A Preliminary Study on the Efficacy of Essential Oils Against Trichoderma longibrachiatum Isolated from an Archival Document in Italy
Abstract
1. Introduction
2. Materials and Methods
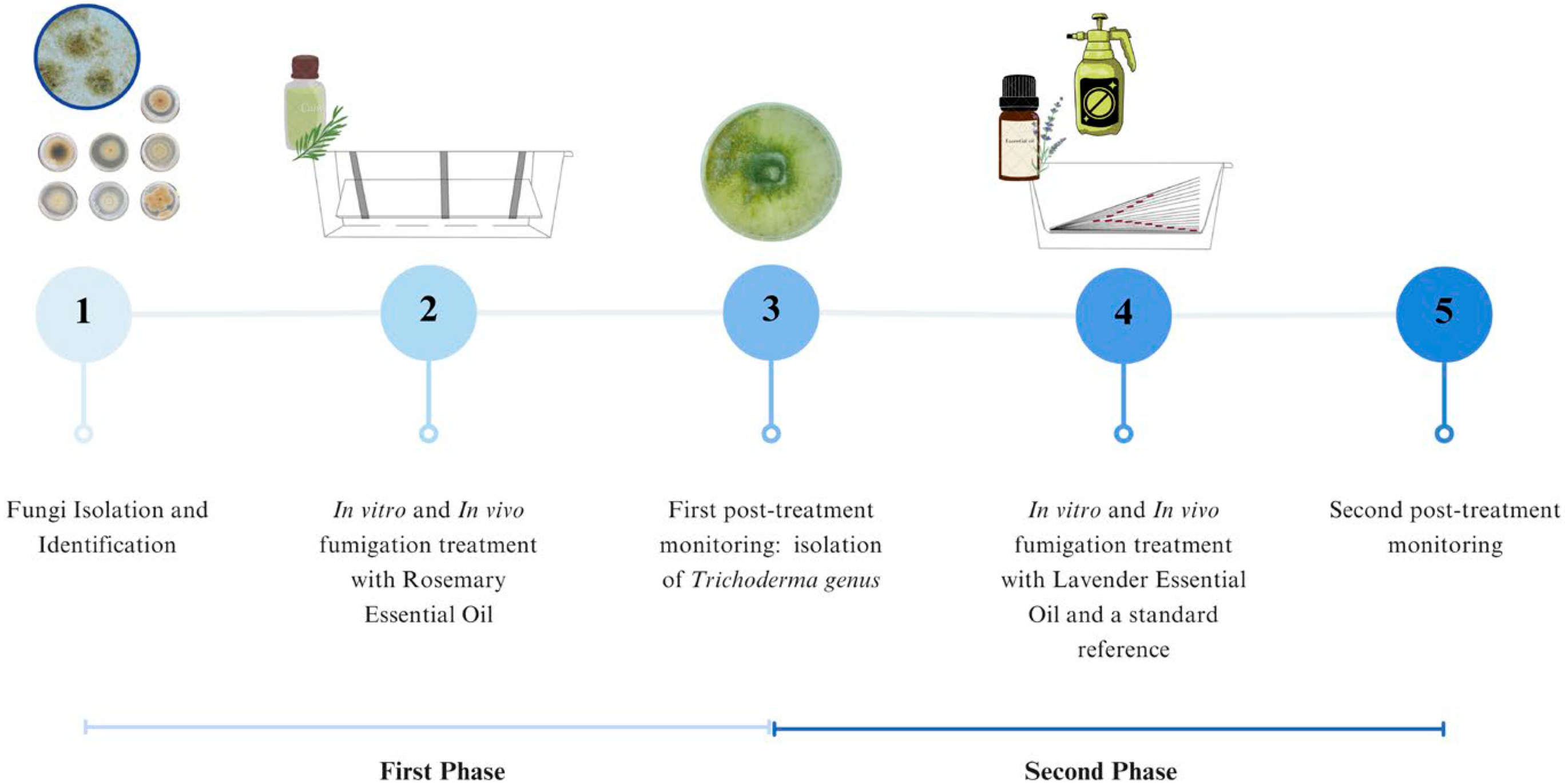
2.1. Experimental Timeline
2.2. Case Study: The Archival Journal
2.3. Fungal Sample Processing and Identification
2.4. In Vitro Antifungal Activity of Rosemary Oil
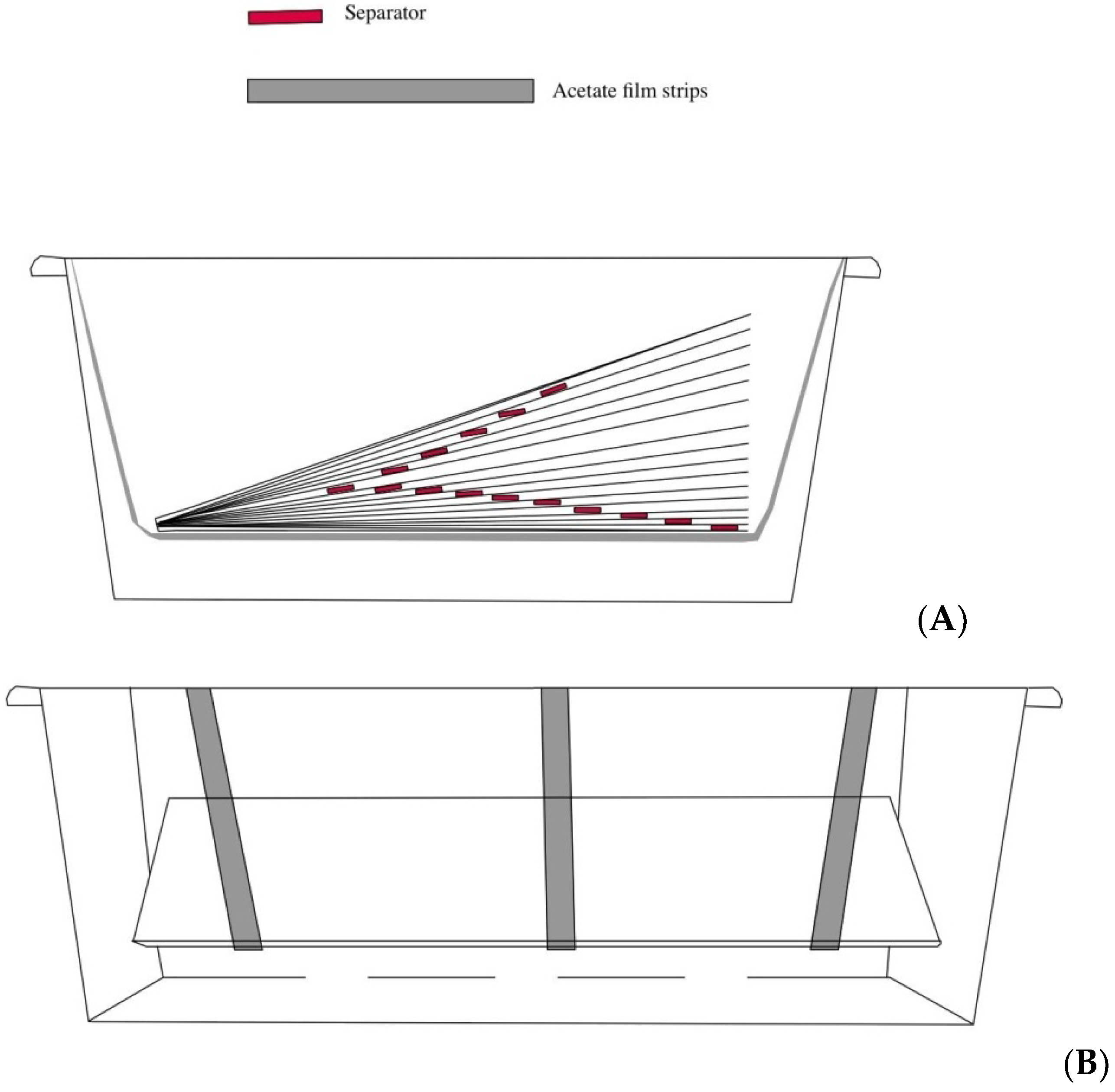
2.5. Evaluation of In Vivo Antifungal Properties of Rosemary Oil
2.6. Post-Treatment Isolation and Morphological Identification
2.7. DNA Extraction, Sequencing, and Phylogenetic Analysis
2.8. New Antifungal Treatment: The Essential Oils and the Commercial Standard
2.9. Colorimetric Analysis
3. Results and Discussion
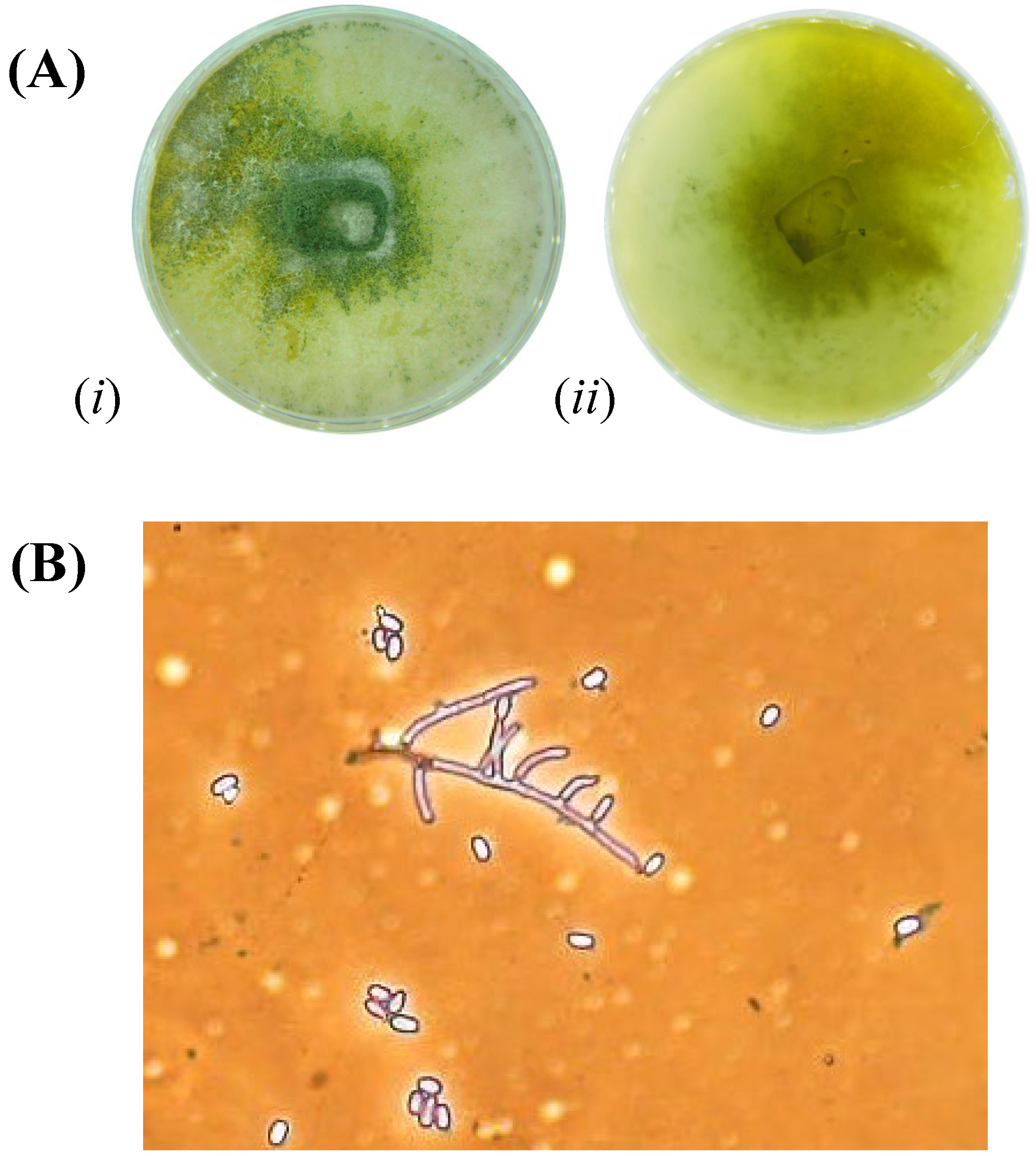
3.1. Fungi Isolation and Identification
3.2. In Vitro and In Vivo Antifungal Activity of Rosemary Oil
3.3. Trichoderma longibrachiatum Identification and Phylogenetic Analysis
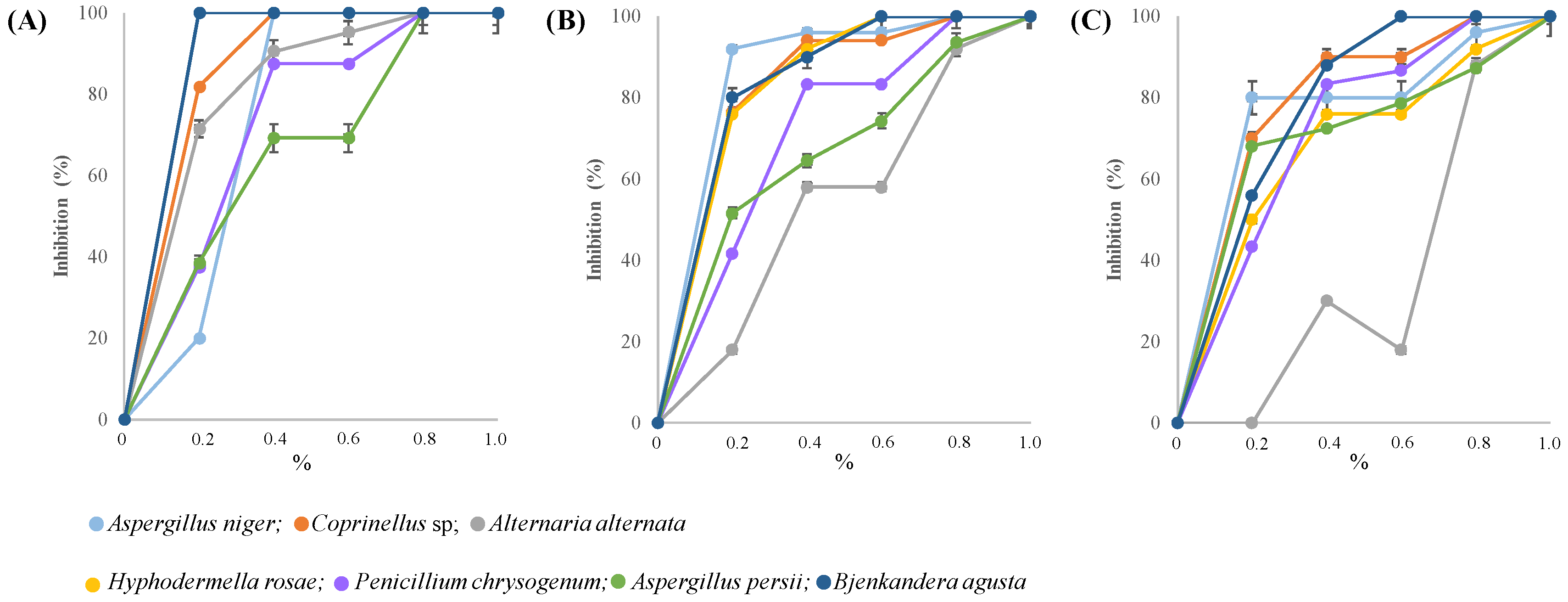
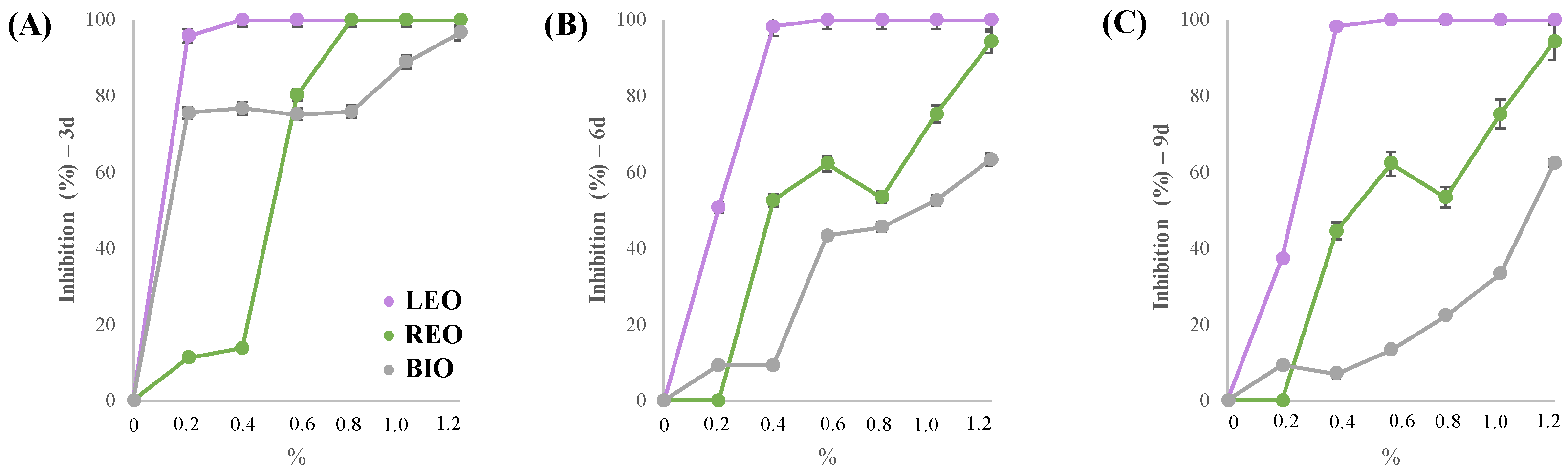
3.4. In Vitro and In Vivo Assessment of Trichoderma longibrachiatum Sensitivity
3.5. Microbiological Monitoring
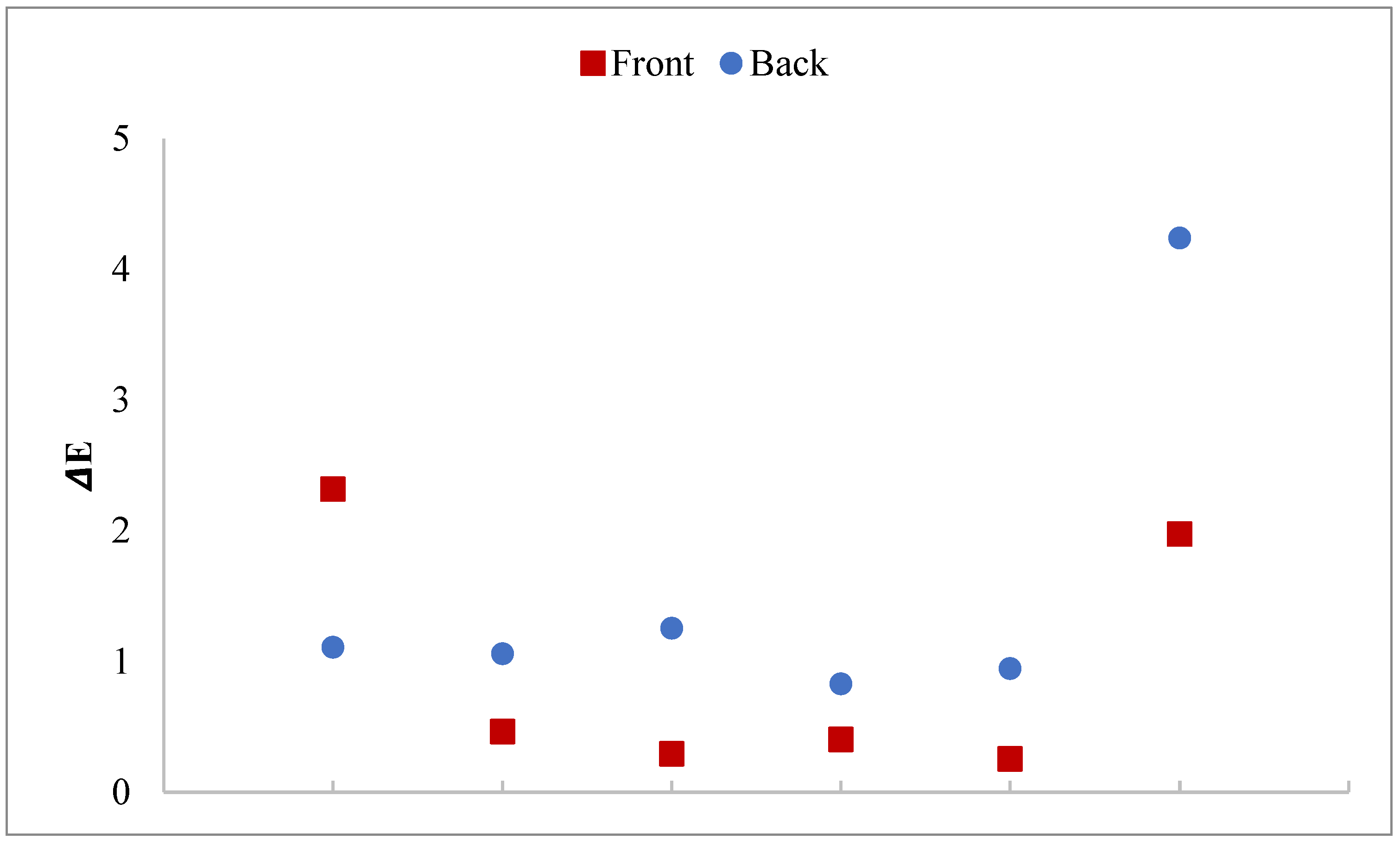
3.6. Data from Colorimetric Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAC | Benzalkonium chloride |
| LEO | Lavender essential oil |
| REO | Rosemary essential oil |
References
- Branysova, T.; Demnerova, K.; Durovic, M.; Stiborova, H. Microbial biodeterioration of cultural heritage and identification of the active agents over the last two decades. J. Cult. Herit. 2022, 55, 245–260. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Sequeira, S. Mycological Studies in Cultural Heritage. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–39. [Google Scholar] [CrossRef]
- Tiano, P. Biodegradation of Cultural Heritage: Decay Mechanisms and Control Methods. ARIADNE 9 Work. Hist. Mater. Their Diagn. 2009, 2, 7–12. [Google Scholar]
- Pinheiro, A.C.; Sequeira, S.O.; Macedo, M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019, 45, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Kredics, L.; Naeimi, S.; Hatvani, L.; Vágvölgyi, C.; Cai, F.; Druzhinina, I.S.; Manczinger, L. “The Good, the Bad and the Ugly” in the shades of green: The genus Trichoderma in the spotlight. Indian Phytopathol. 2021, 74, 403–411. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Kuhls, K.; Lieckfeldt, E.; Samuels, G.J.; Kovacs, W.; Meyer, W.; Petrini, O.; Gams, W.; Börner, T.; Kubicek, C.P. Molecular evidence that the asexual industrial fungus Trichoderma reesei is a clonal derivative of the ascomycete Hypocrea jecorina. Proc. Natl. Acad. Sci. USA 1996, 93, 7755–7760. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Lavédrine, B. Screening for antifungal activity of essential oils and related compounds to control the biocontamination in libraries and archives storage areas. Int. Biodeterior. Biodegrad. 2005, 55, 141–147. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; ’Omez De Saravia, S.G.; Borges, P.; Fung, D.Y.C.; Velge, P. Essential Oils of Plants as Biocides against Microorganisms Isolated from Cuban and Argentine Documentary Heritage. ISRN Microbiol. 2012, 2012, 826786. [Google Scholar] [CrossRef]
- Čabalová, I.; Češek, B.; Mikala, O.; Gojný, J.; Kačík, F.; Tribulová, T. The influence of selected efficient compounds of essential oils for paper protection. J. Cult. Herit. 2019, 37, 148–154. [Google Scholar] [CrossRef]
- Ventorino, V.; La Storia, A.; Robertiello, A.; Corsi, S.; Romano, I.; Sannino, L.; Pepe, O. Fungal Biodeterioration and Preservation of Miniature Artworks. J. Fungi 2023, 9, 1054. [Google Scholar] [CrossRef]
- Tomić, A.; Šovljanski, O.; Nikolić, V.; Pezo, L.; Aćimović, M.; Cvetković, M.; Stanojev, J.; Kuzmanović, N.; Markov, S. Screening of Antifungal Activity of Essential Oils in Controlling Biocontamination of Historical Papers in Archives. Antibiotics 2023, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Menicucci, F.; Palagano, E.; Michelozzi, M.; Ienco, A. Essential Oils for the Conservation of Paper Items. Molecules 2023, 28, 5003. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Mollea, C.; Demichela, M.; Fissore, D. Application of Essential Oils to Control the Biodeteriogenic Microorganisms in Archives and Libraries. Heritage 2022, 5, 2181–2195. [Google Scholar] [CrossRef]
- Paolino, B.; Sorrentino, M.C.; Macchia, A.; Troisi, J.; Zaratti, C.; Hansen, A.; Ilardi, S.; Russo, G.; Lahoz, E.; Pacifico, S. Lavender essential oil for a contactless application for contemporary art conservation: A case study. Npj Herit. Sci. 2025, 13, 8. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Yang, J.; Goksen, G.; Zhang, W. Rosemary essential oil: Chemical and biological properties, with emphasis on its delivery systems for food preservation. Food Control 2023, 154, 110003. [Google Scholar] [CrossRef]
- Paolino, B.; Sorrentino, M.C.; Troisi, J.; Carri, M.D.; Kiselev, P.; Raimondo, R.; Lahoz, E.; Pacifico, S. Lavandula angustifolia mill. for a suitable non-invasive treatment against fungal colonization on organic-media cultural heritage. Herit. Sci. 2024, 12, 53. [Google Scholar] [CrossRef]
- Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. Trichoderma as a human pathogen. In Trichoderma: Biology and Applications; CABI: Wallingford, UK, 2013; pp. 292–313. [Google Scholar] [CrossRef]
- Paolino, B.; Sorrentino, M.C.; Pacifico, S. Greener solutions for biodeterioration of organic-media cultural heritage: Where are we? Herit. Sci. 2024, 12, 334. [Google Scholar] [CrossRef]
- Karbowska-Berent, J.; Górniak, B.; Czajkowska-Wagner, L.; Rafalska, K.; Jarmiłko, J.; Kozielec, T. The initial disinfection of paper-based historic items-Observations on some simple suggested methods. Int. Biodeterior. Biodegrad. 2018, 131, 60–66. [Google Scholar] [CrossRef]
- UNI 8941; Superfici Colorate-Metodi di Misura e Valutazione delle Variazioni di Colore. Milano UNI., Ente Nazionale Italiano di Normazione (UNI): Via Sannio, Italy, 1986.
- Savković, Ž.; Stupar, M.; Unković, N.; Knežević, A.; Vukojević, J.; Grbić, M.L. Fungal Deterioration of Cultural Heritage Objects. In Biodegradation Technology of Organic and Inorganic Pollutants; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Skóra, J.; Gutarowska, B.; Pielech-Przybylska, K.; Stępień, Ł.; Pietrzak, K.; Piotrowska, M.; Pietrowski, P. Assessment of microbiological contamination in the work environments of museums, archives and libraries. Aerobiologia 2015, 31, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bastholm, C.J.; Madsen, A.M.; Andersen, B.; Frisvad, J.C.; Richter, J. The mysterious mould outbreak—A comprehensive fungal colonisation in a climate-controlled museum repository challenges the environmental guidelines for heritage collections. J. Cult. Herit. 2022, 55, 78–87. [Google Scholar] [CrossRef]
- Nol, L.; Henis, Y.; Kenneth, R.G. Biological factors of foxing in postage stamp paper. Int. Biodeterior. Biodegrad. 2001, 48, 98–104. [Google Scholar] [CrossRef]
- Borrego, S.; Lavin, P.; Perdomo, I.; de Saravia, S.G.; Guiamet, P. Determination of Indoor Air quality in archives and biodeterioration of the documentary heritage. ISRN Microbiol. 2012, 2012, 680598. [Google Scholar] [CrossRef]
- Szczepanowska, H.; Lovett, C.M. A Study of the Removal and Prevention of Fungal Stains on Paper. J. Am. Inst. Conserv. 1992, 31, 147–160. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Heude, E.; Lavédrine, B. Isolation and attempts of biomolecular characterization of fungal strains associated to foxing on a 19th century book. J. Cult. Herit. 2007, 8, 126–133. [Google Scholar] [CrossRef]
- Stickings, C.E.; Raistrick, H. Chemistry of the Fungi. Annu. Rev. Biochem. 1956, 25, 225–256. [Google Scholar] [CrossRef]
- Ainsworth, A.M.; Liimatainen, K. Hyphodermella rosae: A “twig-welding” corticioid new to Britain. Field Mycol. 2019, 20, 43–46. [Google Scholar] [CrossRef]
- Schilling, M.; Schilling, M.; Farine, S.; Péros, J.-P.; Bertsch, C.; Gelhaye, E. Wood degradation in grapevine diseases. Adv. Bot. Res. 2021, 99, 175–207. [Google Scholar] [CrossRef]
- Phillips, C.A.; Laird, K.; Allen, S.C. The use of Citri-VTM®—An antimicrobial citrus essential oil vapour for the control of Penicillium chrysogenum, Aspergillus niger and Alternaria alternata in vitro and on food. Food Res. Int. 2012, 47, 310–314. [Google Scholar] [CrossRef]
- Krisch, J. Activity of essential oils in vapor phase against bread spoilage fung. Acta Biol. Szeged. 2013, 57, 9–12. [Google Scholar]
- Melito, S.; Petretto, G.L.; Chahine, S.; Pintore, G.; Chessa, M. Seasonal Variation of Essential Oil in Rosmarinus officinalis Leaves in Sardinia. Nat. Prod. Commun. 2019, 14, 1934578X1986400. [Google Scholar] [CrossRef]
- Rathore, S.; Mukhia, S.; Kapoor, S.; Bhatt, V.; Kumar, R.; Kumar, R. Seasonal variability in essential oil composition and biological activity of Rosmarinus officinalis L. accessions in the western Himalaya. Sci. Rep. 2022, 12, 3305. [Google Scholar] [CrossRef]
- Micić, D.; Đurović, S.; Riabov, P.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinović, B.; Božović, R.; Jovanović, D.; et al. Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods 2021, 10, 2734. [Google Scholar] [CrossRef]
- Yabuki, T.; Miyazaki, K.; Okuda, T. Japanese species of the Longibrachiatum Clade of Trichoderma. Mycoscience 2014, 55, 196–212. [Google Scholar] [CrossRef]
- Haouhach, S.; Karkachi, N.; Oguiba, B.; Sidaoui, A.; Chamorro, I.; Kihal, M.; Monte, E. Three New Reports of Trichoderma in Algeria: T. atrobrunneum, (South) T. longibrachiatum (South), and T. afroharzianum (Northwest). Microorganisms 2020, 8, 1455. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Bölzlbauer, U.M.; Kovacsa, W.; Mach, R.L.; Kuhlsb, K.; Lieckfeldtb, E.; Börnerb, T.; Samuels, G.J. Cellulase production by species of Trichoderma sect. Longibrachiatum and of Hypocrea species with anamorphs referable to Trichoderma sect. Longibrachiatum. Fungal Genet. Biol. 1996, 20, 105–114. [Google Scholar] [CrossRef]
- Sautour, M.; Chrétien, M.; Valot, S.; Lafon, I.; Basmaciyan, L.; Legouge, C.; Verrier, T.; Gonssaud, B.; Abou-Hanna, H.; Dalle, F.; et al. First case of proven invasive pulmonary infection due to Trichoderma longibrachiatum in a neutropenic patient with acute leukemia. J. Mycol. Médicale 2018, 28, 659–662. [Google Scholar] [CrossRef]
- Druzhinina, I.; Kubicek, C.P. Species concepts and biodiversity in Trichoderma and Hypocrea: From aggregate species to species clusters? J. Zhejiang Univ.-Sci. B 2005, 6, 100–112. [Google Scholar] [CrossRef]
- Gordillo-Fuenzalida, F.; Echeverria-Vega, A.; Cuadros-Orellana, S.; Faundez, C.; Kähne, T.; Morales-Vera, R. Cellulases Production by a Trichoderma sp. Using Food Manufacturing Wastes. Appl. Sci. 2019, 9, 4419. [Google Scholar] [CrossRef]
- Hatvani, L.; Homa, M.; Chenthamara, K.; Cai, F.; Kocsubé, S.; Atanasova, L.; Mlinaric-Missoni, E.; Manikandan, P.; Revathi, R.; Dóczi, I.; et al. Agricultural systems as potential sources of emerging human mycoses caused by Trichoderma: A successful, common phylotype of Trichoderma longibrachiatum in the frontline. FEMS Microbiol. Lett. 2019, 366, fnz246. [Google Scholar] [CrossRef] [PubMed]
- Gorski, R.; Krzysztof, S.; Siwulski, M.; Katarzyna, G. Effect of selected natural essential oils on in vitro development of fungus Trichoderma harzianum found in common mushroom (Agaricus bisporus) cultivation. Ecol. Chem. Eng. 2010, 17, 177–185. [Google Scholar]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Parra, C.; Navarro, M.; Blanco, R.; Gea, F. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410. [Google Scholar] [CrossRef]
- Menicucci, F.; Palagano, E.; Michelozzi, M.; Cencetti, G.; Raio, A.; Bacchi, A.; Mazzeo, P.P.; Cuzman, O.A.; Sidoti, A.; Guarino, S.; et al. Effects of trapped-into-solids volatile organic compounds on paper biodeteriogens. Int. Biodeterior. Biodegrad. 2022, 174, 105469. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Savković, Ž.D.; Stupar, M.Č.; Grbić, M.V.L.; Vukojević, J.B. Comparison of anti-Aspergillus activity of Origanum vulgare L. essential oil and commercial biocide based on silver ions and hydrogen peroxide. Acta Bot. Croat. 2016, 75, 121–128. [Google Scholar] [CrossRef]
- Macchia, A.; Aureli, H.; Prestileo, F.; Ortenzi, F.; Sellathurai, S.; Docci, A.; Cerafogli, E.; Colasanti, I.A.; Ricca, M.; La Russa, M.F. In-Situ Comparative Study of Eucalyptus, Basil, Cloves, Thyme, Pine Tree, and Tea Tree Essential Oil Biocide Efficacy. Methods Protocols 2022, 5, 37. [Google Scholar] [CrossRef]
- Isola, D.; Bartoli, F.; Meloni, P.; Caneva, G.; Zucconi, L. Black Fungi and Stone Heritage Conservation: Ecological and Metabolic Assays for Evaluating Colonization Potential and Responses to Traditional Biocides. Appl. Sci. 2022, 12, 2038. [Google Scholar] [CrossRef]
- Tanasă, F.; Nechifor, M.; Teacă, C.-A. Essential Oils as Alternative Green Broad-Spectrum Biocides. Plants 2024, 13, 3442. [Google Scholar] [CrossRef]
- Ilieș, D.C.; Safarov, B.; Caciora, T.; Ilieș, A.; Grama, V.; Ilies, G.; Huniadi, A.; Zharas, B.; Hodor, N.; Sandor, M.; et al. Museal Indoor Air Quality and Public Health: An Integrated Approach for Exhibits Preservation and Ensuring Human Health. Sustainability 2022, 14, 2462. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, B.; Sorrentino, M.C.; Pacifico, S.; Garrigos, M.C.; Riccardi, M.G.; Paradiso, R.; Lahoz, E.; Borriello, G. A Preliminary Study on the Efficacy of Essential Oils Against Trichoderma longibrachiatum Isolated from an Archival Document in Italy. Heritage 2025, 8, 187. https://doi.org/10.3390/heritage8060187
Paolino B, Sorrentino MC, Pacifico S, Garrigos MC, Riccardi MG, Paradiso R, Lahoz E, Borriello G. A Preliminary Study on the Efficacy of Essential Oils Against Trichoderma longibrachiatum Isolated from an Archival Document in Italy. Heritage. 2025; 8(6):187. https://doi.org/10.3390/heritage8060187
Chicago/Turabian StylePaolino, Benedetta, Maria Cristina Sorrentino, Severina Pacifico, Maria Carmen Garrigos, Marita Georgia Riccardi, Rubina Paradiso, Ernesto Lahoz, and Giorgia Borriello. 2025. "A Preliminary Study on the Efficacy of Essential Oils Against Trichoderma longibrachiatum Isolated from an Archival Document in Italy" Heritage 8, no. 6: 187. https://doi.org/10.3390/heritage8060187
APA StylePaolino, B., Sorrentino, M. C., Pacifico, S., Garrigos, M. C., Riccardi, M. G., Paradiso, R., Lahoz, E., & Borriello, G. (2025). A Preliminary Study on the Efficacy of Essential Oils Against Trichoderma longibrachiatum Isolated from an Archival Document in Italy. Heritage, 8(6), 187. https://doi.org/10.3390/heritage8060187









