The Condition of Contemporary Murals in Sun-Exposed Urban Environments: A Model Study Based on Spray-Painted Mock-Ups and Simulated Light Ageing
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Accelerated Daylight Ageing
2.3. Sample Preparation—Substrates
2.4. Methods
2.4.1. Stereomicroscopy
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Atomic Force Microscopy (AFM)
2.4.4. Contact Angle Measurement
2.4.5. Colorimetry
2.4.6. Gloss Measurement
2.4.7. Differential Scanning Calorimetry (DSC)
2.4.8. UV-VIS Spectroscopy
2.4.9. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.10. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS)
3. Results and Discussion
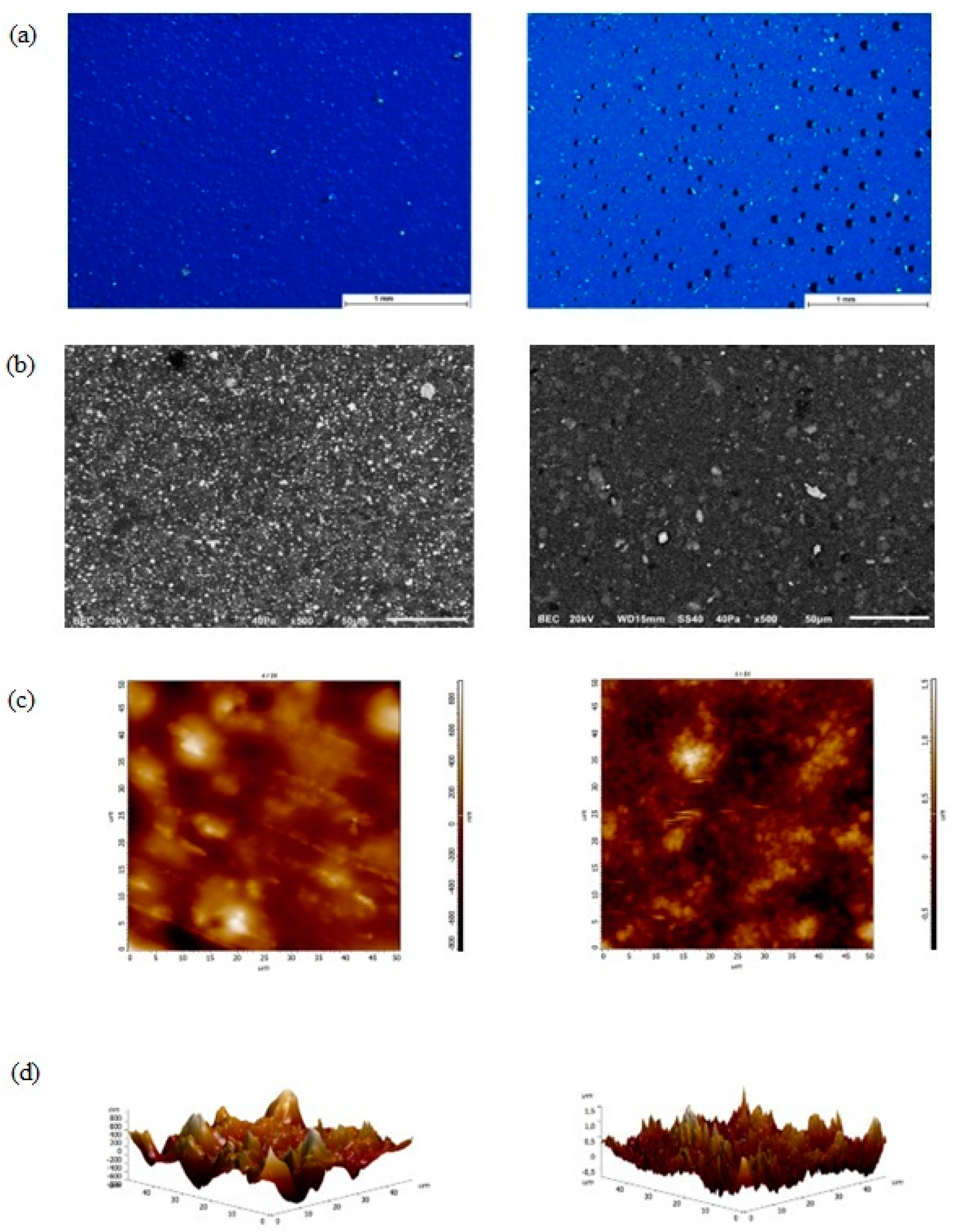
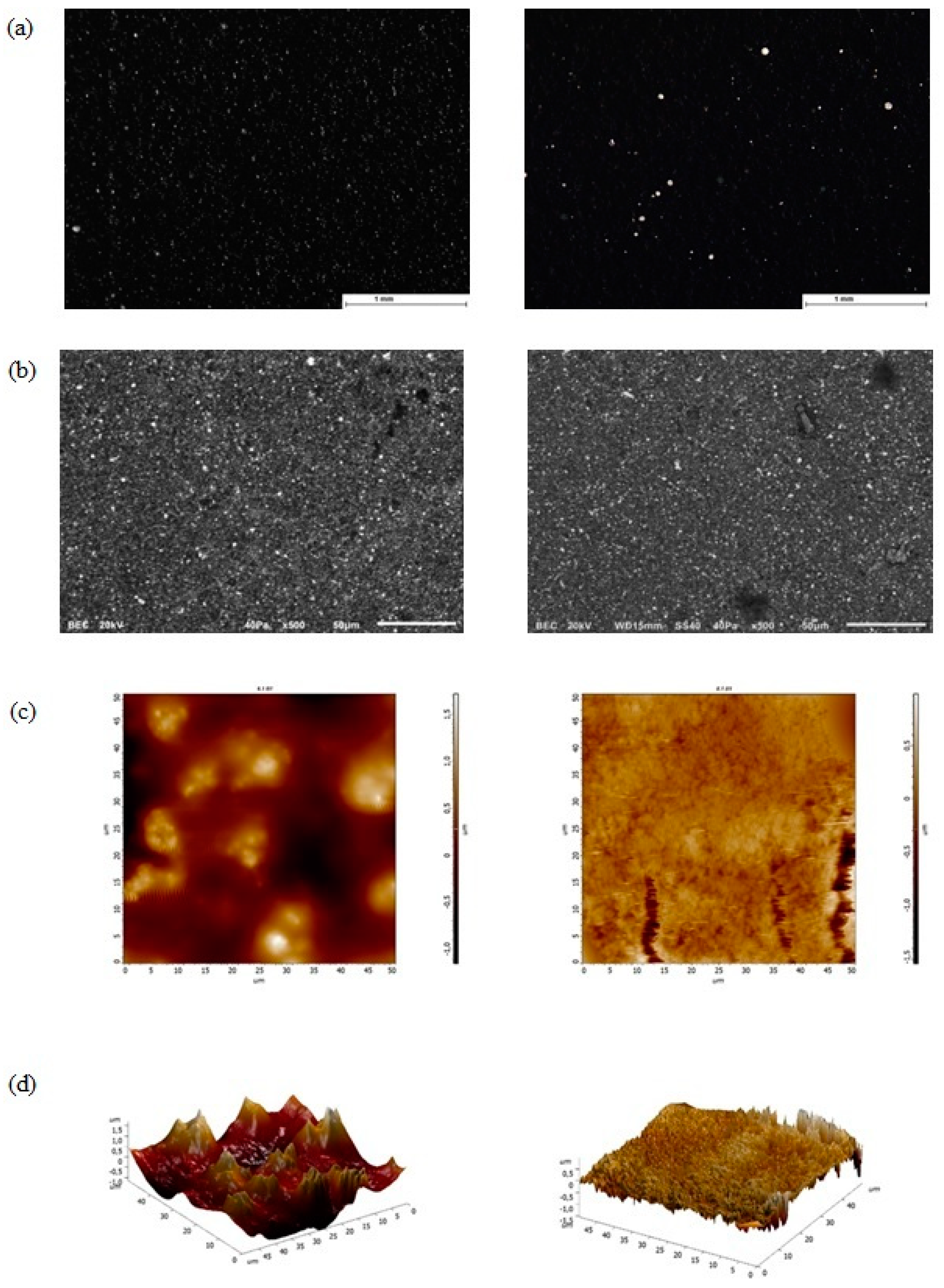
3.1. Morphological Examination with Stereomicroscopy, SEM, AFM
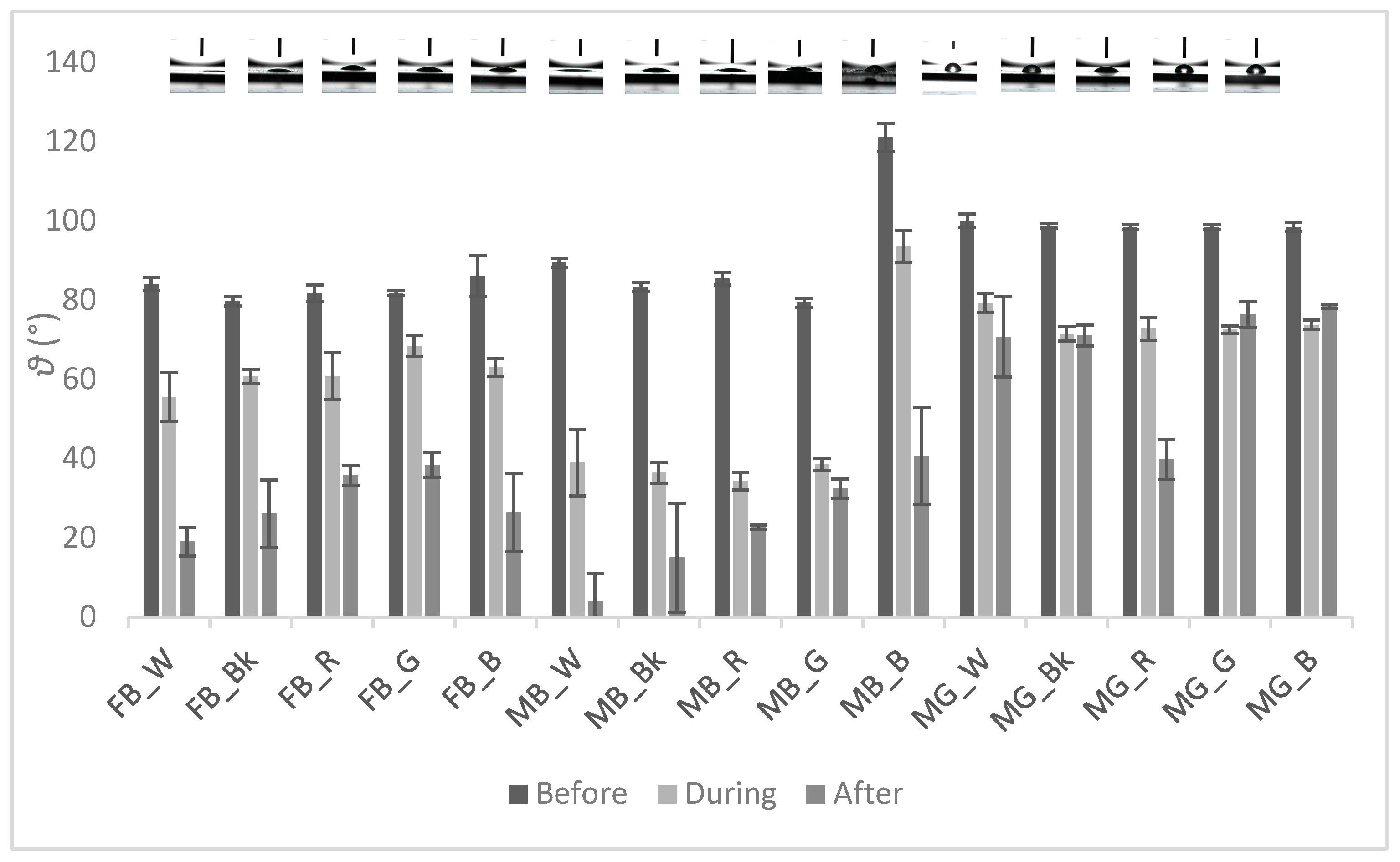
3.2. Contact Angle Measurement
3.3. Colorimetry
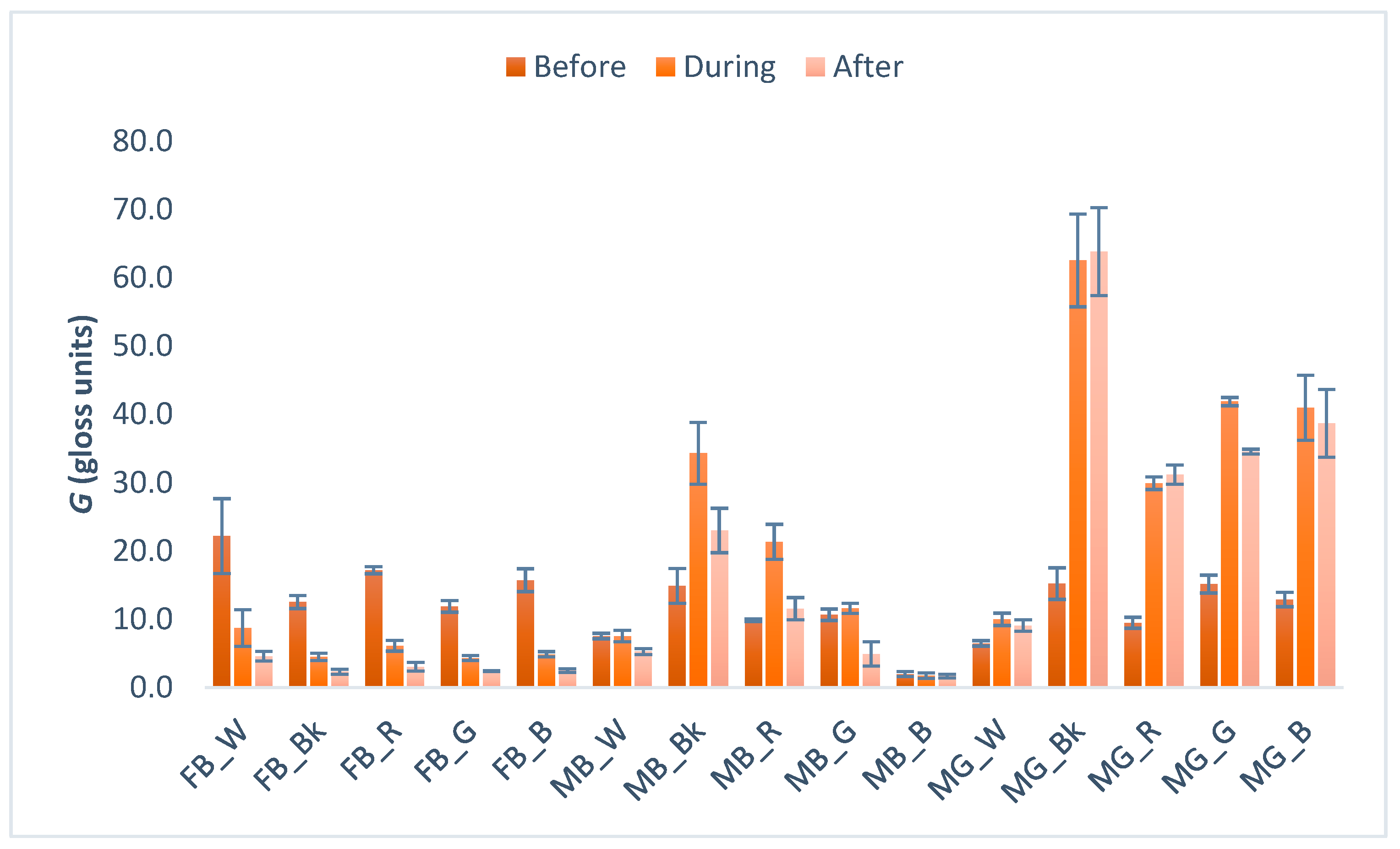
3.4. Gloss Measurement
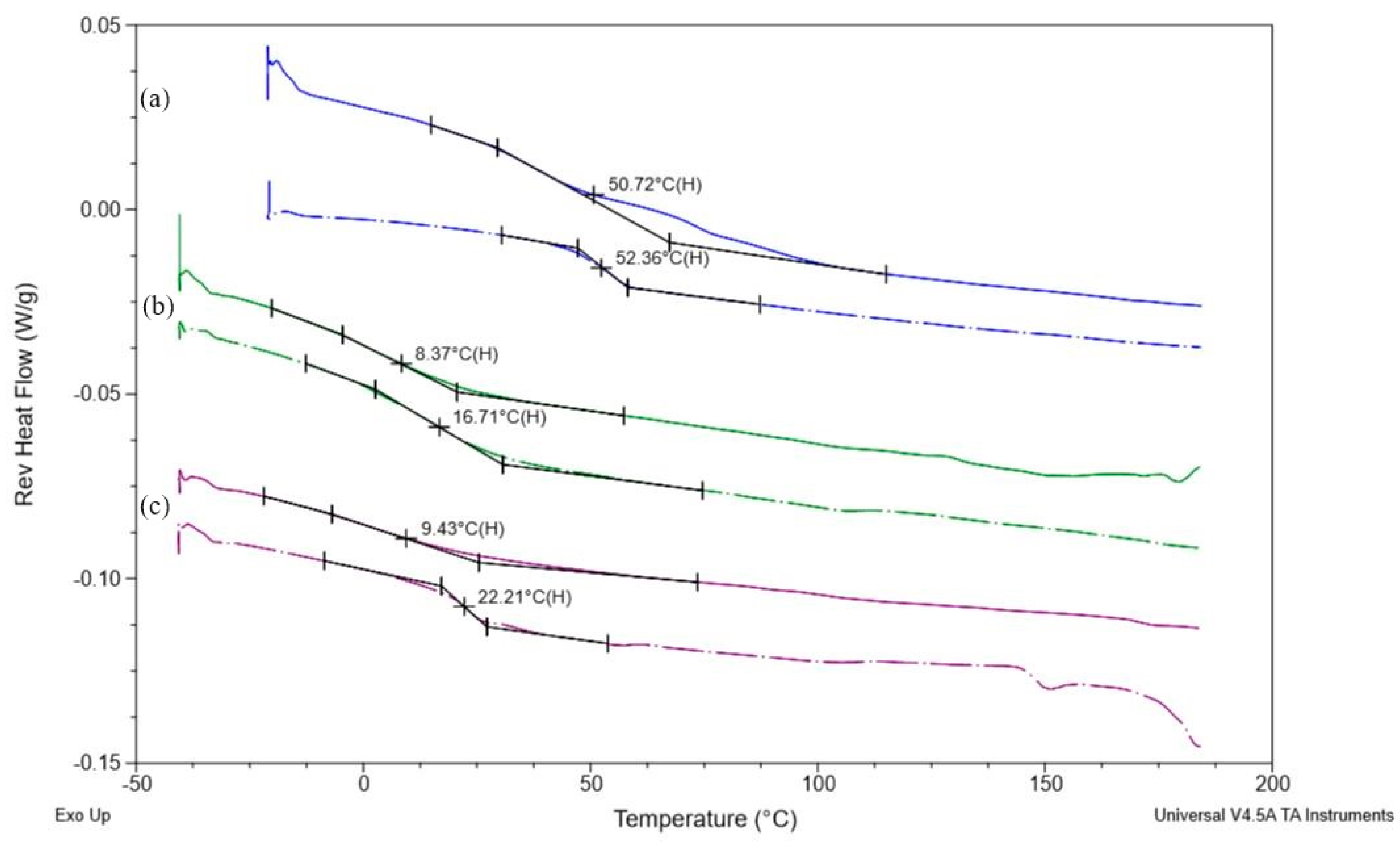
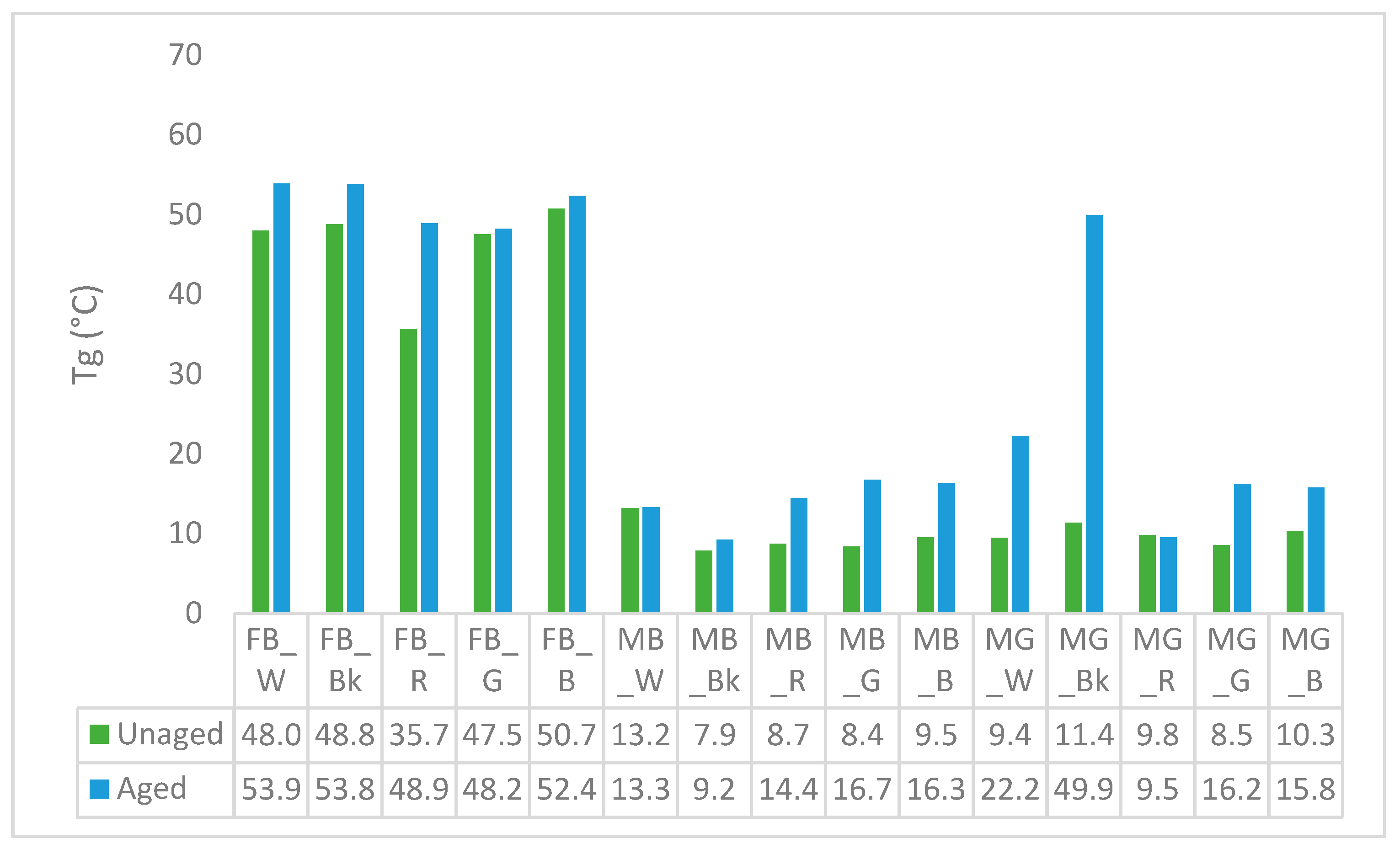
3.5. Differential Scanning Calorimetry (DSC)
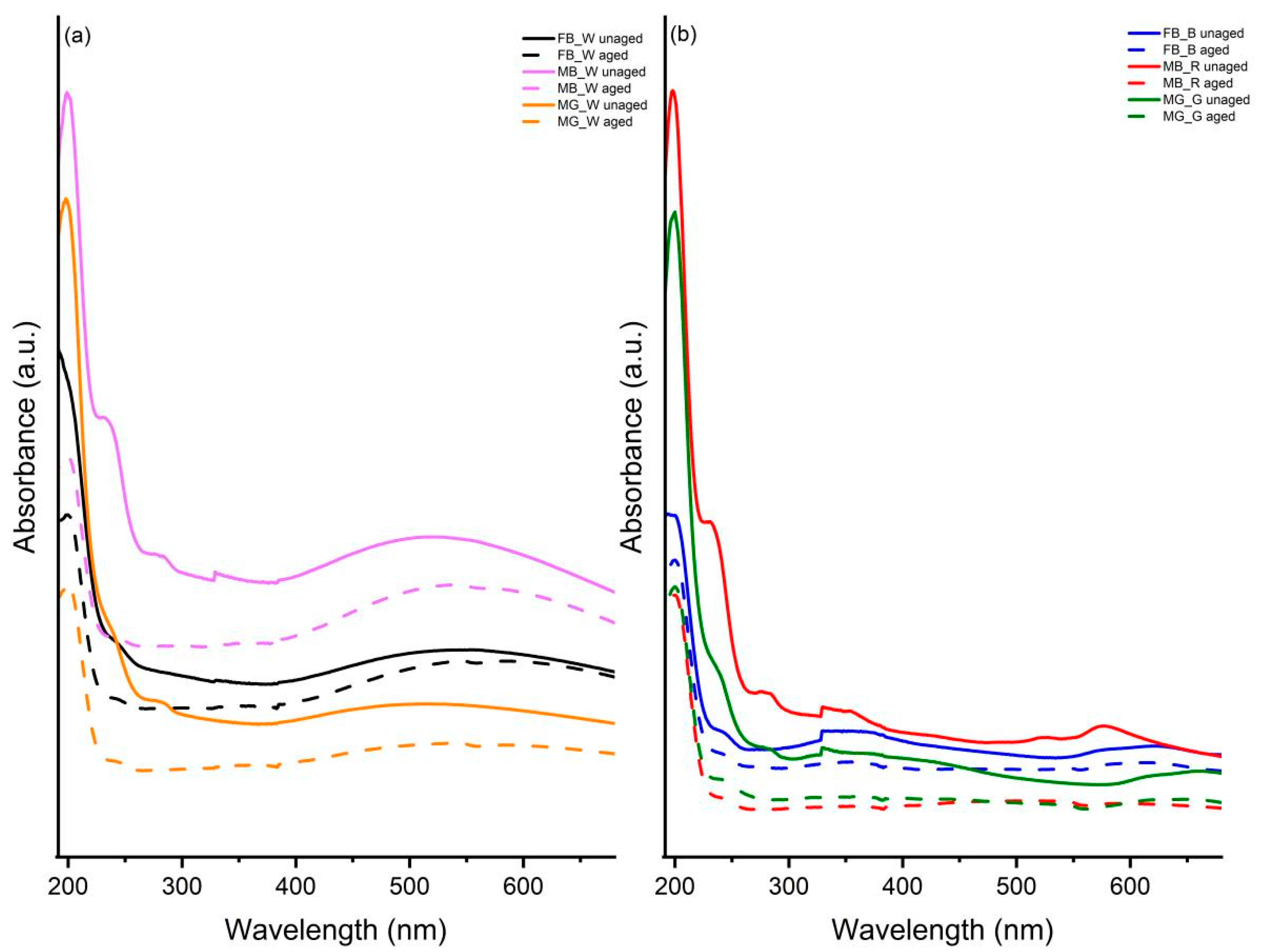
3.6. UV-Vis Spectroscopy
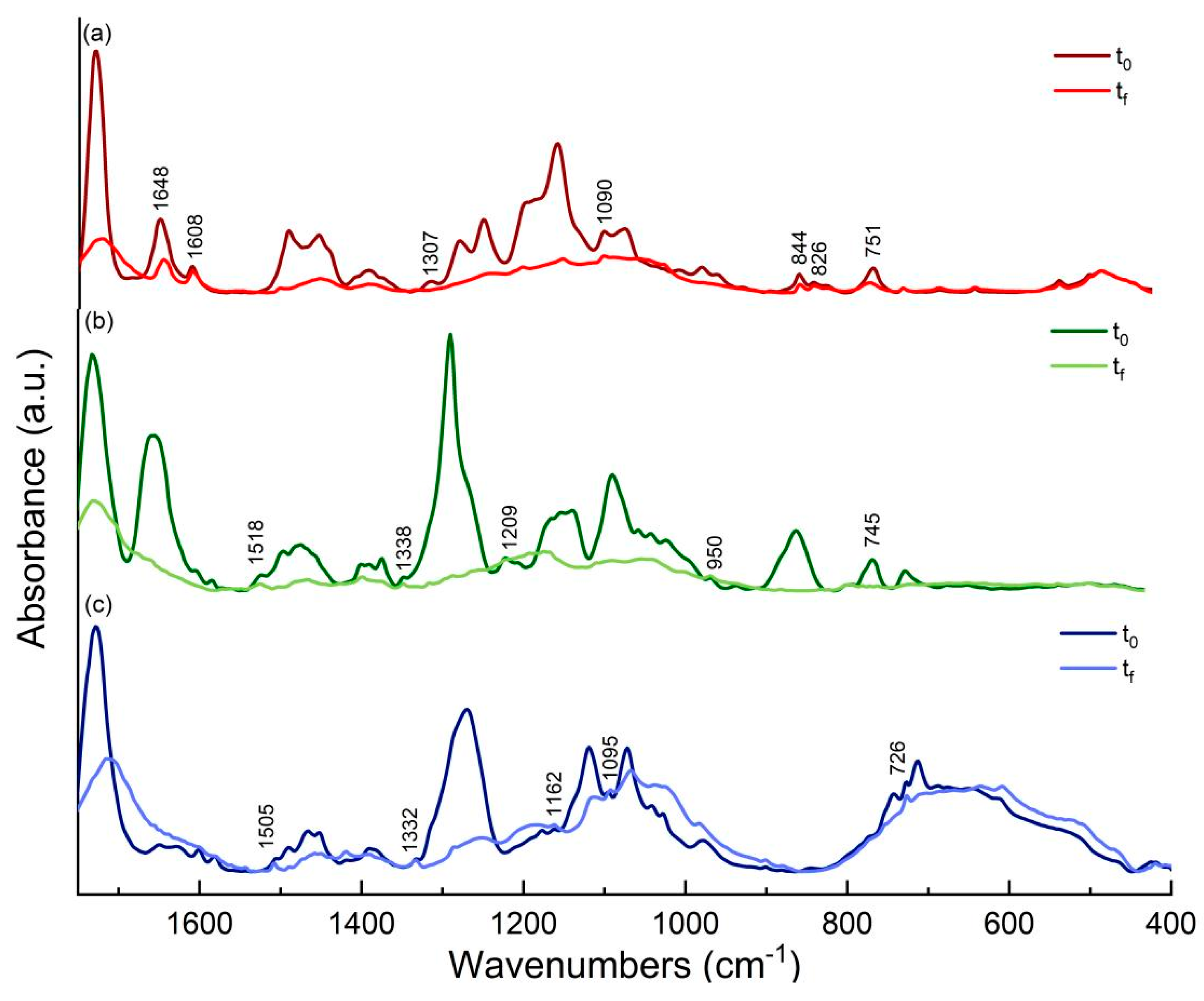
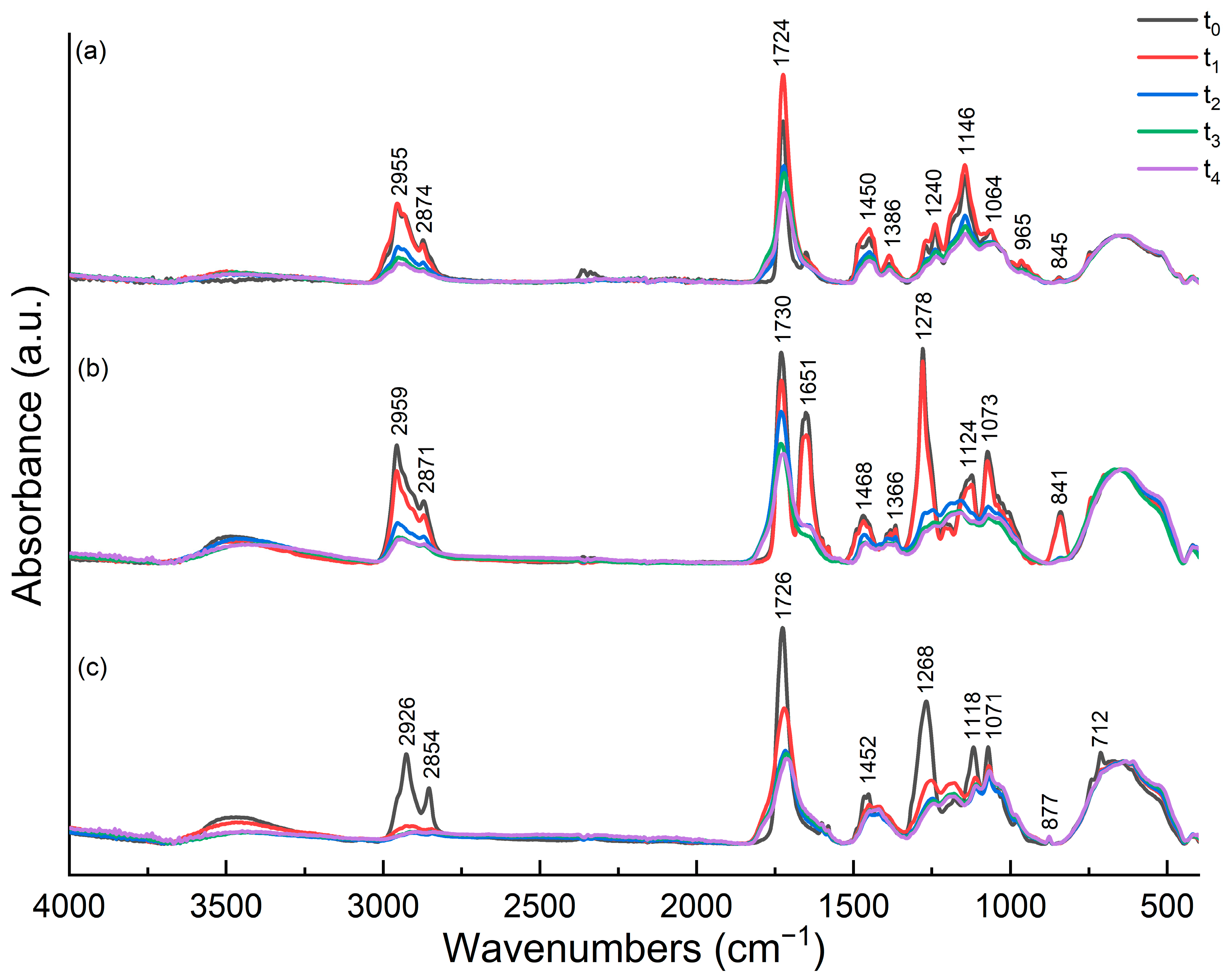
3.7. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)—Discussion on Photo-oxidation Reaction Mechanism of Binders
3.7.1. ATR-FTIR Study of Flame Blue Binders and Pigments
3.7.2. ATR-FTIR Study of Montana Gold Binders and Pigments
3.7.3. ATR-FTIR Study of Montana Black Binders and Pigments
3.7.4. ATR-FTIR Study of Fillers
3.7.5. ATR-FTIR Conclusions
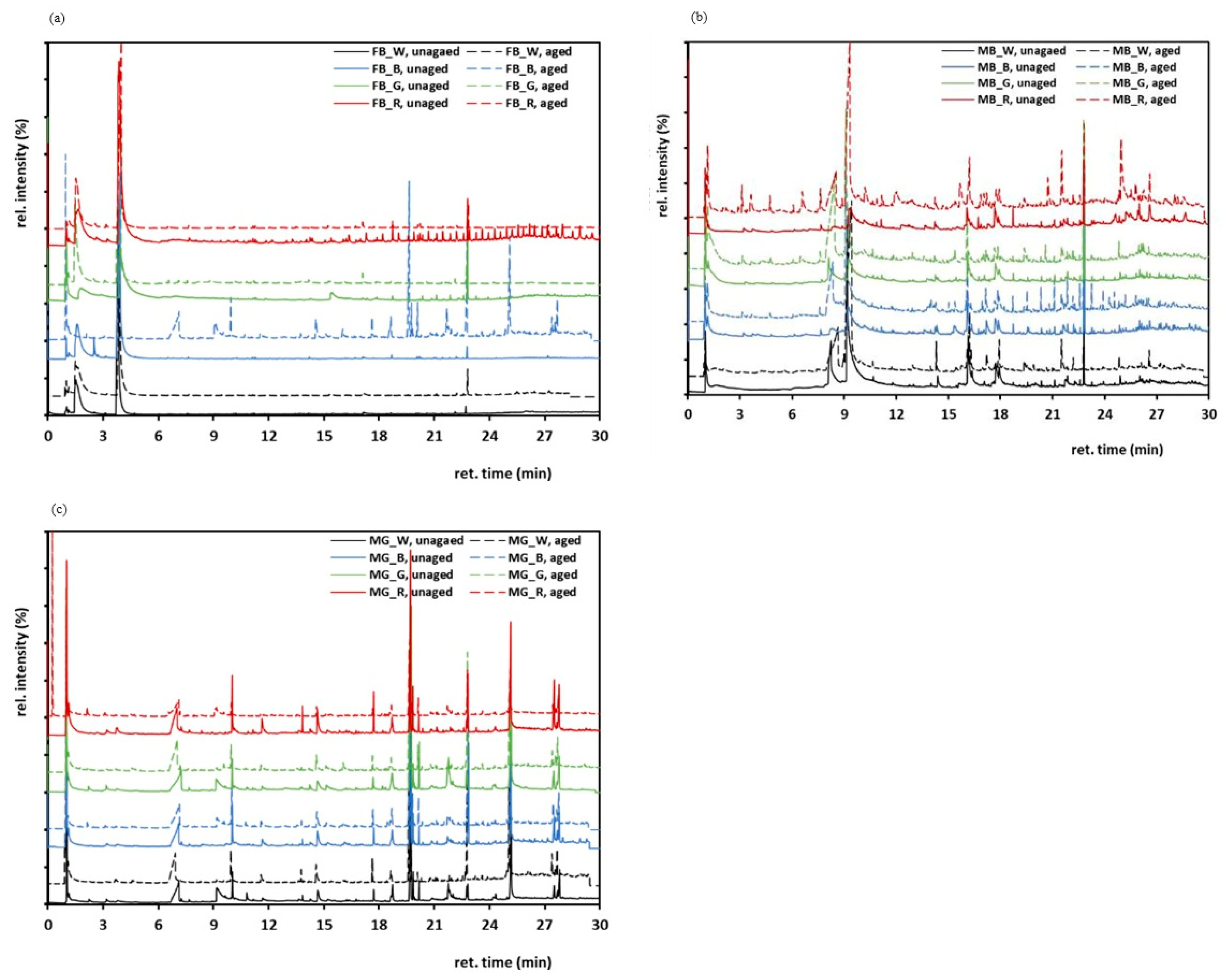
3.8. Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pozo-Antonio, J.S.; Rivas, T.; González, N.; Alonso-Villar, E.M. Deterioration of graffiti spray paints applied on granite after a decade of natural environment. Sci. Total Environ. 2022, 826, 154169. [Google Scholar] [CrossRef]
- Rivas, T.; Alonso-Villar, E.M.; Pozo-Antonio, J.S. Forms and factors of deterioration of urban art murals under humid temperate climate; influence of environment and material properties. Eur. Phys. J. Plus 2022, 137, 1257. [Google Scholar] [CrossRef]
- Cimino, D.; Lamuraglia, R.; Saccani, I.; Berzioli, M.; Izzo, F.C. Assessing the (In)Stability of Urban Art Paints: From Real Case Studies to Laboratory Investigations of Degradation Processes and Preservation Possibilities. Heritage 2022, 5, 581–609. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Papliaka, Z.E.; Andrikopoulos, K.S.; Varella, E.A. Study of the stability of a series of synthetic colorants applied with styrene-acrylic copolymer, widely used in contemporary paintings, concerning the effects of accelerated ageing. J. Cult. Herit. 2010, 11, 381–391. [Google Scholar] [CrossRef]
- Izzo, F.C.; Balliana, E.; Perra, E.; Zendri, E. Accelerated ageing procedures to assess the stability of an unconventional acrylic-wax polymeric emulsion for contemporary art. Polymers 2020, 12, 1925. [Google Scholar] [CrossRef]
- Alonso-Villar, E.M.; Rivas, T.; Pozo-Antonio, J.S. Resistance to artificial daylight of paints used in urban artworks. Influence of paint composition and substrate. Prog. Org. Coat. 2021, 154, 106180. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Pintus, V.; Schreiner, M. Photostability and influence of phthalocyanine pigments on the photodegradation of acrylic paints under accelerated solar radiation. Polym. Degrad. Stab. 2017, 146, 13–23. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, U.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stab. 2006, 91, 3083–3096. [Google Scholar] [CrossRef]
- Chiantore, O.; Lazzari, M. Photo-oxidative stability of paraloid acrylic protective polymers. Polymer 2001, 42, 17–27. [Google Scholar] [CrossRef]
- Cortea, I.M.; Rǎdvan, A.; Vasiliu, C.; Puşcaş, N. Preliminary results of accelerated ageing tests on acrylic art paints. UPB Sci. Bull. Ser. A Appl. Math. Phys. 2014, 76, 215–222. Available online: https://www.scientificbulletin.upb.ro/rev_docs_arhiva/full6f2_394775.pdf (accessed on 15 May 2023).
- Sanmartín, P.; Pozo-Antonio, J.S. Weathering of graffiti spray paint on building stones exposed to different types of UV radiation. Constr. Build. Mater. 2020, 236, 117736. [Google Scholar] [CrossRef]
- Chiantore, O.; Trossarelli, L.; Lazzari, M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer 2000, 41, 1657–1668. [Google Scholar] [CrossRef]
- Ciccola, A.; Guiso, M.; Domenici, F.; Sciubba, F.; Bianco, A. Azo-pigments effect on UV degradation of contemporary art pictorial film: A FTIR-NMR combination study. Polym. Degrad. Stab. 2017, 140, 74–83. [Google Scholar] [CrossRef]
- Melchiorre Di Crescenzo, M.; Zendri, E.; Sánchez-Pons, M.; Fuster-López, L.; Yusá-Marco, D.J. The use of waterborne paints in contemporary murals: Comparing the stability of vinyl, acrylic and styrene-acrylic formulations to outdoor weathering conditions. Polym. Degrad. Stab. 2014, 107, 285–293. [Google Scholar] [CrossRef]
- Ranby, B.; Rabek, J. Photodegradation, Photooxidation, and Photostabilization of Polymers; Wiley: New York, USA, 1975. [Google Scholar]
- Turro, N.J. Modern Molecular Photochemistry. The Benjamin/Cummings Publishing Co., Inc.: Menlo Park, CA, USA, 1978. [Google Scholar]
- Doménech-Carbó, M.T.; Silva, M.F.; Aura-Castro, E.; Fuster-López, L.; Kröner, S.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; Osete-Cortina, L.; Doménech, A.; et al. Study of behaviour on simulated daylight ageing of artists’ acrylic and poly(vinyl acetate) paint films. Anal. Bioanal. Chem. 2011, 399, 2921–2937. [Google Scholar] [CrossRef]
- Ploeger, R.; Chiantore, O. Characterization and Stability Issues of Artists’ Alkyd Paints. Smithson. Contrib. Mus. Conserv. 2012, 3, 89–95. [Google Scholar]
- Ploeger, R.; Musso, S.; Chiantore, O. Contact angle measurements to determine the rate of surface oxidation of artists’ alkyd paints during accelerated photo-ageing. Prog. Org. Coat. 2009, 65, 77–83. [Google Scholar] [CrossRef]
- Duce, C.; Della Porta, V.; Tiné, M.R.; Spepi, A.; Ghezzi, L.; Colombini, M.P.; Bramanti, E. FTIR study of ageing of fast drying oil colour (FDOC) alkyd paint replicas. Spectrochim. Acta—Part. A Mol. Biomol. Spectrosc. 2014, 130, 214–221. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Vladislav, J.M.; Stamenković, J.V.; Stojiljković, D.T. IR-change and colour changes of long-oil air drying alkyd paints as a result of UV irradiation. Prog. Org. Coat. 2012, 73, 401–408. [Google Scholar] [CrossRef]
- Perrin, F.X.; Irigoyen, M.; Aragon, E.; Vernet, J.L. Artificial aging of acrylurethane and alkyd paints: A micro-ATR spectroscopic study. Polym. Degrad. Stab. 2000, 70, 469–475. [Google Scholar] [CrossRef]
- Pagnin, L.; Calvini, R.; Wiesinger, R.; Weber, J.; Schreiner, M. Photodegradation Kinetics of Alkyd Paints: The Influence of Varying Amounts of Inorganic Pigments on the Stability of the Synthetic Binder. Front. Mater. 2020, 7, 600887. [Google Scholar] [CrossRef]
- Anghelone, M.; Stoytschew, V.; Jembrih-Simbürger, D.; Schreiner, M. Spectroscopic methods for the identification and photostability study of red synthetic organic pigments in alkyd and acrylic paints. Microchem. J. 2018, 139, 155–163. [Google Scholar] [CrossRef]
- Ecco, L.G.; Rossi, S.; Fedel, M.; De, F. Color variation of electrophoretic styrene-acrylic paints under field and accelerated ultraviolet exposure. Mater. Des. 2017, 116, 554–564. [Google Scholar] [CrossRef]
- Giacomucci, L.; Toja, F.; Sanmartín, P.; Toniolo, L.; Prieto, B.; Villa, F.; Cappitelli, F. Degradation of nitrocellulose-based paint by Desulfovibrio desulfuricans ATCC 13541. Biodegradation 2012, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berthumeyrie, S.; Collin, S.; Bussiere, P.O.; Therias, S. Photooxidation of cellulose nitrate: New insights into degradation mechanisms. J. Hazard. Mater. 2014, 272, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Shashoua, Y.; Bradley, S.M.; Daniels, V.D. Degradation of cellulose nitrate adhesive. Stud. Conserv. 1992, 37, 113–119. [Google Scholar] [CrossRef]
- Webster, R.I.; Webster, H.F. Light Induced Degradation of Nitrocellulose Lacquer Thin Films for Conservation of Art Objects. MRS Adv. 2017, 2, 3951–3957. [Google Scholar] [CrossRef]
- Neves, A.; Ramos, A.M.; Callapez, M.E.; Friedel, R.; Réfrégiers, M.; Thoury, M.; Melo, M.J. Novel markers to early detect degradation on cellulose nitrate-based heritage at the submicrometer level using synchrotron UV–VIS multispectral luminescence. Sci. Rep. 2021, 11, 20208. [Google Scholar] [CrossRef]
- Selwitz, C. Cellulose Nitrate in Conservation: Research in Conservation; Getty Conservation Institute: Los Angeles, CA, USA, 1988. [Google Scholar]
- Koob, S. The instability of cellulose nitrate adhesives. Conservator 1982, 6, 31–34. [Google Scholar] [CrossRef]
- Farmakalidis, H.V.; Boyatzis, S.; Douvas, A.M.; Karatasios, I.; Sotiropoulou, S.; Argitis, P.; Chryssoulakis, Y.; Kilikoglou, V. The effect of TiO2 component on the properties of acrylic and urea-aldehyde resins under accelerated ageing conditions. Pure Appl. Chem. 2017, 89, 1659–1671. [Google Scholar] [CrossRef]
- Whitmore, P.M.; Colaluca, V.G. The natural and accelerated aging of an acrylic artists’ medium. Stud. Conserv. 1995, 40, 51–64. [Google Scholar] [CrossRef]
- Sanmartín, P.; Cappitelli, F. Evaluation of Accelerated Ageing Tests for Metallic Non-Metallic Graffiti Paints Applied to Stone. Coatings 2017, 7, 180. [Google Scholar] [CrossRef]
- Pagnin, L.; Guarnieri, N.; Izzo, F.C.; Goidanich, S.; Toniolo, L. Protecting Street Art from Outdoor Environmental Threats: What Are the Challenges? Coatings 2023, 13, 2044. [Google Scholar] [CrossRef]
- Pellis, G.; Bertasa, M.; Ricci, C.; Scarcella, A.; Croveri, P.; Poli, T.; Scalarone, D. A multi-analytical approach for precise identification of alkyd spray paints and for a better understanding of their ageing behaviour in graffiti and urban artworks. J. Anal. Appl. Pyrolysis 2022, 165, 105576. [Google Scholar] [CrossRef]
- Marazioti, V.; Douvas, A.M.; Katsaros, F.; Koralli, P.; Chochos, C.; Gregoriou, V.G.; Boyatzis, S.; Facorellis, Y. Chemical characterisation of artists’ spray-paints: A diagnostic tool for urban art conservation. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2023, 291, 122375. [Google Scholar] [CrossRef] [PubMed]
- ISO 16474-2:2013/Amd 1:2022; Paints and Varnishes—Methods of Exposure to Laboratory Light sources—Part 2: Xenon-arc Lamps. International Organization for Standardization: Geneva, Switzerland, 2013.
- Kampasakali, E.; Ormsby, B.; Cosentino, A.; Miliani, C.; Learner, T. A Preliminary Evaluation of the Surfaces of Acrylic Emulsion Paint Films and the Effects of Wet-Cleaning Treatment by Atomic Force Microscopy (AFM). Stud. Conserv. 2013, 56, 216–230. [Google Scholar] [CrossRef]
- de Sá, M.H.; Eaton, P.; Ferreira, J.L.; Melo, M.J.; Ramos, A.M. Ageing of vinyl emulsion paints—An atomic force microscopy study. Surf. Interface Anal. 2011, 43, 1160–1164. [Google Scholar] [CrossRef]
- Sauerbier, P.; Köhler, R.; Renner, G.; Militz, H. Surface activation of polylactic acid-based wood-plastic composite by atmospheric pressure plasma treatment. Materials 2020, 13, 4673. [Google Scholar] [CrossRef]
- European Standard, EN 15802; Conservation of Cultural Property-Test Methods-Determination of Static Contact Angle. IEEE: Washington, DC, USA, 2010.
- ISO/CIE 11664-4:2019; Colorimetry—Part4:CIE1976L*a*b*ColourSpace. International Organization for Standardization/International Commission on Illumination: Geneva, Switzerland, 2019.
- Farbmetrische Bestimmung von Farbmaßzahlen und Farbabständen im angenähert gleichförmigen CIELAB-Farbraum 2007.
- Brischke, C.; Obermeyer, C.; Mengel, U. Outdoor Performance of Artist Colours. Restor. Build. Monum. 2011, 17, 91–102. [Google Scholar] [CrossRef]
- ISO 2813:2014; Paints and Varnishes—Determination of Gloss Values at 20°, 60° and 85°. International Organization for Standardization: Geneva, Switzerland, 2014.
- Pintus, V.; Ploeger, R.; Chiantore, O.; Wei, S.; Schreiner, M. Thermal analysis of the interaction of inorganic pigments with p(nBA/MMA) acrylic emulsion before and after UV ageing. J. Therm. Anal. Calorim. 2013, 114, 33–43. [Google Scholar] [CrossRef]
- Simon, S.L. Temperature-modulated differential scanning calorimetry: Theory and application. Thermochim. Acta 2001, 374, 55–71. [Google Scholar] [CrossRef]
- Picollo, M.; Aceto, M.; Vitorino, T. 5. UV-Vis spectroscopy. In Chemical Analysis in Cultural Heritage; Sabbatini, L., van der Werf, I., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2020; pp. 99–120. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T. Novel analytical methods for characterising binding media and protective coatings in artworks. Anal. Chim. Acta 2008, 621, 109–139. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Tamias, G.A.; Chatzicharistou, E.A.; Achilias, D.S. Thermal Treatment of a Commercial Polycyanoacrylate Adhesive Addressed for Instant Glass Restoration, for Investigating Its Ageing Tolerance. Macromol 2023, 3, 636–652. [Google Scholar] [CrossRef]
- Aguilar-Rodríguez, P.; Mejía-González, A.; Zetina, S.; Colin-Molina, A.; Rodríguez-Molina, B.; Esturau-Escofet, N. Unexpected behavior of commercial artists’ acrylic paints under UVA artificial aging. Microchem. J. 2021, 160, 105743. [Google Scholar] [CrossRef]
- Mejía-González, A.; Zetina, S.; Espinosa-Pesqueira, M.E.; Esturau-Escofet, N. Analysis and Characterization of commercial artists’ acrylic paints and the influence of UV light on aging. Int. J. Polym. Anal. Charact. 2017, 22, 473–482. [Google Scholar] [CrossRef]
- Fardi, T.; Kampasakali, E.; Terzopoulou, Z.; Pintus, V.; Pavlidou, E.; Schreiner, M. A preliminary study on the physicochemical properties of pigmented Sty/nBA/MMA emulsion films: The effect of thermal ageing. Polym. Degrad. Stab. 2018, 158, 157–167. [Google Scholar] [CrossRef]
- de la Rie, E.R.; Michelin, A.; Ngako, M.; Del Federico, E.; Del Grosso, C. Photo-catalytic degradation of binding media of ultramarine blue containing paint layers: A new perspective on the phenomenon of “ultramarine disease” in paintings. Polym. Degrad. Stab. 2017, 144, 43–52. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Barral, D.; Herrera, A.; Elert, K.; Rivas, T.; Cardell, C. Effect of tempera paint composition on their superficial physical properties- application of interferometric profilometry and hyperspectral imaging techniques. Prog. Org. Coat. 2018, 117, 56–68. [Google Scholar] [CrossRef]
- Ormsby, B.; Learner, T. Artists’ acrylic emulsion paints: Materials, meaning and conservation treatment options. AICCM Bull. 2013, 34, 57–65. [Google Scholar] [CrossRef]
- Lazzari, M.; Scalarone, D.; Malucelli, G.; Chiantore, O. Durability of acrylic films from commercial aqueous dispersion: Glass transition temperature and tensile behavior as indexes of photooxidative degradation. Prog. Org. Coat. 2011, 70, 116–121. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Thermal-ageing of paraloid acrylic protective polymers. Polymer. 2000, 41, 6447–6455. [Google Scholar] [CrossRef]
- Hiskia, A.; Mylonas, A.; Papaconstantinou, E. Comparison of the photoredox properties of polyoxometallates and semiconducting particles. Chem. Soc. Rev. 2001, 30, 62–69. [Google Scholar] [CrossRef]
- Rothenberger, G.; Moser, J.; Grätzel, M.; Serpone, N.; Sharma, D.K. Charge Carrier Trapping and Recombination Dynamics in Small Semiconductor Particles. J. Am. Chem. Soc. 1985, 107, 8054–8059. [Google Scholar] [CrossRef]
- Kemp, T.J.; McIntyre, R.A. Influence of transition metal-doped titanium(IV) dioxide on the photodegradation of polyethylene. Polym. Degrad. Stab. 2006, 91, 3020–3025. [Google Scholar] [CrossRef]
- Shang, J.; Chai, M.; Zhu, Y. Solid-phase photocatalytic degradation of polystyrene plastic with TiO2 as photocatalyst. J. Solid. State Chem. 2003, 174, 104–110. [Google Scholar] [CrossRef]
- Pintus, V.; Schreiner, M. Characterization and identification of acrylic binding media: Influence of UV light on the ageing process. Anal. Bioanal. Chem. 2011, 399, 2961–2976. [Google Scholar] [CrossRef] [PubMed]
- Cocca, M.; Arienzo, L.D.; Orazio, L.D.; Gentile, G.; Martuscelli, E. Polyacrylates for conservation: Chemico-physical properties and durability of different commercial products. Polym. Test. 2004, 23, 333–342. [Google Scholar] [CrossRef]
- Perrin, F.X.; Irigoyen, M.; Aragon, E.; Vernet, J.L. Evaluation of accelerated weathering tests for three paint systems: A comparative study of their aging behaviour. Polym. Degrad. Stab. 2001, 72, 115–124. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Schreiner, M. Influence of phthalocyanine pigments on the photo-degradation of alkyd artists’ paints under different conditions of artificial solar radiation. Polym. Degrad. Stab. 2016, 134, 157–168. [Google Scholar] [CrossRef]
- Werner, R.; Krysztafkiewicz, A.; Dec, A.; Jesionowski, T. Effect of surface modification on physicochemical properties of precipitated sodium- Aluminium silicate, used as a pigment in acrylic dispersion paints. Dyes Pigment. 2001, 50, 41–54. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Schreiner, M. A comparison study of alkyd resin used in art works by Py-GC/MS and GC/MS: The influence of aging. J. Anal. Appl. Pyrolysis 2013, 104, 441–447. [Google Scholar] [CrossRef]
- Learner, T.J.S. Analysis of Modern Paints; Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Chrissafis, K.; Pavlidou, E.; Vouvoudi, E.; Bikiaris, D. Decomposition kinetic and mechanism of syndiotactic polystyrene nanocomposites with MWCNTs and nanodiamonds studied by TGA and Py-GC/MS. Thermochim. Acta 2014, 583, 15–24. [Google Scholar] [CrossRef]
- Germinario, G.; van der Werf, I.D.; Sabbatini, L. Chemical characterisation of spray paints by a multi-analytical (Py/GC–MS, FTIR, μ-Raman) approach. Microchem. J. 2016, 124, 929–939. [Google Scholar] [CrossRef]
- Silva, M.F.; Doménech-Carbó, M.T.; Osete-Cortina, L. Characterization of additives of PVAc and acrylic waterborne dispersions and paints by analytical pyrolysis-GC-MS and pyrolysis-silylation-GC-MS. J. Anal. Appl. Pyrolysis 2015, 113, 606–620. [Google Scholar] [CrossRef]
- Jalageri, M.D.; Nagaraja, A.; Puttaiahgowda, Y.M. Piperazine based antimicrobial polymers: A review. RSC Adv. 2021, 11, 15213–15230. [Google Scholar] [CrossRef]




| Sample ID | Manufacturer —Colour | Code Names | Composition | |||
|---|---|---|---|---|---|---|
| Binder * | Synthetic Organic Pigments | Inorganic Pigments and Fillers | ||||
| FB_W | Flame White | Blue | FB-900 Pure White | P(nBMA-MMA)/NC | PW6 PW19 PW21 PW26 | |
| FB_Bk | Flame Black | Blue | FB-904 Deep Black | P(nBMA-MMA)/NC | PW6 PW19 PW26 | |
| FB_R | Flame Red | Blue | FB-312 Fire Red | P(nBMA-MMA)/NC | PR254 | PW6, PW19 PW21 PW26 |
| FB_G | Flame Green | Blue | FB-630 Fern Green | P(nBMA-MMA)/NC | PB15:1 PG7 PY74 | PW6 PW18 PW19 PW21 PW25 PW26 |
| FB_B | Flame Blue | Blue | FB-512 Signal Blue | P(nBMA-MMA)/NC | PB15:1 | PW6 PW19 PW21 PW26 |
| MB_W | Montana Black —White | BLK400-9105 White | Alkyd/NC/Sty | PW6 PW18 PW19 PW21 PW25 PW26 | ||
| MB_Bk | Montana Black —Black | BLK409-9001 Black | Alkyd/NC/Sty | PW6 PW19 PW21 PW26 | ||
| MB_R | Montana Black—Red | BLK400-2093 Code Red | Alkyd/NC/Sty | PR112 | PW6 PW19 PW21 PW26 | |
| MB_G | Montana Black —Green | BLK460-6055 Boston | Alkyd/NC/Sty | PG7 PY74 | PW6 PW19 PW21 PW26 | |
| MB_B | Montana Black—Blue | BLK400-570 Horizon | Alkyd/NC/Sty | PB15:6 | PW6 PW19 PW21 PW26 | |
| MG_W | Montana Gold—White | S9100 Sh. White | P(nBMA-MMA)/NC/Sty ** | PW6 PW19 PW21 | ||
| MG_Bk | Montana Gold— Black | S9000 Sh. Black | P(nBMA-MMA)/NC/Sty ** | PW19 PW26 | ||
| MG_R | Montana Gold—Red | S3000 Sh. Red | P(nBMA-MMA)/NC/Sty | PR112 | PW6 PW19 PW26 | |
| MG_G | Montana Gold—Green | S6020 Sh. Green Dark | P(nBMA-MMA)/NC/Sty ** | PG7 PY74 | PW6 PW19 PW21 | |
| MG_B | Montana Gold—Blue | S5010 Sh. Blue | P(nBMA-MMA)/NC/Sty ** | PB15:6 | PW6 PW19 PW21 PW26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marazioti, V.; Douvas, A.M.; Vouvoudi, E.C.; Bikiaris, D.; Papadokostaki, K.; Nioras, D.; Gogolides, E.; Orfanoudakis, S.; Stergiopoulos, T.; Boyatzis, S.; et al. The Condition of Contemporary Murals in Sun-Exposed Urban Environments: A Model Study Based on Spray-Painted Mock-Ups and Simulated Light Ageing. Heritage 2024, 7, 3932-3959. https://doi.org/10.3390/heritage7080186
Marazioti V, Douvas AM, Vouvoudi EC, Bikiaris D, Papadokostaki K, Nioras D, Gogolides E, Orfanoudakis S, Stergiopoulos T, Boyatzis S, et al. The Condition of Contemporary Murals in Sun-Exposed Urban Environments: A Model Study Based on Spray-Painted Mock-Ups and Simulated Light Ageing. Heritage. 2024; 7(8):3932-3959. https://doi.org/10.3390/heritage7080186
Chicago/Turabian StyleMarazioti, Varvara, Antonios M. Douvas, Evangelia C. Vouvoudi, Dimitrios Bikiaris, Kyriaki Papadokostaki, Dimitrios Nioras, Evangelos Gogolides, Spyros Orfanoudakis, Thomas Stergiopoulos, Stamatios Boyatzis, and et al. 2024. "The Condition of Contemporary Murals in Sun-Exposed Urban Environments: A Model Study Based on Spray-Painted Mock-Ups and Simulated Light Ageing" Heritage 7, no. 8: 3932-3959. https://doi.org/10.3390/heritage7080186
APA StyleMarazioti, V., Douvas, A. M., Vouvoudi, E. C., Bikiaris, D., Papadokostaki, K., Nioras, D., Gogolides, E., Orfanoudakis, S., Stergiopoulos, T., Boyatzis, S., & Facorellis, Y. (2024). The Condition of Contemporary Murals in Sun-Exposed Urban Environments: A Model Study Based on Spray-Painted Mock-Ups and Simulated Light Ageing. Heritage, 7(8), 3932-3959. https://doi.org/10.3390/heritage7080186









