4.1. Swords Manufacturing: Microstructural Analysis
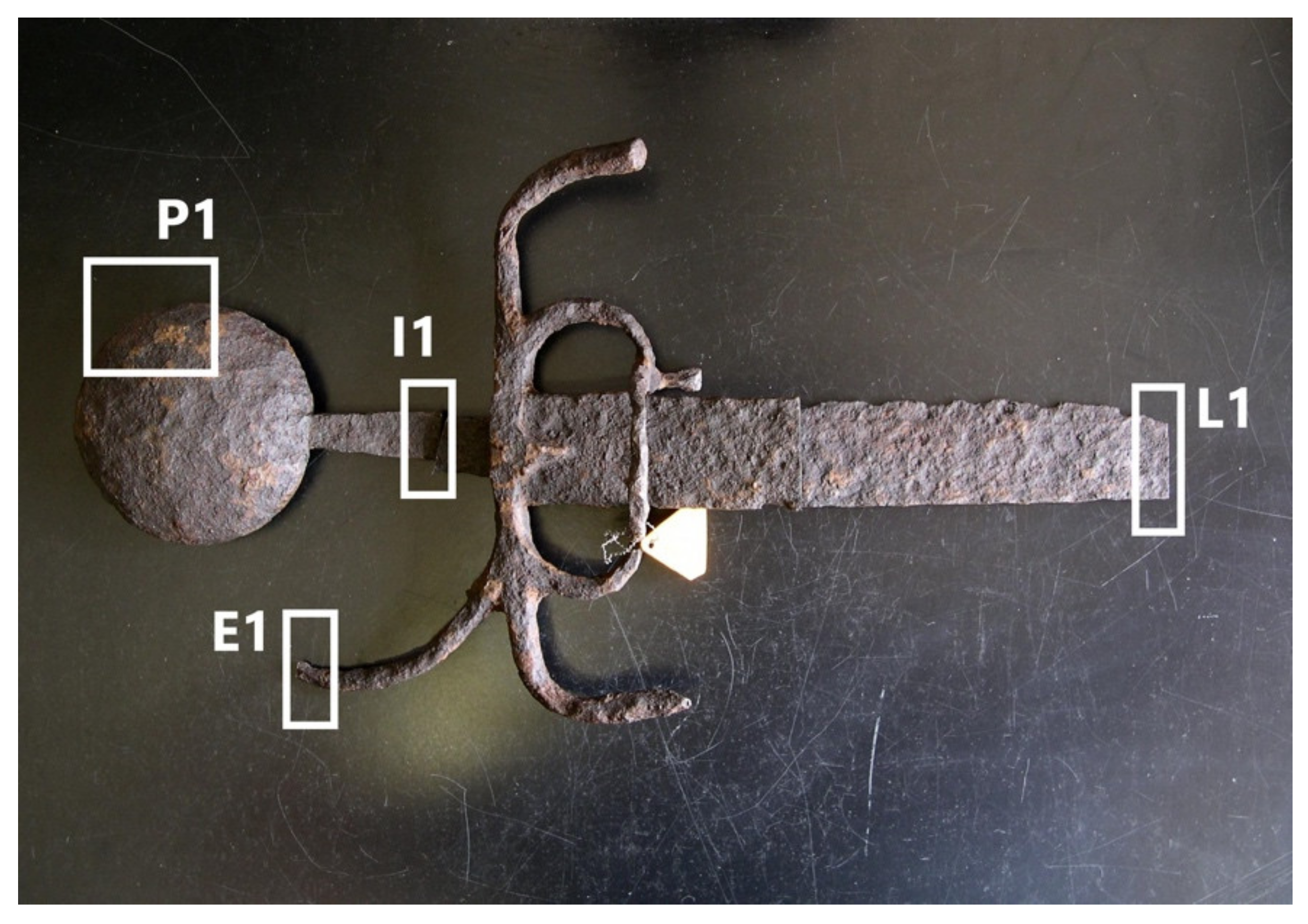
By a visual observation of the samples collected from the two swords, it is possible to infer that the objects reproduce the period style (i.e., Venetian-Lombardy manufacturing, dated between 1480–1490 A.D.) (
Figure 3) [
32,
33], as described in the Materials and Methods section. Interestingly, the pommel P1 (80,5 ∅ × 18 mm) is larger than P2 (55 ∅ × 16 mm) but hollow.
The main function of this tool is balancing the whole sword, as it weighs the same as the rest of the weapon. This outcome is consistent with previous literature studies [
32], considering the total length of the swords (G6′s preserved length is 430 mm and G8′s is 780 mm). It also seems that, for P1, the two hemispheres are joined together (
Figure 3a). Both hilts present curved quillons, useful for protecting against the downward blows; additionally, G6 has the knuckle-guard as further protection against enemies’ blows [
33] (
Figure 3b,d). During the 13th century, the blacksmiths had begun to create ergonomic hilts in different models that go back to the Venetian sword. The G6 quillons shape is quite different from other models, and it could have anticipated the curved quillons present from the 14th century [
33]. To our knowledge, no similar swords have ever been reported and described in the literature so far.
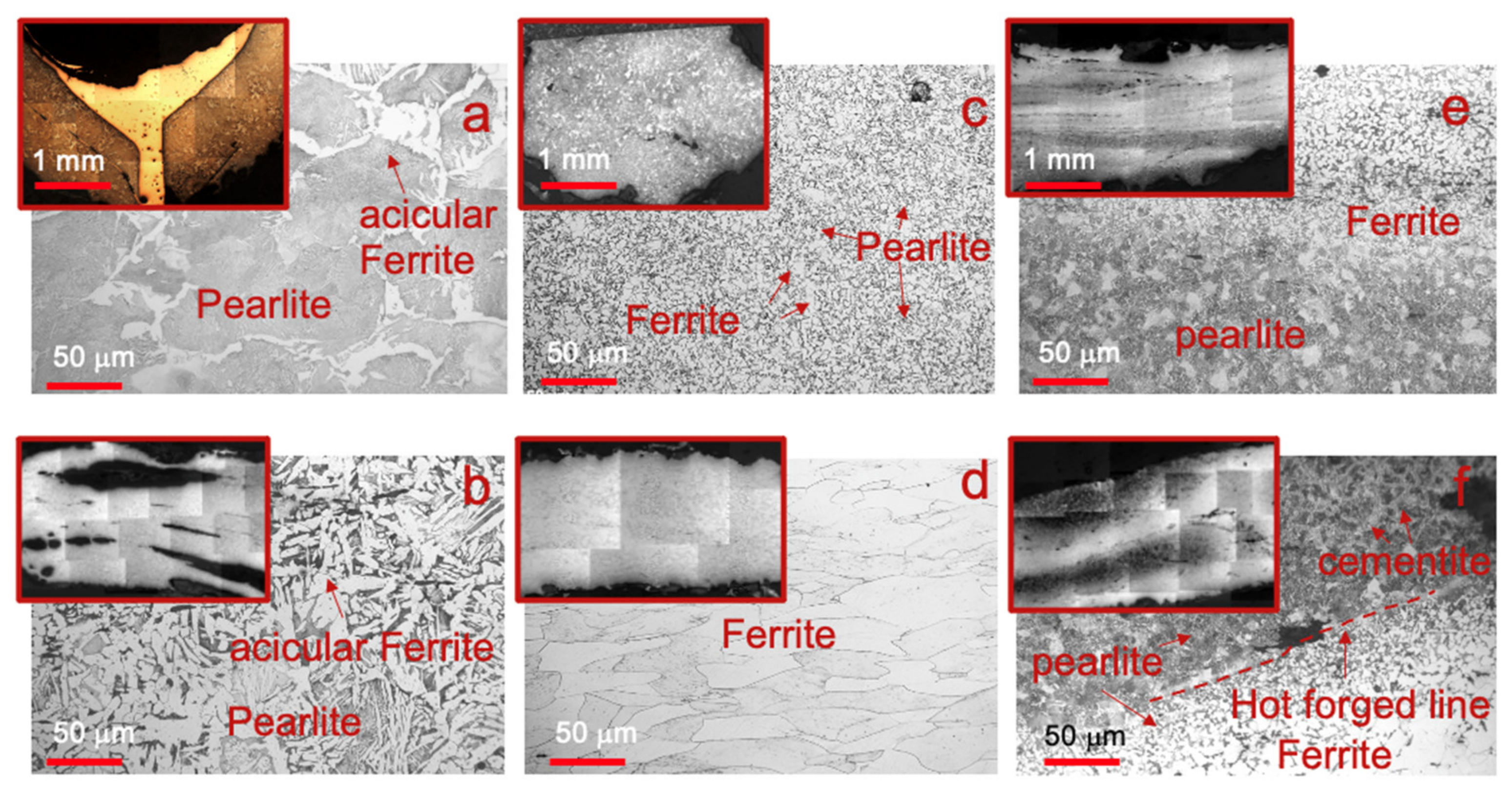
Microscopical observation of all samples revealed several microstructural heterogeneities (
Figure 4), either among the samples or even in the same sample. Microstructural differences were more evident in the hilt and the blade, according to their function.
Both P1 and P2 (
Figure 4a,b) showed predominantly pearlite and acicular (Widmanstätten like) ferrite with rare inclusions distributed on its surface, derived from heating above the austenitization temperature (A3 critical point) and cooling comparable to an air quenching, representing the typical microstructure of forged carbon steel [
1]. Whereas P1 showed an almost pearlitic microstructure, P2 exhibited a fair distribution of pearlite and ferrite, with small areas of cementite [
35]. Probably, both the G6 and the G8 sword’s pommels were warmed up on blazing coals, hammered and air quenched. This kind of forge matches the pommel microstructure and explains the several oxidized areas. It was also possible to observe the application of a copper-based alloy to join the two hemispheres of the pommel P1 (
Figure S2, Supplementary Materials, as later confirmed by the SEM-EDS analyses).
The two hilts’ samples, knuckle-guard (E1) and quillon (E2), showed a microstructure with dominant ferrite and small areas of perlite at the grain boundaries (
Figure 4c,d). The elongated ferrite grains in E2 testify to an isodirectional deformation, followed by annealing due to the absence of strain lines and mechanical twins. The sample E1 showed no forging layers, despite presenting two grain sizes (
Table S1) and internal inclusions. This evidence seems to corroborate the hypothesis proposed by Matteis et al. [
18] that the knuckle-guard was made of a single bar and later repaired. Also, the E2 microstructure, typical of a very low purified carbon steel, suggests the use of a single bar, which was later manufactured into a foil with the procedure used for the pommel.
Rapid quenching effects are only visible on the blade edges and not on the hilts (
Figure 5). As an example, microhardness measurements conducted on specific areas of the tang of the two swords gave average values lower than 210 and 175 HV for G6 and G8, respectively. In addition, the tangs (I1 and I2) differ from the rest of the samples in terms of microstructure and abundance of inclusions. In I1, it is possible to observe (
Figure 2e) two different phases: (i) pearlite and ferrite with diverse grain sizes (
Table S1); (ii) pearlite and cementite. On the other hand, sample I2 (
Figure 4f) showed a higher average grain size (
Table S2) and a sequence of microstructures from the top to the bottom: (i) ferrite and pearlite; (ii) pearlite and cementite; (iii) pearlite and acicular ferrite; (iv) pearlite and cementite. Furthermore, hot-forged lines were observed for both samples.
The G6′s blade (sample L1), single-edged, has a heterogeneous appearance (
Figure 5a–c).
A sequence of microstructures characterizes the sample from the top surface to the bottom: (i) tempered martensite; (ii) ferrite and pearlite; (iii) tempered martensite; (iv) ferrite and pearlite; (v) tempered martensite. The interpretation of tempered martensite is given below: martensite being a solid solution, when present, it would appear as a mechanical mix of acicular grains. The size of such grains depends on the steel composition (e.g., carbon content) and the applied cooling rate due to the cooling medium (e.g., water, oil, etc.). After chemical etching, the martensite might appear dark at the optical microscope, while under the SEM observation it will show only the presence of acicular grains. Conversely, the tempered martensite corresponds to a matrix of body-centered tetragonal martensite, showing precipitated carbides (ε because in defect of C if compared with the classical cementite) [
5,
36,
37]. The SEM images (
Figure 5c) showed that the original martensitic crystal has kept the same boundaries but results now hollow and containing tiny precipitates. If such a microstructure is further annealed, the martensite is transformed into ferrite (changing the grain boundaries and shape) with globular carbides (i.e., cementite) [
36,
37]. The sequence of microstructures observed could be related to several forging and folding steps above the A3 point, and two different cooling rates. The faster cooling rate is compatible with water or oil quenching [
30,
31], the slower could refer to an air quenching. The formation of tempered martensite is probably derived from further heating (probably below 250 °C) and followed by air cooling, which allows for the precipitation of ε carbides (
Figure 5c) [
36]. According to the microstructural features and to the hardness measured by Vickers method, ranging between 480 and 580 HV0.5, it is, therefore, possible to infer that the observed microstructure might result from a temper annealing performed at intermediate temperatures [
3,
36]. Also, a difference was seen between the edge and the back edge (respectively on the right and left side of inset of 5a), with tempered martensite on the first, and pearlite and cementite on the latter. A hypothesis for the presence of cementite in the back-edge is as follows: during the forging, the blacksmith left one side of the blade on the blazing coals longer than the other side and applied an air cooling. Accordingly, on the back-edge, quenched structures with an excess of carbon were produced, that formed grain boundary cementite. This hypothesis is also supported by the microhardness measurements (
Table S3) with harder areas (480 HV 0.5 to 580 HV 0.5), corresponding to tempered martensite [
4,
36]. Hot-forged lines were also observed at the core of the blade (
Figure 5a,b). They clearly define the folding procedure applied during hot-forging by the blacksmith.
The G8′s blade samples (L2I and L2P), double-edged, are sampled near the grip and the edge, respectively, to observe how the microstructure differs between the
forte and the
debole (
Figure 5d–i). It appears that the alloy is made of iron with different carbon content along the transversal axis (
Figure 5d,g). The hardness has irregular values, and it could be the result of different procedures: 530 HV 0.5 compared to 300 HV 0.5 [
4]. Sample L2I is hollowed and has visible hot-forged lines (
Figure 5e), which draw the forge folds and divide the microstructure from side to side into: (i) tempered martensite; (ii) pearlite and ferrite; (iii) tempered martensite; (iv) pearlite and acicular ferrite; (v) tempered martensite (
Figure 5f). The abundance of small inclusions near the hot-forged lines could be related to the supplementation of wet clays during the hot-forging that protects the material from oxidation and carburization [
34]. In the sample L2P, the hot-forged lines are defined clearly by two kinds of microstructures (
Figure 5h): (i) ferrite and pearlite at the center; (ii) tempered martensite on the edges (
Figure 5i). The carburized layer, which is visible in L1, is not detected in L2P, thus confirming the use of a different procedure for the sword. The hardness profile of this sample is consistent with what was already observed for L1: in the core, the blade measures between 150 and 250 HV 0.5 and at the edges between 550 HV 0.5 and 600 HV 0.5 (
Table S3) [
4,
37].
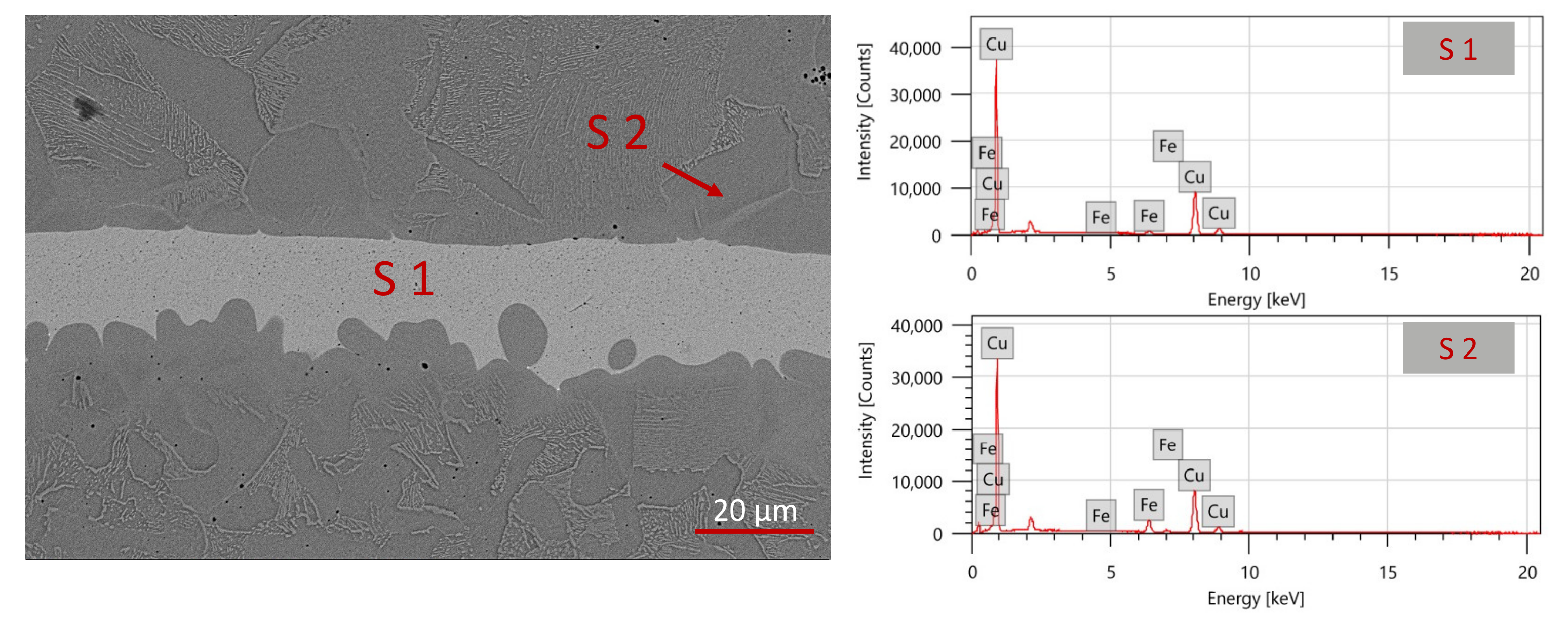
The SEM-EDS analyses provided information on the nature of the joint (
Figure 6 and
Figure 7 and
Table 3 and
Table 4). The main element detected is copper, with a low fraction of iron (average 3 wt.%, spectrum 1 in
Table 3).
Figure 6 shows the difference in composition of the two parts (copper joint and iron), as reflected in a variation in greyscale, and the presence of solid-state interdiffusion (spectrum 1 and 2).
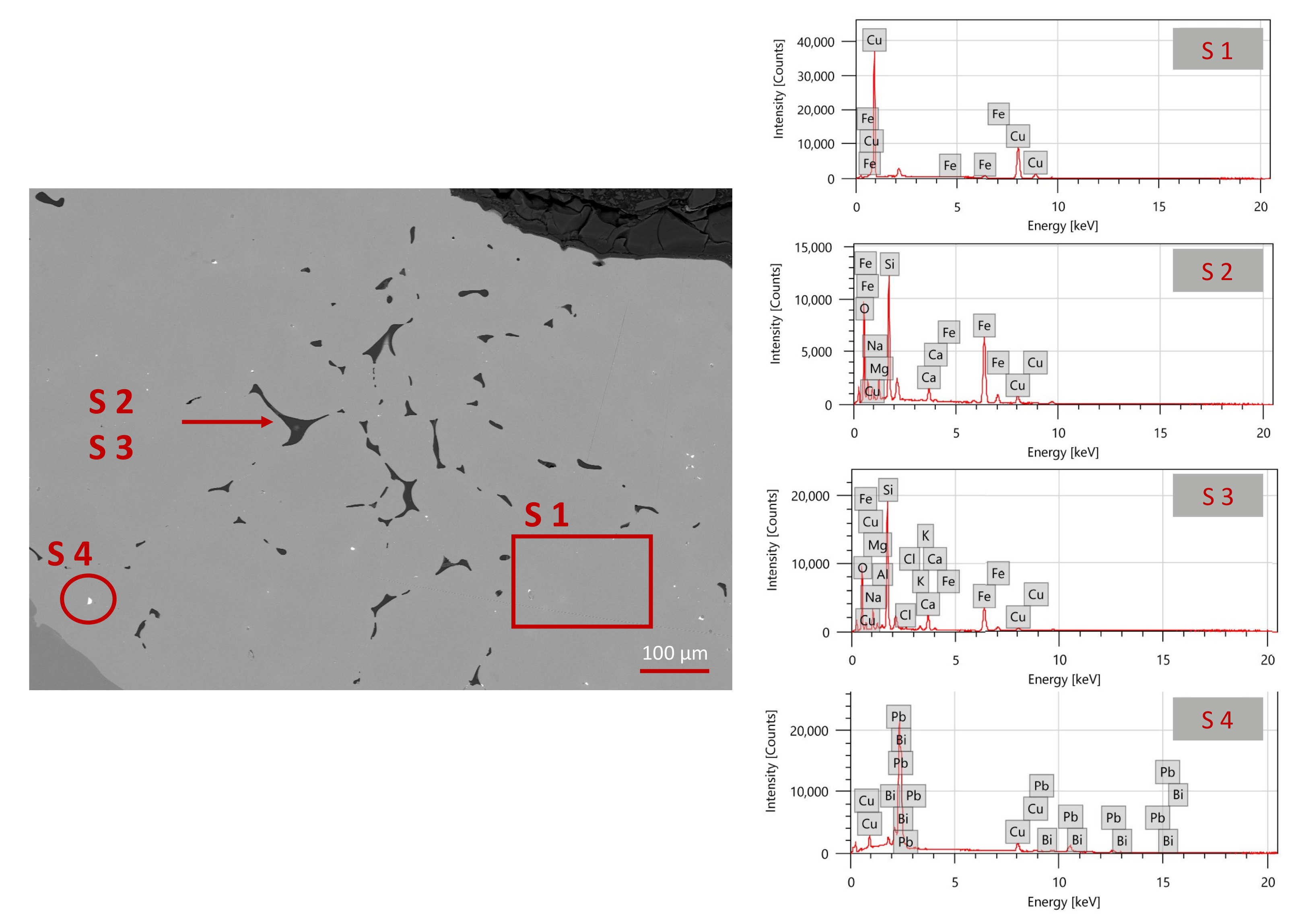
The analysis of the microstructure (
Figure 7) showed that the alloy appears homogeneous, with a few domains containing lead and bismuth (spectrum 4 in
Table 4), usually associated with copper in Cu-bearing mineral assemblage. In addition, dendritic grains are distinguished by the characteristic shape and silicate inclusions are observed at the grain boundaries (
Figure 7). The hypothesized procedure is as follows: copper chips or grains were used to join the two hemispheres of the pommel using a clay shell that helped preserve the heat. The shell was supposedly warmed up above 1080 °C (copper melting temperature) and air-cooled [
38]. This allowed copper and iron to diffuse easily. The detected silicate-based inclusions (as displayed by the EDS spectra 02 and 03 in
Figure 7 and
Table 4), support this specific procedure. To our knowledge, such a material was never used on components of the swords coming from this period and the correct interpretation of these results would require further studies. However, it is not possible to exclude an interpretation either towards the innovation of the blacksmiths welding technique or a more recent reparation.
4.2. Provenance of the Swords: Analysis of Inclusions
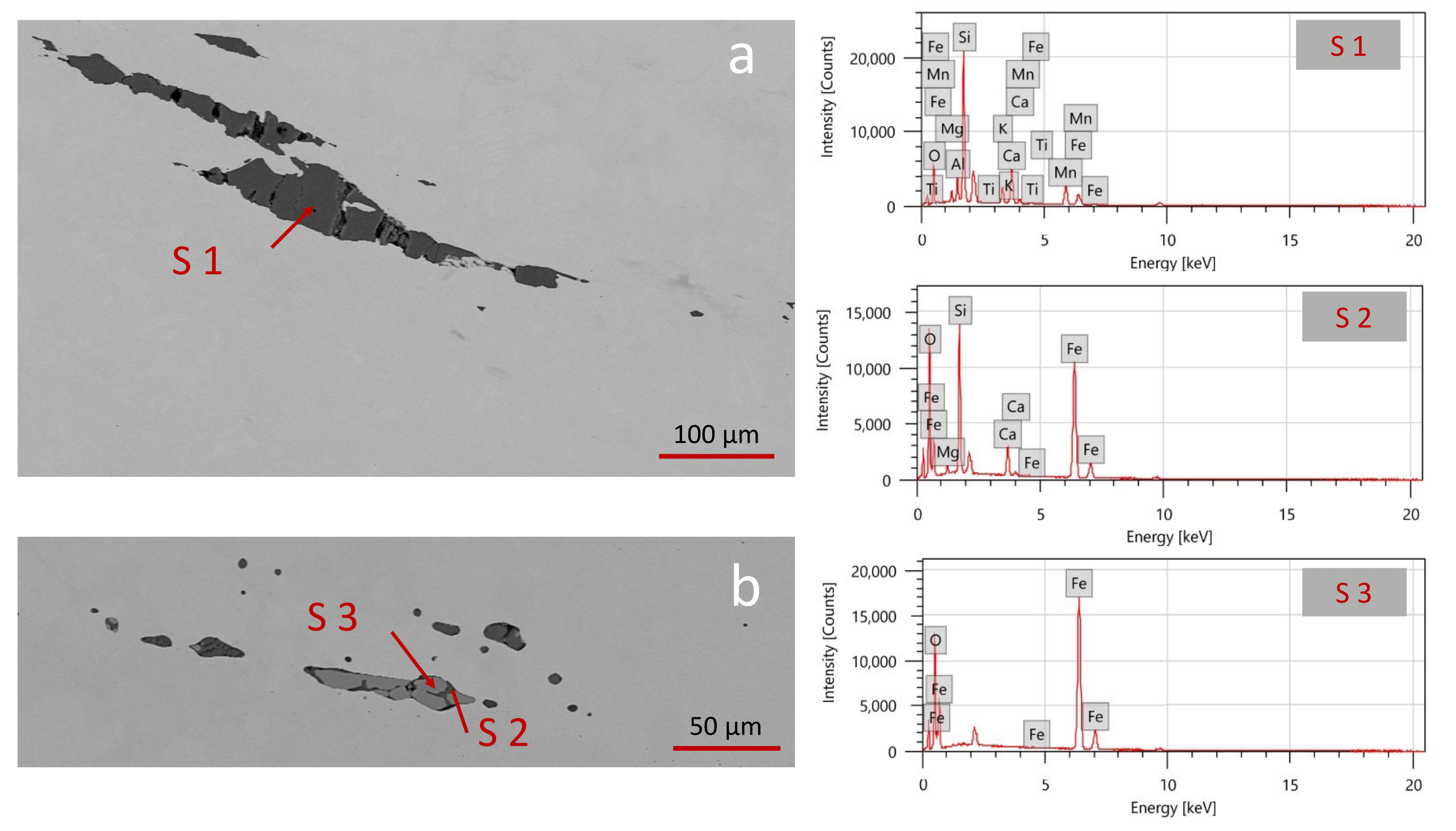
The nature and distribution of inclusions can provide evidence on the provenance of raw materials and ores used for manufacturing. Both swords show the same results for the inclusions in terms of morphology and composition. Almost all of them appear elongated along the manufacturing direction and either uniform (usually with deep grey tones in BSE) or heterogeneous. Two differently shaped and sized inclusions were detected through SEM-EDS, as visible in
Figure 8.
Figure 8a displays the so-called bloomery or finery slags (according to the reducing process), which are bigger and homogeneously distributed on the samples. They generally give information about the provenance and the nature of iron ores [
20].
Often, the inclusions contain small crystals (from 30 to 50 μm), with a polygonal or round shape. From the compositional analysis (
Figure 8a and
Table 5), elements like Si, Mg, Al, K, Ca, Ti, Mn, and Fe were detected. This indicates that they are made of silicates, with an important fraction of Mn, being this element knowingly a marker for bloomery slags [
20]. It is known that Mn is preferably reduced during the smelting process and only a small fraction of Mn is re-oxidized in the finery process.
Figure 8b displays the smithing slags, derived from the hot-forging process, recognizable due to their morphology and location near the hot-forged lines observed through LOM [
20]. They are smaller (around 100 μm) and elongated along the manufacturing direction. According to the literature, they are mainly composed of silicate minerals (clays) or ground bones [
39]. As expected, analysis of the domains shown in
Figure 8b confirms the presence of elements such as Si, Mg, Ca, and Fe. However, the compositional analysis suggests the presence of two distinct phases: the dark matrix in BSE is composed of silicates, the same composition of the inclusions in L1; the bright crystals are iron oxides. These outcomes confirm the aforementioned hypothesis of using wet clays to protect the iron from further oxidation. During this process, wustite or FeO (spectrum 3) is formed in lack of oxygen. A subsequent reaction with the silicate-based clay promoted the formation of fayalite Fe
2SiO
4 (spectrum 2).
To unambiguously distinguish bloomery from finery slags, Williams et al. suggested a protocol based on chemical quantitative analyses [
20]. The compositions observed with the EDS analysis were used to set the WDS analysis, to obtain quantitative results in terms of chemical composition expressed as oxide wt%. According to Williams et al. [
20], a direct process of production displays ratios of K
2O:MgO and/or Al
2O
3:CaO higher than those of SiO
2:Al
2O
3. In addition, large quantities of Mn must be present in the silicates inclusions. Otherwise, iron is derived from an indirect process of production [
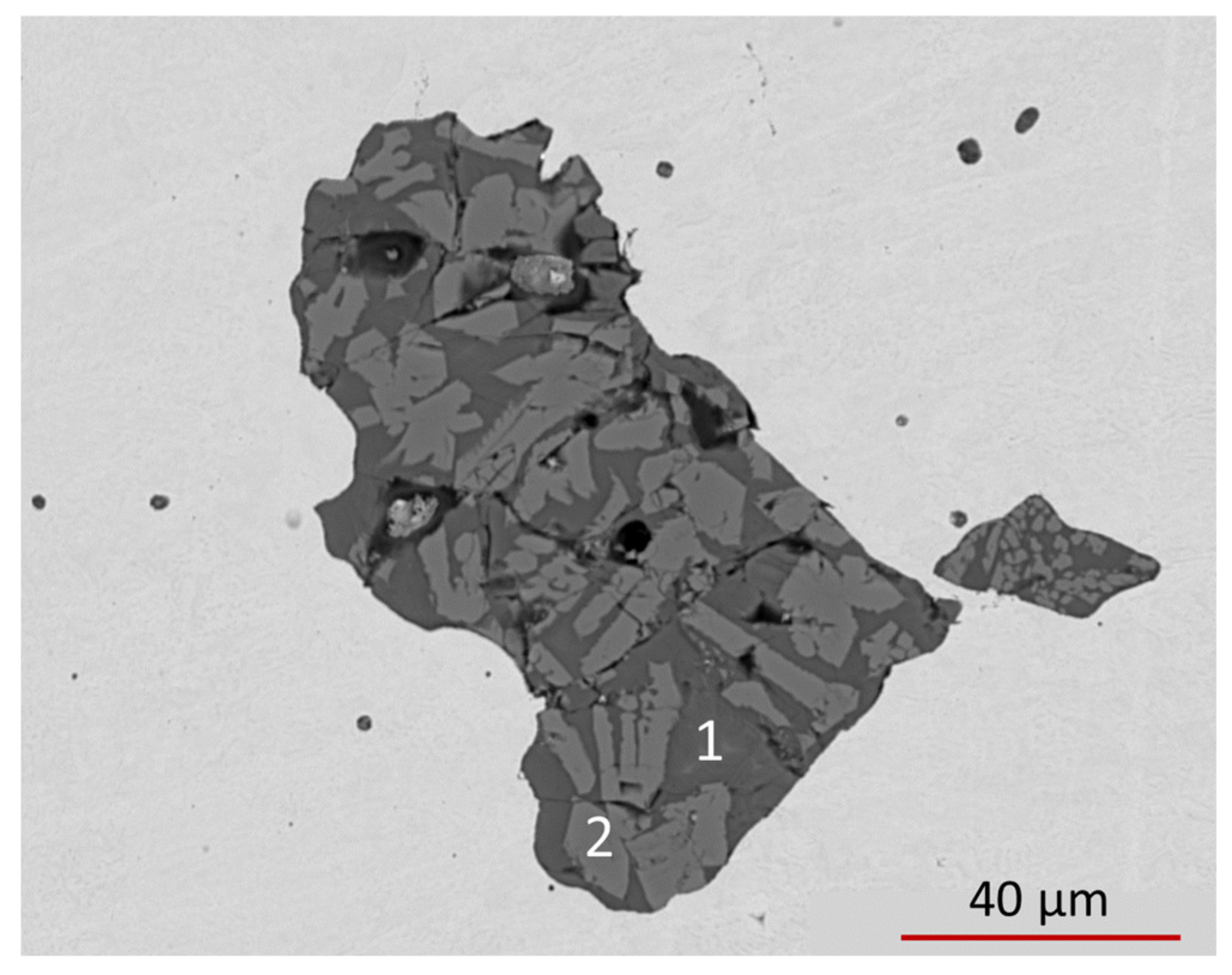
20]. We then selected some relatively large inclusions, a reference one shown in
Figure 9, to perform EMPA-WDS analyses and to apply the Williams et al. protocol. As shown by the spot analyses in
Table 6, EMPA-WDS quantitative results confirm the heterogeneous nature of the inclusion. The chemical data pertaining to the ref. #1 in
Table 6 could be reasonably ascribed to an Mn-rich olivine (e.g., with the general formula ((Fe
2+,Mn,Mg)
2(SiO
4)) or to an amorphous silicate with similar composition, whereas data pertaining to ref. #2 point to glass, with a complex composition incompatible with the common rock-forming silicates. Unfortunately, the small dimensions of the inclusions do not permit a characterization based on X-ray diffraction methods, which would be decisive for the unambiguous identification of the Mn-rich olivine and of the glass.
Applying the Williams et al. protocol, results are described in
Table 7. For some parts of the blade, the SiO
2:Al
2O
3 ratio is higher than that of K
2O:MgO (blade G6, pommel P1, knuckle guard E1, and quillon E2) and for other samples, the value is lower (pommel P2 and blade G8).
Historically, both reduction processes were common practices in the 14th–17th centuries [
28] and according to the outcomes obtained, the process applied for the reduction of the swords was both direct (G8) and indirect (G6). In addition, for the sword G8, the SiO
2:Al
2O
3 and K
2O:MgO ratio values seem at first contradictory considering the different components. However, these outcomes are plausible if we consider that a direct process of production was applied for the blades and an indirect one for the hilt. Consequently, we could only consider a possible replacement of the hilt according to the changing fashion of the time [
24].
To gather information on the iron ores, the composition of the inclusions was correlated to the minerals of the ores exploited to produce iron from the 13th century and coming from the Venetian area [
24]. The most important iron-bearing mineral of the Brescia and Bergamo territories, probably exploited since the 6th–7th century (Lombard times), is represented by siderite occurring at the mines of Val Trompia and Val Camonica (near Brescia), and Val di Scalve-Val Seriana (near Bergamo). Siderite is an iron carbonate (FeCO
3), which contains up to 48 wt% of Fe. These mines consist of layers of (mainly Triassic) metasomatic carbonate rocks (limestones, dolostones, and mudstone), with different siderite content. The richest levels contain even up to ca. 75 wt% in siderite. A common feature of the siderite from these ores is the high fraction of Mn. A similar geological scenario occurs even in the Dolomites area, for example in the Fursil mine, in which Mn-rich siderite occurs [
31,
40,
41]. Other minerals of industrial interest occur with siderite, which has been exploited over time: in Val Trompia, for example, where fluorite (CaF
2), barite (BaSO
4), silver-bearing galena (PbS), chalcopyrite (CuFeS
2), tetrahedrite ((Cu,Fe)
12Sb
4S
13), and sphalerite (ZnS) occur. Therefore, even copper is a metal occurring in the minerals available and exploitable at the Val Trompia mines, but not at all the other mines of the Brescia and Bergamo territory. The co-presence of Pb (along with Bi or S) observed in some of the chemical analyses of this study is compatible with the mineralogical assemblage of the ore.
The metals used to produce the swords in this study are available in the Brescia and Bergamo territories. The chemical compositions of the previously described inclusions cannot be used as markers for unambiguous identification of the parent ores: Si, Mg, Al, K, Ca, Ti, Mn, and Fe, which are compatible with the mineralogical assemblages of the metasomatic carbonate rocks (limestones, dolostones, and mudstone), found rich of siderite, from the different mines. The multi-elemental composition of the (likely amorphous) inclusions makes it difficult to reconstruct the melting temperature.