The Pigments of the Painter Fleury Richard (1777–1852), a Model for Multidisciplinary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Pigmented Powders
2.1.2. Paintings
- Princess Elizabeth, sister of King Louis XVI, distributing milk (c. 1816), with analysis of red, green, white, and blue zones;
- Henri IV at Gabrielle d’Estrées (c. 1810–1812), with analysis of red, green, and white zones;
- The Painter and his family (c. 1817 or 1822), with analysis of red, green, and white zones.
2.2. Analytical Techniques
2.2.1. X-ray Fluorescence (XRF)
2.2.2. X-ray Powder Diffraction (XRD)
2.2.3. Infrared Absorption (FTIR)
2.2.4. Micro-Raman
2.2.5. Color Images Analyses
3. Results
3.1. Raw Pigmented Powders
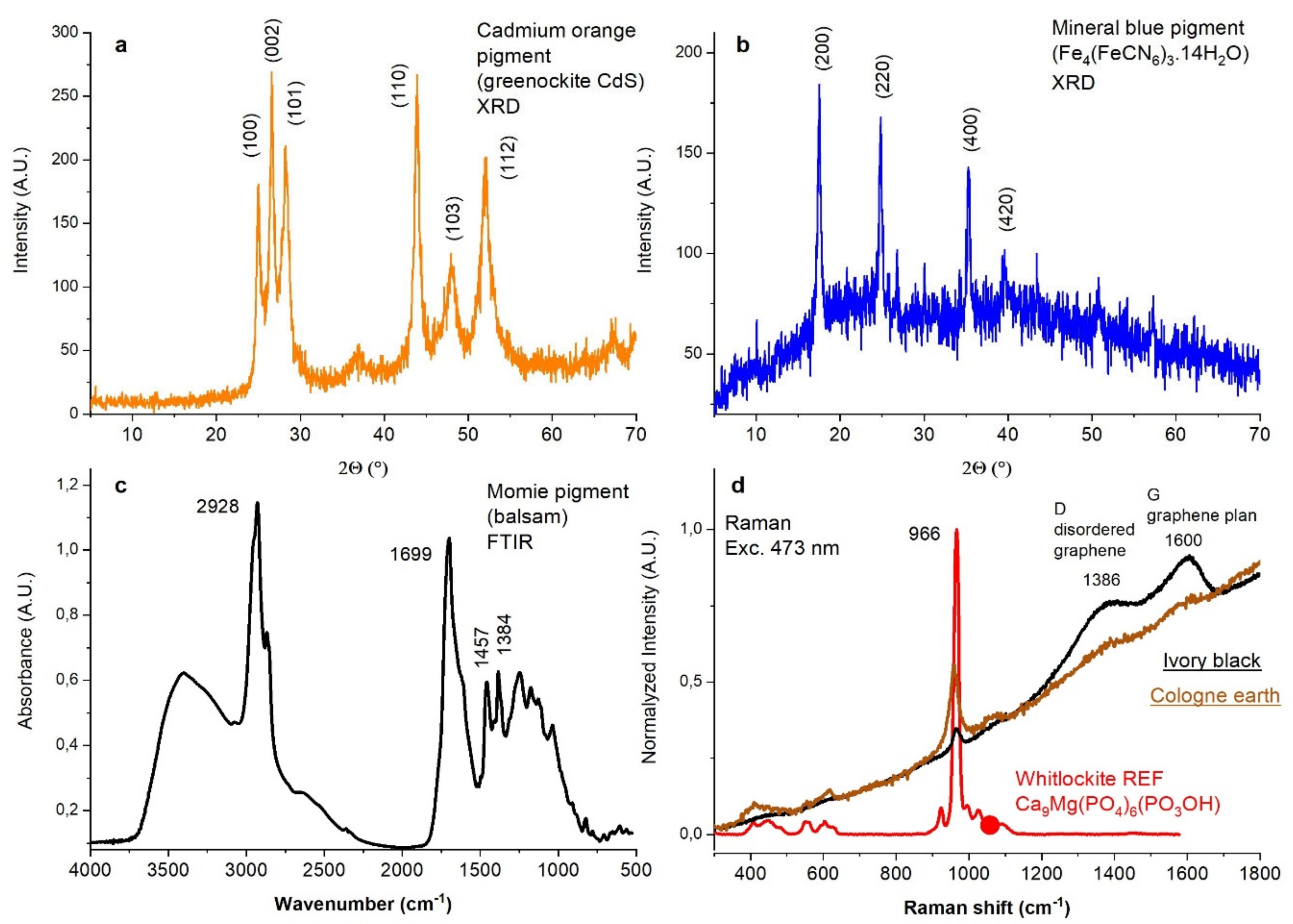
3.1.1. Yellow and Orange Powders
3.1.2. Red Powders
3.1.3. Blue Powders
3.1.4. Green Powders
3.1.5. Brown Powders
3.1.6. Black Powders
3.1.7. White Powders
3.2. The Paintings
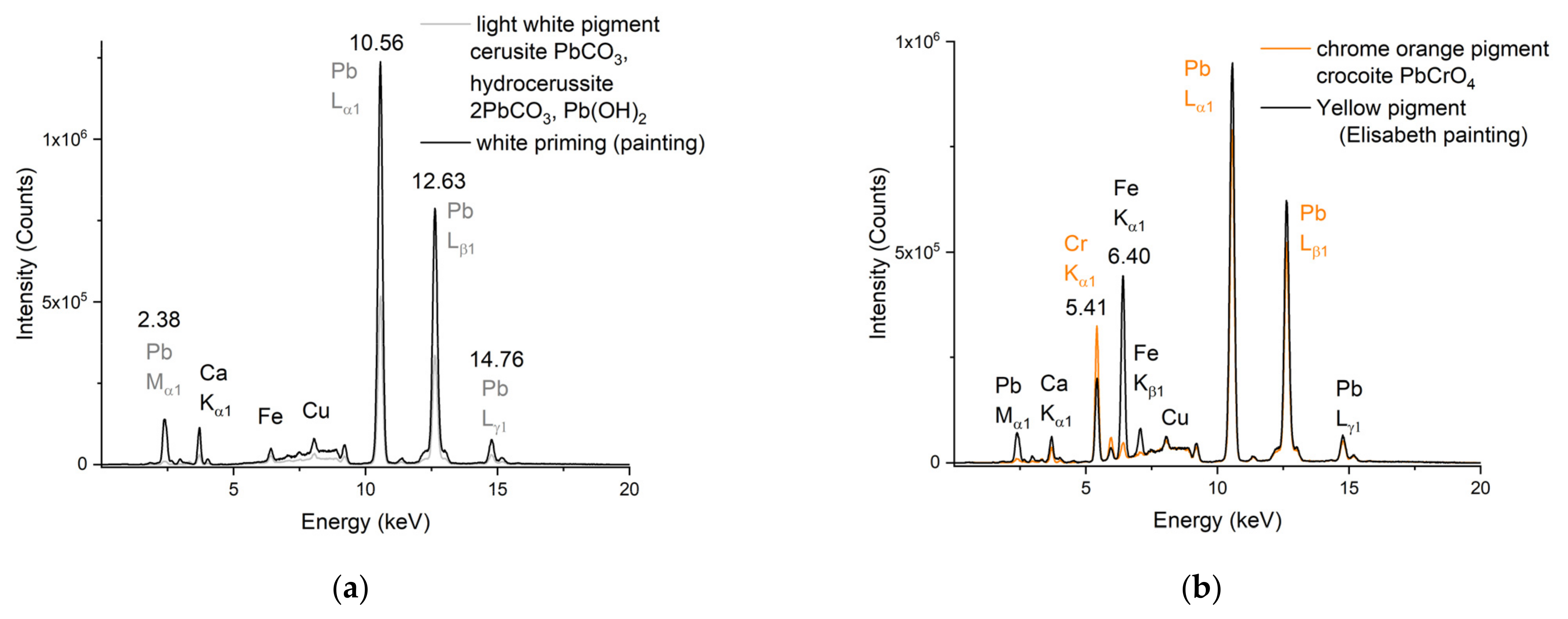
3.2.1. Layers of Oil-Based Primer Paint
3.2.2. The Yellow-Orange Areas
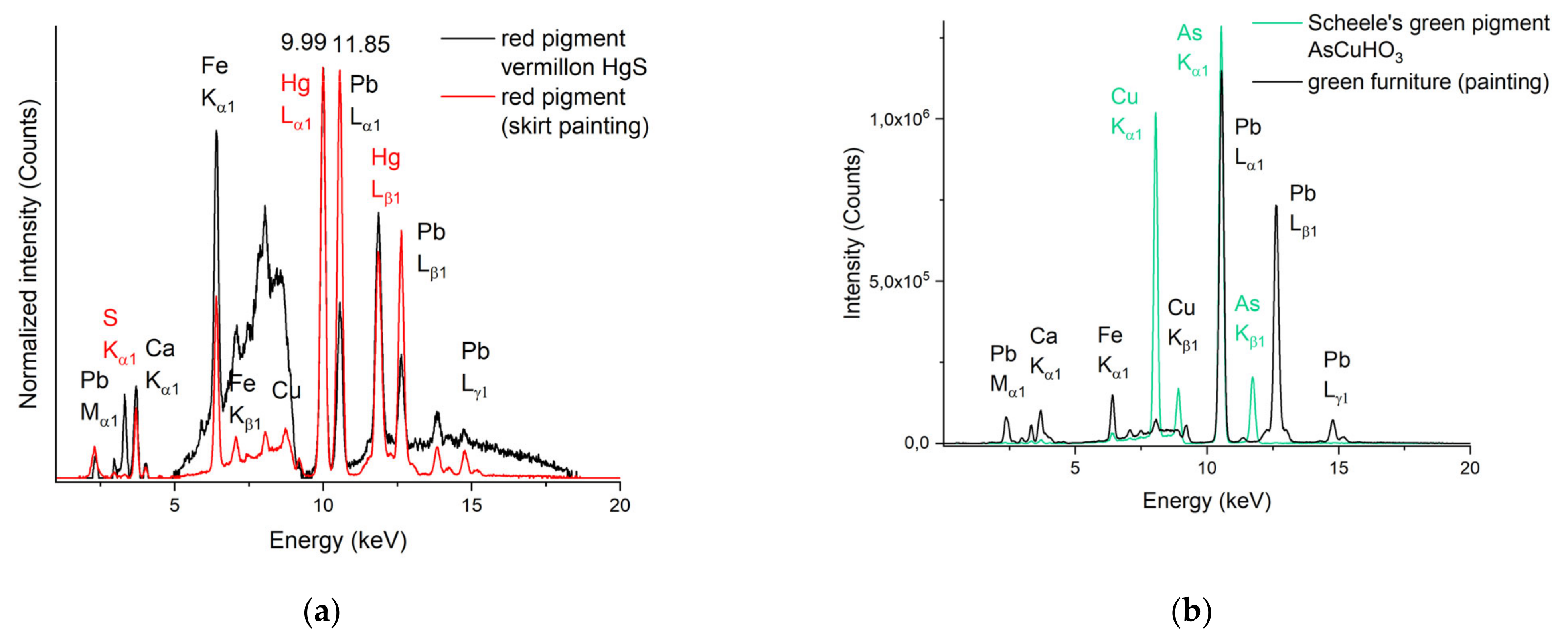
3.2.3. The Red Zones
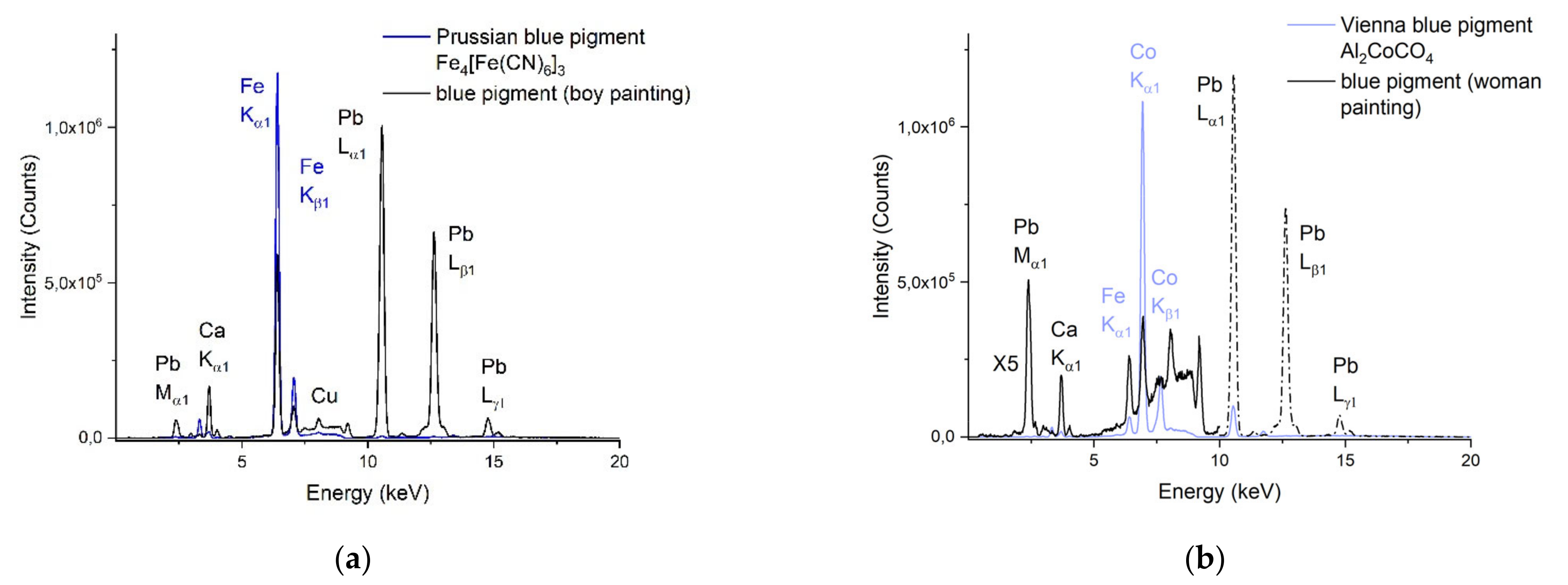
3.2.4. The Blue Zones
3.2.5. The Green Zones
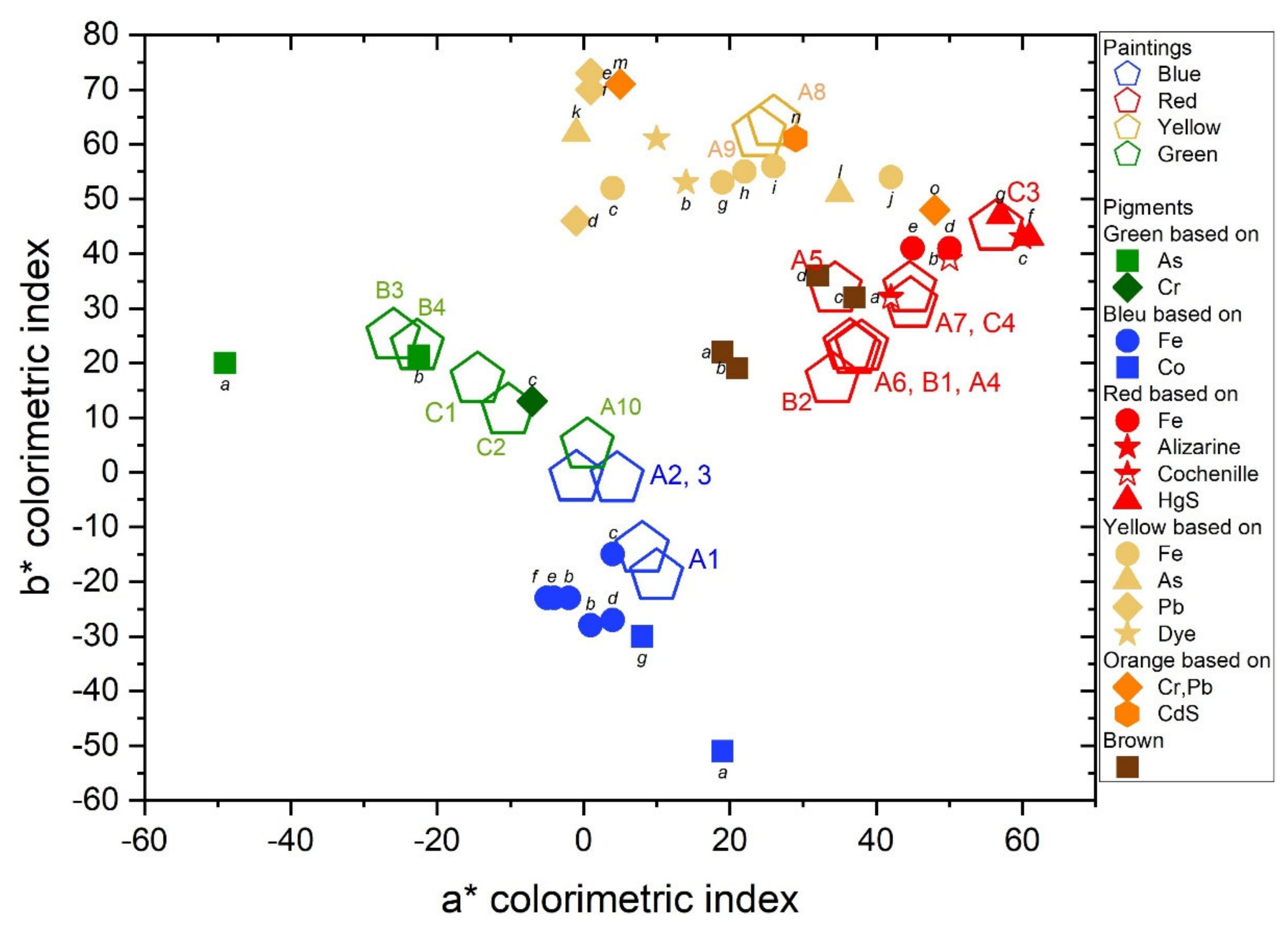
3.3. Colorimetric Analysis of Raw Pigmented Powders and Paints
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bann, S.; Paccoud, S. L’Invention du Passé (Tome 2). Histoires de Cœur et d’Epée en Europe 1802–1850; Musée des Beaux-Arts et Malakoff, Hazan: Malakoff, France, 2014; catalogue d’exposition, Lyon. [Google Scholar]
- Bouquier, G. Rapport et Projet de Décret, Relatifs à la Restauration des Tableaux et Autres Monuments des Arts, Formant la Collection du Muséum National; Convention Nationale: Paris, France, 1794; pp. 2–7; 6 messidor an II (24 juin 1794). In L’Art Social de la Révolution à la Grande Guerre: Anthologie de Textes Sources; McWilliam, N., Méneux, C., Ramos, J., Eds.; l’Institut National d’Histoire de l’Art, PUR: Paris, France, 2014. [Google Scholar]
- Guichard, C. Palettes et tableaux: Des laboratoires de la couleur? Dix-Huit. Siècle 2019, 51, 187–204. [Google Scholar] [CrossRef]
- Richard, F. Mes Souvenirs Rassemblés et Mis en Ordre, 1847–1849; illustrated autograph manuscript; Musée des Beaux-Arts: Lyon, France, unpublished work.
- Pernety, A.-J. Dictionnaire Portatif de Peinture, Sculpture et Gravure; Avec un Traité Pratique des Différentes Manières de Peindre, dont la Théorie est Développée dans les Articles qui en Sont Susceptibles; Bauche: Paris, France, 1757. [Google Scholar]
- Mérimée, J.-F.-L. De la Peinture à l’Huile, ou des Procédés Matériels Employés dans ce Genre de Peinture, depuis Hubert et Jean Van Eyck Jusqu’à nos Jours; Mme Huzard: Paris, France, 1830. [Google Scholar]
- Sofio, S. Les marchands de couleurs au XIXe siècle, artisans ou experts? (Paris, Tours). Ethnol. Fr. 2017, 47, 75–86. [Google Scholar] [CrossRef]
- Bruyère, G. Analytic Inventory Established 1990. In L’Invention du Passé; [CD-ROM] accompanying the catalogue; Musée des Beaux-Arts: Lyon, France, 1990. [Google Scholar]
- Roth-Meyer, C. Les Marchands de Couleurs à Paris au XIXe Siècle. Ph.D. Thesis, Université Paris IV, Paris, France, 2004. [Google Scholar]
- Rand, J.G. Improvement in the Construction of Vessels or Apparatus for Preserving Paint. Patent 2252, 11 September 1841. [Google Scholar]
- Bouvier, P.-L. Manuel des Jeunes Artistes et Amateurs en Peinture; F.-G. Levrault: Paris, France, 1827. [Google Scholar]
- Ricci, C.; Borgia, I.; Brunetti, B.G.; Miliani, C.; Sgamellotti, A.; Seccaroni, C.; Passalacqua, P. The Perugino’s palette: Integration of an extended in situ XRF study by Raman spectroscopy. J. Raman Spectrosc. 2004, 35, 616–621. [Google Scholar] [CrossRef]
- Stanzani, E.; Bersani, D.; Lottici, P.P.; Colomban, P. Analysis of artist’s palette on a 16th century wood panel painting by portable and laboratory Raman instruments. Vib. Spectrosc. 2016, 85, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Mulholland, R.; Howell, D.; Beeby, A.; Nicholson, C.E.; Domoney, K. Identifying eighteenth century pigments at the Bodleian library using in situ Raman spectroscopy, XRF and hyperspectral imaging. Herit. Sci. 2017, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Leuchs, J.C. Traité Complet des Propriétés, de la Préparation et de l’Emploi des Matières Tinctoriales et des Couleurs. Fabrication des Couleurs; Malher et Cie: Paris, France, 1829. [Google Scholar]
- Althen, J. Mémoire Sur la Culture de la Garance; Impr. Royale: Paris, France, 1772. [Google Scholar]
- Kraft, A. On the discovery and history of Prussian blue. Bull. Hist. Chem. 2008, 33, 61–67. [Google Scholar]
- Thénard, L.J. Considérations générales sur les Couleurs, suivies d’un procédé pour préparer une couleur bleue aussi belle que l’outremer. J. Mines 1804, XV, 128–136. [Google Scholar]
- Ball, P. Histoire Vivante des Couleurs: 5000 Ans de Peinture Racontée par les Pigments; Hazan: Paris, France, 2010; pp. 232–234. [Google Scholar]
- Petit, J.; Roire, J.; Valot, H. Encyclopédie de la Peinture: Formuler, Fabriquer, Appliquer; Puteaux, EREC: Paris, France, 1999; p. 378. [Google Scholar]
- Bibliothèque Nationale de France. L’emploi du cyanogène et de ses dérivés à l15a guerre. In Le Génie Civil: Revue Générale des Industries Françaises et Etrangères; Bibliothèque Nationale de France: Paris, France, 1919; p. 91. [Google Scholar]
- Thorpe, E. A Dictionary of Applied Chemistry; Longmans, Green & Co.: New York, NY, USA, 1921; Volume 1. [Google Scholar]
- Berrie, B.H. Prussian blue. In Artists’ Pigments: A Handbook of their History and Characteristics; National Gallery of Art: Washington, DC, USA, 1997; Volume 3. [Google Scholar]
- Beaugrand, E. Des Différentes Sortes d’Accidents Causés par les Verts Arsenicaux Employés dans l’Industrie; Rapport présenté à la Commission d’hygiène et de salubrité du 5ème arrondissement; Plon: Paris, France, 1859. [Google Scholar]
- Riffault, J.; Vergnaud, A.-D. Nouveau Manuel Complet du Peintre en Bâtiments, du Fabricant de Couleurs, du Doreur, du Vernisseur, du Vitrier et de l’Argenteur; Roret: Paris, France, 1843. [Google Scholar]
- Cersoy, S.; Martinetto, P.; Bordet, P.; Hodeau, J.L.; Van Elslandec, E.; Walter, P. Identifying and quantifying amorphous and crystalline content in complex powdered samples: Application to archaeological carbon blacks. J. Appl. Cryst. 2016, 49, 585–593. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Ramirez-Gutierrez, C.F.; del Real, A.; Rubio-Rosas, E.; Rodriguez-García, M.E. Study of bovine hydroxyapatite obtained by calcination at low heating rates and cooled in furnace air. J. Mater. Sci. 2016, 51, 4431–4441. [Google Scholar] [CrossRef]
- Gonzalez, V.; van Loon, A.; Stephen, S.W.T.; Noblea, P.; Keunea, K. Synchrotron micro-XRD and micro-XRD-CT reveal newly formed lead–sulfur compounds in Old Master paintings. J. Anal. At. Spectrom. 2020, 35, 2267. [Google Scholar] [CrossRef]
- Yao, Z.; Qi, J.; Wang, L. Isolation, fractionation and characterization of melanin-like pigments from chestnut (Castanea mollissima) shells. J. Food Sci. 2012, 77, C671–C676. [Google Scholar] [CrossRef] [PubMed]
- Gettens, R.J.; Kühn, H.; Chase, W.T. Lead white. Stud. Conserv. 1967, 12, 125–139. [Google Scholar]
- Gonzales, V.; Calligaro, T.; Wallez, G.; Eveno, M.; Toussaint, K.; Menu, M. Composition and microstructure of the lead white pigment in Masters paintings using HR Synchrotron XR. Microchem. J. 2016, 125, 43. [Google Scholar] [CrossRef]
- Perego, F. Dictionnaire des Matériaux du Peintre; Belin: Paris, France, 2007. [Google Scholar]
- Kühn, H. Zinc White. In Artists’ Pigments: A Handbook of Their History and Characteristics; National Gallery of Art: Washington, DC, USA, 1986; Volume 1. [Google Scholar]
- Garofano, I.; Perez-Rodriguez, J.L.; Robador, M.D.; Duran, A. An innovative combination of non-invasive UV–Visible-FORS, XRD and XRF techniques to study Roman wall paintings from Seville, Spain. J. Cult. Herit. 2016, 22, 1028–1039. [Google Scholar] [CrossRef]
- Gil, M.; Serrao, V.; Carvalho, M.L.; Longelin, S.; Dias, L.; Cardoso, A.; Caldeira, A.T.; Rosado, T.; Mirao, J.; Candeias, A.E. Material and Diagnostic Characterization of 17th Century Mural Paintings by Spectra-Colorimetry and SEM-EDS: An Insight Look at Jose de Escovar Workshop at the CONVENT of Na Sra da Saudac¸ao (Southern Portugal). Color Res. Appl. 2014, 39, 288. [Google Scholar] [CrossRef]
- Kampasaki, E.; Varella, E.A. The Russian avant-garde painting palette: Documentary and physicochemical codification of organic colorants. J. Cult. Herit. 2008, 9, 77–88. [Google Scholar] [CrossRef]
- Bacci, M.; Casini, A.; Cucci, C.; Picollo, M.; Radicati, B.; Vervat, M. Non-invasive spectroscopic measurements on the Il ritratto della figliastra by Giovanni Fattori: Identification of pigments and colorimetric analysis. J. Cult. Herit. 2003, 4, 329–336. [Google Scholar] [CrossRef]
- Lefort, J. Chimie des Couleurs pour la Peinture a L’eau et a l’Huile Comprenant l’Historique, la Synonymie, les Propriétés Physiques et Chimiques, la Préparation, les Variétés, les Falsifications, l’Action Toxique et l’Emploi de Couleurs Anciennes et Nouvelles; Victor Masson: Paris, France, 1855. [Google Scholar]




| Name | Pigment | Additive | L*; a*; b* |
|---|---|---|---|
| Petite pierre jaune [Small yellow stone] | Asisite Pb7O8Cl2, leadhilite Pb4(SO4)(CO3)2(OH)2 | Hydrocerussite 2PbCO3 Pb(OH)2 |  |
| Jaune de Vienne [Vienna yellow] | Crocoite PbCrO4 | Cerussite PbCO3 |  |
| Crôme Citron [Lemon chrome] | Crocoite PbCrO4 |  | |
| Pierre Jaune [Yellow Stone] | Goethite FeO(OH) | Quartz SiO2, kaolinite Al2Si2O5(OH)4 |  |
| Ocre jaune [Yellow Ochre] | Goethite FeO(OH) | quartz, SiO2 |  |
| Ocre de Rue (or Ru) [Ru ochre] | Goethite FeO(OH) | Quartz SiO2, barite BaSO4 |  |
| Jaune Marron [Brown Yellow] | Jarosite KFe3(SO4)2(OH)2 |  | |
| Orpin jaune [orpiment] | Orpiment As2S3 |  | |
| Sans nom ‘marron’ [No name brown] | Arsenolite As2O3, orpiment As2S3 |  | |
| Crôme orange [chrome orange] | Crocoite PbCrO4 |  | |
| Cadmium | Greenockite CdS |  | |
| Sans nom ‘orange’ [No name Orange] | Minium Pb2+2Pb4+O4 or Pb3O4 |  | |
| Lacque de Gaude [Gaude lake] | Reseda Luteola Luteolin? | Quartz SiO2 |  |
| Laque Jaune [Yellow lake] | Reseda Luteola Luteolin? | Quartz SiO2, gypsum CaSO4.2H2O |  |
| Jaune clair n°1 [Light yellow] | Organic lake? |  |
| Name | Pigment | Additive | L*; a*; b* |
|---|---|---|---|
| Brun Rouge d’angleterre d’Antheaume [Antheaume’s English redbrown] | Hematite Fe2O3 |  | |
| Rouge Mars [Mars red] | Hematite Fe2O3 |  | |
| Vermillon [Vermillion] | Vermillon HgS |  | |
| Vermillon de la Chine [Vermillion from China] | Vermillon HgS |  | |
| Laque de Garance pour Mer [Madder lake] | Alizarin? |  | |
| Galet rouge [Red pebble] | Alizarin? | Gibbsite Al(OH)3, calcite CaCO3 (chalk) |  |
| Laque magnifique [Magnificent lake] | Carminic acid (cochineal) |  |
| Name | Pigment | Additive | L*; a*; b* |
|---|---|---|---|
| Bleu de Vienne [Vienna blue] | Cobalt blue Al2CoO4 | Silica SiO2 |  |
| Bleu mineral [Mineral blue] | Prussian blue Fe4[FeCN6]3.14H2O |  | |
| Bleu de Prusse [Prussian blue] | Prussian blue Fe4[FeCN6]3.14H2O |  | |
| Sans nom bleu [No name blue] | Prussian blue Fe4[FeCN6]3.14H2O | Gypsum CaSO4.2H2O or alunite KAl3(SO4)2(OH)6 |  |
| Bleu de Ciel [Sky blue] | Prussian blue Fe4[FeCN6]3.14H2O |  | |
| Laque Bleue de Garance Inaltérable [Blue madder lake] | Prussian blue Fe4[FeCN6]3.14H2O |  | |
| Cobalt | Al2CoO or CoCO3? | Hydrocerussite Pb3(CO3)2(OH)2 or leadhilite Pb4SO4(CO3)2(OH)2 |  |
| Name | Pigment | Additive | L*; a*; b* |
|---|---|---|---|
| Sans nom vert [No name Green] | Arsenolite As2O3, trippkeite CuAs2O4, emerald green C2H3As3Cu2O8 |  | |
| Vert de Scheele [Scheele’s green] | Cu(AsO2)2 or tyrolite CaCu5(AsO4)2(CO3)(OH)4.6H2O | ? |  |
| Sans nom vert foncé [No name dark green] | Eskolaite Cr2O3 | Hydrocerussite 2PbCO3 Pb(OH)2 |  |
| Name | Pigment | Additive | L*; a*; b* |
|---|---|---|---|
| Terre de Cologne (Mr. Dechazelle) [Mr. Dechazelle’s Cologne earth] | Carbon C, hematite Fe2O3 | SiO2, whitlockite Ca3(PO4)2 |  |
| Terre de Sienne brulée Antheaume [Burnt Sienna] | Hematite Fe2O3 | Clay montmorillonite |  |
| Couleur d’Appiani [Appiani’s color] | Clay? |  | |
| Momie [Mummy] | Canadian balm (bitumen?) | Silica SiO2 |  |
| Name | Pigment | Additive | Picture |
|---|---|---|---|
| Noir de vigne [Vine charcoal] | Carbon C | Calcite CaCO3, quartz SiO2 |  |
| Noir d’ivoire antheaume [Ivory black] | Carbon C | Whitlockite Ca3(PO4)2 |  |
| Grand noir (de Mr) [Great black] | Carbon C | Whitlockite Ca9.5MgP7O28 |  |
| Terre de Cassel [Cassel earth] | Walnut brown (melanin-like) | Silica SiO2 |  |
| Name | Pigment | Additive | Picture |
|---|---|---|---|
| Blanc léger [Light white] | Cerusite PbCO3, hydrocerussite 2PbCO3, Pb(OH)2 or plumbonacrite Pb5(CO3)3O(OH)2 |  | |
| Blanc de Cremnitz [Cremnitz white] | Hydrocerussite 2PbCO3, Pb(OH)2 |  | |
| Blanc d’argent (de Bellot) [Silver-white] | Hydrocerussite 2PbCO3, Pb(OH)2 |  | |
| Blanc [White] | Potassium hydrogenotartrate “wine tart” C4H5KO6 |  | |
| Grosse pierre blanche [Big white pebble] | Calcite CaCO3 (chalk) | Quartz SiO2 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carole, D.; Wicky, E.; Bensalah-Ledoux, A.; Paccoud, S.; Le Luyer, C.; Pillonnet, A.; Panczer, G. The Pigments of the Painter Fleury Richard (1777–1852), a Model for Multidisciplinary Study. Heritage 2022, 5, 1276-1294. https://doi.org/10.3390/heritage5020066
Carole D, Wicky E, Bensalah-Ledoux A, Paccoud S, Le Luyer C, Pillonnet A, Panczer G. The Pigments of the Painter Fleury Richard (1777–1852), a Model for Multidisciplinary Study. Heritage. 2022; 5(2):1276-1294. https://doi.org/10.3390/heritage5020066
Chicago/Turabian StyleCarole, Davy, Erika Wicky, Amina Bensalah-Ledoux, Stéphane Paccoud, Cécile Le Luyer, Anne Pillonnet, and Gérard Panczer. 2022. "The Pigments of the Painter Fleury Richard (1777–1852), a Model for Multidisciplinary Study" Heritage 5, no. 2: 1276-1294. https://doi.org/10.3390/heritage5020066
APA StyleCarole, D., Wicky, E., Bensalah-Ledoux, A., Paccoud, S., Le Luyer, C., Pillonnet, A., & Panczer, G. (2022). The Pigments of the Painter Fleury Richard (1777–1852), a Model for Multidisciplinary Study. Heritage, 5(2), 1276-1294. https://doi.org/10.3390/heritage5020066







