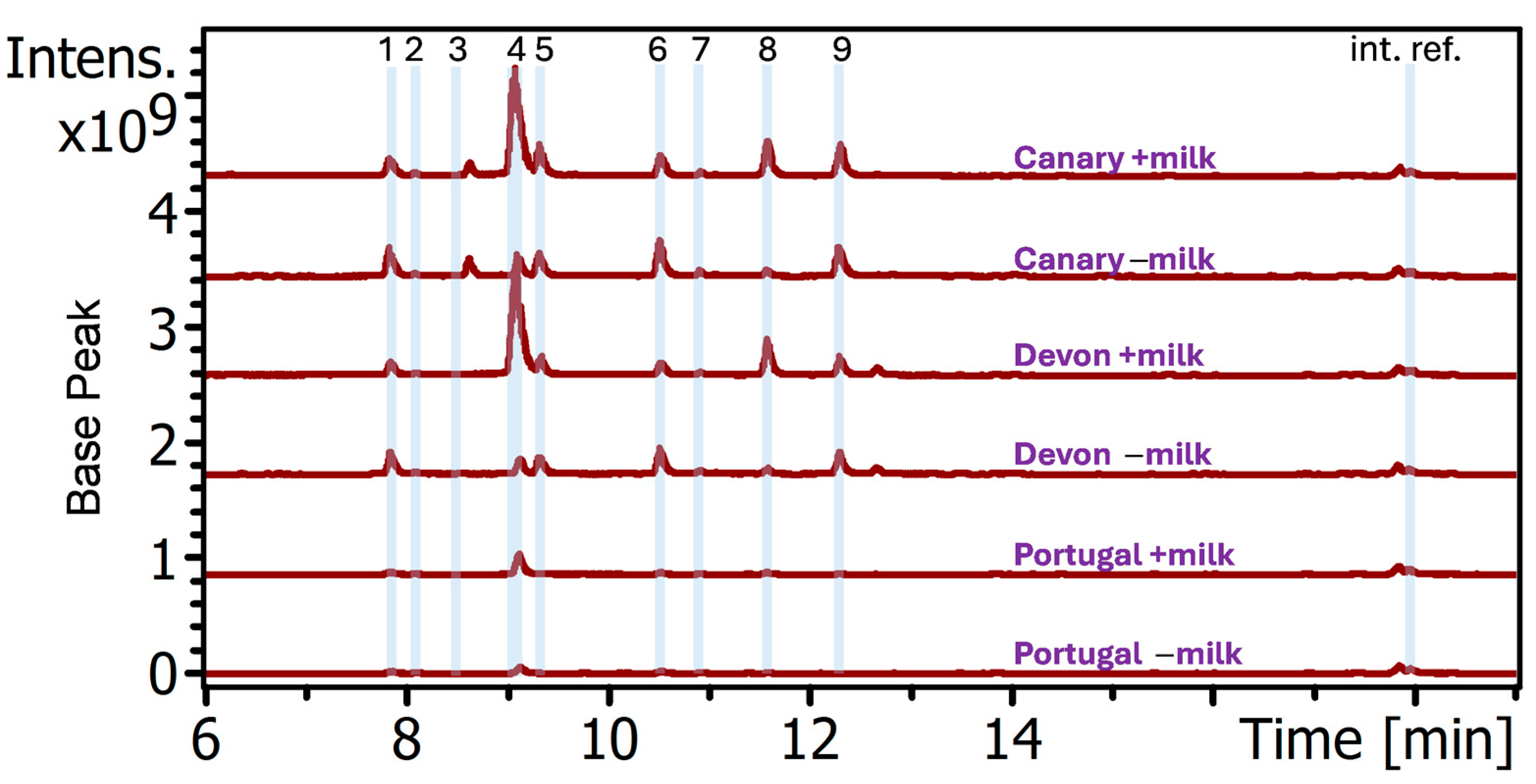
To this end, the sparse instructions of recipe S109 were interpreted to create protocols for orchil dyeing with goat milk using present-day materials. Between 2022 and 2023, several sets of wool were prepared identically with alum mordant and were subsequently dyed with and without goat milk added to orchil dyebaths prepared separately with lichens from three different sources. The yarns dyed in orchil baths with milk were found to be visually darker for all three preparations, irrespective of lichen origin.
Since the chemical basis of milk’s effects on orchil dyeing, as far as we know, has not been described, for this study, extracts of the dyed yarns were made and subjected to analysis by liquid chromatography-diode-array-detector-mass spectrometry (LC-DAD-MS). Given the authors’ previous experience in detection of the group of nine “primary” orceins shown in
Figure 1 [
8] that are typically detected on orchil dyed textiles [
9,
10,
11], the goal of this preliminary study was to focus on these nine orceins and assess how milk affected their internal relative ratios in the extracts. Secondarily, since wool “fermentation” has previously been found to cause wool cuticle damage resulting in enhanced dye uptake [
12], preliminary investigations by scanning electron microscopy were conducted on some samples of the orchil dyed yarns to see if milk in the dyebath led to morphological changes in the wool fibers that might indicate cutaneous breakdown leading to better dye uptake.
2. Materials and Methods
2.1. Chemicals
Oxalic acid, dihydrate (99+% extra pure,129601000) was obtained from Acros Organics, Morris Plains, NJ, USA. Anhydrous pyridine (99.5+%, 43799) was purchased from Alfa Aesar, Ward Hill, MA, USA. Acetonitrile (Optima LC/MS grade, A944-1) and formic acid (Optima LC/MS grade, A117-50) were from Fisher Scientific, Fair Lawn, NJ, USA. Tectochrysin (phyproof®, 83915) was sourced from Phytolab Gmbh & Co., Vestenbergsgreuth, Germany. Orcein described as consisting of numerous compuonds (synthetic, 2140863066) was obtained from TCI, Portland, OR, USA. Water for chemical analysis (18 MΩ) was purified through a Milli-Q® Direct 8 Water Purification System outfitted with an LC-Pak cartridge (MilliporeSigma, Burlington, MA, USA).
2.2. Dyestuffs and Materials Used in Dyeing
Lichens used in this work are pictured in
Figure 2.
Roccella canariensis from Lanzarote, one of the Canary Islands (hitherto referred to as lichen Canary), was provided courtesy of Juan Cazorla.
Lasallia pustulata was collected both from Devon, UK, by one of the authors (I.W.) and from Portugal by an anonymous donor (hitherto referred to as lichen Devon and lichen Portugal, respectively).
Household ammonia (Kleen Off) and pasteurized full cream goat milk (Waitrose), respectively, were purchased from the Co-op, in Hatherleigh and Waitrose in Okehampton, Devon, UK. Urine and hearth ash were from anonymous donors, and alum (potassium aluminum sulfate) was purchased from DT Craft and Design, Sale, Greater Manchester. Universal Indicator Paper was from Zoic Paleo Tech, UK. Rainwater used for dye preparation and in all the dyebaths was collected from roof run-off.
2.3. Dyeing
The vagueness of the recipe S109 required some interpretation using knowledge and experience in craft dyeing by one of the authors (I.W.) to produce the workable dyebaths. Orchil dyes were prepared by soaking 5 g of finely ground lichen in 500 mL of either stale urine or ammonia:rainwater (1:3) at ambient temperature (16 °C) with periodic agitation until the purple color was formed. Undyed, commercial two-ply woolen yarn from the same cone was used for all sample preparations. The yarn was prepared with sea salt and ashes, mordanted with alum, rinsed, and wetted out as needed prior to dyeing.
Focusing on addressing the effects of milk on dyeing, two groups of dyeings were prepared for each lichen species, with and without milk added to the dyebath for different durations as summarized in
Table 1.
For dyeing with milk, an aliquot of 40 mL of each dye was mixed with 20 mL of goat milk. For dyeing without milk, the same volume of dye was used without further addition. Dyeings were performed at ambient temperature (16 °C), a decision based on personal experience in dyeing and knowledge of contemporary Bedouin practices. For each condition, yarns of 15 cm length were soaked in dyebaths for the indicated time, washed extensively with water, and dried. For the timed dyeings listed in group 2, one yarn of 15 cm in length was used with (+) and another without (−) goat milk for all the time points. After the indicated days in the dyebath, the yarn was removed from each dyebath, approximately 2 cm was cut from each yarn, washed, dried, and the remaining yarn returned to the dyebath. The process was repeated until the end of 28 days. The pH of the dyebaths was measured with Universal Indicator Paper at the end of the dyeing period only for those in group 1 as indicated in the above
Table 1.
2.4. Dye Extraction
Depending on dye content, between 0.5 and 2 mg of yarn was extracted. For orchil dyed yarns, extraction was performed at 80 °C for 1 h in 0.50 mL MeOH-water (1:1) containing 10% formic acid and 4 mM oxalic acid. A suitable amount of tectochrysin was added to track recovery similar to the approach used by Villela et al. [
13]. The unusually high formic acid concentration was needed to overcome issues relating to analytical sample dissolution created by the presence of milk. The extracts in group 1 were dried under vacuum at 4 °C and redissolved in 100 µL MeOH-water(1:1) containing 10% formic acid for analysis. Extracts in group 2 were analyzed without further manipulation. Between 10 and 25 µL of each sample was analyzed by LC-DAD-MS.
2.5. LC-DAD-MS
All dye analyses were performed on a Bruker instrument consisting of an Elute autosampler, UPLC pump, column heater, DAD, and an amaZon Speed ion trap MS equipped with electrospray ionization (ESI). LC separation of orchil dye components was effected using a Kinetex 1.7 µm C18 100 Å 100 × 2.1 mm column eluted with an aqueous-acetonitrile (ACN) system containing 0.1% formic acid at 0.4 mL/min and 40 °C using a linear ACN gradient from 15–55% over 20 min.
For analyte detection, the DAD was set to scan the range 200–800 nm at 1 nm bandwidth at 10 Hz. For MS, ESI used nitrogen gas both as nebulizer gas at 40.0 psi and as drying gas at 12 L/min and 160 °C; ion capillary was set at 4500 V with end plate offset at 500 V. The ion trap was set to operate in the ultrascan mode and positive ionization at 32,500 m/z per sec, covering the m/z range 70–1500, and tuned at m/z 350, together with ion charge control limited at 70,000 or maximum accumulation time 5 ms, and reporting the average of 3 scans.
2.6. Data Analysis
To evaluate the effect of the milk, the relative amounts of the nine orceins were determined. Each of the analytes shown in
Figure 1 were identified by their mass and UV─vis spectra as performed previously [
8]. For quantitation, the extracted ion chromatogram of the monoisotopic ion of each analyte was displayed and the identified peaks integrated using the “compound manually” feature in the Bruker Data Analysis software package version 6.2.
2.7. Scanning Electron Microscopy (SEM)
The morphologies of dyed fibers were examined using a Zeiss EVO MA15 scanning electron microscope operated in variable pressure mode at 45 Pa of room air. A variable pressure secondary electron detector (VPSE) was used to acquire images of uncoated yarn fibers mounted on a graphite stub using carbon tape. The beam was operated at an accelerating voltage of 5 keV, 500 Pa current, and a sample working distance of 9.5 mm. The SEM was controlled using Zeiss SmartSEM software v7. Four locations on each sample were randomly selected at 70× magnification for high resolution imaging at 1200×.
4. Discussion
This work presents preliminary data showing that the presence of milk in orchil dyebaths as described in the Stockholm Papyrus recipe 109 selectively enhanced the binding to wool yarns of two of the nine primary orceins. Moreover, dyeing proceeded for 28 days under a nonsterile condition in nutrient-rich dyebaths containing ammonia and milk, during which time the milk curdled. Therefore, the observed selective enhancement of dye binding to wool could be due to a combination of multiple physical, chemical, and biological factors. Since as far as we know, this is the first chemical study of the effects of milk in orchil dyeing, it is unknown which factors are responsible for the enrichment in wool binding of selective orceins. The relevance of some of these factors are discussed below along with hypotheses presented for the possible mechanism involved intended for framing future studies.
4.1. Formation of Orcein–Protein Complex
One possibility is the formation of certain orcein complexes involving milk components that in turn are preferentially deposited on the wool. Given that milk is a complicated mixture consisting of fat, proteins, and non-protein nitrogen fractions such as vitamins and peptides [
14], each fraction could contain entities having some effects contributing to increased dye binding to wool.
While the role of milk fat, lactose, and other small molecules cannot be ruled out, it may be argued that given the presence of multiple hydroxyl, amino, and imino substituents on a phenozaxone scaffold, orceins are not likely to be sufficiently hydrophobic to form tight complexes with fat. Furthermore, orcein complexes with hydrophilic small molecules such as lactose, if formed, would likely be removed by washing due to the expected water solubility. Therefore, the contribution of fat and small molecules to the observed enhanced orcein binding to wool in the presence of milk may not be important.
Given that orceins dye wool and are used in histology to stain protein targets such as elastin [
15], the observed color enrichment mediated by milk proteins seems more likely. The data obtained show that milk enhanced yarn binding involved selectively only two of the nine orceins, namely orcein 4 (α-aminoorcein) and orcein 8 (α-hydroxyorcein), which are the smaller derivatives of the nine orceins (
Figure 1). This selectivity suggests that the binding entities may be proteins that contain certain nonrandom binding sites for these two orceins, perhaps engendered by the folding of their polypeptide. Additionally, the apparent correlation of higher orcein enrichment observed for yarns dyed darker may indicate a certain degree of concentration dependence. Specifically, the higher enrichment promoted by milk occurred in the darker colored yarns dyed by lichens Devon and Canary than in those by lichen Portugal, as described in
Section 3.1. It is generally assumed by dyers that color intensity of dyed yarns correlates with colorant concentration in the dyebath. Therefore, the yarns dyed lightly (i.e., the yarns dyed by lichen Portugal) would have been performed in baths of lower orcein concentration than the yarns dyed intensely (i.e., those dyed by lichen Devon and Canary). It may be inferred that lower orcein concentrations in the lichen Portugal would lead to lower amounts of orcein–protein complexes formed and thus to a smaller enrichment on the yarn as was observed. This would also be consistent with what is expected for mass action driven ligand binding to proteins.
In both cow and goat milk, 20% of total proteins are represented by the eight whey proteins and the remaining 80% are made up of the four homologous polypeptides α-S1, α-S2, β-, and ĸ-casein [
16,
17]. These four caseins in raw milk predominantly associate with each other to form micelles [
16,
17,
18]. Aside from the role of binding colloidal calcium phosphate, casein micelles can reversibly entrap drugs and many other bioactive molecules [
19,
20,
21]. Based on this property, caseins have been exploited as molecular delivery vehicles for bioactive compounds such as pharma- and nutraceuticals [
22,
23]. Furthermore, the micellar caseins are in equilibrium with a minor fraction of free caseins in solution and can also bind other non-casein proteins such as the denatured forms of whey thereby incorporating them into the micelle [
18]. Thus, the possible formation of some sort of orcein–casein complex(es) that may in turn bind to wool could lead to dye enrichment on the yarn. However, this may not be the sole mechanism of dye uptake enhancement.
4.2. Effects of Cutaneous Degradation
Another factor to consider is the cutaneous integrity of wool, which if it is intact would normally serve as a barrier to dye uptake into the fiber [
12]. Degradation of the wool cuticle could be caused by microbial growth in the nutrient-rich dyebath, leading to increased dye adsorption. A recent study of fibers from the 2400-year-old Pazyryk carpet [
12] found evidence that the cutaneous layer of wool was broken down, possibly due to the yarns having been intentionally subjected to fermentation prior to dyeing to facilitate dye penetration. Such fermentation techniques to promote color enhancement have continued to be employed by dyers well into modern times [
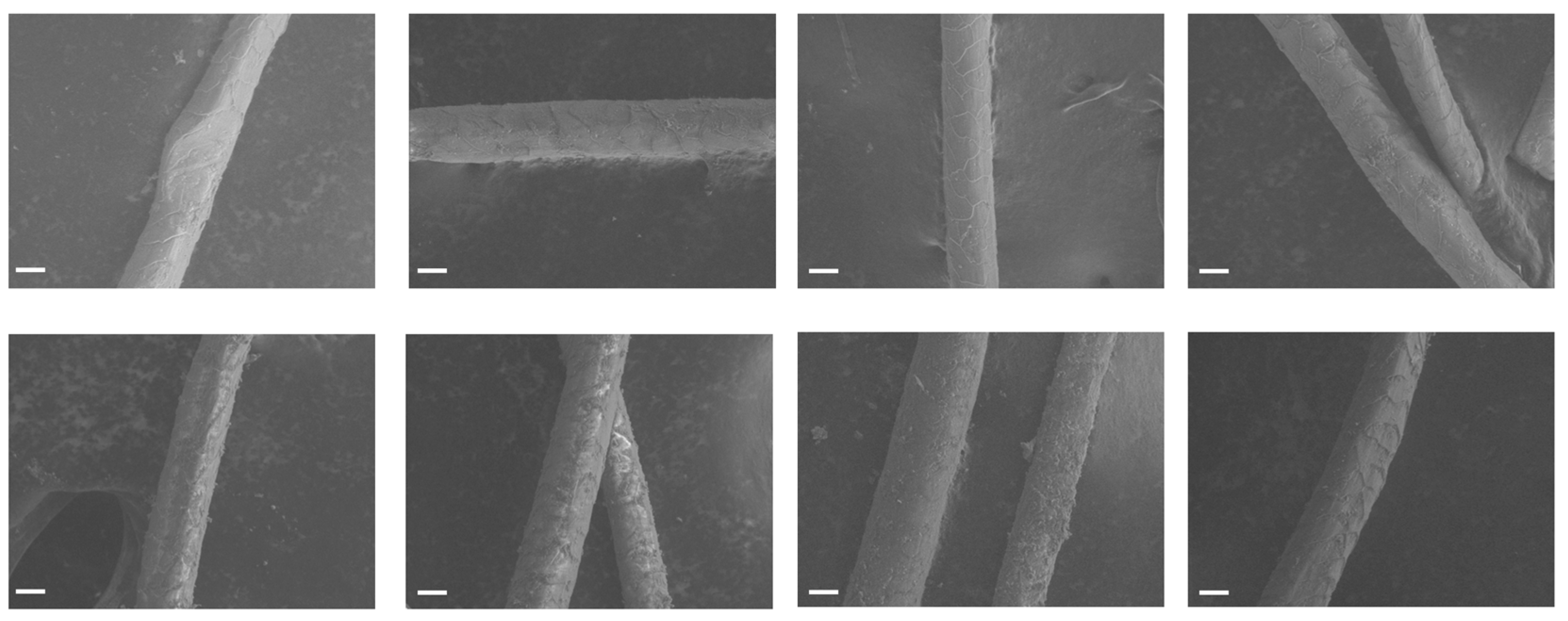
12]. Though not an exhaustive study, the SEM images reported here (
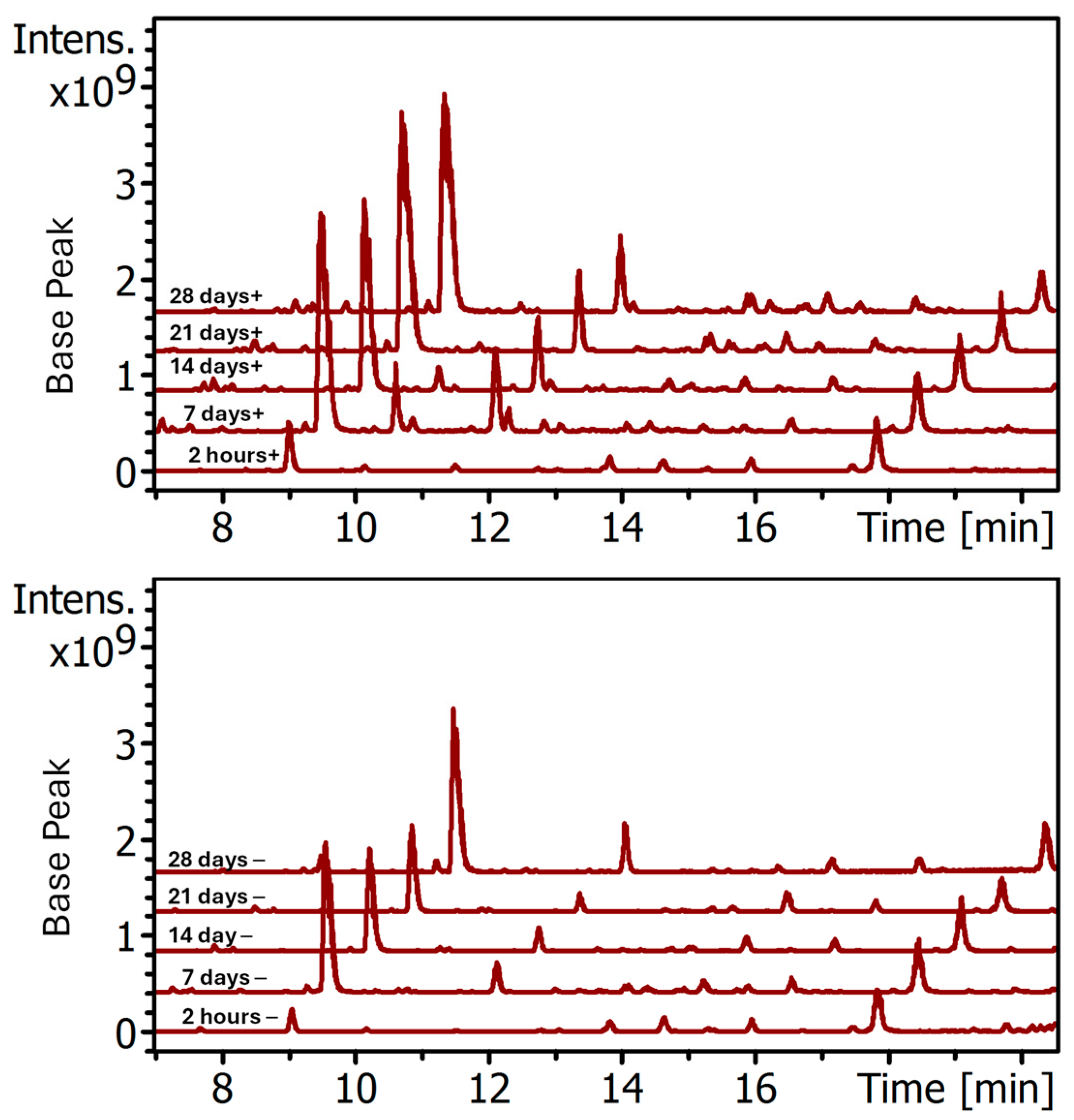
Figure 9) support this hypothesis that goat milk in the dye bath leads to further fiber biodeterioration allowing for better dye penetration into the yarns. However, it is hard to imagine that such breakdown of the cutaneous barrier allowed selective dye penetration of only two of the nine orceins, as has been observed here. Furthermore, the time course study reported in
Figure 6,
Figure 7 and
Figure 8 in
Section 3.2 shows that even within two hours, well before significant microbial growth could have taken place, the presence of milk already made a difference in the color of the yarn. Even so, prolonged soaking in a dyebath with microbial growth could result in compromise to cutaneous layer that may lower the barrier of the uptake of free and protein-bound orceins into the wool fiber and further contribute to the increased level of the two orceins in the wool fiber as well as the overall color intensity of the yarn.
4.3. Effect of pH
Yet another factor that may be relevant is the acidity of the dyebath, since this may affect the ionic state of the dyes resulting in changes in their affinity for wool. The final pH values of the dyebaths shown in
Table 1 for the samples described in
Section 3.1 were one to two units lower in the dyebaths containing milk. Recent studies have shown that the pH-dependent ionization profile for orcein 8 is complex, depending both on dye concentration and temperature [
24]. How pH affects the binding of orcein 8 on wool or other proteins is not readily predictable. On the other hand, for orcein 4, the transition from its mono-cation to neutral form and then to mono-anion are characterized by pKa 3.5 and 11.8, respectively [
25]. Given that the pH measured for the dyebaths were between 6 and 9, which are more than 2 pH units from the reported pKa values, this would argue that the observed pH difference alone is unlikely to have a significant impact on the affinity of orcein 4 to wool and other proteins.
4.4. Time-Dependent Change in Orcein Content in Dyebath
One additional potential factor to consider is that milk might alter the relative ratios of the orceins in the dye bath. In the histological applications of orceins, it has been noticed that the quality of a stained specimen is somewhat dependent on the age of the orcein stain [
26]. In the present study, the dyeing process went on for 28 days, during which time the relative dye component makeup could change. From the elegant work of Musso et al. as reviewed by Beecken [
9], it is now understood that the orcein colorants are derived from orcinol precursors hydrolyzed from various lichen esters during the extraction process by ammonia and oxygen. During the preparation of orchil it is unclear if all the precursors extracted from lichen were exhaustively consumed when the bath was used for dyeing. If substantial amounts of precursors remained in the dyebath, they might somehow be further converted by milk into α-aminoorcein and α-hydroxyorcein during the 28 days of dyeing that eventually led to the corresponding increase in amounts being deposited on the dyed wool. Interestingly, natural milk does contain lactoperoxidase [
27], but its role in furthering the oxidation of precursor intermediates preferentially to the two orcein remains to be investigated. However, the observation described in
Section 3.2 that the impact of milk in the dyebath is observable within a week would seem to contradict the likelihood of such age-related change in orcein content as a main cause of selective enhanced orcein dye binding to wool. For a better understanding, future studies should include analysis of the dyebath liquor along with the dyed yarns.
5. Conclusions
We have analyzed wool sample pairs dyed with orchil prepared from different sources of lichens with and without the use of goat milk as described in the Leyden and Stockholm papyri. Using either urine or ammonia as the nitrogen source, we reproducibly observed that milk present in the dyebath enhanced the selective binding of two of the nine primary orceins to wool. Additionally, microscopic analysis of the wool fiber suggests a certain degree of cutaneous damage that is more extensive in the milk treated sample. Recognizing the chemical complexity of the dyebath, it is possible that multiple factors may be responsible for the selective enhanced binding. The hypothesis proposed suggests the formation of dye complexes selectively involving orceins 4 and 8 with milk proteins such as the caseins, which could in turn bind to wool proteins. Additionally, these complexes, as well as free orceins, could better penetrate the yarn when the wool’s cutaneous structure has been further compromised in a bioactive milk containing dyebath.
Milk and textiles are still very much linked in modern times. In addition to adding milk to the dyebath, since the 1930s, milk proteins have been considered both as raw material for creating wool fiber substitutes as well as a modifier of different textiles to improve durability and tactile feel [
28,
29]. In particular, it was found that milk protein impregnation facilitated dye uptake onto the textile. Today, the use of milk in craft dyeing is still very much in vogue among natural dyers, not just animal milk, but also soy milk [
30,
31]. Further study of the interaction of dyes with milk protein and yarns is justified to provide a better understanding of the scientific basis behind the use of these ordinary food products in art and textiles, both historically and in modern times.