Abstract
The painting The Descent from the Cross, painted in 1620 by Pedro Nunes (1586–1637), presents two large figures with orange-coloured fabrics with conservation problems. Through the analysis of two samples with several analytical techniques, especially scanning electron microscopy combined with X-ray spectroscopy and Raman microscopy, it was possible to conclude that the orange colour is due to a complex artificial pigment made of amorphous arsenic sulphide. It essentially consists of spherical particles obtained by sublimation and condensation, possibly from orpiment, which ended up being joined with irregularly shaped particles resulting from crushing of the residual fraction obtained by solidification and fusion. This is a rare documented case of the extensive use of artificial arsenic sulphides in European easel painting, especially outside Italy. The conservation problems can be explained by the great sensitivity of the arsenic sulphides to photodegradation and the formation of powdery compounds.
1. Introduction
A large-scale application of an orange paint with an unusual surface appearance was found on The Descent from the Cross, a large panel (460 cm × 304 cm) painted in 1620 by Pedro Nunes (1586–1637) for the chapel of Esporão in Évora’s cathedral, Portugal [1] (Figure 1). This orange paint was found in the draperies of two apostles, the one standing tall to the right of the composition being more than two meters high.

Figure 1.
The Descent from the Cross by Pedro Nunes, 1620, Esporão Chapel, Évora’s Cathedral, Portugal, (a) and a surface detail of the orange cloak (marked with a white rectangle) under incident (b) and raking light (c).
These areas raised interest, firstly, by the conservation problem that they exhibited, and secondly, by the material responsible for the orange colour. One of the initial hypotheses was that it was realgar, an orange-coloured pigment little used in European easel painting [2], particularly in Portugal [3], even though it fits well with the Mannerist palette, characterised by contrasts between complementary acid colours, namely, orange and blue, on the one hand, and rose and green, on the other. Pedro Nunes was a painter who, for some years, learnt the art in Rome [1], and, therefore, associated with this hypothesis, there was an additional reason of interest: to know whether or not the evident Italian artistic influences in the painting were accompanied by the same influences regarding the materials used, in particular, the realgar, which, at the time, seems to have mainly be used in Italy [2,4] p. 128.
In order to investigate these questions, microsamples were collected from the orange areas and analysed with microscopic and spectroscopic techniques. The results obtained, which are presented below, turned out to be much more interesting than initially anticipated, as they showed the use, in an extensive area, of a material still little documented in European easel painting—an artificial arsenic sulphide.
2. Methods
The painting was subject to a visual inspection of the surface in situ under incident and raking light. Digital photographs were taken with a Canon G15 camera. Two samples were collected from the medium (#01) and light (#02) tints of the cloak of the apostle standing to the right of the composition, in areas exhibiting an irregular grainy surface.
Part of each sample was sandwiched between two rigid transparent polymethyl methacrylate (PMMA) cubes with the self-curing resin Spofacryl from SpofaDental and polished as a cross-section. The cross-sections were examined with optical microscopy in reflection mode (OM), under incident visible (OM-Vis) and ultraviolet radiation (OM-UV). A Leica DM2500 microscope was used with an excitation filter BP 340–380, a dichromatic mirror, and a suppression filter LP 425. Digital images were taken with a Leica digital camera DFC290HD. The optical properties of the artificial arsenic sulphide were further investigated using fragments of sample #02 examined in petrographic microscope Leica DM2700P coupled with a Flexacam C3 camera.
Subsequently, the uncoated cross-sections as well as loose fragments of sample #02 were analysed with scanning electron microscopy with energy dispersive X-ray spectrometry on a scanning electron microscope Phenom Pro-X, operated at 15 kV (SEM-EDS). The SEM-EDS microscope uses a charge reduction mode via a low vacuum sample holder. In addition to X-ray data, backscattered electron images (SEM-BSE) were used.
Micro-Raman spectroscopy (µ-RS) was used in the paint cross-sections using a Raman spectrometer Horiba XPlora equipped with a diode laser of 10.3 mW operating at 785 nm, coupled to an Olympus microscope. Raman spectra were acquired in extended mode in the 100–1500 cm–1 region. The laser was focused with an Olympus 50× lens, 10% of the laser power on the sample surface (5 s exposure, 5 cycles of accumulation). The spectra were analysed with the equipment software (LabSPEC 5 from Horiba Jobin Yvon, France) and the Spectragryph (v. 2.15, Dr. Friedrich Menges, Oberstdorf, Germany) application.
Micro X-ray diffraction was performed using a Bruker AXS D8 Discover diffractometer with a Cu Kα radiation source and a Bruker LynxEye energy dispersive one-dimensional detector (µ-XRD). The top layer of the orange paint was separated from the sample, and the diffractogram was acquired in the interval 5–60° (2Θ) with a step of 0.05° and 4 s per step. EVA software (with ICDD PDF X-ray patterns database) was used for the identification.
3. Results
3.1. Colour and Morphology
To the naked eye, the medium and light tints of the orangey garments of the apostle bending over the cross and the one standing to the right of the composition exhibited an irregular surface with small spherical nodules protruding from within the paint layer and leaving an open crater whenever lost (Figure 1). Areas of the garments with this specific surface texture had a muted orange to ochre yellowish colour and absorbed the varnish coating that protects the painting, creating matt areas perceived as stains over the surface.
The two cross-sections examined under the optical microscope showed a similar structure with a first underlayer of ochre colour with a thickness of 25–30 µm, covered by a bright orange paint, of around the same thickness, with bright orange particles without UV fluorescence (Figure 2). Among these particles, it is possible to distinguish spherical particles and particles of irregular or undefined shapes (OM). The former have a diameter of up to about 15 μm, while irregularly shaped particles can, in some cases, reach 40 μm in their largest dimension. Some of the spherical particles show a brighter outer shell than the core (Figure 2).
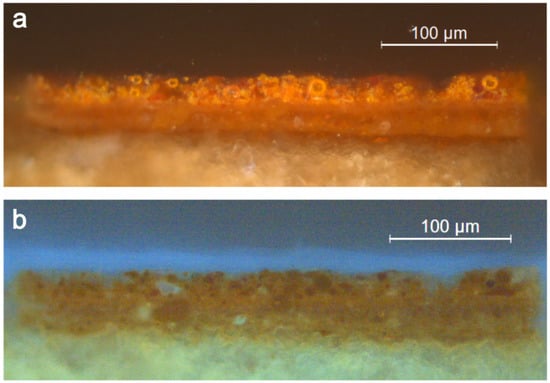
Figure 2.
Cross-section of sample #01; OM under incident light (a) and UV radiation (b).
Examination of the top bright orange layer under the petrographic microscope reveals that some particles show intense green (Figure 3a) or blue (Figure 3b) interference colours.
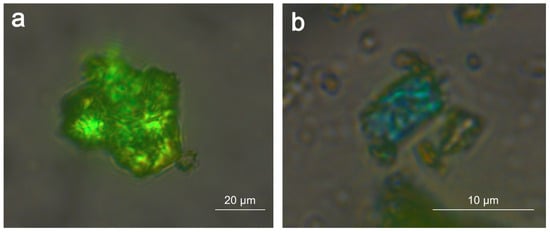
Figure 3.
Example of particles with orange colour showing interference colours in the petrographic microscope.
These interference colours, the particles’ orange colour, and the absence of UV fluorescence are compatible with the minerals realgar or pararealgar, both of natural origin [5]. However, the spherical shape of some of them is a clear indication of artificial arsenic sulphide particles obtained by sublimation [6].
Focussing on the upper orange bright paint layer, SEM-BSE images show a large number of spherical particles with widely varying sizes ranging from, at least, 3 to 15 μm (Figure 4). Some of them are clearly crushed as a result of the grinding of the pigment (Figure 4a,b). In addition to these, particles with other shapes and also with a wide range of sizes are observed. The latter often reveal cracks of variable thickness. In a significant number of cases, the separation of the fragments resulting from the cracking seems to have occurred after the formation of the paint layer, due to the relative position that the various fragments from the same particle maintain (Figure 4b–d).
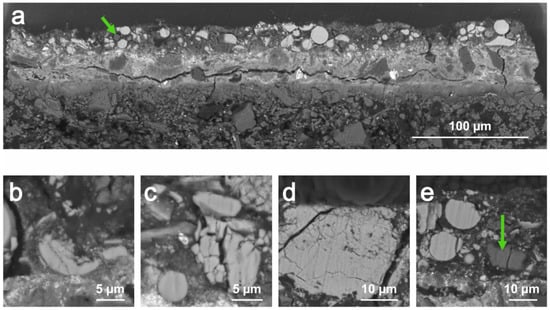
Figure 4.
SEM-BSE images from cross-sections of sample #01 (a) and sample #02 (b–e). The green arrows highlight particles from the orange upper layer exhibiting a lower mean atomic number.
Contrary to what could be expected from the observations with the petrographic microscope, namely, the interference colours, no marks were detected on the orange particles that could clearly be related to crystalline structures. An exception is a particle observed on the surface of sample #02, which appears to have a foliated structure (Figure 5a).

Figure 5.
SEM-EDS images of sample #02 showing degraded particles (a,b), a crystalline particle ((a), green arrow) and an amorphous phase (c).
The SEM-EDS results (see below) show that the orange particles have a high arsenic and sulphur content, as with the apparently amorphous phase seen in Figure 5c. Regardless of their shape, these particles are homogenous, and have well-defined borders and a similar average atomic number in the SEM-BSE images (Figure 4). In sample #02, some particles have brighter areas in the outer layer (Figure 5b and Figure 6), but this should not imply a mean atomic number superior to that of the interior since, as suggested by the SEM-EDS results (see below), this may simply be an artefact of the particles morphology. Moreover, some of these loose sample particles have a heterogeneous structure coated on the outside by a homogeneous and compact material without crystalline forms, which in many particles appears to be in a degraded state (Figure 5a). In the upper part of the stratigraphic sections, it is possible to observe spherical particles with the rim (outer layer) degraded on the side facing the painting surface (Figure 6). They appear to have a narrow surface rim with a higher atomic number than the core, but the As and S concentration profiles obtained for some particles show no variation inside them (see below).
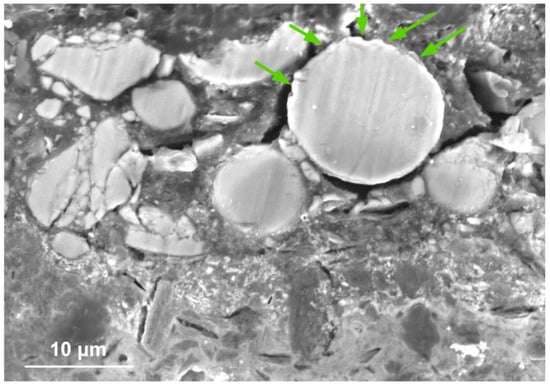
Figure 6.
Spherical particles, in sample #01, with the rim facing towards the surface of the sample showing signs of corrosion (noted with green arrows).
Finally, SEM-BSE images of the top orange layer expose a few irregularly shaped particles displaying a lower atomic number than the aforementioned particles (Figure 4a,e).
3.2. Elemental Composition
The SEM-EDS results show that the bright orange upper paint layer is mainly composed of arsenic-rich components. It is over an underpaint formed essentially by compounds of Fe, Si, Al, Ca, and Pb, that is, a clay earth pigment with some ochre-iron-rich particles and, probably, some white lead.
A total of 115 point analyses were performed by SEM-EDS in the orange layer (the vast majority) and in the ochre layer of the two samples, and in general, the elements As, S, C, and O correspond to almost all the detectable and quantified elements (Figure 7). In these points, the molar ratio As:S is, on average, 59:41, with an excess of arsenic in relation to the theoretical ratio of realgar or pararealgar (50:50), the ratio actually determined in realgar samples (48:52) [7], the theoretical ratio of orpiment (40:60), or for other arsenic sulphide compounds. The repeated analyses at different points of some particles, in general, did not show significant random errors, but no results were obtained to allow for an estimation of the systematic errors. However, the results obtained for a particle (Figure 4d) of pararealgar (identified by Raman spectroscopy) suggest that the actual ratio is lower than the values obtained by SEM-EDS (see below). This problem can be explained by the semi-quantitative nature of the SEM-EDS results. In this situation, the corrected molar ratio between As and S will in fact be around 47:53.
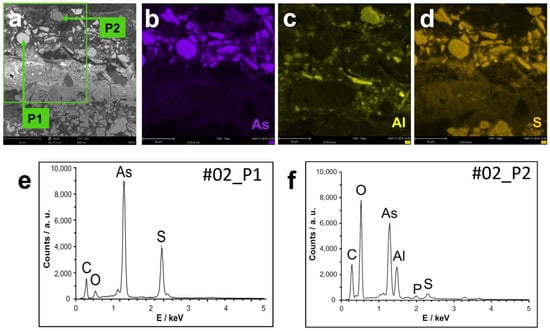
Figure 7.
SEM back-scattered electron image of the details of sample #02 (a); SEM-EDS maps of As (b), Al (c), and S (d); and SEM-EDS spectra (e,f) of points P1 and P2 located in (a).
For the spherical particles, the average value of the molar fraction of As is slightly lower than in the other particles and, above all, has a much smaller variation. The average ratio obtained for these particles (54:46) is similar to that of a glass used as a pigment in an 18th century sculpture, prepared from pararealgar [8], but this does not appear to be a common value. However, if we consider that the actual As values are lower than the values obtained by SEM-EDS (as described above), to those spherical particles corresponds a corrected As:S molar fraction of about 42:58, which is already comparable to usual glasses [9].
In the spherical particles, no significant difference was found between the inner and outer surface (interior and the exterior surface), regardless of its state. The situation was confirmed both through point analysis and concentration profiles (Figure 8).
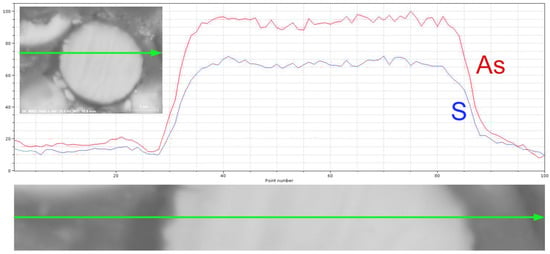
Figure 8.
Line scan of spherical particles from sample #01.
Among the non-spherical particles, there is a significant number with a composition comparable to that of the spherical particles, but there is also a significant number with a very different composition, in particular with a reduced proportion of sulphur, an element that is not even detectable in many of these cases. One of the cases is the irregular and fractured particle at the paint surface, with a low average atomic number, which the SEM-EDS results suggest could be arsenolite (As2O3) (Figure 7e).
For the crystalline-flake-like material observed in Figure 5a, the molar ratio As:S is 51:49 according to the two analyses performed. In reality, the ratio is about 39:61 if we take into account the correction mentioned above, a value that, together with the lamellar structure of the particle, suggests that it is orpiment.
Where, besides As, S, C and O, other elements were detected with significant concentration, these were generally Ca, Si, and Al, and less frequently K, P, Na, Fe, and Pb. None of the 115 analyses detected elements that appear associated with some specific arsenic minerals, such as antimony or selenium [10].
The analyses in which significant concentrations of Pb were detected correspond to points in the underlying ochre layer, mainly from areas with small particles. In these points, regardless of whether they correspond to these particles or to the matrix surrounding them, a significant correlation was found between the Pb and the As concentration (Figure 9), which suggests that the two elements are combined in the same compound, namely, a lead arsenate.
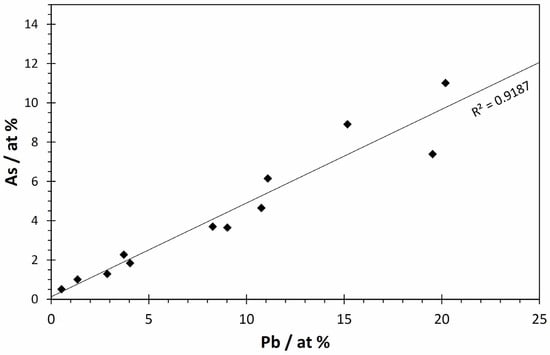
Figure 9.
Relationship between atomic concentration of lead and arsenic at the points analysed by SEM-EDS in the underlying ochre layer (n = 12).
3.3. Structural Data
In the X-ray diffraction spectra obtained for the paint surface, only anhydrite and quartz are detectable, and no peaks due to arsenic compounds are evident, which is an indication of the essentially amorphous nature of these compounds. This result is in agreement with the generality of the numerous Raman spectra obtained, where a very broad band is observed at about 345 cm–1, characteristic of arsenic sulphides in a glassy state [11].
This band at about 345 cm–1 is the dominant band in most of the Raman spectra (Figure 10), in which, in some of them, we also observe weak and equally broad bands at about 190, 221, 236, 277, 298, 318, 350, and 360 cm–1. The spectra (a) and (c) in Figure 10 are examples, respectively, of the cases of lowest and highest visibility of these weak bands, which often appear in glassy arsenic sulphides [11] and correspond to crystalline As4S4 (~190, ~221, ~236, ~277, ~350 and ~360 cm–1) and As2S3 (~297 and ~318 cm–1) nanophases that form in the glassy matrix [12]. These spectra were obtained both for spherical particles and for fragments with other shapes. In the case of the spheres for which spectra were obtained for the interior and for the surface ring, no significant differences were detected between these zones (Figure 10a,b), except, in some cases, a slight decrease in amorphous features inside. According to the literature, some of the mentioned bands, namely, those appearing at about 190 and 360 cm–1, have an intensity that increases with the molar fraction of As [12].
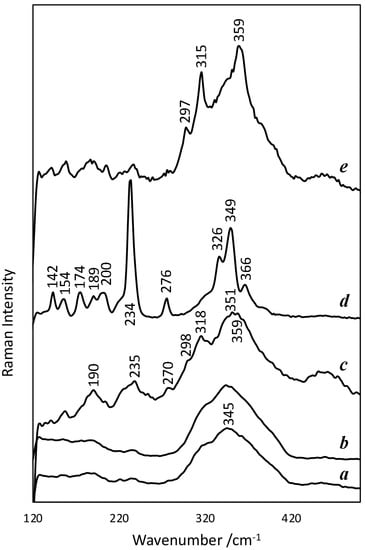
Figure 10.
Raman spectra restricted to the 100–500 cm−1 region of the acquired extended range: (a) centre of the spherical particle, of sample #01, visible in the upper-right-hand corner of Figure 4a (analysis As6i); (b) surface ring of the same particle (analysis As6e); (c) irregularly shaped particle from sample #02 obtained by fragmentation (analysis As15); (d) particle of sample #02, visible in Figure 4d (analysis As11); (e) centre of a spherical particle of sample #02 (analysis As13i). Spectra offset for clarity.
The main exception to the spectra with amorphous characteristics is the one obtained for a large, non-spherical particle on the surface of the stratum, exposed to the outside and with a large number of cracks (Figure 4d): a very intense and thin band at 234 cm–1 and others, also well defined, at 142, 154, 174, 189, 200, 276, 326, 349 and 366 cm–1, correspond to the spectrum of the pararealgar (Figure 10d) [8,13]. Such particles, which are a minority, explain the observations made with the petrographic microscope. For the mentioned particle of pararealgar, according to the SEM-EDS analyses, the molar ratio between As and S is 62:38 (average of four point analyses), which corresponds to an As value clearly higher than expected for this mineral (50:50). If we consider that this is a relative systematic error, this means that the other ratios obtained by SEM-EDS are also higher than the real values and should be corrected accordingly (the ratio is corrected by first considering that a quotient of 1.63 corresponds to a quotient of 1 and then normalising the two terms of the quotient so that their sum is 100).
Another Raman spectrum different from most spectra was obtained for a spherical particle apparently equal to others that gave rise to typical spectra of glassy materials. In this case, the most intense bands of orpiment, namely, at 297, 315 and 359 cm–1 [11] (Figure 10e), are superimposed on the spectrum with amorphous characteristics. This situation is observed in the spectrum obtained at the centre of the particle, but is much less noticeable in the spectrum obtained at the surface, which differs little from the most frequent spectra.
4. Discussion
4.1. Material Significance
The results obtained by microscopy and Raman spectroscopy show that the orange colour of Pedro Nunes’s painting results from a complex mixture of arsenic compounds in which an arsenic sulphide glass obtained by sublimation and condensation is predominant. First of all, the results obtained demonstrate, as others have already mentioned [11], that it is not possible to identify an arsenic pigment only by its colour: in this case, despite the orange colour that, without detailed results, would be attributed to the realgar, the results prove that it is not this pigment.
An arsenic sulphide glass obtained by sublimation, such as the one identified in the painting, characterised by spherical particles, could be obtained by procedures described in ancient treatises, especially of mineralogy [14] pp. 57–58; [15] pp. 57–58; [16] pp. 106–107 or pharmacy [17] pp. 254–255; [18] pp. 889–890. The process involved heating in a closed apparatus, usually of ceramic, arsenolite, and sulphur in some cases, or orpiment in others. Despite the possibility of collecting only the fraction resulting from sublimation, the ancient procedures also led to the formation of a fraction obtained by fusion. This situation is described in several works of the 18th century. For example: “The factitious kind is made of orpiment melted and boiled for some time in subliming vessels, by which the yellow flowers are raised to the upper part of the vessels, and the mass remaining at the bottom, being condensed by cold, becomes of a red colour, like cinnabar, and is called realgar” [15] p. 163. In the present case, the mixture found with amorphous spherical particles and irregular fragments can be explained by the insufficient separation of the two materials, which would be more probable in the case of the use of a simpler apparatus, such as that described by Georgius Agricola in 1546 for the preparation of an orange pigment from orpiment: “Medium small particles of the later are placed in an earthen jar and then the mouth of it is sealed. The jar is placed in a furnace for five hours and the mineral will then have the colour of realgar” [14] pp. 57–58. In fact, the mixing of different phases was necessarily a consequence of the procedure to which, according to the Spanish painter Antonio Palomino in 1724, some people subjected the orpiment: “burning the pigment in a little glass vial. Then they break up the vial and grind it with white wine, so that the glass serves as a drier for the orpiment” [19] p. 165. A similar but more detailed description is given in a book of secrets published in Portugal at the end of the century: “Put the orpiment in one of these glasses that have little difference both in height and diameter, cover it leaving a small hole so that it does not crack, and put it in a bain-marie until the orpiment melts and rises in steam, which will run down again through the glass around the neck; then take the glass off the fire, let it cool, and break it” [20] p. 41.
In such a situation, the treatment should not have the direct intention of obtaining a new material, but simply of obtaining a more intense colour for a material that was considered to be the same. In this respect, it is important to note that in Iberian Peninsula treatises, the same name jalde, which corresponds to what nowadays is called orpiment, is used for two different materials: the first is yellow (jalde), and the second, obtained by heating the yellow, is orange (jalde queimado or burnt jalde). The use of this nomenclature has already been highlighted in the aforementioned treatise by Antonio Palomino (1724) [21] p. clxviii, as well as in two other Spanish treatises, that of Francisco Pacheco (1646) and that of an anonymous author (1656) [22] p. 175, but there is also a Portuguese reference, even older (though similar to Pacheco’s), in the painting treatise by Filipe Nunes, published in 1615: “burn the jalde stone, just as you by it, over the fire in an iron spoon, or in a little pot lid. It should be heated over smokeless embers, and when it begins to trickle like honey it is properly heated” [19] p. 4. According to a Portuguese source from the same period, which is similar to others in this respect, the red colour resulting from the heating of the orpiment would be more intense the longer the heating time [23] p. 43v.
In the case of the painting under study, the material used would result from the vigorous grinding of the mixture obtained, which is confirmed by the fragmented spheres, an operation that would be dispensable in the case of using only the fraction obtained by sublimation.
Regarding the raw material employed, the results are not clear.
The alteration of arsenic pigments by the action of light is a well-known fact of realgar with the formation of orpiment, having pararealgar as intermediate [24], and of orpiment with the formation of arsenolite, in both cases with evident consequences in terms of alteration of the paint colours [25]. In the painting under study, pararealgar and possibly orpiment were detected, but the crystalline forms observed suggest that they did not result from transformation occurring in situ [22] p. 172. Furthermore, neither realgar nor arsenolite were detected as being associated with pararealgar, nor was arsenolite detected as being associated with orpiment. Thus, the results suggest that these minerals did not result from alteration processes in the paint, but were part of the mixture of materials used as pigment. Considering, on the one hand, the composition of the glass spheres with a molar ratio As:S of about 42:58 (relatively close to 40:60, the ideal fraction of the orpiment) and the detection of Raman bands due to the orpiment and, on the other hand, the fact that the sublimation process is congruent [26] and, therefore, the proportion between the two elements is maintained, one can hypothesise that the raw material is essentially orpiment, but the presence of the pararealgar crystals remains to be explained. One possibility is that the painter mixed the two materials in order to obtain the desired colour, but this is only a hypothesis that the results obtained do not allow us to discuss.
The hypothesis that the main material results from the sublimation of orpiment seems to correspond to the origin of the type I defined by Grundmann and Richter, but the yellow colour and the characteristic Raman bands of this type [6] are not in conformity with the orange pigment under study.
The problems detected on the paint surface were not directly addressed, but the fact that the changes detected by SEM-BSE in the spherical particles occur predominantly on the surface side of the paint suggests that they result from the photodegradation and formation of powdery compounds, as reported in other cases [25,27]. It can be noted that even though glassy arsenic compounds have higher light resistance than natural crystalline materials in the short term, in the long term, this is not the case [28].
The presence of arsenic compounds in the underlying layer, when there is no evidence of the use of arsenic pigments there, and the relationship found between this element and lead (Figure 9), whose presence in that layer must have originated from the use of lead white, suggest that a reaction occurred between the lead white and the arsenic ions coming from the superficial layer as a result of the degradation of the arsenic pigments, similarly to what was found in another work, although in that case, the reaction involved aluminium and the formation of aluminium arsenate [29]. In the case of the painting under study, the process can be related to the degraded appearance of the exterior of some spherical particles of the superficial layer (Figure 5) and can be justified by periods of high relative humidity inside the church, a condition that facilitates the formation of mobile forms of arsenic oxides [29].
4.2. Historical Significance
Although several studies on arsenic pigments have been recently published, these pigments have had little use in Western easel painting [2]. This recent interest does not have to do, therefore, with their historical importance. It is mainly due to the discovery, provided by the development of analytical equipment, that, contrary to the idea that was common until recently, several arsenic compounds, either natural or artificial, were used as pigments, besides the usually mentioned orpiment and realgar. To these developments contributed, firstly, the studies by Grundmann and collaborators [6,22,30,31,32], which, through documentary research, reconstructions, and sample characterisations, have shown the existence and use of a wide diversity of artificial compounds, many of them amorphous in nature. Likewise contributing to this were a number of case studies of different kinds of works in which these artificial pigments have been identified, as can be seen in a recent review, which includes the literature on the material alteration [25].
Characteristic of the palette of the 16th century Venetian School painters [13,33,34], arsenic sulphide pigments became increasingly used in the 17th century, not only by Italian but by Northern painters as well [27,29,35,36]. Artificial arsenic sulphide pigments of different and sometimes complex compositions have been identified more recently in a painting by Tintoretto, in a few works by 17th century Dutch and Flemish artists, and in two German sculptures [8,30,31,37]. With the exception of Tintoretto’s painting [31], this type of pigment has, however, mostly been identified in small passages, notably in still life paintings [35,38].
The number of Portuguese works in which yellow or orange arsenic pigments have been identified is extremely small: to the rare cases detected in a survey made in 2007 [3], only a few more can be added at present [39,40,41,42,43,44] and they do not exclusively concern easel painting. However, as said, these pigments, under the name of jalde, were mentioned in Filipe Nunes’ treatise on painting, published in 1615 [19]. Plus, these materials were relatively common, as we can see from a law of 1521, which stipulated that they could only be sold by apothecaries, and moreover, only to those who needed them because of their profession [45] p. 316–317. This determination was maintained in the 1603 legislation [46] p. 1240. In the 18th century, under the name of arsenic and rosalgar, they appear among the main imported materials that were used in painting [47], but such materials, in general, were not used exclusively in this activity. In the mid-18th century, according to the official price on which taxes were paid, burnt jalde was 50% more expensive than jalde (respectively 120 and 80 reis per arrátel) [48] p. 39, but the two were more expensive than many other pigments, contrary to what happened in other places [6]. The contrast between the small number of paintings in which arsenic sulphides were identified and the extensive references to them in treatises on pharmacy [17] leads to the conclusion that their use in Portugal was mainly for medical purposes.
Regardless of the doubts about the origin or the preparation form of the pigment used by Pedro Nunes, the results obtained are sufficiently clear to show the importance of the case.
First of all, this painting contributes to the knowledge of the diversity of materials actually accessible to artists in Portugal, particularly in a period when Northern European influences had given way to predominantly Italian influences. Besides adding another example to the small number of known cases of arsenic sulphide use in the country, it is the first in which an artificial form is identified.
From a European perspective, the use of arsenic sulphides in large areas, as happens in this painting, in a way is closer to the Italian practice exemplified by Tintoretto’s work and enriches the perspective on regional diversities, even if the data are still insufficient to understand what the situation really is. This case shows that in the Mannerist period, the influences of the main artistic centres in peripheral areas were not limited to strictly stylistic features. Or, considering a Baroque sculpture with popular characteristics where an arsenic sulphide was also used in an extensive area [43], does it integrate a regional tradition not yet detected? These are questions that this case raises and that deserve investigation.
In any case, this painting contributes to the knowledge of the diversity of artificial arsenic sulphides used in European painting, a subject about which very little is still known [25].
5. Conclusions
In the painting The Descent from the Cross, executed in 1620 by Pedro Nunes, an orange pigment corresponding to an artificial arsenic sulphide was used to a significant extent. It is a complex material, formed essentially by a mixture of amorphous materials where spherical particles, up to 15 μm in diameter, seem to predominate. These particles were obtained, probably from orpiment, by a process of sublimation and condensation, which ended up being combined with irregularly shaped particles, possibly resulting from the residual fraction obtained by solidification and fusion. According to current knowledge about artificial arsenic sulphides, this is a rare case in European easel painting.
The problems observed on the surface of the painting, which were at the origin of this material characterisation study, can be explained by the great susceptibility to photo-oxidation of arsenic sulphides, which, in this case, is evidenced by the alteration of the pigment particles that is more accentuated on the side closest to the painting surface.
Author Contributions
Conceptualisation, H.P.M.; methodology, H.P.M., A.J.C., S.V. and C.M.; investigation, H.P.M., S.V., C.M. and A.J.C.; writing—original draft preparation, A.J.C. and H.P.M.; writing—review and editing, A.J.C., H.P.M., S.V. and C.M.; funding acquisition, H.P.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundação para a Ciência e Tecnologia (FCT), Portugal [Grants SFRH/BPD/109296/2015 & DL57/2016/CP1372/CT0012], Fundo Social Europeu (FSE), ERIHS.pt [ALT20-03-0145-FEDER-022115 (Program Alentejo2020)], and HERCULES Strategic Plan [UIDB/04449/2020 e UIDP/04449/2020].
Data Availability Statement
The data that support the findings of this study are available from H.P.M. upon reasonable request.
Acknowledgments
The authors wish to acknowledge Sr. Cónego Eduardo Pereira da Silva, Rita Vaz Freire, Yigit Helvaci, Nuno Carriço, Jorge Faria, Luís Dias, Massimo Beltrame, and José Silva.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Serrão, V. Pedro Nunes (1586–1637)—Um notável pintor maneirista eborense. A Cid. Évora 1988–1993, 45–50, 105–137. [Google Scholar]
- FitzHugh, E.W. (Ed.) Orpiment and realgar. In Artists’ Pigments. A Handbook of Their History and Characteristics; National Gallery of Art: Washington, DC, USA, 1997; Volume 3, pp. 47–79. [Google Scholar]
- Cruz, A.J. A cor e a substância: Sobre alguns pigmentos mencionados em antigos tratados portugueses de pintura—Pigmentos amarelos. Artis Rev. Inst. História Arte Fac. Let. Lisb. 2007, 6, 139–160. [Google Scholar]
- Hall, M.B. Color and Meaning. Practice and Theory in Renaissance Painting; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Eastaugh, N.; Walsh, V. The Pigment Compendium. Optical Microscopy of Historic Pigments; Elsevier Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Grundmann, G.; Richter, M. Types of dry-process artificial arsenic sulphide pigments in cultural heritage. In Fatto d′Archimia. Los Pigmentos Artificiales en las Técnicas Pictóricas; Egido, M.D., Kroustallis, S., Eds.; Ministerio de Educación, Cultura y Deporte: Madrid, Spain, 2012; pp. 119–144. [Google Scholar]
- Douglass, D.L.; Shing, C.; Wang, G. The light-induced alteration of realgar to pararealgar. Am. Mineral. 1992, 77, 1266–1274. [Google Scholar]
- Vermeulen, M.; Saverwyns, S.; Coudray, A.; Janssens, K.; Sanyova, J. Identification by Raman spectroscopy of pararealgar as a starting material in the synthesis of amorphous arsenic sulfide pigments. Dye. Pigment. 2018, 149, 290–297. [Google Scholar] [CrossRef]
- Golovchak, R.; Shpotyuk, O.; McCloy, J.S.; Riley, B.J.; Windisch, C.F.; Sundaram, S.K.; Kovalskiy, A.; Jain, H. Structural model of homogeneous As–S glasses derived from Raman spectroscopy and high-resolution XPS. Philos. Mag. 2010, 90, 4489–4501. [Google Scholar] [CrossRef]
- Bonazzi, P.; Bindi, L. A crystallographic review of arsenic sulfides: Effects of chemical variations and changes induced by exposure to light. Z. Für Krist. 2008, 223, 132–147. [Google Scholar] [CrossRef]
- Vermeulen, M.; Palka, K.; Vlček, M.; Sanyova, J. Study of dry- and wet-process amorphous arsenic sulfides: Synthesis, Raman reference spectra, and identification in historical art materials. J. Raman Spectrosc. 2019, 50, 396–406. [Google Scholar] [CrossRef]
- Georgiev, D.G.; Boolchand, P.; Jackson, K.A. Intrinsic nanoscale phase separation of bulk As2S3 glass. Philos. Mag. 2003, 83, 2941–2953. [Google Scholar] [CrossRef]
- Trentelman, K.; Stodulski, L.; Pavlosky, M. Characterization of Pararealgar and Other Light-Induced Transformation Products from Realgar by Raman Microspectroscopy. Anal. Chem. 1996, 68, 1755–1761. [Google Scholar] [CrossRef]
- Agricola, G. De Natura Fossilium (Textbook of Mineralogy); Dover Publications: New York, NY, USA, 2004. [Google Scholar]
- Geoffroy, E.-F. A Treatise of the Fossil, Vegetable, and Animal Substances, That Are Made Use of in Physick; W. Innys and R. Manby: London, UK, 1736. [Google Scholar]
- Biringuccio, V. The Pirotechnia; Smith, C.S., Gnudi, M.T., Eds.; Dover Publications: New York, NY, USA, 1990. [Google Scholar]
- Sarmento, J.D.C. Materia Medica; London, UK, 1735. [Google Scholar]
- Charas, M. Pharmacopée Royale Galénique et Chymique; Chez Anisson & Posuel: Paris, France, 1676. [Google Scholar]
- Veliz, Z. (Ed.) Artists′ Techniques in Golden Age Spain. Six Treatises in Translation; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Ferreira, S.T. Segredos Necessarios para os Officios, Artes, e Manufacturas; Office de Simão Thaddeo Ferreira: Lisboa, Portugal, 1794; Volume 2. [Google Scholar]
- Merrifield, M.P. (Ed.) Medieval and Renaissance Treatises on the Arts of Painting; Dover Publications: New York, NY, USA, 1999. [Google Scholar]
- Richter, M.; Grundmann, G.; Loon, A.V.; Keune, K.; Boersma, A.; Rapp, K. The occurrence of artificial orpiment (dry process) in northern European painting and polychromy and evidence in historical sources. In Auripigment/Orpiment. Studien zu Dem Mineral Und Den Künstlichen Produkten/Studies on the Mineral and the Artificial Products; Schuller, M., Emmerling, E., Nerdinger, W., Eds.; Verlag Anton Siegl: München, Germany, 2007; pp. 169–188. [Google Scholar]
- Leão, D.N.D. Descripção do Reino de Portugal; Iorge Rodriguez: Lisboa, Portugal, 1610. [Google Scholar]
- Jovanovski, G.; Makreski, P. Intriguing minerals: Photoinduced solid-state transition of realgar to pararealgar—Direct atomic scale observation and visualization. Chem. Texts 2020, 6, 5. [Google Scholar] [CrossRef]
- Gliozzo, E.; Burgio, L. Pigments—Arsenic-based yellows and reds. Archaeol. Anthropol. Sci. 2022, 14, 4. [Google Scholar] [CrossRef]
- Emelina, A.L.; Alikhanian, A.S.; Steblevskii, A.V.; Kolosov, E.N. Phase diagram of the As-S system. Inorg. Mater. 2007, 43, 95–104. [Google Scholar] [CrossRef]
- Simoen, J.; De Meyer, S.; Vanmeert, F.; de Keyser, N.; Avranovich, E.; Van der Snickt, G.; Van Loon, A.; Keune, K.; Janssens, K. Combined Micro- and Macro scale X-ray powder diffraction mapping of degraded Orpiment paint in a 17th century still life painting by Martinus Nellius. Herit. Sci. 2019, 7, 83. [Google Scholar] [CrossRef]
- Vermeulen, M.; Janssens, K.; Sanyova, J.; Rahemi, V.; McGlinchey, C.; De Wael, K. Assessing the stability of arsenic sulfide pigments and influence of the binding media on their degradation by means of spectroscopic and electrochemical techniques. Microchem. J. 2018, 138, 82–91. [Google Scholar] [CrossRef]
- Keune, K.; Mass, J.; Meirer, F.; Pottasch, C.; van Loon, A.; Hull, A.; Church, J.; Pouyet, E.; Cotte, M.; Mehta, A. Tracking the transformation and transport of arsenic sulfide pigments in paints: Synchrotron-based X-ray micro-analyses. J. Anal. At. Spectrom. 2015, 30, 813–827. [Google Scholar] [CrossRef]
- Grundmann, G.; Richter, M. Current Research on Artificial Arsenic Sulphide Pigments in Artworks: A Short Review. Chimia 2008, 62, 903–907. [Google Scholar] [CrossRef]
- Grundmann, G.; Ivleva, N.; Richter, M.; Stege, H.; Haisch, C. The rediscovery of sublimed arsenic sulphide pigments in painting and polychromy: Applications of Raman microspectroscopy. In Studying Old Master Paintings. Technology and Practice; Spring, M., Ed.; Archetype Publications: London, UK, 2011; pp. 269–276. [Google Scholar]
- Grundmann, G.; Rötter, C. Artificial orpiment: Microscopic, diffractometric and chemical characteristics of synthesis products in comparison to natural orpiment. In Auripigment/Orpiment. Studien zu Dem Mineral Und Den Künstlichen Produkten/Studies on the Mineral and the Artificial Products; Schuller, M., Emmerling, E., Nerdinger, W., Eds.; Verlag Anton Siegl: München, Germany, 2007; pp. 105–136. [Google Scholar]
- Penny, N.; Spring, M. Veronese’s paintings in the National Gallery. Techniques and materials: Part II. Natl. Gallery Tech. Bull. 1996, 17, 32–55. [Google Scholar]
- Dunkerton, J.; Spring, M. Titian’s Painting Technique to c.1540. Natl. Gallery Tech. Bull. 2013, 34, 4–31. [Google Scholar]
- Wallert, A. (Ed.) Still Life: Techniques and Style. An Examination of Paintings from the Rijksmuseum; Rijksmuseum: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Keune, K.; Mass, J.; Mehta, A.; Church, J.; Meirer, F. Analytical imaging studies of the migration of degraded orpiment, realgar, and emerald green pigments in historic paintings and related conservation issues. Herit. Sci. 2016, 4, 10. [Google Scholar] [CrossRef]
- van Loon, A.; Noble, P.; Krekeler, A.; Van der Snickt, G.; Janssens, K.; Abe, Y.; Nakai, I.; Dik, J. Artificial orpiment, a new pigment in Rembrandt’s palette. Herit. Sci. 2017, 5, 26. [Google Scholar] [CrossRef]
- De Keyser, N.; Van der Snickt, G.; Van Loon, A.; Legrand, S.; Wallert, A.; Janssens, K. Jan Davidsz. de Heem (1606–1684): A technical examination of fruit and flower still lifes combining MA-XRF scanning, cross-section analysis and technical historical sources. Herit. Sci. 2017, 5, 38. [Google Scholar] [CrossRef]
- Antunes, V.; Candeias, A.; Oliveira, M.J.; Carvalho, M.L.; Dias, C.B.; Manhita, A.; Francisco, M.J.; Costa, S.; Lauw, A.; Manso, M. Uncover the mantle: Rediscovering Gregório Lopes palette and technique with a study on the painting “Mater Misericordiae”. Appl. Phys. A 2016, 122, 965. [Google Scholar] [CrossRef]
- Muralha, V.S.F.; Miguel, C.; Melo, M.J. Micro-Raman study of Medieval Cistercian 12–13th century manuscripts: Santa Maria de Alcobaça, Portugal. J. Raman Spectrosc. 2012, 43, 1737–1746. [Google Scholar] [CrossRef]
- Correia, A.M.; Oliveira, M.J.V.; Clark, R.J.H.; Ribeiro, M.I.; Duarte, M.L. Characterization of Pousão pigments and extenders by micro-X-ray diffractometry and infrared and Raman microspectroscopy. Anal. Chem. 2008, 80, 1482–1492. [Google Scholar] [CrossRef]
- Correia, A.M.; Clark, R.J.; Ribeiro, M.I.; Duarte, M.L. Pigment study by Raman microscopy of 23 paintings by the Portuguese artist Henrique Pousão (1859–1884). J. Raman Spectrosc. 2007, 38, 1390–1405. [Google Scholar] [CrossRef]
- Barata, C.; Carballo, J.; Cruz, A.J.; Coroado, J.; Araújo, M.E.; Mendonça, M.H. Caracterização através de análise química da escultura portuguesa sobre madeira de produção erudita e de produção popular da época barroca. Química Nova 2013, 36, 21–26. [Google Scholar] [CrossRef]
- Maltieira, R.; Calvo, A.; Cunha, J. Primórdios da pintura sobre tela em Portugal. Contributos para a sua conservação através de um estudo técnico e material. ECR Estud. Conserv. Restauro 2014, 6, 163. [Google Scholar] [CrossRef][Green Version]
- Ordenações Manuelinas. Livro V; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1984.
- Ordenações Filipinas. Livros IV e V; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1985.
- Cruz, A.J. A proveniência dos pigmentos utilizados em pintura em Portugal antes da invenção dos tubos de tintas: Problemas e perspectivas. In As Preparações na Pintura Portuguesa. Séculos XV e XVI; Serrão, V., Antunes, V., Seruya, A.I., Eds.; Faculdade de Letras da Universidade de Lisboa: Lisboa, Portugal, 2013; pp. 297–306. [Google Scholar]
- Pauta e Alvará de Sua Confirmação do Consulado Geral de Sahida, e Entrada na Casa da India; Off. de Joseph da Costa Coimbra: Lisboa, Portugal, 1756.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).