1. Introduction
Parietal art, including cave paintings and rock art, is one of the most widespread and information-rich archaeological artefacts. Such prehistoric drawings covering rock outcrops occur in more than 120 countries of the world across diverse geographical, geomorphological, and climatic environments, from caves and grottos to mountain plateaus, from tundras to deserts [
1].
Many ancient and prehistoric Ural pictographs, also called “pisanitsy” or “painted rocks”, are located on rock outcrops typically occurring along the banks of rivers (Chusovaya, Tagil, Rezh, Neyva, Ufa, Iset’, Irbit, Belaya, Yuruzan’ and others) and—less often—lakes, covering a total area extending from north to south for about 800 km [
1].
Current ideas about the dating of Ural parietal art works are mainly based on analogies with dated archaeological items and rock figures obtained from other regions; radiocarbon datings have so far been obtained only for the paintings from the Ignatievskaya cave. However, by carefully cross-analyzing figurative and non-figurative motifs, it is possible to determine the time of creation of the Ural pictographs as having mainly occurred during the Eneolithic and Bronze Ages [
1].
At present, more than 90 sites with prehistoric parietal art have been discovered in the Urals, with two main localization areas: on the eastern slope of the Middle Urals along the rivers Tagil (20 pictographs), Neiva and Rezh; and on the western Southern Urals slope along the rivers Ai and Yuryuzan [
1].
Pictographic art has been applied to various rocks characteristic of one or another part of the Urals; thus, the river banks of the western slope are predominantly composed of limestone, while on the eastern slope granites, syenites, gabbro and diorites prevail along with gneisses and shales [
1]. In contrast to the multiple techniques applied for creating images in Siberia or the European North, such as drawing with paint, embossing, engraving, grinding and their combinations, Uralian pictographs (with a few exceptions) present monochromatic drawings [
1].
Color is an extremely important aspect of the study of art and archaeological objects. By identifying the nature of the pigments used in archaeological artefacts, we gain an insight into their provenance, manufacture, use, exchange, alterations, as well as solutions to issues occurring due to the changes in pigment colors over time, and hence, problems with their conservation and restoration [
2]. From archaeological and historical perspectives, a study of the composition of colorants can reveal the level of technology necessary for their creation, lead to a deeper understanding of the compositional styles and chronology of rock paintings, and form a basis for an identification of the cultural traditions of their creators [
3].
The introduction of the physicochemical and geochemical methods and approaches into the studies of cultural heritage objects and archaeological artefacts has found application in the investigation of rock art [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. Recipes of prehistoric paintings frequently include inorganic substances, mainly red-orange, black and white pigments that might have been mixed with organic binders of vegetable or animal origin and extenders in order to achieve the particular hue [
1,
2,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. Since most natural organic molecules cannot be used as colorants in artworks due to their weathering and fading with light, reactions with other chemicals (oil, resins, substrates, etc.) of the artwork itself or pollutants, the minerals have played a major role in the use of pigments due to higher stability [
2]. Natural-colored minerals are known from prehistoric times (red and yellow ochres, black manganese oxides, carbon black). Ferric oxide monohydrate Fe
2O
3∙H
2O or FeOOH (goethite), when mixed with silica and clay, provides a yellow color (yellow ochre), while red ochre, which may be produced by heating the yellow ochre to anhydrous ferric oxide Fe
2O
3 (hematite), may also occur naturally [
2,
14,
15]. The pigments used for obtaining white color are calcite, or white clay or shale [
14,
15], or beeswax [
12]. Manganese oxides and carbon are responsible for black color [
2,
12,
14,
15].
One of the imperative challenges when sampling the rock art objects involves maintaining the intactness of the pictograph, ideally using in situ non-destructive techniques as demonstrated for the first time by Tournié et al. [
15] for on-site Raman spectroscopic studies of San rock art (South Africa) and now widely applied in studies of rock art (see the reviews by Rusaki and Vandenabeele [
17] and Hernanz et al. [
4]). If the extraction of samples is justified and unavoidable (dating, GC–MS), these must be minimized so as to take only micro-specimens from areas affected by exfoliation, flaking or spallation areas of the painting panel surface in such a way that the extraction area is negligible to the naked eye [
4]. Here, samples must be removed with extreme care to avoid contamination using sterile surgical blades or Dremel micro-tools. Very small granules of pigment (<1 mm
2) may be extracted, together with rock flakes and accretions. In general, their removal should have a minimal effect on the pictographs and their surrounding area. In parallel to sampling the pictorial areas, samples from their surroundings should also be collected in order to compare the geological setting both with and without the paint layer [
4].
Further laboratory investigation of such micro-samples (some millimeters in size and tens of milligrams by weight) involves selecting from a number of microspectroscopic techniques, including those with higher degree of visualization (imaging and mapping): even a single mineral grain can possess great informational potential, whether in terms of morphological features, elemental distributions, or structural irregularities.
Representing a scientifically elegant technique for pigment analysis of archaeological materials, Raman spectroscopy is used in an ever-growing number of research areas in cultural heritage (archaeometry and analysis of artefacts) [
2,
4]. This is a rapid, non-destructive vibrational spectroscopic technique for studying various mineral and organic species with high spatial resolution (~1 μm), requiring minimal sample preparation and offering the ability to perform Raman imaging and hypermapping operations using hydrated and dehydrated specimens.
The molecular and mineralogical characterization of rock art materials by Raman spectroscopy may be complemented by other microscopic techniques, such as scanning electron microscopy combined with energy-dispersive X-ray spectrometry or electron probe microanalysis with wavelength-dispersive X-ray spectrometry, which provide images (in back-scattered and secondary electron modes) and elemental composition, as well as, using graphical software, elemental maps.
Known and hypothetical organic binding agents in rock art include plant juices or oils, urine, animal fat, bone marrow, blood, eggs (yolk and/or albumen), as well as human saliva [
4,
18,
19,
20]. While organic binding agents are typically contemporaneous with paint production, these materials often degrade over time and become difficult to characterize [
18]. Lipids (fats, waxes and resins) can survive for longer periods of time due to their hydrophobic properties, i.e., they are not readily dissolved in water. The need to study the low contents of preserved lipid components in archaeological samples necessitates the development and application of suitable extraction, purification and analysis methods by gas chromatography–mass spectrometry (GC–MS), the method most often used for qualitative and quantitative analysis of complex organic mixtures, including those of archaeological origin [
4,
6,
21,
22,
23].
This article develops the microanalytical approach to the studies of colorants from a number of Uralian cave paintings and pictographs (Ignatievskaya cave and Idrisovskaya II and Zmiev Kamen’ pictographs), applied earlier to the investigation of prehistoric and experimental (30 years old) drawings from the Dvuglazy Kamen’ pictograph (Neyva River, middle Urals [
6]. The approach employs the combination of the SEM-EDS analysis of elemental composition and Raman spectroscopic identification of mineral composition and GC–MS determination of the organic compounds (fatty acids) of the microscopic colorant samples.
As well as adding to the existing knowledge of compositions of inorganic mineral pigments and possible extenders added to the colorant mixtures and that of the organic compounds comprising the binder, it is hoped that the reported investigation will serve to refine and critically revise our earlier results obtained from the Ignatievskaya cave [
24] and Zmiev Kamen’ pictograph [
3].
2. Materials and Methods
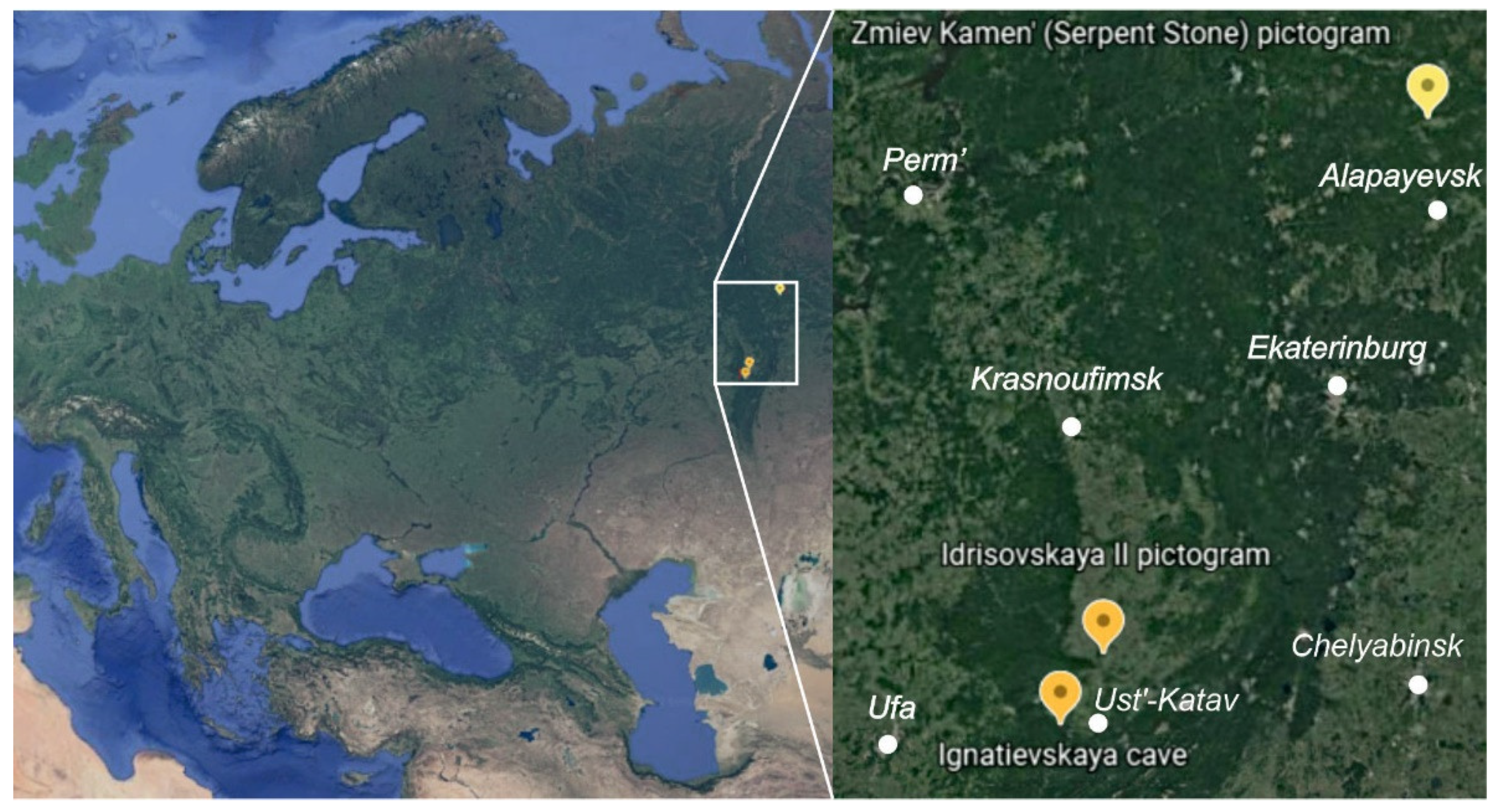
The elemental, mineral and organic composition of the paintings at three parietal art sites across the Southern and Middle Urals (Russia), along with their corresponding rock panels (substrates), was investigated using microspectroscopic and GC–MS techniques (
Figure 1).
The Ignatievskaya (Yamazy-Tash) cave is located in the northwestern foothills of the southern Ural Mountains (the Katav-Ivanovsk district of the Chelyabinsk region, Russia), on the right bank of the Sim River, a tributary of the Belaya River. Forming the largest cave in the karst region, it is situated in Devonian limestones. Along with the neighboring Kapova (Shulgan-Tash) cave, it hosts the easternmost occurrences of Paleolithic cave art in Europe. The Ignatievskaya cave became world famous after the discovery of ancient drawings in 1980 by Russian archaeologists V.T. Petrin, S.E. Chairkin and V.N. Shirokov [
25,
26]. It preserves representational images of a mammoth and a horse, as well as various fantastic and composite animals, anthropomorphic creatures and non-figurative motifs.
Three early conventional
14C datings of charcoal and bones from the cultural layer yielded Upper Paleolithic ages (18.3–15.4 cal ka BP [
25]). The chronology was recently refined by accelerator mass spectrometry (AMS) dating of charcoal and bones from the cultural layers associated with artistic activity, with four dates constraining the time of its accumulation to 17.4–16.3 cal ka BP [
27]. The dates are consistent with paleontological evidence from the cultural layer, represented by Late Pleistocene fauna [
25] and pollen characteristic of uniform, forest-free vegetation of a periglacial steppe [
28]. Upper Paleolithic dates are broadly consistent with
230Th dates obtained on flowstone underlying and covering paintings [
29]. Although three earlier
14C determinations made directly on the charcoal paintings returned Mesolithic rather than Upper Paleolithic dates (8.9–6.6 cal ka BP [
30]), they appeared younger than the flowstone that formed after both red and black paintings were made [
29]. At the time of publication, Steelman et al. [
30] acknowledged that their dates appear “unexpectedly recent” (p. 347) and offered four explanations of the inconsistency, three of which questioned the presumed Paleolithic antiquity of paintings, while one suggested a conflation of two painting episodes. Later, the images of the Ice Age were also found in the Serpievskaya II (Kolokol’naya) cave, near the Ignatievskaya cave [
26,
31].
The Idrisovskaya II open-air pictograph is located in the Salavat district of Bashkortostan, Russia, on the left bank of the Yuryuzan river (
Figure 1). It is situated in Lower and Middle Carboniferous limestones. The archaeological site at the Idrisovskaya cave is correlated with the early Late Paleolithic [
32,
33]. Drawings of animals, anthropomorphic creatures and non-figurative motifs are located at the bottom of the rock shelter under the Idrisovskaya cave. Excavations revealed a sacrificial site including bone remains of animals and humans, fragments of ocher and pieces of hematite cut with ancient saws, stone products and the fragments of clay pottery from the Chalcolithic, Bronze and Iron Ages [
32].
The Zmiev Kamen’ open-air pictograph is located in the granite and amphibolite outcrops on the left bank of the Tagil river near the village of Machnevo (Sverdlovsk region, Russia) (
Figure 1). The pictograph is characterized by the numerous drawings combined into 9 groups demonstrating animals, geometric figures and signs, anthropomorphic creatures and non-figurative motifs [
1,
3,
34].
During the sample collection, conservation concerns and ethical considerations were taken into account. To ensure minimal intervention, microscopic fragments (several mm in size, weighing 10–100 mg) were carefully scraped from cracks in the rock or the thickest paint layers using a medical scalpel in order to protect the integrity of drawings. Where possible, two or more samples were taken from different areas of a painting.
Fragments of red and black colorants were analyzed: the Great Hall of the Ignatievskaya cave (red colorant from the panel near the cruciform sign and near a small red horse, black colorant from an arrow-shaped sign, black matter of natural origin from a rock crack); the Idrisovskaya II pictograph (red colorant from the pictograph); the Zmiev Kamen’ (red colorant from the pictograph). Along with the colorants, the underlying rock substrate was analyzed in all cases.
Raman spectra and SEM-EDS analyses were obtained in the Geoanalitik shared research facilities of the IGG UB RAS. The re-equipment and comprehensive development of the Geoanalitik shared research facilities of the IGG UB RAS is generously supported by a grant of the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-680).
For scanning electron microscopic (SEM) studies, the samples were mounted onto conductive carbon adhesive tape and carbon-sputtered. The remaining un-sputtered portions of samples were used for the purposes of Raman microspectroscopic investigation.
SEM images, EDS analyses and elemental maps of samples were obtained using a JEOL JSM-6390LV scanning electron microscope equipped with an INCA Energy 450 X-Max EDS spectrometer and AZtecOne software with an accelerating voltage of 20 kV and an exposure time of 5 ms per pixel.
The mineral phases were identified using a LabRAM HR800 Evolution confocal Raman spectrometer equipped with an Olympus BX-FM optical microscope. An Olympus 50× objective (numerical aperture = 0.7), a diffraction grating of 600 gr/mm and an electrically cooled CCD detector were used for recording. The spectrometer calibration was guided along the Rayleigh line and the emission lines of a neon lamp. The spatial resolution was up to 1 μm. Laser power of about 5–10 mW was applied behind the objective lens for excitation by He-Ne (633 nm) and Ar- (488 nm) gas lasers. Acquisition time was 5–50 s per 10–50 accumulations depending on the degree of fluorescence displayed by the samples. As a result, Raman spectra were obtained in the range of 150–2000 cm−1. In fact, the spectra were recorded covering the range of Rayleigh scattering to control the positions of the vibrational modes. A useful Raman signal was recorded against the background of an extended growing bright photoluminescent signal of a mixed cumulative nature. The resulting superimposed spectra were processed; the low-energy range from ~100–150 cm−1 was cut off and the background was subtracted using a linear function. The identification of mineral phases was carried out using the KnowItAll (Bio RAD) database integrated into the spectrometer software and the RRUFF.INFO database.
The chromatographic analyses were performed at a facility hosted by the Ural Federal University. For the determination of fatty acid methyl esters (FAME) by gas chromatography with mass-spectrometric detector, sample weights of 10–100 mg were used. Fatty acids (FAs) were extracted from the weighed samples using a chloroform: methanol (2:1) mixture. To assist their dissolution, they were placed into an ultrasound bath for 20 min. The samples were centrifuged for 10 min at 3000 rpm. The extract obtained was placed into vacuum to remove the solvent. The dry residue was dissolved in acetonitrile and derivatized with dimethylformamide-dimethyl acetal. To perform gas chromatography–mass spectrometry (GC–MS) using a Perkin Elmer Clarus 600T mass spectrometer, the samples were injected into a capillary column in splitless mode (Elite-5MS 30 m × 250 μm); the thickness of the stationary phase layer was 0.25 μm. The injector temperature was 200 °C with a 1 min evaporation phase. The GC program consisted in a uniform increase in temperature from 30 to 300 °C at a rate of 10 °C/min, followed by an isothermal period at T = 300 °C for 5 min. The total time of the analysis was 32 min. MS was conducted in the mode of electron impact ionization (EI) at a gas chromatographic interface temperature of 200 °C; the cathode temperature was 180 °C, while the ion source voltage was 70 eV. Mass spectra were obtained in the range of the mass-to-charge ratio of 35–400 a.m.u. The peaks were identified using the integral mass spectral library and literature data.
4. Discussion
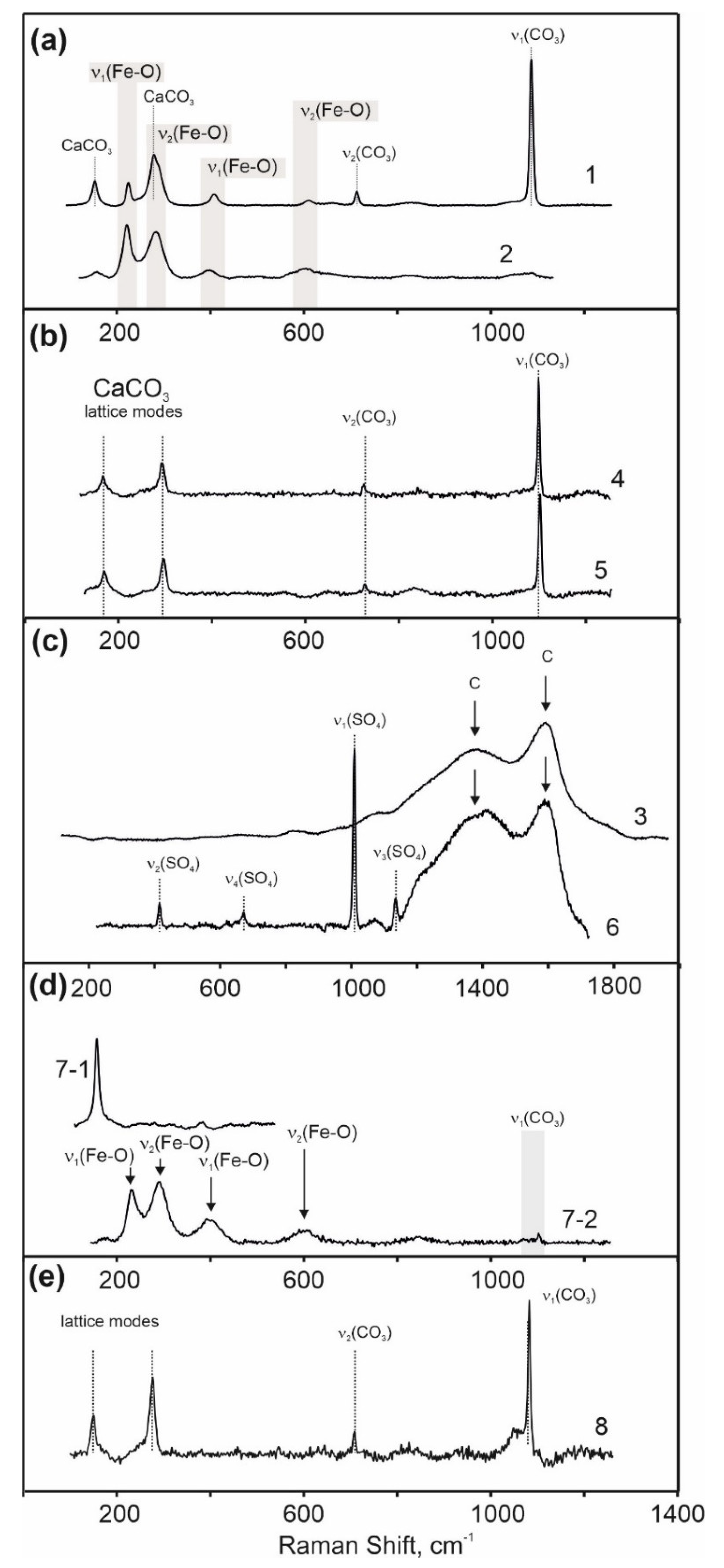
The results of microanalytical study of the mineral and elemental composition of colorants demonstrated that the principal component of the red colorants from the Ignatievskaya cave and the Idrisovskaya II and Zmiev Kamen’ pictographs was hematite. The set of characteristic bands of the Fe-O vibration in hematite at 225, 245, 290, 412, 500 and 611 cm
−1 was registered in the Raman spectra (
Figure 4,
Figure 7b and
Figure 10). No bands corresponding to other possible iron oxides such as magnetite (310, 540 and 665–670 cm
−1) and hydroxides such as goethite (244, 299, 385, 480, 548 and 681 cm
−1) were observed in the spectra [
35,
36]. Hematite was also found in the red colorant from the Dvuglazy Kamen’ (Two-Eyed Stone) pictograph (Neyva River, Middle Urals) studied earlier [
6].
However, the line broadening in the hematite spectra observed for the red colorants from the Ignatievskaya cave and the Idrisovskaya II pictograph (
Figure 4a and
Figure 7b) indicates local disorder possibly correlated to the insertion of substituting ions (Al
3+ or Ti
2+) into the Fe(O)
6 octahedra constituting hematite structure [
37]. Titanium was apparently derived from the ancient gabbroids and gabbronorites (1390 to 1350 Ma) of the ultramafic-mafic layered intrusions of the Kusa-Kopan complex and associated large magmatic deposits of ilmenite and Ti-magnetite ores located 100 km further east [
38]. Although the comparison of Ti and Fe maps in
Figure 3 supports Ti-for-Fe substitution in hematite for the colorants from the Ignatievskaya cave and the Idrisovskaya II pictograph, it was rather difficult to define the Ti/Fe ratio due to the surface roughness of the unpolished samples. Moreover, there were some more factors responsible for the irregularities of the crystalline arrangement of hematite, such as heat treatment, grinding, biodegradation and weathering [
39]. Thus, the Raman spectrum of the apparently weathered reference hematite found in the excavations at the Idrisovskaya cave was characterized by the line broadening similar to that of red colorants. The sub-micrometer sizes of Fe-bearing pigments demonstrated in BSE images and EDS maps (
Figure 2,
Figure 3,
Figure 5,
Figure 6,
Figure 8 and
Figure 9) can also result in local disordering and line broadening.
Although many authors report rapid transformations of iron oxides during Raman analysis due to the in situ laser irradiation effects (such as heating and oxidation), the data on the laser powers used to avoid such effects are quite controversial. While some recommend that measurements should be performed at the lowest possible laser power of 0.01–0.5 mW [
12,
35,
36,
39], others demonstrate that hematite bands in the spectra of maghemite (γ -Fe
2O
3) and magnetite (Fe
3O
4) begin to appear when the laser power is increased above 15–20 mW [
40,
41]. The laser power applied in our studies was below 10 mW when excited by 488 nm and below 5 mW when excited by 633 nm, respectively, which could not promote substantial processes of phase transition and/or oxidation. In general, this corresponded to the laser powers used for the Raman spectroscopy of rock art (0.1–10 mW) [
5,
14,
15,
42] not affecting the signal quality of other informative bands in the Raman spectra.
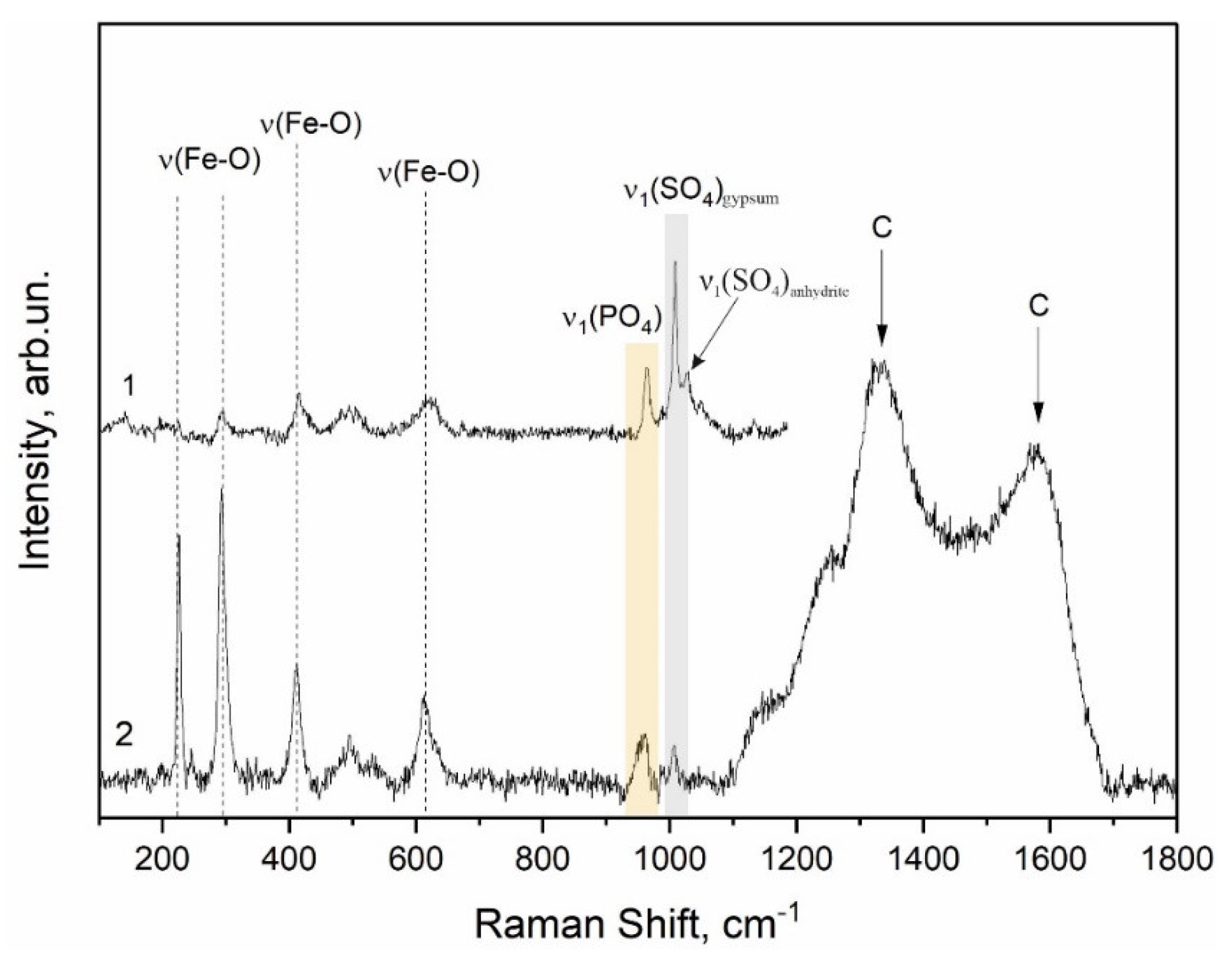
The red pigment of the Zmiev Kamen’ pictograph as composed of two components, hematite and black carbon, which were confirmed by Raman spectroscopy (
Figure 10). The band at 1338 cm
−1 corresponded to the stretching vibrations in the planar graphite structure and the band at 1558 cm
−1 appeared as a result of defects of graphite structure and the presence of heteroatoms [
12,
43]. These relatively broad bands suggested that the crystallite size of the black pigment was small and the broadening of peaks indicated the high degree of disorder. The band of the ν
1(PO
4) vibration at ~960 cm
−1 observed in the spectrum indicated the possible use of calcinated bones (bone black) as black pigment. H. Gomes et al. [
12] observed the combination of carbon and iron oxides in the red colorant from the rock art paintings in Gode Roriso (Ethiopia), and indicated the thermal treatment of the hematite with a carbonaceous material resulting in the partial reduction of iron (III) oxide to iron (II) oxide by carbon, which was confirmed by the presence of hematite and magnetite and carbon bands in the Raman spectra of the red colorant.
Since no reduced forms of iron were found in the studied pigments, it was not possible to reliably ascertain if the raw material for pigment was hematite or goethite, whether the process of heating (if any) took place in an oxidizing or reducing atmosphere, or whether the hematite and bone black powders were mixed with each other in the absence of heating. However, taking into account the finds of hematite pieces in the excavations of the Idrisovskaya cave and the overall availability of hematite from the nearby supergene deposits characterized by the presence of oxidized iron deposits (so-called “iron hats”) and the iron deposits of the Alapaevsk type (hematite–limonite ores and pisolitic iron ores) in the Southern and Middle Uralian regions under consideration [
6], we can assume that no heating was needed in order to obtain the bright red pigment.
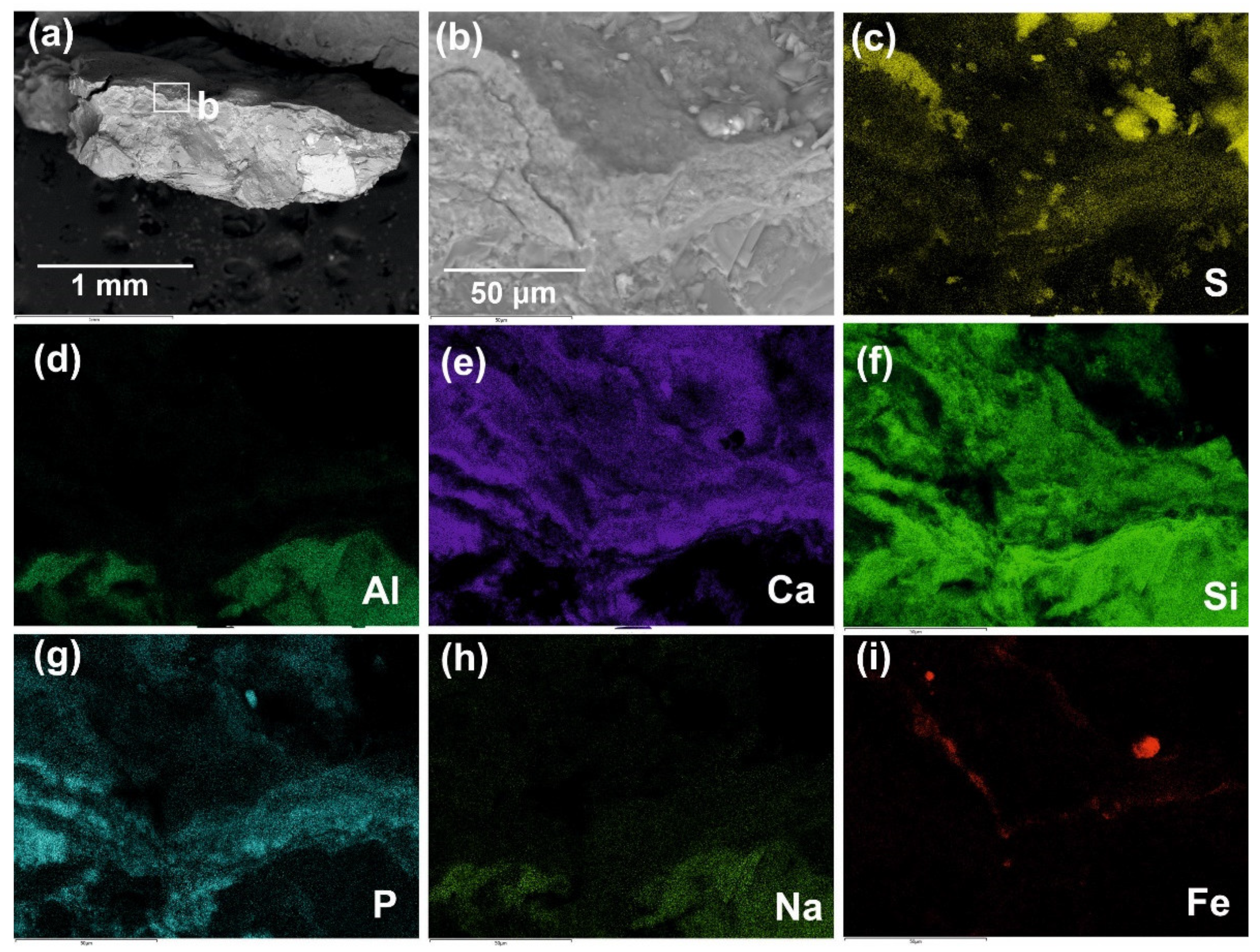
We compared the obtained results with the earlier data on ICP-MS microelement and phase mineral composition and thermal analysis of the red colorant for the Zmiev Kamen’ pictograph [
3]. The results of the phase mineral analysis (powder X-ray diffraction) of powdered samples of rock panel with red colorant were as follows, wt.%: crystal quartz 15–20%; gypsum < 5%; calcite < 5%; clay minerals (chlorite and/or hydrous micas) 5–10%; amphibole (up to 50% of hornblende confirmed by the endothermic effect at 1174 °C) [
3]. This was in a rather satisfactory agreement with the results of SEM-EDS and Raman spectroscopy. However, no significant amounts of iron minerals (as well as bone apatite) were found either by XRD or ICP-MS, which supports a conclusion about the minor role of iron minerals in the preparation of colorants [
3]. Indeed, the iron content in the red colorant of the Zmiev Kamen’ pictograph observed in the present study was visually lower than in the red colorants from the Ignatievskaya cave and Idrisovskaya II pictograph (see Fe elemental maps in
Figure 2c,
Figure 3d,
Figure 5f,
Figure 6,
Figure 8i and
Figure 9e), possibly due to the mixing with black carbon pigment. Although Fe mineral phases were below the limits of detection by XRD (0.61–0.78 wt.% of Fe as determined by ICP-MS) [
3], Raman spectroscopy is sufficiently sensitive to reveal the peaks of Fe-O vibrations in hematite (and P-O vibrations in apatite) even following dilution with bone black; the iron content in the resulting pigment is sufficient for the red color to persist in the colorant. However, the distribution of iron across the surface of colorant and its confinement to the circular areas of peeled colorant layers points to the repeated application of an iron-containing substance rather than the natural distribution of Fe-containing mineral grains (see for comparison the black pigment of the Ignatievskaya cave,
Figure 3d). The fairly low mass losses of organic matter in the red colorant determined by thermal analysis in the temperature range of 250–500 °C (0.5–0.9 wt.%) [
3] were consistent with the presence of inorganic compounds and bone black in the colorant.
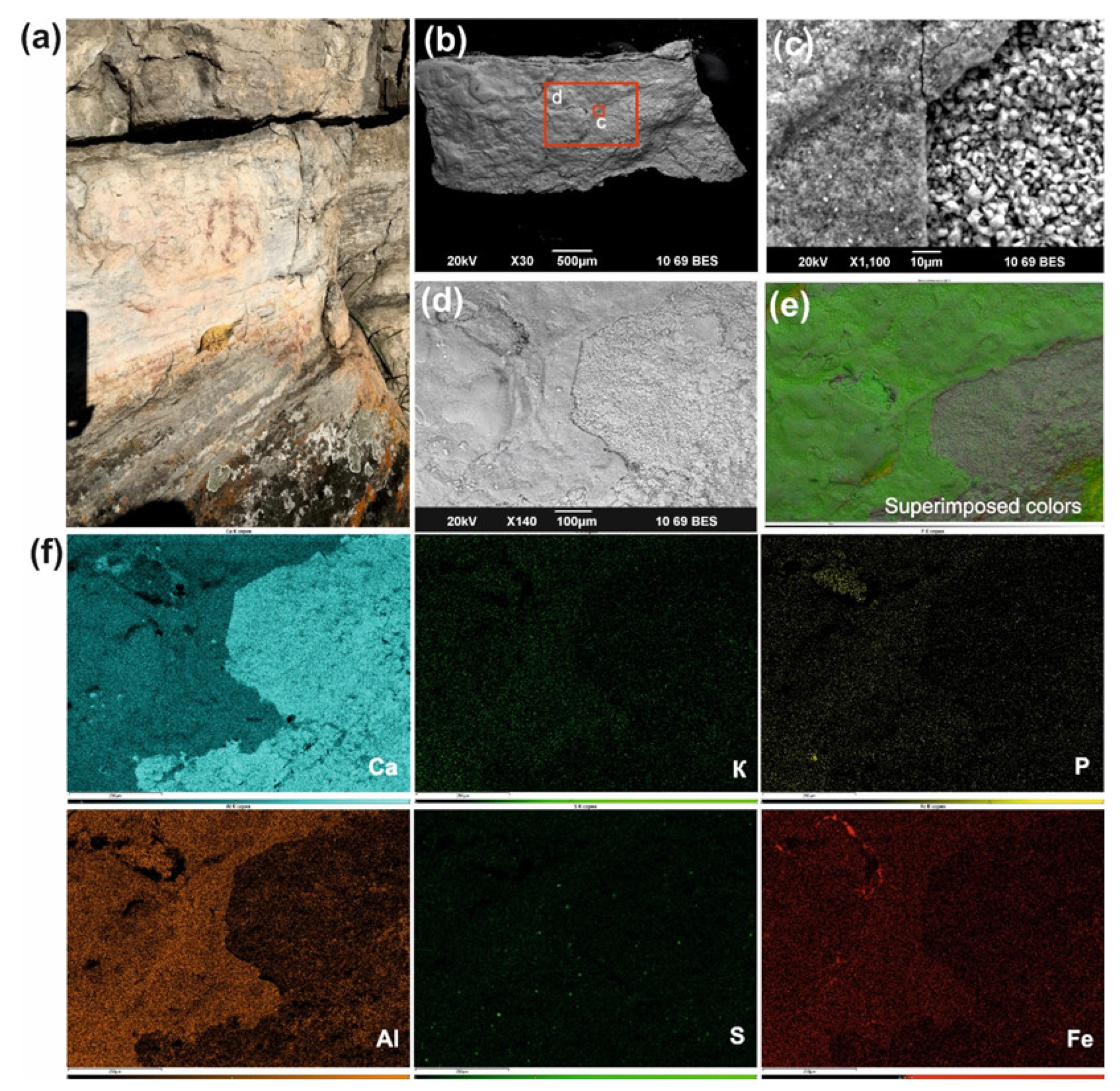
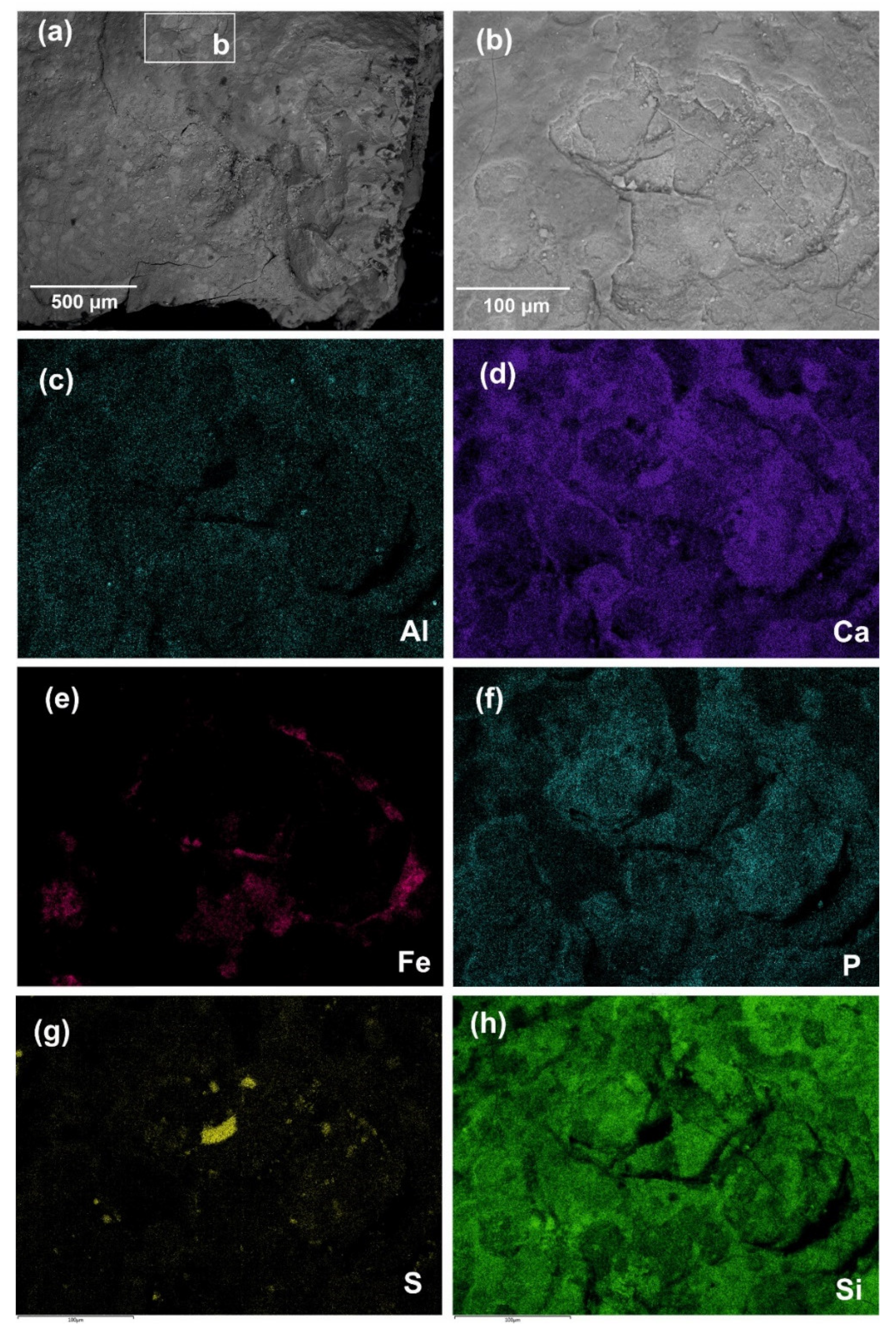
Earlier, V.S. Zhitenev identified the composition of the red colorants from the Ignatievskaya cave by SEM-EDS, XRF spectrometry and X-ray diffraction as quartz, calcite and clay minerals, along with traces of gypsum and insignificant content of iron, and suggested the use of feldspar as an extender [
24]. We suppose that the quartz, calcite and feldspar (
Figure 2c) originated from the rock panel rather than comprising an artificial addition to the colorant, while the gypsum was more of an authigenic mineral, i.e., forming crystal encrustations on the rock and colorant surface (
Figure 8). This was clearly demonstrated by the underlying rock panel composed of calcite and dolomite (
Figure 5d–f and
Figure 6) for the colorant from the Idrisovskaya II pictograph. The feldspar was distributed across the colorant surface in the form of rare individual grains (
Figure 2c and
Figure 3d) in sufficiently small amounts as to preclude the likelihood of its artificial addition in high amounts as an extender.
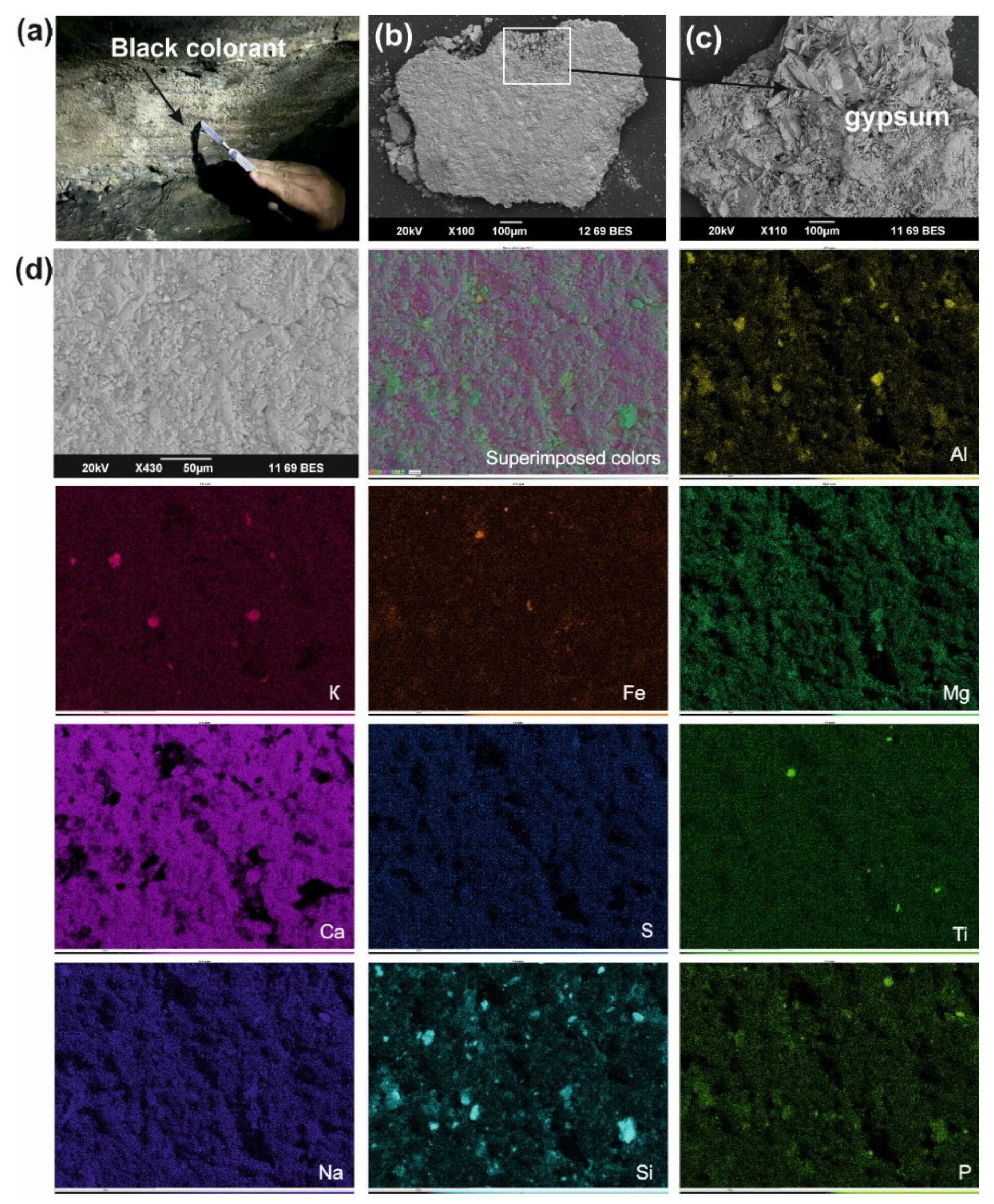
The fine-grained (<1–5 μm in size) hydromicous mineral containing iron (hematite) found in the red colorants apparently could originate from the mixing of hematite particles with clayey extender for the Ignatievskaya cave and Idrisovskaya II pictographs, and with addition of bone black into the colorant mixture for the Zmiev Kamen’ pictograph. Thus, the presence of iron minerals as inorganic pigments in red colorants was confirmed; the rather small amounts can be possibly explained as due to mixing with the clayey extender in order to achieve the desired hue. Even such small amounts of hematite possess strong coloring properties to provide the bright color to the masterworks of parietal art.
The principal pigment in the black colorant of the drawings from the Ignatievskaya cave was carbon, as confirmed by the Raman spectroscopy. The absence of Raman bands attributable to organic residuals indicated that the black pigment was fully fired in a reducing atmosphere [
12,
43]. Moreover, the absence of phosphate functional group at 960 cm
−1 in the Raman spectrum (
Figure 4), which excluded the use of calcinated bones (bone black) in preparing the black pigment (
Figure 5), rather suggested the use of charcoal of vegetal origin; this was supported by the finds of charcoal pieces during excavations carried out in the Ignatievskaya cave [
25,
26,
29].
Authigenic minerals that form in situ within the cave sediments, as opposed to those that are transported into the cave, can be used to reconstruct the ancient chemical environments in the sediments [
44]. Each mineral forms under a specific set of conditions, and hence its presence is indicative of the conditions that prevailed at the time of formation.
Bicarbonate waters equilibrated with carbonate host rocks of the caves drain the sediments over long periods of geological time to move the water–rock equilibria toward a higher alkalinity of pH = 7.0–8.5, favoring the stabilization of carbonates [
45] in the form of speleothems (stalactites and stalagmites, moon milk) (
Figure 4b,e).
Phosphates, which are among the most common, widespread and manifold authigenic minerals (about sixty mineral species), form by interaction of clayey matter, aluminous silicate rocks and/or limestone with solutions enriched in phosphate derived from bat or bird guano during its bacterial and fungal degradation [
44,
45]. Since many of the authigenic phosphates have relatively poor order at the atomic level, and/or very small crystal sizes and irregular distribution across the surface [
44], they are not easily detected by Raman spectroscopy. For this reason, scanning electron microscopy and elemental analyses using an energy dispersive spectrometer can be of great assistance.
Thus, phosphorus distribution across the P elemental maps in the colorants from the Ignatievskaya cave (
Figure 3d) might be connected with the formation of Ca-bearing phosphates such as crandallite CaAl
3(PO
4)
2(OH)
5·H
2O; ardealite Ca
2(SO
4)(HPO
4)·4H
2O; brushite CaHPO
4·2H
2O; whitlockite Ca
9(Mg,Fe
2+)(PO
4)
6(HPO
4); montgomeryite Ca
4(Mg,Fe)Al
4(PO
4)
6(OH)
4·12H
2O and dahllite Ca
5(PO
4,CO
3)
3(OH), or K,Na,Al-bearing phosphates such as taranakite (K,Na,NH
4)
3(Al,Fe
3+)
5(HPO
4)
6(PO
4)
2·8H
2O); leucophosphite (KFe
3+2(PO
4)
2(OH)·2H
2O); tinsleyite K(Al,Fe)
2(PO4)
2(OH)·2H
2O and variscite Al(PO
4)·2H
2O [
44,
45]. Ardealite, a sulfate phosphate (Ca
2(SO
4)(HPO
4)·4H
2O), may form early during the process in limestone of dry caves and sometimes coexists with gypsum (CaSO
4·2H
2O) [
45].
Similar to their formation in cave environments, authigenic phosphates may form on the rock surfaces exposed to the impact of atmospheric factors, such as humidity, temperature, dust and sunlight. Phosphorus from soil and, to a lesser extent, bird excrements can produce phosphate-enriched media resulting in the formation of phosphates. Moreover, the apatite from the bone black in the red colorant from the Zmiev Kamen’ pictograph could have been an additional source of phosphorus.
The whitish substance filling the micro-cracks on the surface of red colorant from the Idrisovskaya II pictograph was identified as Ca(C
2O
4)·2H
2O weddellite (
Figure 7a). The calcium oxalate minerals whewellite Ca(C
2O
4)·H
2O and weddellite Ca(C
2O
4)·(2 + x)H
2O are common on rock surfaces and may encapsulate and stabilize the paints of pictographs, protecting them from weathering and fixing the colorants on the rock panel [
4,
5,
13,
15,
46]. Oxalates are generally associated with prehistoric pictographs but also exist on natural rock outcrops, both those in open air and protected from atmospheric precipitation surfaces.
The origin of whewellite and weddellite on exposed surfaces is not fully understood. Proposed sources include natural processes such as biological activity of organisms living on or within the stone, reactions of organic compounds in rain or aerosols at the atmosphere/rock boundary, as well as reactions and/or deposition of dissolved organic matter in runoff, and animal urine [
4,
5,
13,
15,
46].
Earlier weddellite has been found in another open-air pictograph, Dvuglazy Kamen’ (Neyva River, Middle Urals) whose rock massive is composed of silicified limestone [
6]. Although the substrates on which oxalates are known to occur include granite, marble, limestone and sandstone, no weddellite has been observed in the colorant of the Zmiev Kamen’ pictograph granite rock panel.
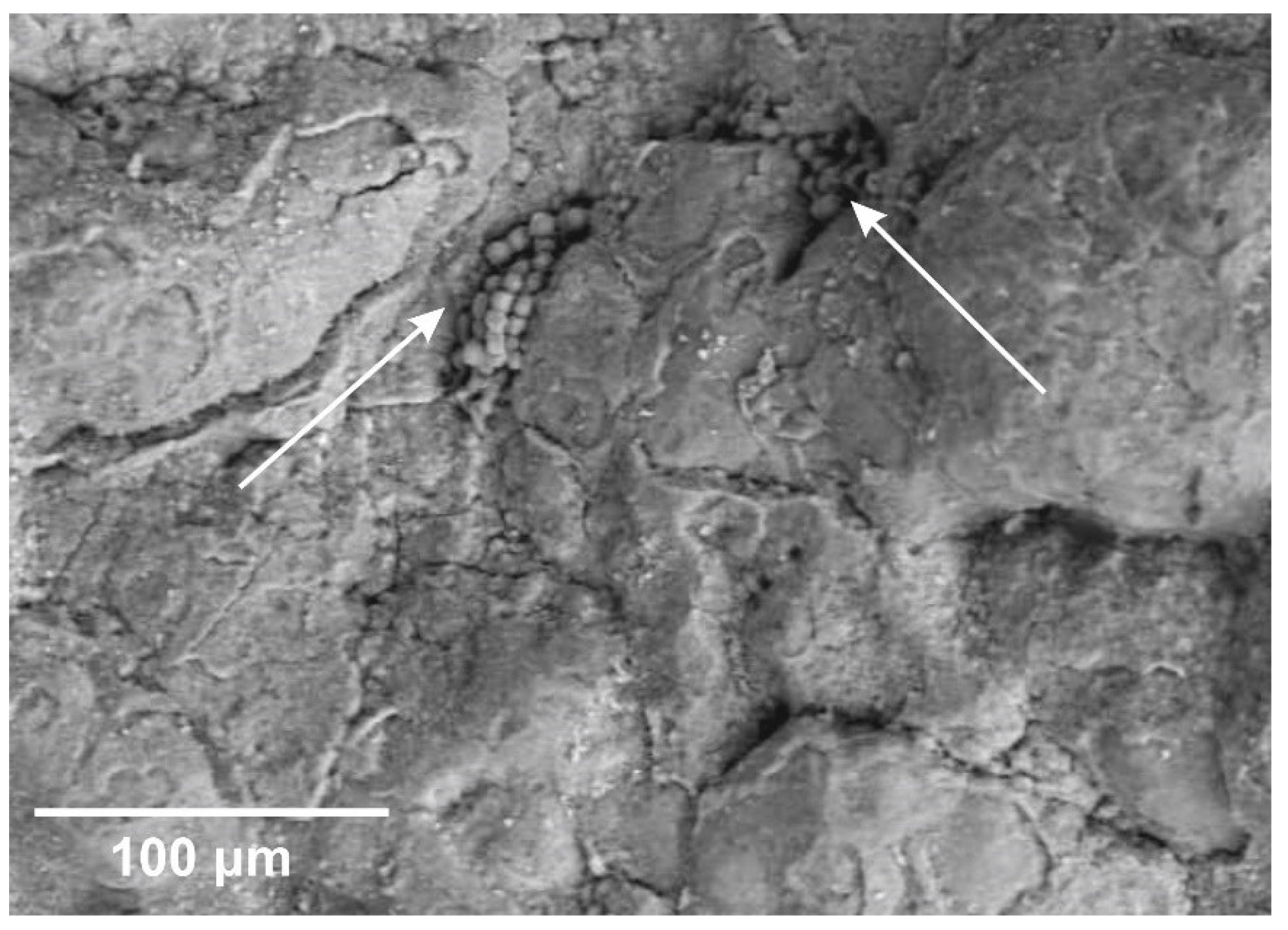
One of the principal reasons for oxalate formation can be the presence of lichens— symbiotic associations of fungi (mycobionts) and microscopic green algae and/or cyanobacteria (photobionts)—which can be highly rock-specific, like the lichen calcareus L.
Aspicilia calcarea (L.), and produce pruina (a wax-like protective coating made of weddellite) [
4,
6,
13]. Here, the possible lithobiontic community could be made up of a great variety of microorganisms, lichens, and cyanobacteria [
47]. Their traces were clearly visible on the rock surface of the Idrisovskaya II pictograph (
Figure 11).
All studied sites (both cave and open-air) were characterized by the formation of authigenic gypsum as encrustations covering the colorant layer (
Figure 3c,
Figure 4c,
Figure 6,
Figure 8c and
Figure 10). Several possible mechanisms of gypsum formation on the limestone surface are described: (i) gypsum crystallization due to the evaporation of sulfate-rich pore waters percolating through the limestone strata; (ii) sulfur oxide migration from the dry and wet atmospheric precipitation (probably predominating mechanism for open-air pictographs); and (iii) artificial application of sulfur-containing organic compounds with the binder of colorant (tallow or bone marrow) [
6,
16].
The phosphate assemblages can coexist with gypsum in caves [
45,
48]. Since the process of phosphoritization of the organic remains present in the unconsolidated deposits (bone detritus, bat guano and coprolites) in the Ignatievskaya cave was accompanied by the acidification of the environment, the reaction of H
2SO
4 with carbonate substrate resulted in the formation of gypsum [
48]. The subaerial phosphorite-gypsum deposits in the Ignatievskaya cave indicate the relatively warm conditions of the arid Mikulian Interglacial climate [
48].
Moreover, CaSO
4 anhydrite is identified in the Raman spectrum of the red colorant from the Zmiev Kamen’ (
Figure 10). The simultaneous presence of easily hydrated–dehydrated minerals such as gypsum CaSO
4·2H
2O and anhydrite CaSO
4, as well as whewellite Ca(C
2O
4)·H
2O and weddellite Ca(C
2O
4)·(2 + x)H
2O, may indicate the alternation of dry and wet climatic conditions during the formation of oxalate and gypsum encrustations over the rock surface for open-air pictographs [
13,
15]. However, for the Ignatievskaya cave, with its rather stable temperature and humidity regime (t = 5.0 °C and f = 99% in summer, t = 3.5° C and f = 78% in winter, and t = 6.0 °C and f = 99% in autumn), this seems highly unlikely [
48].
The absence of Raman bands attributable to organic residuals in studied colorants indicates the significant degree of its decomposition and structural transformation. Thus, based on the results of the chromatographic analysis of fatty acids, which was ultra-sensitive to the organic remains, we have attempted to evaluate the organic composition of red colorants.
The recipes of prehistoric paintings most frequently include organic binders of vegetable or animal origin such as plant juices or oils, urine, animal fat, bone marrow, blood, eggs (yolk and/or albumen), as well as human saliva, in order to form a stable, homogeneous, flexible and viscous paste with the pigment and extender [
4,
7,
16,
18,
19,
20]. The basis of such binders is generally composed of proteinaceous, lipidic, glucidic (plant gums) or waxy (beeswax) materials. H. Gomes et al., found the remains of beeswax in the white colorant from the rock art paintings in Gode Roriso (Ethiopia) [
12].
Triglycerides are the main form of lipids in living organisms and consist of a glycerol molecule combined with three fatty acids, comprising esters of glycerin with saturated and unsaturated fatty acids in long linear chains [
49]. Lipids are sensitive to chemical and biological alteration processes; for example, hydrolytic degradation releases fatty acids from triacylglycerols and β-oxidation, while reduction processes lead to the loss of unsaturated acids [
19,
49,
50]. In most of the archaeological pigment samples, the main components of the residual organic phase are comprised of free fatty acids and their salts, indicating that the originally present triacylglycerols underwent hydrolysis and reacted with the mineral matrix of the samples [
19].
Despite the large number of naturally occurring fatty acids (more than 1000), only some of them were found in significant quantities. The most common saturated fatty acids of both animal and plant origin were unbranched carbon chain compounds containing 16 and 18 carbon atoms: palmitic (C16:0) and stearic (C18:0) acids, which are most often preserved in organic residues, since unsaturated fatty acids are quite easily decomposed [
19,
49,
50]. Given that short- and medium-chain fatty acids arising from oxidation processes are more soluble in percolating ground water than long-chain fatty acids, degraded lipids in archaeological samples are identified by high concentrations of palmitic (C16:0) and stearic (C18:0) acids [
19].
Since no fatty acids were found in the rock substrate of the painting from the Ignatievskaya cave, taphonomic contamination of the colorant can be excluded. The fatty acid distributions with a greater abundance of palmitic acid (C16:0) than stearic acid (C18:0), together with minor oleic acid (C18:1) obtained for the colorant (
Table 1), are typical of degraded animal fats [
19,
20]. The presence of odd-carbon-numbered, straight-chain components—specifically C15:0 and C17:0—point to a ruminant origin [
51].
The use of the P/S ratio (ratio of mass fractions of palmitic and stearic acids) proposed to discriminate fatty substances by their origin [
6,
51,
52,
53] is debated in the literature due to their high concentration either in plant or in animal sources; moreover, the significant amounts of C16:0 and C18:0 FAs could also be the result of the conversion of unsaturated FAs to SFAs [
23,
54]. Thus, only a combination of criteria including fatty acids distribution, abundances of minor components, such as odd-carbon number branched-chain fatty acids, and positional isomers of unsaturated compounds, enables distinctions to be made among different types of animal fats [
23]. Based on the concept that further FFAs or different ratios could provide a more reliable and accurate identification strategy, the following ratio (C15:0 + C17:0)/(C12:0 + C14:0 + C16:0 + C18:0) was proposed for discriminating between monogastric (<0.04) and ruminant (> 0.04) animal fats residues [
55,
56].
The calculated ratio (C15:0 + C17:0)/(C12:0 + C14:0 + C16:0 + C18:0) for the organic fraction of the red colorant from the Ignatievskaya cave was equal to 0.10, indicating the presence of ruminant fat. However, the ratios of palmitic to stearic (P/S) and palmitic to myristic (P/M) acids calculated for the colorant from the Ignatievskaya cave were P/S = 5.8 and P/M = 7.6, which are quite atypical for adipose and dairy fats known for domesticated ruminants [
53]. A comparison with the bone marrow from the metatarsal bones of wild ruminants—European fallow deer (P/S = 5.5 and P/M = 6.7) [
57], North American elk and mule deer (P/S = 4.4 − 3.5 and P/M = 7.7 − 19.4) [
58]—demonstrated that binder for the red pigment could have originated from the bone marrow of wild ruminants like roe deer, moose or other members of the deer (
Cervidae) family. The use of bone marrow of guanaco from Patagonia was suggested in the archaeological pigment residues from one of the most remote regions of the planet (Beagle Channel region, Tierra del Fuego, Southern South America) [
19].
Since forming the components of the cellular membranes of autotrophic bacteria, the presence of monounsaturated fatty acids in the red colorant from the Ignatievskaya cave may be due to the activity of the cave bacterial communities, such as sulfur and sulfide oxidizers, iron and manganese oxidizers, sulfate reducers, as well as nitrifiers, that appear to be abundant in cave environments [
59]. These bacteria play a significant role in the dissolution of limestone in caves with hydrogen sulfide-rich waters, contributing to cave enlargement, as well as having possible biocorrosive potential to cause irreversible damage to the rock art paintings due to the degradation and discoloration of painting pigments [
59].
The cis-7-hexadecenoic (hypogeic) acid has been isolated from the strains of the genus
Nitrospira, a kind of nitrite oxidizing autotrophic bacteria [
60]. Such bacteria have been found among the bacterial communities on Paleolithic paintings and surrounding rock walls in two Spanish caves (Llonín and La Garma) [
59].
Although the substrate of the Zmiev Kamen’ pictograph contained myristic, palmitic and stearic acids, their content was rather low to significantly affect the fatty acid composition of corresponding red pigment. Moreover, the substrate lacked MUFA (palmitoleic and oleic acids), saturated odd-chain pentadecanoic (C15:0) and even-chain (C20:0) arachidic fatty acids, as well as branched-chain fatty acids (iC15:0 isopentadecanoic and iC16:0 isopalmitic) found in the colorant. This difference suggests that the presence of organic fraction in the colorant was not the result of taphonomic contamination.
The branched-chain fatty acids and MUFA are well known as bacterial markers [
19,
51]. As discussed above, the presence of microorganisms within the lithobiontic communities inhabiting the rock surfaces of open-air pictographs is quite common. However, these compounds also occur in the fats of ruminant animals.
The calculated ratio (C15:0 + C17:0)/(C12:0 + C14:0 + C16:0 + C18:0) for the organic fraction of the red colorant from the Zmiev Kamen’ pictograph was equal to 0.01, which indicates the presence of monogastric animal fat. Moreover, it corresponded to the ratio (0.01) obtained for the experimental rock painting drawn with ground ochre mixed with pig lard on a stone in 1992 and left in a grotto near the Dvuglazy Kamen’ pictograph (Neyva River, Middle Urals, Russia) and analyzed in 2021 [
6].
5. Conclusions
Studies of mineral, elemental and organic phase composition of the colorant micro-samples from the drawings of Ignatievskaya cave and Idrisovskaya II and Zmiev Kamen’ pictographs (Southern and Middle Urals, Russia) were carried out using a set of microspectroscopic methods with high spatial resolution (SEM-EDS and Raman spectroscopy). The fatty acid composition of the organic phase was analyzed by GC–MS following methylation.
The red colorants were characterized by the presence of fine-grained (< 1–5 μm in size) hydromicous mineral containing iron, apparently originating from the thorough grinding and mixing of hematite particles with clayey extender for the Ignatievskaya cave and Idrisovskaya II pictographs, and the addition of bone black into the red colorant mixture for the Zmiev Kamen’ pictograph. The principal pigment in the black colorant of the drawings from the Ignatievskaya cave was charcoal of vegetal origin. No traces of thermal treatment were found for inorganic pigment (hematite).
The proportions of fatty acids derived from the colorants corresponded to monogastric animal fat (possibly pig (boar) adipose tissue (lard) for the red colorant from the Zmiev Kamen’ pictograph) and ruminant fat (possibly bone marrow) for the red colorant from the Ignatievskaya cave), which could well have been added into the colorant mixture as an organic binder.
The technology of colorant manufacture likely involved thorough grinding and mixing of hematite with an organic binder of animal origin and clayey extender in order to achieve the desired hue and intensity when applying the colorant in layers (Idrisovskaya II and Zmiev Kamen’ pictographs).
The development of authigenic phosphate mineralization is inherent both to cave- and open-air sites resulting in the formation of a number of Ca-bearing and K,Na,Al-bearing phosphates due to the availability of phosphorus released in the process of bat/bird guano—or, less commonly, bone biodegradation—to produce acid solutions enriched in phosphate that dissolve limestone and apatite.
All studied sites (both cave and open-air) were characterized by the formation of authigenic gypsum in the form of encrustations covering the colorant layer. In the Ignatievskaya cave the phosphoritization of the organic remains in the unconsolidated deposits (bone detritus, bat guano and coprolites) was accompanied by the acidification of environment and gypsum crystallization. In the case of the open-air pictographs, gypsum forms as a result of migrations of sulfur oxides with dry and wet atmospheric precipitation and limestone and granite dissolution. Moreover, the sulfur from organic binder (S-enriched triglycerides from adipose fat and bone marrow) and activity of sulfur oxidizing bacteria could be the additional source for gypsum crystallization in all colorants.
The activity of autotrophic cave and lithobiontic microbial communities (and sulfate reducers, nitrifiers, etc.) was observed even on areas visually free from bacterial colonies. Evidence of calcium oxalate encrustation, formed as a result of the biological activities of microorganisms (lichens, cyanobacteria) from the lithobiontic community of limestone rock, was found in the images of the Idrisovskaya II pictograph. Although no oxalates were found on its surface, the presence of lithobiontic microorganisms on the granite substrate of the Zmiev Kamen’ pictograph, as confirmed by GC–MS, demonstrated the high specificity of certain microorganisms to rock composition.
The simultaneous presence of easily hydrated–dehydrated minerals, such as gypsum and anhydrite (Zmiev Kamen’ pictograph), and calcium oxalates (Idrisovskaya II pictograph) indicated the alternation of dry and wet climatic conditions during the formation of mineral encrustations covering the rock surface of open-air pictographs, while the cave environment was more generally characterized by highly stable temperature and humidity regimes.