Chemo-Mineralogical Changes in Six European Monumental Stones Caused by Cyclic Isothermal Treatment at 600 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Petrographic Characterisation of the Lithotypes
2.2. Calcination
2.3. Analytical Techniques
3. Results and Discussion
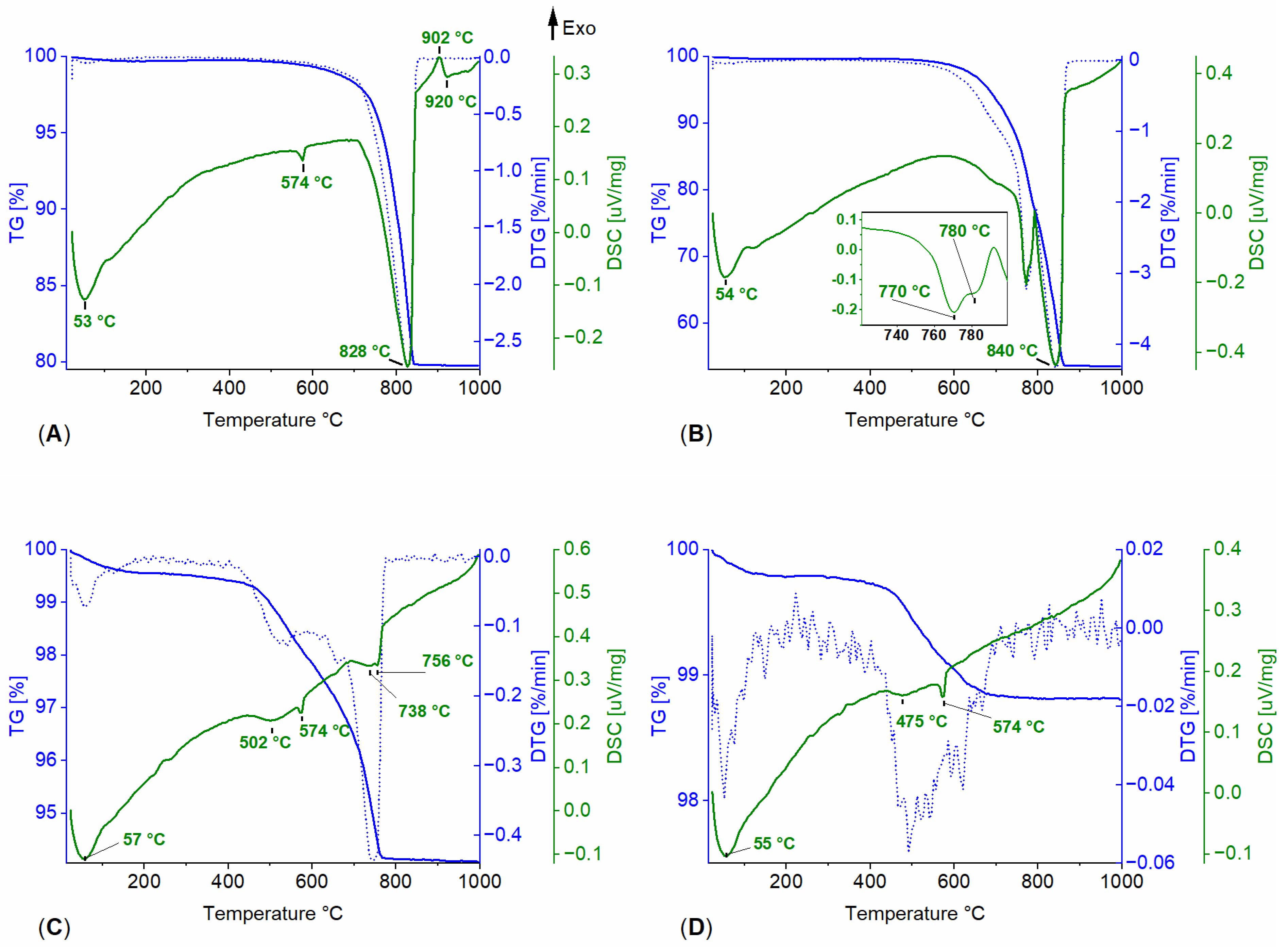
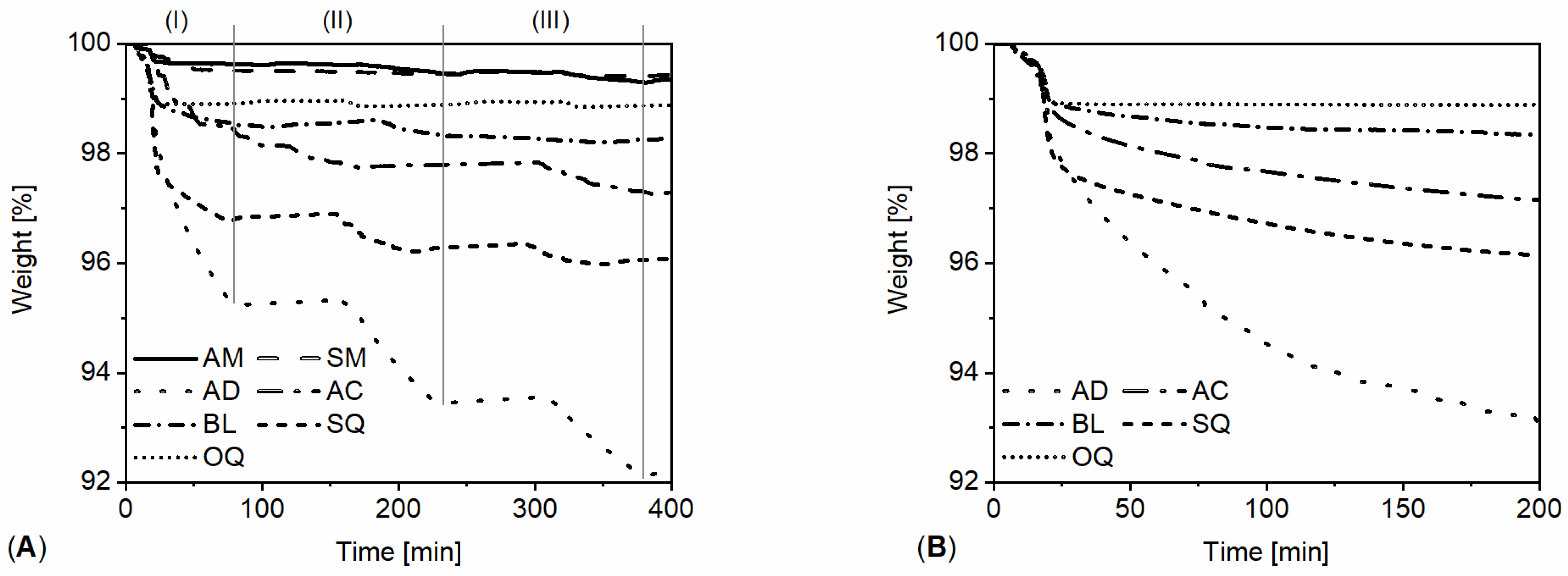
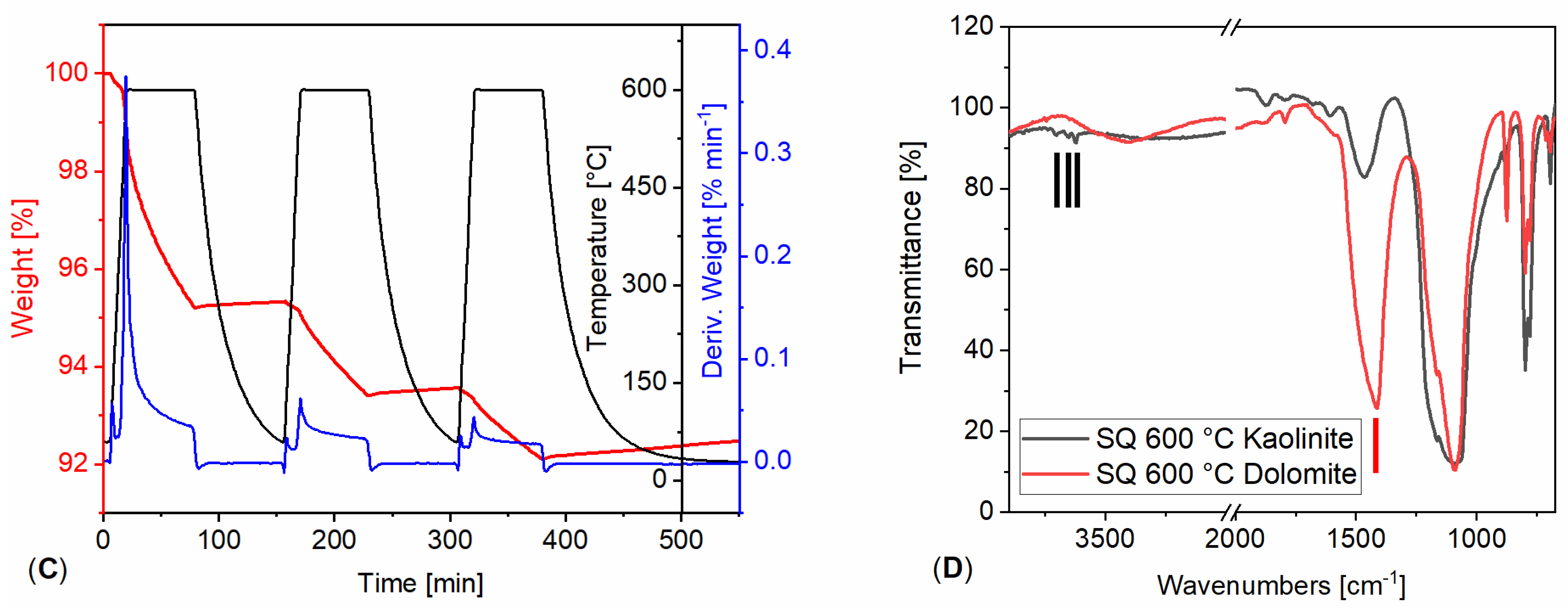
3.1. Powdered Sample Analysis: Simultaneous Thermal Analysis, Cyclic Thermal Gravimetric Analysis, and Fourier-Transform Infrared Spectroscopy
3.2. Solid-Sample Analysis: In Situ X-Ray Diffraction, Colour Measurements, and Microscopy
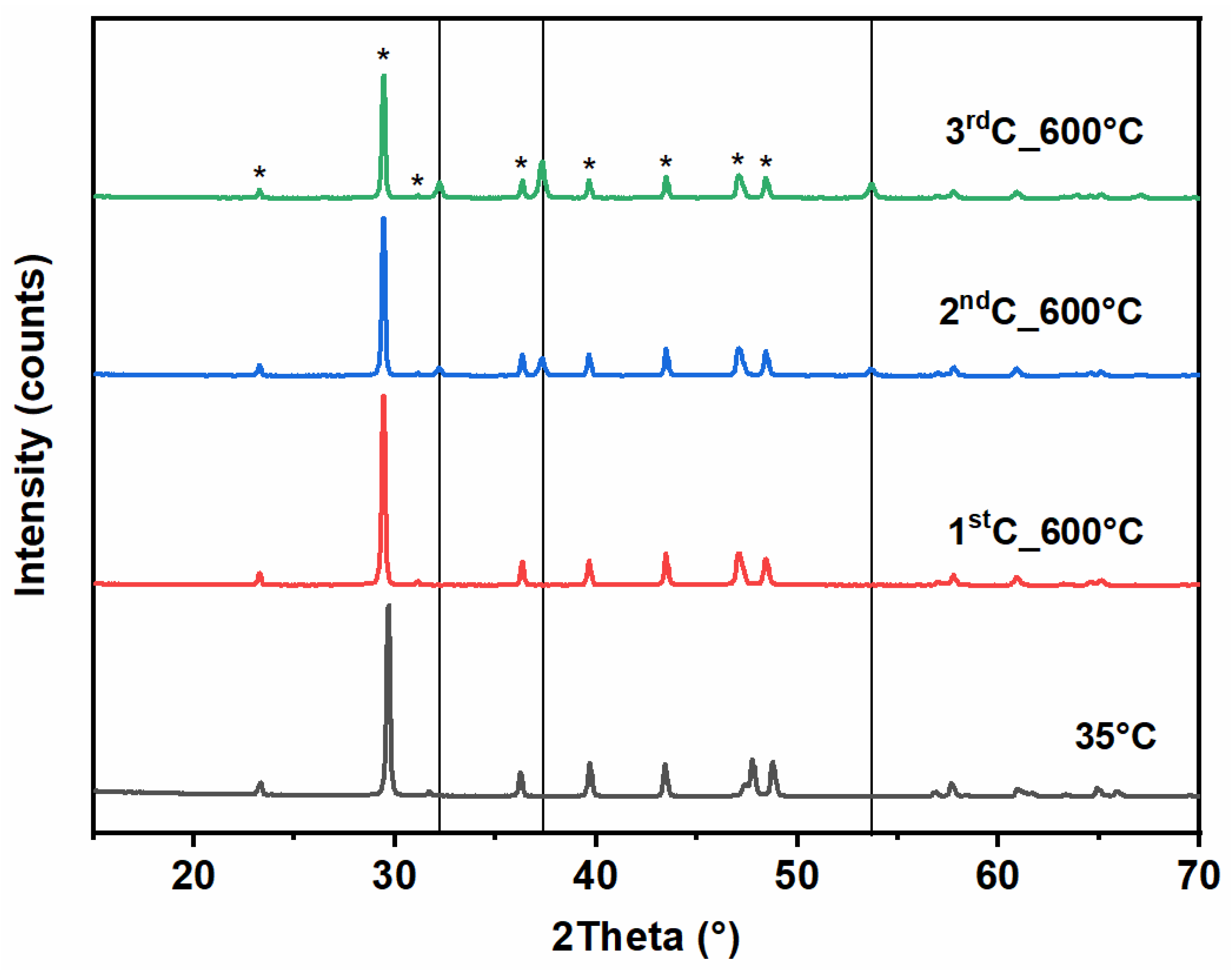
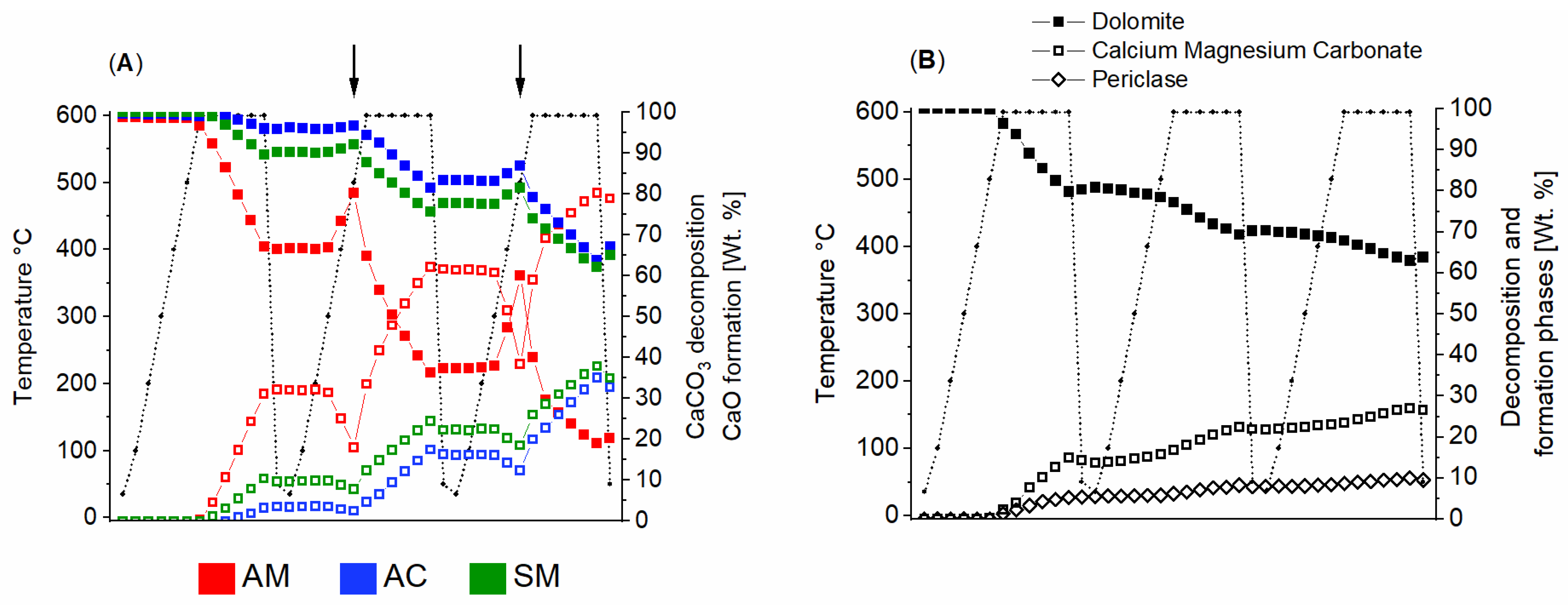
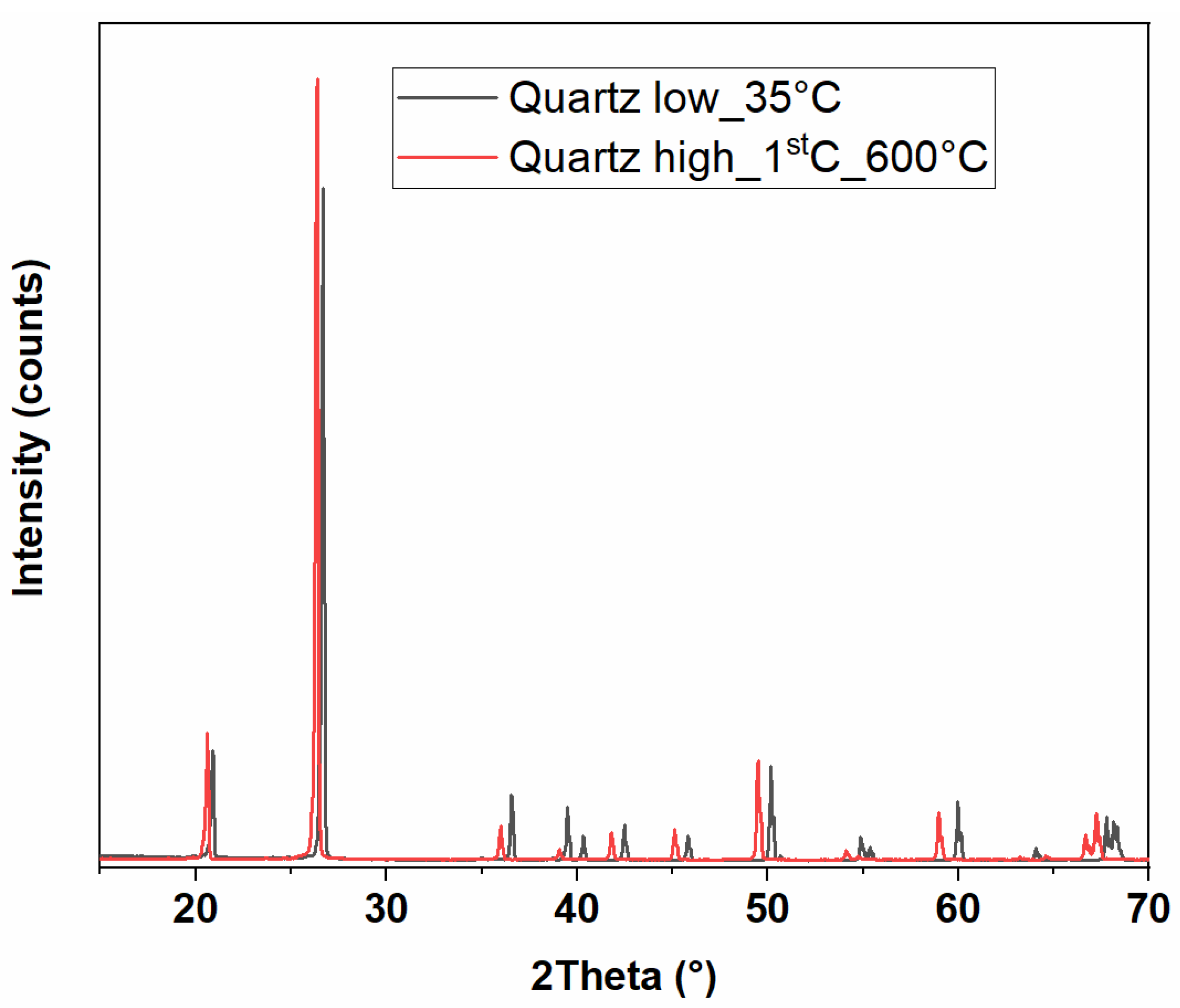
3.2.1. X-Ray Diffraction
3.2.2. Colour Measurements
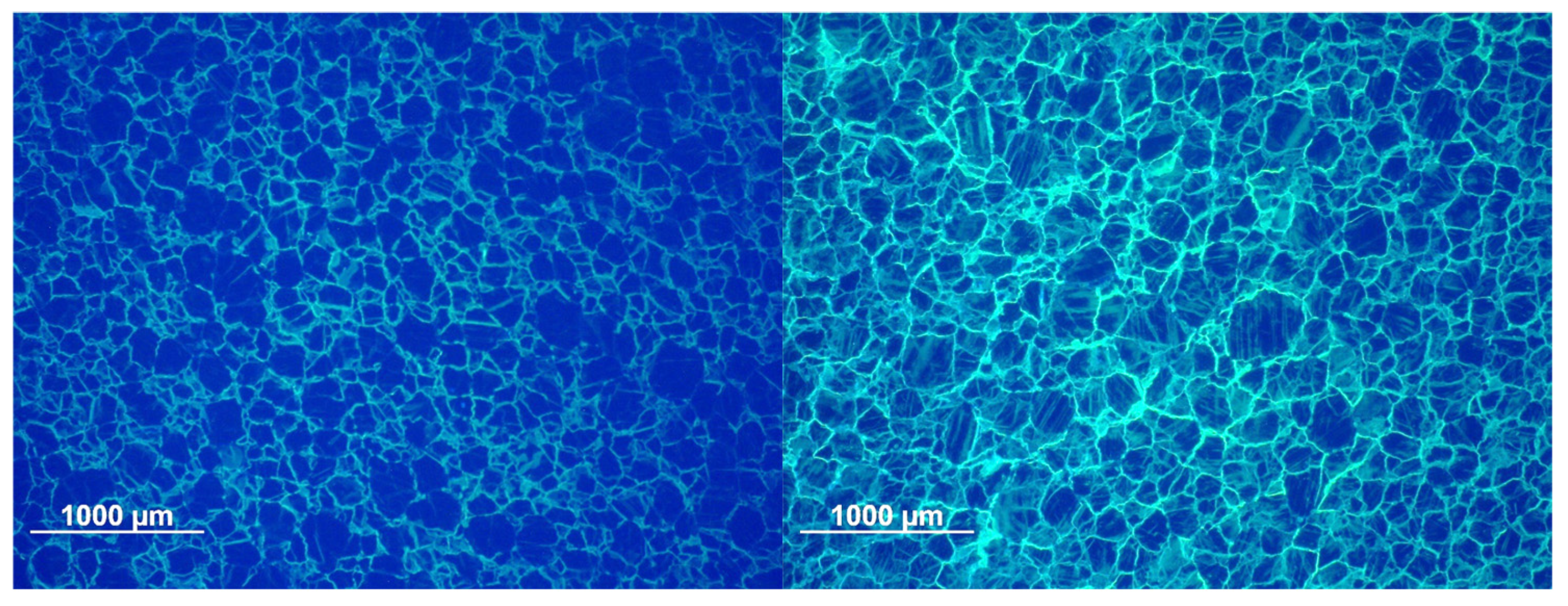
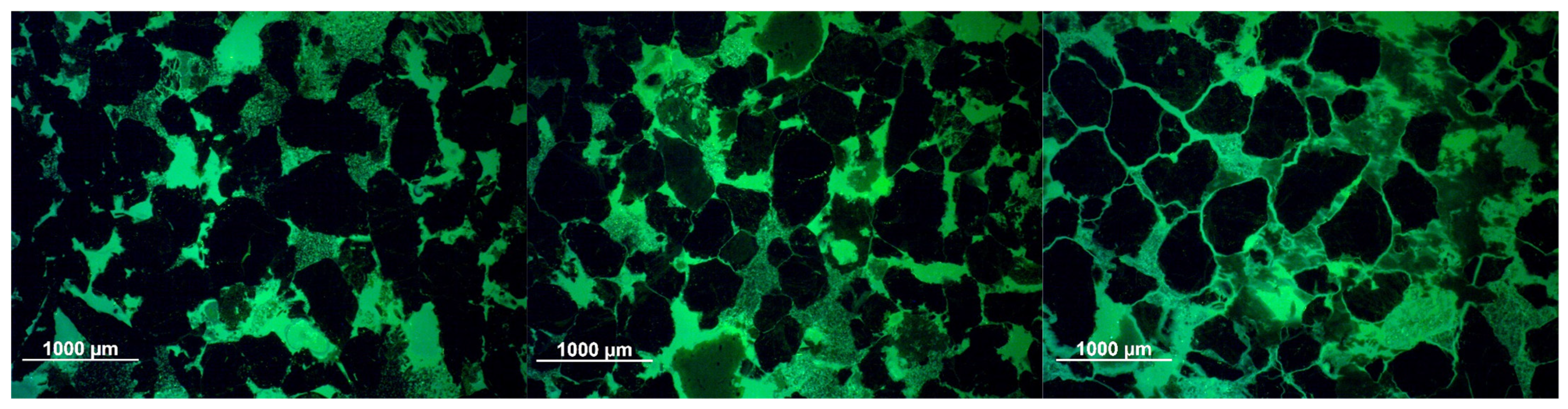
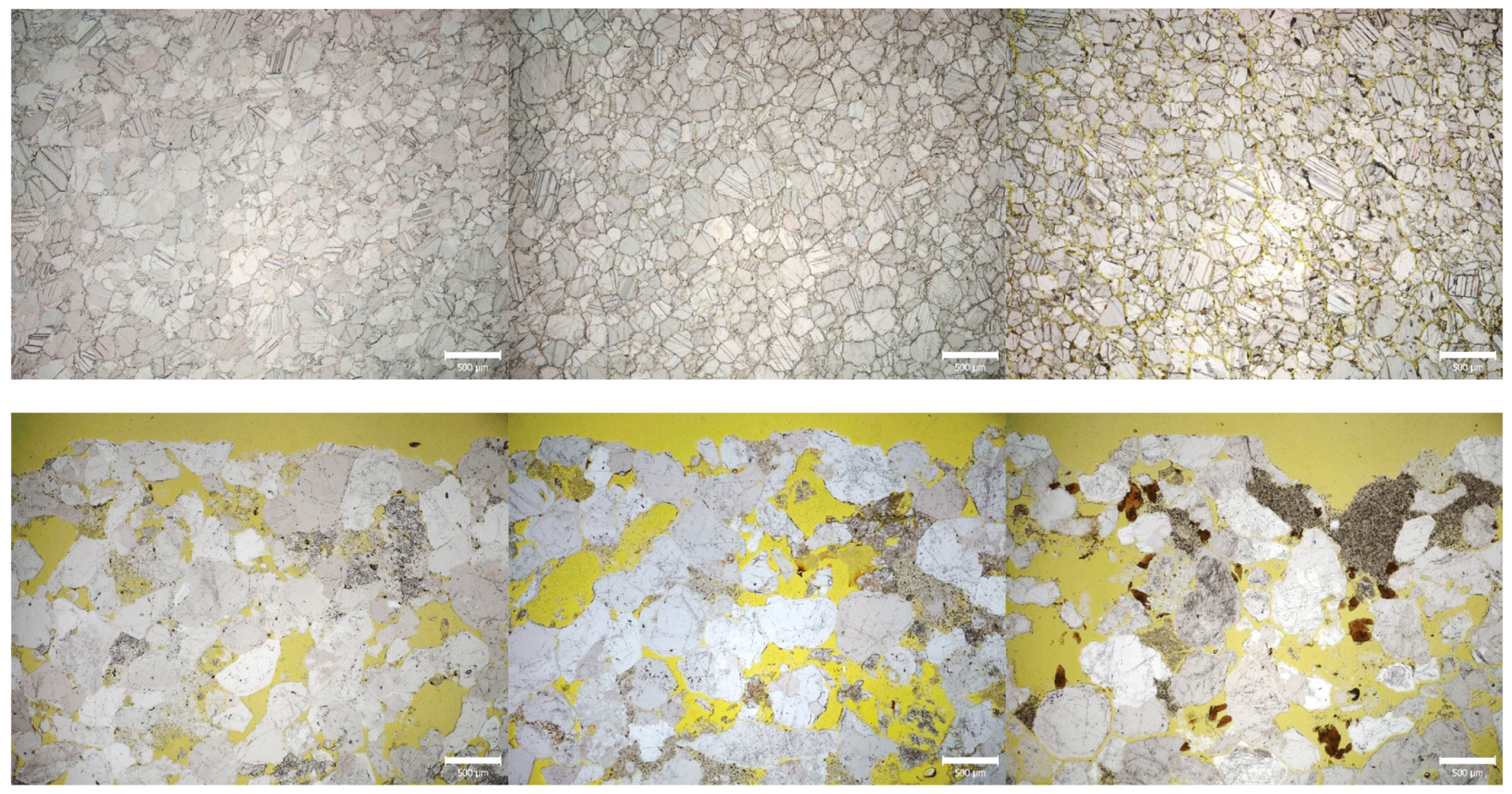
3.2.3. Optical Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gomez-Heras, M.; McCabe, S.; Smith, B.J.; Fort, R. Impacts of Fire on Stone-Built Heritage. J. Archit. Conserv. 2009, 15, 47–58. [Google Scholar] [CrossRef]
- Garlock, M.; Paya-Zaforteza, I.; Kodur, V.; Gu, L. Fire hazard in bridges: Review, assessment and repair strategies. Eng. Struct. 2012, 35, 89–98. [Google Scholar] [CrossRef]
- Vazzoler, J.d.S.; Vieira, G.L.; Teles, C.R.; Degen, M.K.; Teixeira, R.A. Investigation of the potential use of waste from ornamental stone processing after heat treatment for the production of cement-based paste. Constr. Build. Mater. 2018, 177, 314–321. [Google Scholar] [CrossRef]
- Tang, C.-S.; Cui, Y.-J.; Tang, A.-M.; Shi, B. Experiment evidence on the temperature dependence of desiccation cracking behavior of clayey soils. Eng. Geol. 2010, 114, 261–266. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milano, A.; Russo, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Appl. Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Cerantola, V.; Bykova, E.; Kupenko, I.; Merlini, M.; Ismailova, L.; McCammon, C.; Bykov, M.; Chumakov, A.I.; Petitgirard, S.; Kantor, I.; et al. Stability of iron-bearing carbonates in the deep Earth’s interior. Nat. Commun. 2017, 8, 15960. [Google Scholar] [CrossRef]
- Sanjurjo-Sánchez, J.; Gomez-Heras, M.; Fort, R.; Alvarez de Buergo, M.; Izquierdo Benito, R.; Bru, M.A. Dating fires and estimating the temperature attained on stone surfaces. The case of Ciudad de Vascos (Spain). Microchem. J. 2016, 127, 247–255. [Google Scholar] [CrossRef]
- Gatta, T.; Gregori, E.; Marini, F.; Tomassetti, M.; Visco, G.; Campanella, L. New approach to the differentiation of marble samples using thermal analysis and chemometrics in order to identify provenance. Chem. Cent. J. 2014, 8, 35. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Wang, C.-C.; Hou, M.; Li, A. Recent progress in instrumental techniques for architectural heritage materials. Herit. Sci. 2019, 7, 1–50. [Google Scholar] [CrossRef]
- Tretiach, M.; Bertuzzi, S.; Candotto Carniel, F. Heat shock treatments: A new safe approach against lichen growth on outdoor stone surfaces. Env. Sci. Technol. 2012, 46, 6851–6859. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Scherer, G.W.; Naidu, S. Artificial weathering of stone by heating. J. Cult. Herit. 2013, 14, E85–E93. [Google Scholar] [CrossRef]
- Ban, M.; De Kock, T.; Ott, F.; Barone, G.; Rohatsch, A.; Raneri, S. Neutron Radiography Study of Laboratory Ageing and Treatment Applications with Stone Consolidants. Nanomaterials 2019, 9, 635. [Google Scholar] [CrossRef]
- Martinho, E.; Mendes, M.; Dionisio, A. 3D imaging of P-waves velocity as a tool for evaluation of heat induced limestone decay. Constr. Build. Mater. 2017, 135, 119–128. [Google Scholar] [CrossRef]
- Ozguven, A.; Ozcelik, Y. Effects of high temperature on physico-mechanical properties of Turkish natural building stones. Eng. Geol. 2014, 183, 127–136. [Google Scholar] [CrossRef]
- Tian, H.; Kempka, T.; Xu, N.-X.; Ziegler, M. Physical Properties of Sandstones After High Temperature Treatment. Rock. Mech. Rock. Eng. 2012, 45, 1113–1117. [Google Scholar] [CrossRef]
- Wang, F.; Frühwirt, T.; Konietzky, H. Influence of repeated heating on physical-mechanical properties and damage evolution of granite. Int. J. Rock. Mech. Min. 2020, 136, 104514. [Google Scholar] [CrossRef]
- Vagnon, F.; Colombero, C.; Colombo, F.; Comina, C.; Ferrero, A.M.; Mandrone, G.; Vinciguerra, S.C. Effects of thermal treatment on physical and mechanical properties of Valdieri Marble–NW Italy. Int. J. Rock. Mech. Min. 2019, 116, 75–86. [Google Scholar] [CrossRef]
- McCabe, S.; Smith, B.J.; Warke, P.A. Exploitation of inherited weakness in fire-damaged building sandstone: The ‘fatiguing’ of ‘shocked’ stone. Eng. Geol. 2010, 115, 217–225. [Google Scholar] [CrossRef]
- Delegou, E.T.; Apostolopoulou, M.; Ntoutsi, I.; Thoma, M.; Keramidas, V.; Papatrechas, C.; Economou, G.; Moropoulou, A. The Effect of Fire on Building Materials: The Case-Study of the Varnakova Monastery Cells in Central Greece. Heritage 2019, 2, 1233–1259. [Google Scholar] [CrossRef]
- Rosenholtz, J.L.; Smith, D.T. Linear thermal expansion of calcite, var. Iceland spar, and Yule Marble. Am. Mineral. J. Earth Planet. Mater. 1949, 34, 846–854. [Google Scholar]
- Martinho, E.; Dionísio, A. Assessment Techniques for Studying the Effects of Fire on Stone Materials: A Literature Review. Int. J. Archit. Herit. 2018, 14, 275–299. [Google Scholar] [CrossRef]
- Hajpál, M.; Török, A. Mineralogical and colour changes of quartz sandstones by heat. Env. Geol. 2004, 46, 311–322. [Google Scholar] [CrossRef]
- Martínez-Ibáñez, V.; Benavente, D.; Hidalgo Signes, C.; Tomás, R.; Garrido, M.E. Temperature-Induced Explosive Behaviour and Thermo-Chemical Damage on Pyrite-Bearing Limestones: Causes and Mechanisms. Rock. Mech. Rock. Eng. 2020, 54, 219–234. [Google Scholar] [CrossRef]
- Erasmus, E. The influence of thermal treatment on properties of kaolin. Hem. Ind. 2016, 70, 595–601. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Hu, Y. Investigation of the thermal behaviour and decomposition kinetics of kaolinite. Clay Miner. 2018, 50, 199–209. [Google Scholar] [CrossRef]
- Hartlieb, P.; Toifl, M.; Kuchar, F.; Meisels, R.; Antretter, T. Thermo-physical properties of selected hard rocks and their relation to microwave-assisted comminution. Miner. Eng. 2016, 91, 34–41. [Google Scholar] [CrossRef]
- Heaney, P.J. Structure and chemistry of the low-pressure silica polymorphs. In Silica: Physical Behavior, Geochemistry, and Materials Applications; Walter de Gruyter GmbH: Berlin, Germany; Munich, Germany; Boston, MA, USA, 1994; pp. 1–40. [Google Scholar]
- Chakrabarti, B.; Yates, T.; Lewry, A. Effect of fire damage on natural stonework in buildings. Constr. Build. Mater. 1996, 10, 539–544. [Google Scholar] [CrossRef]
- Kristóf-Makó, É.; Juhász, A. The effect of mechanical treatment on the crystal structure and thermal decomposition of dolomite. Thermochim. Acta 1999, 342, 105–114. [Google Scholar] [CrossRef]
- De Aza, A.H.; Rodríguez, M.A.; Rodríguez, J.L.; De Aza, S.; Pena, P.; Convert, P.; Hansen, T.; Turrillas, X. Decomposition of dolomite monitored by neutron thermodiffractometry. J. Am. Ceram. Soc. 2002, 85, 881–888. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Luque, A.; Rodriguez-Navarro, A.B.; Ortega-Huertas, M. Thermal decomposition of calcite: Mechanisms of formation and textural evolution of CaO nanocrystals. Am. Mineral. 2009, 94, 578–593. [Google Scholar] [CrossRef]
- Shahraki, B.; Mehrabi, B.; Gholizadeh, K.; Mohammadinasab, M. Thermal behavior of calcite as an expansive agent. J. Min. Metall. B Metall. 2011, 47, 89–97. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Kudlacz, K.; Ruiz-Agudo, E. The mechanism of thermal decomposition of dolomite: New insights from 2D-XRD and TEM analyses. Am. Mineral. 2012, 97, 38–51. [Google Scholar] [CrossRef]
- Valverde, J.M.; Perejon, A.; Medina, S.; Perez-Maqueda, L.A. Thermal decomposition of dolomite under CO2: Insights from TGA and in situ XRD analysis. Phys. Chem. Chem. Phys. 2015, 17, 30162–30176. [Google Scholar] [CrossRef]
- McIntosh, R.; Sharp, J.; Wilburn, F. The thermal decomposition of dolomite. Thermochim. Acta 1990, 165, 281–296. [Google Scholar] [CrossRef]
- Kieslinger, A. Zerstörungen an Steinbauten: Ihre Ursachen und ihre Abwehr; Franz Deuticke: Leipzig, Germany, 1932. [Google Scholar]
- Biró, A.; Hlavička, V.; Lublóy, É. Effect of fire-related temperatures on natural stones. Constr. Build. Mater. 2019, 212, 92–101. [Google Scholar] [CrossRef]
- Praticò, Y.; Ochsendorf, J.; Holzer, S.; Flatt, R.J. Post-fire restoration of historic buildings and implications for Notre-Dame de Paris. Nat. Mater. 2020, 19, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Siegesmund, S.; Snethlage, R. Stone in architecture: Properties, Durability; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- De Kock, T.; Turmel, A.; Fronteau, G.; Cnudde, V. Rock fabric heterogeneity and its influence on the petrophysical properties of a building limestone: Lede stone (Belgium) as an example. Eng. Geol. 2017, 216, 31–41. [Google Scholar] [CrossRef]
- Graue, B.J. Stone Deterioration and Replacement of Natural Building Stones at the Cologne Cathedral-A Contribution to the Preservation of Cultural Heritage. Doctoral Degree, Georg-August-Universität Göttingen, Göttingen, Germany, 2013. [Google Scholar]
- Rohatsch, A. Neogene Bau-und Dekorgesteine Niederösterreichs und des Burgenlandes. In “Junge” Kalke, Sandsteine und Konglomerate—Neogen; Schwaighofer, B., Eppensteiner, W., Eds.; Mitteilungen IAG BOKU: Vienna, Austria, 2005; pp. 27–31. [Google Scholar]
- De Kock, T.; Boone, M.; Dewanckele, J.; De Ceukelaire, M.; Cnudde, V. Lede Stone: A Potential “global Heritage Stone Resource” from Belgium. Episodes 2015, 38, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Graue, B.; Siegesmund, S.; Middendorf, B. Quality assessment of replacement stones for the Cologne Cathedral: Mineralogical and petrophysical requirements. Env. Earth Sci. 2011, 63, 1799–1822. [Google Scholar] [CrossRef]
- Ehling, A.; Kaur, G.; Wyse Jackson, P.N.; Cassar, J.; Aparecida Del Lama, E.; Heldal, T. The First 55 IUGS Heritage Stones. 2024. Available online: https://iugs-geoheritage.org/designations-stones/ (accessed on 1 February 2025).
- Lobarinhas, R.; Dionísio, A.; Paneiro, G. High Temperature Effects on Global Heritage Stone Resources: A Systematic Review. Heritage 2024, 7, 6310–6342. [Google Scholar] [CrossRef]
- Ban, M.; Baragona, A.; Ghaffari, E.; Weber, J.; Rohatsch, A. Artificial aging techniques on various lithotypes for testing of stone consolidants. In Proceedings of the Science and Art: A Future for Stone: Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Volume 1; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; pp. 253–260. [Google Scholar]
- Standard EN 15886; Conservation of cultural Property. Colour Measurement of Surfaces. CEN Brussels: Bruxelles, Belgium, 2010.
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Faber, J.; Fawcett, T. The powder diffraction file: Present and future. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 325–332. [Google Scholar] [CrossRef]
- Kabekkodu, S.N.; Faber, J.; Fawcett, T. New Powder Diffraction File (PDF-4) in relational database format: Advantages and data-mining capabilities. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 333–337. [Google Scholar] [CrossRef]
- Wendlandt, W.W. Thermal Methods of Analysis; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Haines, P.J. Thermal Methods of Analysis: Principles, Applications and Problems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Haaland, M.M.; Friesem, D.E.; Miller, C.E.; Henshilwood, C.S. Heat-induced alteration of glauconitic minerals in the Middle Stone Age levels of Blombos Cave, South Africa: Implications for evaluating site structure and burning events. J. Archaeol. Sci. 2017, 86, 81–100. [Google Scholar] [CrossRef]
- Mashlan, M.; Martinec, P.; Kašlík, J.; Kovářová, E.; Scucka, J. Mössbauer study of transformation of Fe cations during thermal treatment of glauconite in air. In Proceedings of AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2012; pp. 169–173. [Google Scholar]
- Fanning, D.S.; Keramidas, V.Z.; El-Desoky, M.A. Micas. In Minerals in Soil Environments; Wiley: Hoboken, NJ, USA, 1989; Volume 1, pp. 551–634. [Google Scholar]
- Stoch, L. Significance of structural factors in dehydroxylation of kaolinite polytypes. J. Therm. Anal. Calorim. 1984, 29, 919–931. [Google Scholar] [CrossRef]
- Heide, K.; Földvari, M. High temperature mass spectrometric gas-release studies of kaolinite Al2[Si2O5(OH)4] decomposition. Thermochim. Acta 2006, 446, 106–112. [Google Scholar] [CrossRef]
- Singh, V.; Tathavadkar, V.; Denys, M.B.; Venugopal, R. Application of quartz inversion phenomenon in mineral processing–A case study of siliceous manganese ores. Miner. Eng. 2012, 32, 8–11. [Google Scholar] [CrossRef]
- Ungár, T. Industrial Applications of X-ray Diffraction; Chung, F.H., Smith, D.K., Eds.; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Sippel, J.; Siegesmund, S.; Weiss, T.; Nitsch, K.H.; Korzen, M. Decay of natural stones caused by fire damage. Geol. Soc. Lond. Spec. Publ. 2007, 271, 139–151. [Google Scholar] [CrossRef]
- Ban, M.; Luxbacher, T.; Lützenkirchen, J.; Viani, A.; Bianchi, S.; Hradil, K.; Rohatsch, A.; Castelvetro, V. Evolution of calcite surfaces upon thermal decomposition, characterized by electrokinetics, in-situ XRD, and SEM. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624. [Google Scholar] [CrossRef]
- Dionisio, A.; Sequeirabraga, M.; Waerenborgh, J. Clay minerals and iron oxides-oxyhydroxides as fingerprints of firing effects in a limestone monument. Appl. Clay Sci. 2009, 42, 629–638. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review—Calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Ban, M.; Mascha, E.; Weber, J.; Rohatsch, A.; Rodrigues, J.D. Efficiency and Compatibility of Selected Alkoxysilanes on Porous Carbonate and Silicate Stones. Materials 2019, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Ban, M. Chemo-Mineralogical and Petrophysical Alterations on Lithotypes Due to Thermal Treatment Before Stone Consolidation. Doctoral dissertation, Technische Universität Wien, Vienna, Austria, 2021. [Google Scholar] [CrossRef]




| Stone | Cal | Dol | Qtz | Kao | Phy | Goeth | KFSP | Pl |
|---|---|---|---|---|---|---|---|---|
| Apuan Marble (AM) | *** | tr | tr | tr | ||||
| St. Margarethen (SM) | *** | tr | ||||||
| Ajarte Dolostone (AD) | *** | tr | ||||||
| Ajarte Calcite (AC) | *** | tr | tr | |||||
| Balegem (BL) | *** | *** | * | * | ||||
| Schlaitdorf (SQ) | * | *** | ** | tr | tr | tr | ||
| Obernkirchen (OQ) | *** | * | tr | tr | * |
| Stone | LOI * | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Na2O | Remaining ** |
|---|---|---|---|---|---|---|---|---|---|
| Apuan Marble (AM) | 43.30 | 0.09 | 0.06 | 0.04 | 0.73 | 55.40 | 0.01 | 0.00 | <0.44 |
| St. Margarethen (SM) | 43.50 | 0.28 | 0.07 | 0.31 | 0.67 | 54.58 | 0.01 | 0.00 | <0.60 |
| Ajarte Dolostone (AD) | 46.30 | 1.30 | 0.52 | 0.18 | 20.21 | 31.91 | 0.08 | 0.06 | <0.35 |
| Ajarte Calcite (AC) | 42.00 | 2.66 | 1.13 | 0.21 | 0.71 | 52.51 | 0.21 | 0.03 | <0.59 |
| Balegem (BL) | 22.40 | 45.53 | 1.25 | 1.16 | 0.40 | 27.94 | 0.69 | 0.15 | <0.52 |
| Schlaitdorf (SQ) | 1.36 | 95.09 | 3.85 | 0.17 | 0.04 | 0.05 | 0.62 | 0.05 | <0.07 |
| Obernkirchen (OQ) | 1.11 | 95.95 | 3.22 | 0.18 | 0.03 | 0.03 | 0.25 | 0.06 | <0.38 |
| Stone | 600 °C Isothermal (TGA) | 600 °C Non-Isothermal (STA) | Residual Mass (STA) | |
|---|---|---|---|---|
| 40 °C min−1 3 Cycles at 60 min | 40 °C min−1 1 Cycle at 180 min | 10 °C min−1 22 to 600 °C | 10 °C min−1 at 1000 °C | |
| Apuan Marble | 0.705 | N/A | 0.13 | 55.92 |
| St. Margarethen | 0.627 | N/A | 0.40 | 55.93 |
| Ajarte Dolostone | 7.891 | 7.301 | 1.38 | 53.51 |
| Ajarte Calcite | 3.033 | 2.840 | 0.84 | 58.19 |
| Balegem | 1.971 | 1.815 | 0.65 | 79.76 |
| Schlaitdorf | 4.013 | 3.845 | 2.16 | 94.09 |
| Obernkirchen | 1.122 | 1.117 | 0.96 | 98.81 |
| Stone | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|
| AM | 9.17 ± 0.02 | 0.91 ± 0.01 | 2.31 ± 0.02 | 9.50 |
| SM | −15.62 ± 0.16 | −2.60 ± 0.1 | −14.38 ± 0.23 | 21.38 |
| AD | −12.13 ± 0.03 | 1.91 ± 0.16 | −3.02 ± 0.15 | 12.65 |
| AC | −11.4 ± 0.04 | 0.58 ± 0.01 | −2.53 ± 0.03 | 11.69 |
| BL | −6.85 ± 0.08 | 3.90 ± 0.08 | 3.10 ± 0.11 | 8.47 |
| SQ | −3.35 ± 0.76 | 5.92 ± 0.65 | 3.68 ± 0.08 | 7.73 |
| OQ | −3.24 ± 0.93 | 5.68 ± 0.16 | 5.60 ± 0.01 | 8.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanek, M.; Wriessnig, K.; Artner, W.; Pintér, F.; Ottner, F. Chemo-Mineralogical Changes in Six European Monumental Stones Caused by Cyclic Isothermal Treatment at 600 °C. Heritage 2025, 8, 107. https://doi.org/10.3390/heritage8030107
Urbanek M, Wriessnig K, Artner W, Pintér F, Ottner F. Chemo-Mineralogical Changes in Six European Monumental Stones Caused by Cyclic Isothermal Treatment at 600 °C. Heritage. 2025; 8(3):107. https://doi.org/10.3390/heritage8030107
Chicago/Turabian StyleUrbanek, Matea, Karin Wriessnig, Werner Artner, Farkas Pintér, and Franz Ottner. 2025. "Chemo-Mineralogical Changes in Six European Monumental Stones Caused by Cyclic Isothermal Treatment at 600 °C" Heritage 8, no. 3: 107. https://doi.org/10.3390/heritage8030107
APA StyleUrbanek, M., Wriessnig, K., Artner, W., Pintér, F., & Ottner, F. (2025). Chemo-Mineralogical Changes in Six European Monumental Stones Caused by Cyclic Isothermal Treatment at 600 °C. Heritage, 8(3), 107. https://doi.org/10.3390/heritage8030107







