Predicted Dynamic of Biodeterioration in Cultural Heritage Stones Due to Climate Changes in Humid Tropical Regions—A Case Study on the Rhodotorula sp. Yeast
Abstract
:1. Introduction
- (i)
- It affects highly populated areas with considerable building density.
- (ii)
- Thanks to the high average annual temperature and humidity, the tropical humid climates clearly offer ideal conditions for the growth of stone colonizers.
- (i)
- Will climate change favor or reduce the growth of Rhodotorula sp. on limestone substrates?
- (ii)
- Given the escalating levels of atmospheric CO2 and temperatures, is there an anticipated change in the biodegradation caused by Rhodotorula sp.?
Stone Materials Biodeterioration Due to Rhodotorula sp. Yeast
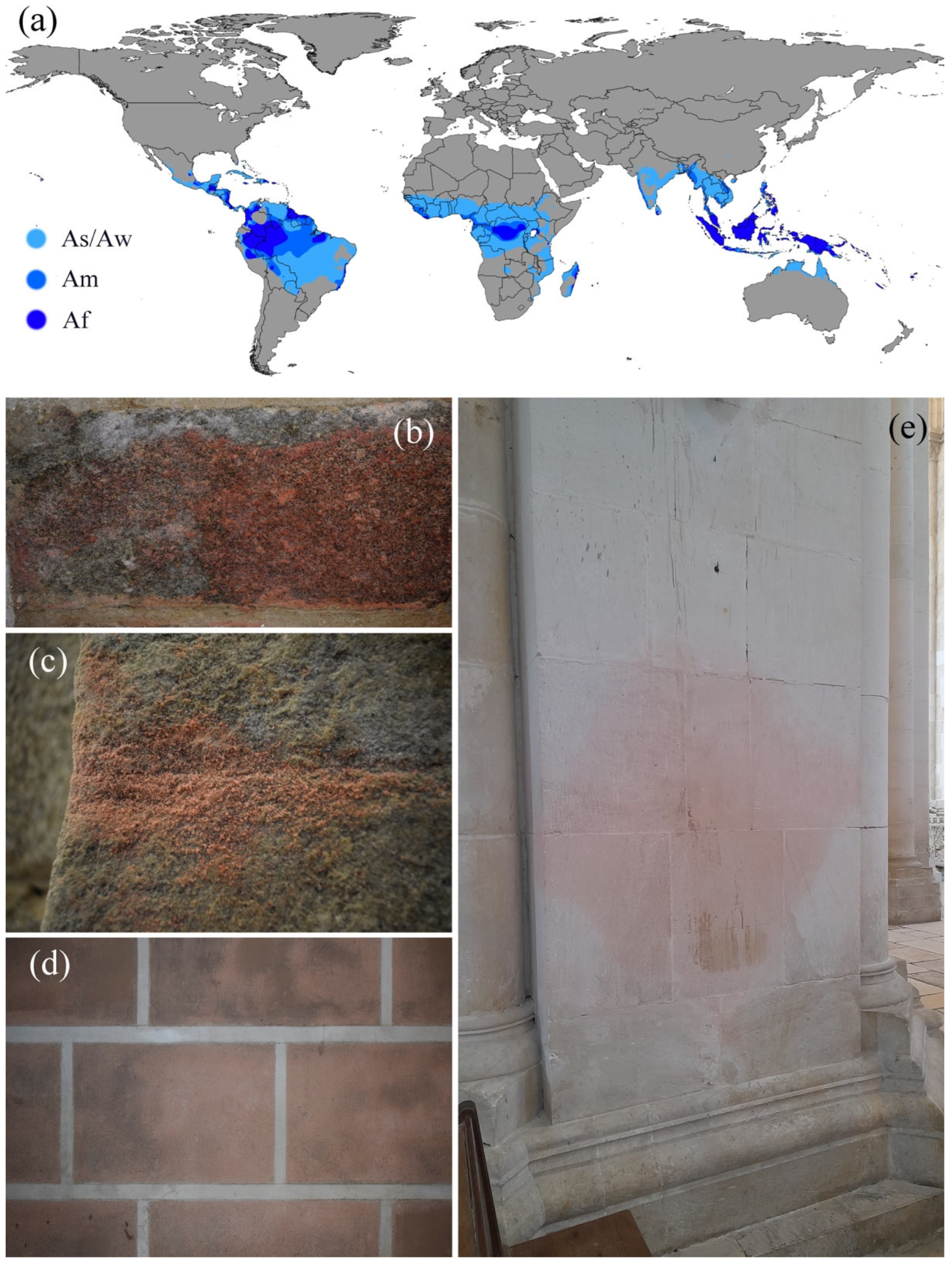
2. Materials and Methods
2.1. Materials
2.2. Methods
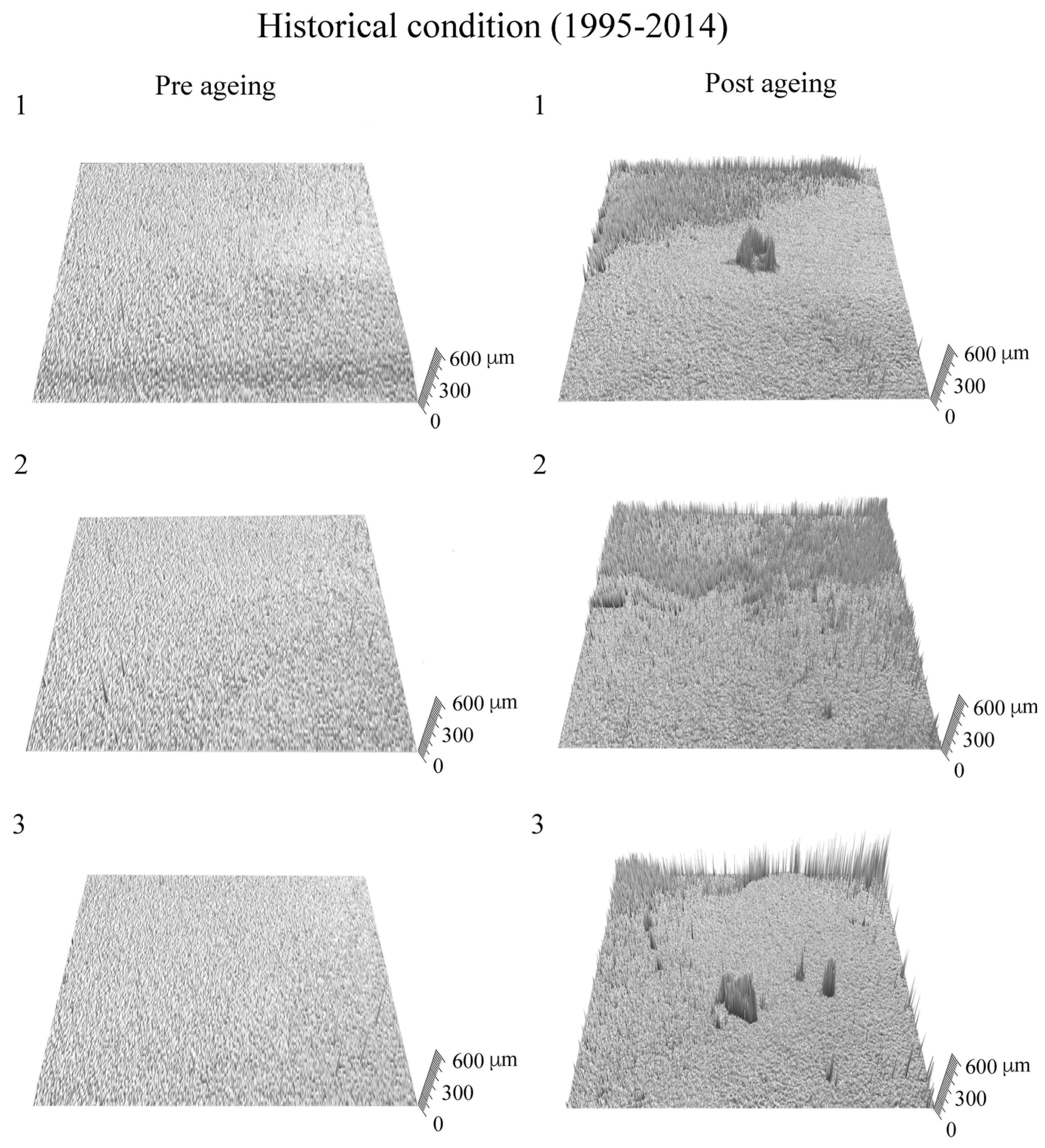
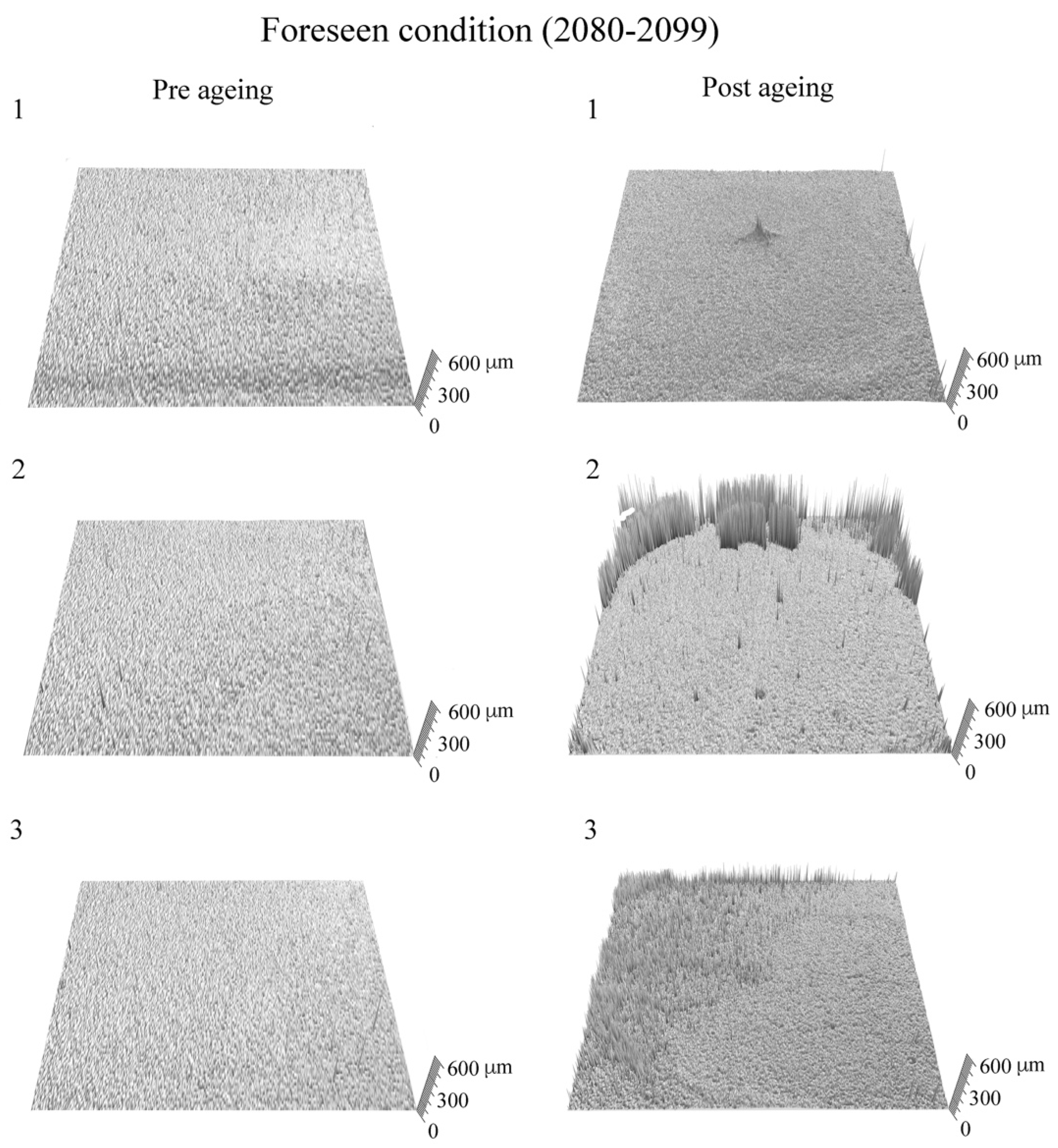
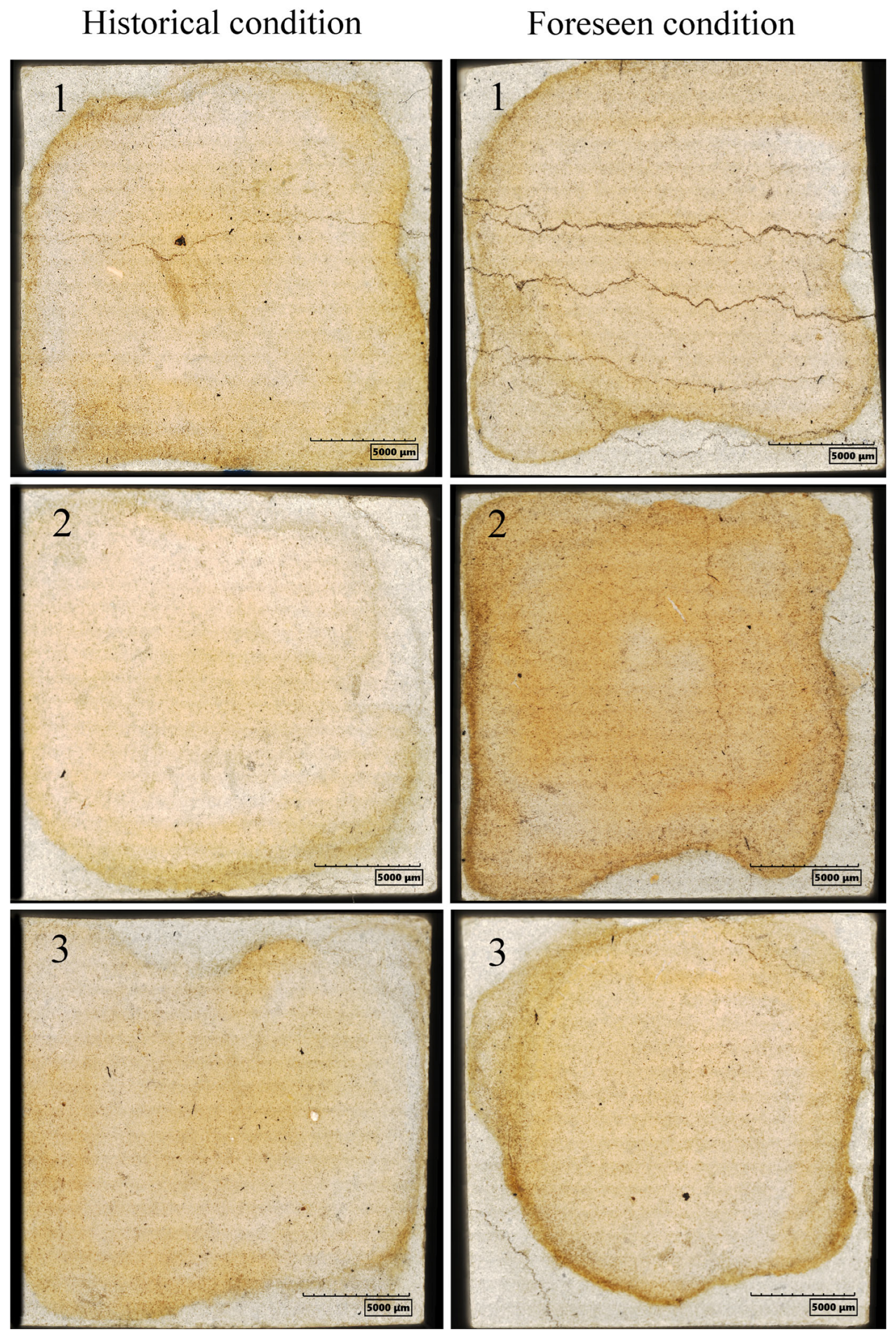
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pires, V.; Amaral, P.M.; Simão, J.A.R.; Galhano, C. Experimental Procedure for Studying the Degradation and Alteration of Limestone Slabs Applied on Exterior Cladding. Environ. Earth Sci. 2022, 81, 59. [Google Scholar] [CrossRef]
- Sitzia, F. Climate Change and Cultural Heritage: From Small- to Large-Scale Effects—The Case Study of Nora (Sardinia, Italy). Heritage 2022, 5, 3495–3514. [Google Scholar] [CrossRef]
- Sitzia, F.; Lisci, C.; Pires, V.; Alves, T.; Mirão, J. Laboratorial Simulation for Assessing the Performance of Slates as Construction Materials in Cold Climates. Appl. Sci. 2023, 13, 2761. [Google Scholar] [CrossRef]
- Dias, L.; Pires, V.; Sitzia, F.; Lisci, C.; Candeias, A.; Caldeira, A.T.; Mirão, J. Evaluating the Biosusceptibility of Natural Stone as an Supporting Tool to Prevent Cultural Heritage Biodeterioration. Eur. Phys. J. Plus 2023, 138, 570. [Google Scholar] [CrossRef]
- Addesso, R.; Baldantoni, D.; Cubero, B.; De La Rosa, J.M.; González Pérez, J.A.; Tiago, I.; Caldeira, A.T.; De Waele, J.; Miller, A.Z. A Multidisciplinary Approach to the Comparison of Three Contrasting Treatments on Both Lampenflora Community and Underlying Rock Surface. Biofouling 2023, 39, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Prieto, B.; Vázquez-Nion, D.; Fuentes, E.; Durán-Román, A.G. Response of Subaerial Biofilms Growing on Stone-Built Cultural Heritage to Changing Water Regime and CO2 Conditions. Int. Biodeterior. Biodegrad. 2020, 148, 104882. [Google Scholar] [CrossRef]
- Schimel, D.S. Terrestrial Ecosystems and the Carbon Cycle. Glob. Change Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Korner, C. Biosphere Responses to CO2 Enrichment. Ecol. Appl. 2000, 10, 1590–1619. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Hamilton, J.G.; Naidu, S.L.; Thomas, R.B.; Andrews, J.A.; Finzi, A.; Lavine, M.; Matamala, R.; Mohan, J.E.; Hendrey, G.R.; et al. Net Primary Production of a Forest Ecosystem with Experimental CO2 Enrichment. Science 1999, 284, 1177–1179. [Google Scholar] [CrossRef]
- Querijero-Palacpac, N.M.; Martinez, M.R.; Boussiba, S. Mass Cultivation of the Nitrogen-Fixing Cyanobacterium Gloeotrichia Natans, Indigenous to Rice-Fields. J. Appl. Phycol. 1990, 2, 319–325. [Google Scholar] [CrossRef]
- Huertas, E.; Montero, O.; Lubián, L.M. Effects of Dissolved Inorganic Carbon Availability on Growth, Nutrient Uptake and Chlorophyll Fluorescence of Two Species of Marine Microalgae. Aquac. Eng. 2000, 22, 181–197. [Google Scholar] [CrossRef]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.R.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil Fertility Limits Carbon Sequestration by Forest Ecosystems in a CO2-Enriched Atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef]
- Morales, E.; Rodríguez, M.; García, D.; Loreto, C.; Marco, E. Crecimeinto, Producción de Pigmentos y Exopolisacáridos de La Cianobacteria Anabaena Sp. PCC 7120 En Función Del PH y CO2. Interciencia 2002, 27, 373–378. [Google Scholar]
- Singh, S.P.; Singh, P. Effect of CO2 Concentration on Algal Growth: A Review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Fujibayashi, M.; Niu, Q.; Tanaka, N.; Nishimura, O. Response of Microalgae to Elevated CO2 and Temperature: Impact of Climate Change on Freshwater Ecosystems. Environ. Sci. Pollut. Res. 2016, 23, 19847–19860. [Google Scholar] [CrossRef]
- Palmqvist, K. Carbon Economy in Lichens. New Phytol. 2000, 148, 11–36. [Google Scholar] [CrossRef]
- Collins, S.; Bell, G. Evolution of Natural Algal Populations at Elevated CO2. Ecol. Lett. 2006, 9, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Viles, H.A.; Cutler, N.A. Global Environmental Change and the Biology of Heritage Structures. Glob. Change Biol. 2012, 18, 2406–2418. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The Shared Socio-Economic Pathway (SSP) Greenhouse Gas Concentrations and Their Extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E.; Uslu, H.; Ozbek, A.; Ipekoglu, B.; Ocak, I.; Hasenekoglu, I. The Accelerating Effects of the Microorganisms on Biodeterioration of Stone Monuments under Air Pollution and Continental-Cold Climatic Conditions in Erzurum, Turkey. Sci. Total Environ. 2006, 364, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Jimenez, C. Deposition of Anthropogenic Compounds on Monuments and Their Effect on Airborne Microorganisms. Aerobiologia 1995, 11, 161–175. [Google Scholar] [CrossRef]
- Del Monte, M. Il Biodegrado Dei Monumenti in Pietra: I Licheni e i “Segni El Tempo”. Il Geologo dell’Emilia Romagna 2007, VII, 11–55. [Google Scholar]
- Bonazza, A.; Messina, P.; Sabbioni, C.; Grossi, C.M.; Brimblecombe, P. Mapping the Impact of Climate Change on Surface Recession of Carbonate Buildings in Europe. Sci. Total Environ. 2009, 407, 2039–2050. [Google Scholar] [CrossRef]
- Gómez-Bolea, A.; Llop, E.; Ariño, X.; Saiz-Jimenez, C.; Bonazza, A.; Messina, P.; Sabbioni, C. Mapping the Impact of Climate Change on Biomass Accumulation on Stone. J. Cult. Herit. 2012, 13, 254–258. [Google Scholar] [CrossRef]
- Cutler, N.A.; Oliver, A.E.; Viles, H.A.; Ahmad, S.; Whiteley, A.S. The Characterisation of Eukaryotic Microbial Communities Onsandstone Buildings in Belfast, UK, Using TRFLP and 454 Pyrosequencing. Int. Biodeterior. Biodegrad. 2013, 82, 124–133. [Google Scholar] [CrossRef]
- Miller, A.Z.; Rogerio-Candelera, M.A.; Dionísio, A.; Macedo, M.F.; Saiz-Jiménez, C. Evaluación de La Influencia de La Rugosidad Superficial Sobre La Colonización Epilítica de Calizas Mediante Técnicas Sin Contacto. Mater. Constr. 2012, 62, 411–424. [Google Scholar] [CrossRef]
- Miller, A.Z.; Dionísio, A.; Laiz, L.; Macedo, M.F.; Saiz-Jimenez, C. The Influence of Inherent Properties of Building Limestones on Their Bioreceptivity to Phototrophic Microorganisms. Ann. Microbiol. 2009, 59, 705–713. [Google Scholar] [CrossRef]
- Prieto, B.; Silva, B. Estimation of the Potential Bioreceptivity of Granitic Rocks from Their Intrinsic Properties. Int. Biodeterior. Biodegrad. 2005, 56, 206–215. [Google Scholar] [CrossRef]
- Cennamo, P.; Caputo, P.; Marzano, C.; Miller, A.Z.; Saiz-Jimenez, C.; Moretti, A. Diversity of Phototrophic Components in Biofilms from Piperno Historical Stoneworks. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 720–729. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of Building Stones: A Review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pitzurra, L.; Moroni, B.; Nocentini, A.; Sbaraglia, G.; Poli, G.; Bistoni, F. Microbial Growth and Air Pollution in Carbonate Rock Weathering. Int. Biodeterior. Biodegrad. 2003, 52, 63–68. [Google Scholar] [CrossRef]
- Rosado, T.; Reis, A.; Mirão, J.; Candeias, A.; Vandenabeele, P.; Caldeira, A.T. Pink! Why Not? On the Unusual Colour of Évora Cathedral. Int. Biodeterior. Biodegrad. 2014, 94, 121–127. [Google Scholar] [CrossRef]
- Durgenova, A.; Piksayakina, A.; Bogatov, V.; Salman, A.L.; Erofeev, V. The Economic Damage from Biodeterioration in Building Sector. IOP Conf. Ser. Mater. Sci. Eng. 2019, 698, 077020. [Google Scholar] [CrossRef]
- Dias, L.; Rosado, T.; Battacharia, S.; Candeias, A.; Teresa Caldeira, A.; Mirão, J. Assessing Aesthetic and Structural Deterioration in Historic Buildings. In Proceedings of the 9th Euro-American Congress on Construction Pathology, Rehabilitation Technology and Heritage Management, Granada, Spain, 13–16 September 2022; pp. 596–605. [Google Scholar]
- Gaylarde, C.C.; Baptista-Neto, J.A. Microbiologically Induced Aesthetic and Structural Changes to Dimension Stone. npj Mater. Degrad. 2021, 5, 33. [Google Scholar] [CrossRef]
- Moliné, M.; Libkind, D.; Van Broock, M. Production of Torularhodin, Torulene, and β-Carotene by Rhodotorula Yeasts. Methods Mol. Biol. 2012, 898, 275–283. [Google Scholar] [CrossRef]
- Bartoli, F.; Ellwood, N.T.W.; Bruno, L.; Ceschin, S.; Rugnini, L.; Caneva, G. Ecological and Taxonomic Characterisation of Trentepohlia Umbrina (Kützing) Bornet Growing on Stone Surfaces in Lazio (Italy). Ann. Microbiol. 2019, 69, 1059–1070. [Google Scholar] [CrossRef]
- Ekendahl, S.; O’Neill, A.H.; Thomsson, E.; Pedersen, K. Characterisation of Yeasts Isolated from Deep Igneous Rock Aquifers of the Fennoscandian Shield. Microb. Ecol. 2003, 46, 416–428. [Google Scholar] [CrossRef]
- Geiger, R. Classificação Climática de Köppen-Geiger. Creat. Commons Attrib. Alike 3.0 Unported 1936. Available online: https://creativecommons.org/licenses/by-sa/3.0/ (accessed on 20 October 2023).
- Ribeiro, F. Pedra de Ançã: Contribuição Para a Classificação “Global Heritage Stone Resource”. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2017. [Google Scholar]
- Columbu, S.; Palomba, M.; Sitzia, F.; Carcangiu, G.; Meloni, P. Pyroclastic Stones as Building Materials in Medieval Romanesque Architecture of Sardinia (Italy): Chemical-Physical Features of Rocks and Associated Alterations. Int. J. Archit. Herit. 2020, 16, 49–66. [Google Scholar] [CrossRef]
- Ferreira, M.; Lidia, G.; Delgado Rodrigues, J. The Ançã Limestones, Coimbra, Portugal. In Proceedings of the EGU General Assembly 2016, Wien, Austria, 17–22 April 2016. [Google Scholar]
- EN 15803:2010; Conservation of Cultural Property-Test Methods-Determination of Water Vapour Permeability (Δp). Comité Européen de Normalisation: Bruxelles, Belgium, 2010.
- EN 1926:2006; Natural Stone Test Methods-Determination of Uniaxial Compressive Strength. Comité Européen de Normalisation: Bruxelles, Belgium, 2006.
- Dias, L. STONECOLOR: Color of Commercial Marbles and Limestone–Causes and Changes. Ph.D. Thesis, University of Evora, Évora, Portugal, 2021. [Google Scholar]
- Climate Change Knowledge Portal CCKP. Available online: https://climateknowledgeportal.worldbank.org (accessed on 20 October 2023).
- Instituto Nacional de Meteorologia INMET. Available online: https://portal.inmet.gov.br (accessed on 20 October 2023).
- European Union’s Earth Observation Programme—Copernicus. Available online: https://www.copernicus.eu/en (accessed on 20 October 2023).
- Fratini, F.; Rescic, S.; Tiano, P. A New Portable System for Determining the State of Conservation of Monumental Stones. Mater. Struct. Constr. 2006, 39, 139–147. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, G.; Zhou, L. Heterotrophic Microorganism Rhodotorula Mucilaginosa R30 Improves Tannery Sludge Bioleaching through Elevating Dissolved CO2 and Extracellular Polymeric Substances Levels in Bioleach Solution as Well as Scavenging Toxic DOM to Acidithiobacillus Species. Water Res. 2010, 44, 5423–5431. [Google Scholar] [CrossRef] [PubMed]
- Urano, N.; Shirao, A.; Naito, Y.; Okai, M.; Ishida, M.; Takashio, M. Molecular Phylogeny and Phenotypic Characterization of Yeasts with a Broad Range of PH Tolerance Isolated from Natural Aquatic Environments. Adv. Microbiol. 2019, 9, 56. [Google Scholar] [CrossRef]
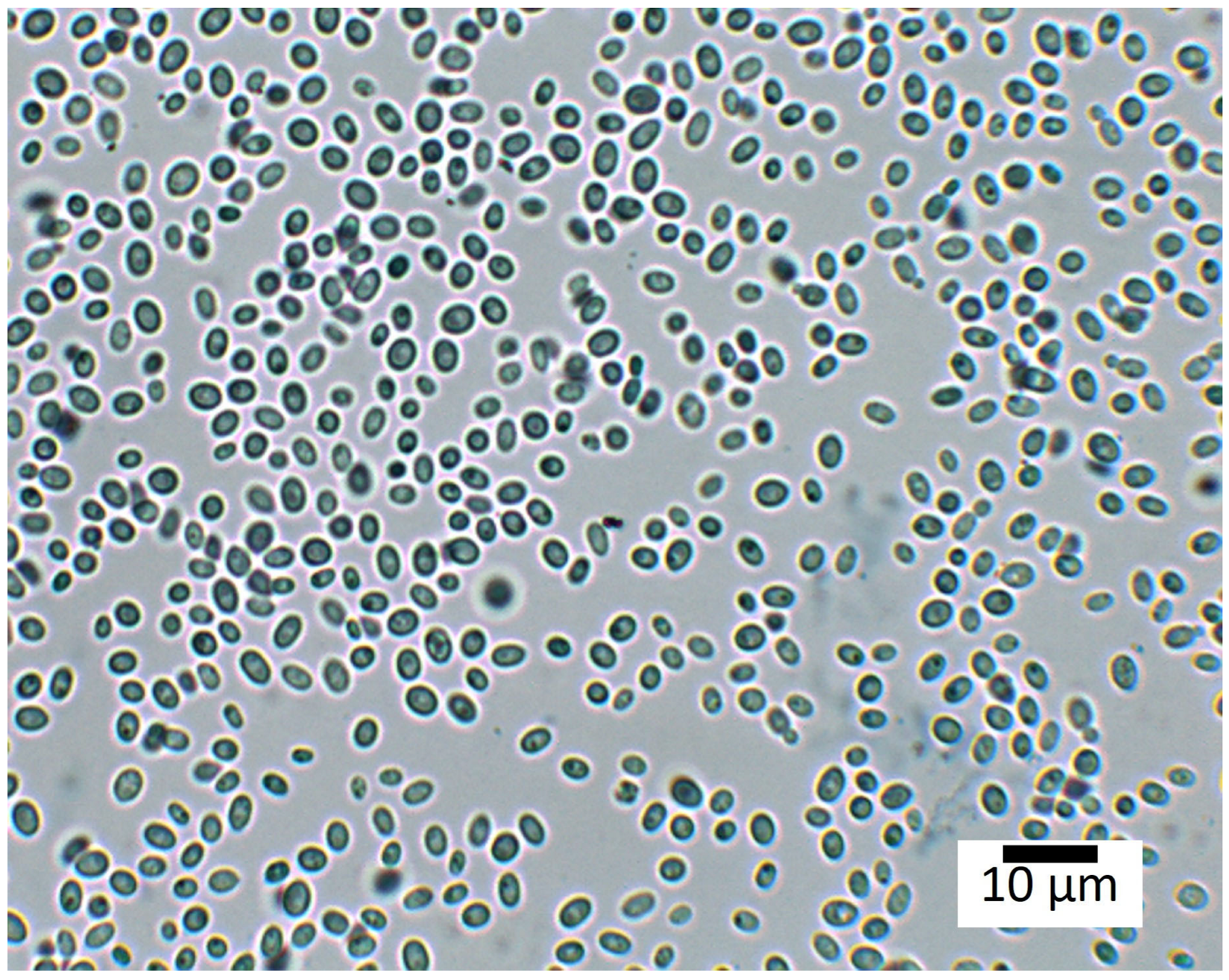

| Closest Related Type Strain on Basis of ITS | Family | Class | Phylum |
|---|---|---|---|
| Rhodotorula mucilaginosa | Sporidiobolaceae | Microbotryomycetes | Basidiomycota |
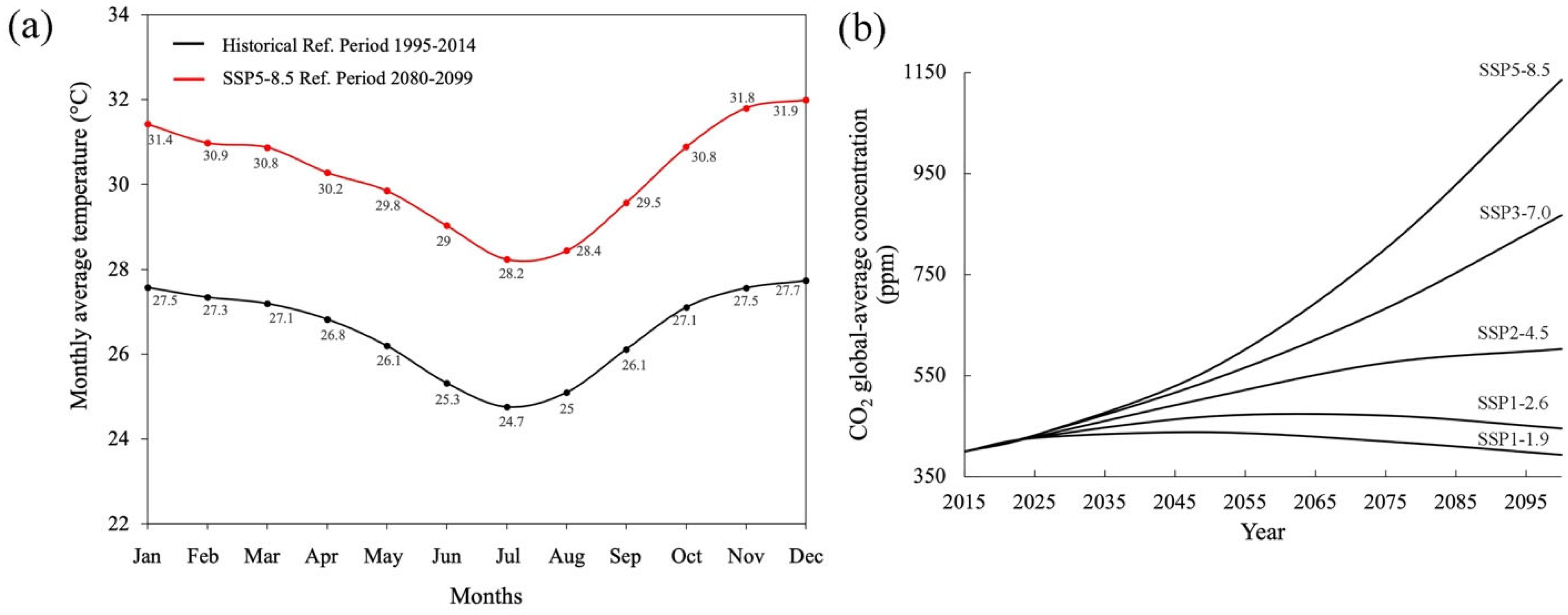
| Paraiba Region (Brazil) | Historical Conditions (Period 1995–2014) | Foreseen Conditions, CMIP 6, IPCC SSP5-8.5 Scenario (Period 2080–2099 AD), Figure 3a,b | ||||
|---|---|---|---|---|---|---|
| Average Annual Temperature | Average Annual Relative Humidity | CO2 Atmospheric Concentration | Average Annual Temperature | Average Annual Relative Humidity | CO2 Atmospheric Concentration (ppm) | |
| 26.5 °C | 74.9% | ~420 ppm | 30.2 °C | 78.2% | ~1135 ppm | |
| Physical–Mechanical Feature | Range |
|---|---|
| Real density (g/cm3) | 2.72 < ρR < 2.73 |
| Bulk density (g/cm3) | 2.05 < ρB < 2.17 |
| Effective porosity (%) | 18.7 < Φ0 < 22.4 |
| Closed porosity (%) | 0.54 < ΦC < 1.91 |
| Total immersion coefficient (%) | 8.1 < CIW < 12.2 |
| Point load strength index (MPa) | 1.62 < Is50 < 2.63 |
| Compressive strength (MPa) | 21.7 < σC < 24.4 |
| Thermal conductivity (W/m⋅K) | 1.64 < k < 1.78 |
| Volume heat capacity (J/m3⋅K) | 2.04 × 106 < s < 2.11 × 106 |
| Thermal diffusivity (m2/s) | 0.78 × 10−6 < α < 0.84 × 10−6 |
| Leeb D hardness (HLD) | 547 < LH < 604 |
| Vapor permeability (g/m⋅s⋅Pa) | 1.8 × 10−11 < kV < 2.1 × 10−11 |
| P wave speed (m/s) | 4850 < VP < 5102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitzia, F.; Lisci, C.; Pires, V.; Dias, L.; Mirão, J.; Caldeira, A.T. Predicted Dynamic of Biodeterioration in Cultural Heritage Stones Due to Climate Changes in Humid Tropical Regions—A Case Study on the Rhodotorula sp. Yeast. Heritage 2023, 6, 7727-7741. https://doi.org/10.3390/heritage6120406
Sitzia F, Lisci C, Pires V, Dias L, Mirão J, Caldeira AT. Predicted Dynamic of Biodeterioration in Cultural Heritage Stones Due to Climate Changes in Humid Tropical Regions—A Case Study on the Rhodotorula sp. Yeast. Heritage. 2023; 6(12):7727-7741. https://doi.org/10.3390/heritage6120406
Chicago/Turabian StyleSitzia, Fabio, Carla Lisci, Vera Pires, Luís Dias, José Mirão, and Ana Teresa Caldeira. 2023. "Predicted Dynamic of Biodeterioration in Cultural Heritage Stones Due to Climate Changes in Humid Tropical Regions—A Case Study on the Rhodotorula sp. Yeast" Heritage 6, no. 12: 7727-7741. https://doi.org/10.3390/heritage6120406
APA StyleSitzia, F., Lisci, C., Pires, V., Dias, L., Mirão, J., & Caldeira, A. T. (2023). Predicted Dynamic of Biodeterioration in Cultural Heritage Stones Due to Climate Changes in Humid Tropical Regions—A Case Study on the Rhodotorula sp. Yeast. Heritage, 6(12), 7727-7741. https://doi.org/10.3390/heritage6120406











