Unexpected Findings in 16th Century Wall Paintings: Identification of Aragonite and Unusual Pigments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Infrared Spectroscopy
2.3. X-ray Diffraction
2.4. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
2.5. Optical Microscopy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearce, M. Reconstructing past transapennine routes: The Trebbia valley. Reconstr. Past Transapennine Routes Trebbia Val. 2002, 6, 181–183. [Google Scholar]
- Wilson, M.J. Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Chapman & Hall: London, UK, 1994; ISBN 9780412533808. [Google Scholar]
- Bikiaris, D.; Daniilia, S.; Sotiropoulou, S.; Katsimbiri, O.; Pavlidou, E.; Moutsatsou, A.P.; Chryssoulakis, Y. Ochre-differentiation through micro-Raman and micro-FTIR spectroscopies: Application on wall paintings at Meteora and Mount Athos, Greece. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 3–18. [Google Scholar] [CrossRef]
- Bugini, R.; Corti, C.; Folli, L.; Rampazzi, L. Unveiling the Use of Creta in Roman Plasters: Analysis of Clay Wall Paintings from Brixia (Italy). Archaeometry 2017, 59, 84–95. [Google Scholar] [CrossRef]
- Crupi, V.; La Russa, M.F.; Venuti, V.; Ruffolo, S.; Ricca, M.; Paladini, G.; Albini, R.; Macchia, A.; Denaro, L.; Birarda, G.; et al. A combined SR-based Raman and InfraRed investigation of pigmenting matter used in wall paintings: The San Gennaro and San Gaudioso Catacombs (Naples, Italy) case. Eur. Phys. J. Plus 2018, 133, 369. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments; Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments; Butterworth-Heinemann: Oxford, UK, 2008; ISBN 9780750689809. [Google Scholar]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999; ISBN 0892364696. [Google Scholar]
- Bugini, R.; Corti, C.; Folli, L.; Rampazzi, L. Roman Wall Paintings: Characterisation of Plaster Coats Made of Clay Mud. Heritage 2021, 4, 48. [Google Scholar] [CrossRef]
- Montoya, C.; Lanas, J.; Arandigoyen, M.; Navarro, I.; García Casado, P.J.; Alvarez, J.I. Study of ancient dolomitic mortars of the church of Santa Maria de Zamarce in Navarra (Spain): Comparison with simulated standards. Thermochim. Acta 2003, 398, 107–122. [Google Scholar] [CrossRef]
- Igea, J.; Lapuente, P.; Martínez-Ramírez, S.; Blanco-Varela, M.T. Characterization of mudejar mortars from St. Gil Abbot church (Zaragoza, Spain): Investigation of the manufacturing technology of ancient gypsum mortars. Mater. Constr. 2012, 62, 515–529. [Google Scholar] [CrossRef]
- Biscontin, G.; Pellizon Birelli, M.; Zendri, E. Characterization of binders employed in the manufacture of Venetian historical mortars. J. Cult. Herit. 2002, 3, 31–37. [Google Scholar] [CrossRef]
- Crupi, V.; D’Amico, S.; Denaro, L.; Donato, P.; Majolino, D.; Paladini, G.; Persico, R.; Saccone, M.; Sansotta, C.; Spagnolo, G.V.; et al. Mobile Spectroscopy in Archaeometry: Some Case Study. J. Spectrosc. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Zhang, K.; Grimoldi, A.; Rampazzi, L.; Sansonetti, A.; Corti, C. Contribution of thermal analysis in the characterization of lime-based mortars with oxblood addition. Thermochim. Acta 2019, 678, 178303. [Google Scholar] [CrossRef]
- Zhang, K.; Corti, C.; Grimoldi, A.; Rampazzi, L.; Sansonetti, A. Application of Different Fourier Transform Infrared (FT-IR) Methods in the Characterization of Lime-Based Mortars with Oxblood. Appl. Spectrosc. 2019, 73, 479–491. [Google Scholar] [CrossRef]
- Žigovečki Gobac, Ž.; Posilović, H.; Bermanec, V. Identification of biogenetic calcite and aragonite using SEM. Geol. Croat. 2009, 62, 201–206. [Google Scholar] [CrossRef]
- Roy, A. Artists’ Pigments: A Handbook of Their History and Characteristics; Oxford University Press: London, UK, 1993; Volume 2, ISBN 9780894681899. [Google Scholar]
- Aroke, U.O.; Abdulkarim, A.; Ogubunka, R.O. Fourier-transform Infrared Characterization of Kaolin, Granite, Bentonite and Barite. ATBU J. Environ. Technol. 2013, 6, 42–53. [Google Scholar]
- Ranalli, G.; Bosch-Roig, P.; Crudele, S.; Rampazzi, L.; Corti, C.; Zanardini, E. Dry biocleaning of artwork: An innovative methodology for Cultural Heritage recovery? Microb. Cell 2021, 8, 91–105. [Google Scholar] [CrossRef]
- Edreira, M.C.; Feliu, M.J.; Fernández-Lorenzo, C.; Martín, J. Roman wall paintings characterization from Cripta del Museo and Alcazaba in Mérida (Spain): Chromatic, energy dispersive X-ray fluorescence spectroscopic, X-ray diffraction and Fourier transform infrared spectroscopic analysis. Anal. Chim. Acta 2001, 434, 331–345. [Google Scholar] [CrossRef]
- Gebremariam, K.F.; Kvittingen, L.; Banica, F.-G. Physico-Chemical Characterization of Pigments and Binders of Murals in a Church in Ethiopia. Archaeometry 2016, 58, 271–283. [Google Scholar] [CrossRef]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; Getty Conservation Institute: Los Angeles, CA, USA, 2002; ISBN 0892366389. [Google Scholar]
- Setti, M.; Lanfranchi, A.; Cultrone, G.; Marinoni, L. Archaeometric investigation and evaluation of the decay of ceramic materials from the church of Santa Maria del Carmine in Pavia, Italy. Mater. Constr. 2012, 62, 79–98. [Google Scholar] [CrossRef]
- Gabrielli, N. Technical-scientific investigations to detect the temporal vicissitudes of the funeral monument of Innocent VIII (Giovanbattista Cibo, 1484–1492), compared with that of Sixtus IV (Francesco della Rovere 1471–1484), both made by Antonio del Pollaiolo. Nat. Prod. Res. 2019, 33, 926–936. [Google Scholar] [CrossRef]
- Otero, V.; Campos, M.F.; Pinto, J.V.; Vilarigues, M.; Carlyle, L.; Melo, M.J. Barium, zinc and strontium yellows in late 19th–early 20th century oil paintings. Herit. Sci. 2017, 5, 46. [Google Scholar] [CrossRef]
- Fulton, E.L.; Newman, R.; Woodward, J.; Wright, J. The Methods and Materials of Martin Johnson Heade. J. Am. Inst. Conserv. 2002, 41, 155. [Google Scholar] [CrossRef]
- Klyachkovskaya, E.V.; Kozhukh, N.M.; Rozantsev, V.A.; Gaponenko, S.V. Layer-by-Layer Laser Spectrum Microanalysis of Easel-Painting Materials. J. Appl. Spectrosc. 2005, 72, 371–375. [Google Scholar] [CrossRef]
- Vetter, W.; Schreiner, M. A Fiber Optic Reflection-UV/Vis/NIR-System for Non-Destructive Analysis of Art Objects. Adv. Chem. Sci. 2014, 3, 7–14. [Google Scholar]
- Grifoni, E.; Briganti, L.; Marras, L.; Orsini, S.; Colombini, M.P.; Legnaioli, S.; Lezzerini, M.; Lorenzetti, G.; Pagnotta, S.; Palleschi, V. The chemical-physical knowledge before the restoration: The case of “The Plague in Lucca”, a masterpiece of Lorenzo Viani (1882–1936). Herit. Sci. 2015, 3, 26. [Google Scholar] [CrossRef]
- Romano, F.P.; Caliri, C.; Nicotra, P.; Di Martino, S.; Pappalardo, L.; Rizzo, F.; Santos, H.C. Real-time elemental imaging of large dimension paintings with a novel mobile macro X-ray fluorescence (MA-XRF) scanning technique. J. Anal. At. Spectrom. 2017, 32, 773–781. [Google Scholar] [CrossRef]
- Cristea-Stan, D.; Constantinescu, B. Studies on pigments of religious mural paintings using a portable X-ray Fluorescence spectrometer—The cases of Urechesti-Cicanesti Arges and Icoanei Bucuresti churches. Proc. Rom. Acad. Ser. A 2019, 20, 347–352. [Google Scholar]
- Regazzoni, L.; Cavallo, G.; Biondelli, D.; Gilardi, J. Microscopic Analysis of Wall Painting Techniques: Laboratory Replicas and Romanesque Case Studies in Southern Switzerland. Stud. Conserv. 2018, 63, 326–341. [Google Scholar] [CrossRef]
- Kriznar, A.; Ruiz-Conde, A.; Sánchez-Soto, P.J. Microanalysis of Gothic mural paintings (15th century) in Slovenia: Investigation of the technique used by the Masters. X-ray Spectrom. 2008, 37, 360–369. [Google Scholar] [CrossRef]
- Piovesan, R.; Mazzoli, C.; Maritan, L.; Cornale, P. Fresco and lime-paint: An experimental study and objective criteria for distinguishing between these painting techniques. Archaeometry 2012, 54, 723–736. [Google Scholar] [CrossRef]
- Mugnaini, S.; Bagnoli, A.; Bensi, P.; Droghini, F.; Scala, A.; Guasparri, G. Thirteenth century wall paintings under the Siena Cathedral (Italy). Mineralogical and petrographic study of materials, painting techniques and state of conservation. J. Cult. Herit. 2006, 7, 171–185. [Google Scholar] [CrossRef]
- Mora, P.; Mora, L.; Philippot, P. La Conservazione delle Pitture Murali; Editrice Compositori: Bologna, Italy, 1999; ISBN 8877941839. [Google Scholar]
- Rampazzi, L. Calcium oxalate films on works of art: A review. J. Cult. Herit. 2019, 40, 195–214. [Google Scholar] [CrossRef]
- Dilaria, S. Costruire ingegnosamente riutilizzando materiali poveri. L’impiego di conchiglie a fini edilizi ad Aquileia tra età repubblicana e tarda antichità. REUDAR. Eur. J. Rom. Archit. 2017, 1, 25. [Google Scholar] [CrossRef][Green Version]
- Asscher, Y.; van Zuiden, A.; Elimelech, C.; Gendelman, P.; Ad, U.; Sharvit, J.; Secco, M.; Ricci, G.; Artioli, G. Prescreening Hydraulic Lime-Binders for Disordered Calcite in Caesarea Maritima: Characterizing the Chemical Environment Using FTIR. Radiocarbon 2020, 62, 527–543. [Google Scholar] [CrossRef]
- Brysbaert, A. Lapis Lazuli in an Enigmatic ‘Purple’ Pigment from a Thirteenth-Century BC Greek Wall Painting. Stud. Conserv. 2006, 51, 252–266. [Google Scholar] [CrossRef]
- Mazzocchin, G.; Del Favero, M.; Tasca, G. Analysis of Pigments from Roman Wall Paintings Found in the “Agro Centuriato” of Julia Concordia (Italy). Ann. Chim. 2007, 97, 905–913. [Google Scholar] [CrossRef]
- Mazzocchin, G.A.; Vianello, A.; Minghelli, S.; Rudello, D. Analysis of roman wall paintings from the thermae of ‘Iulia Concordia’. Archaeometry 2010, 52, 644–655. [Google Scholar] [CrossRef]
- Duran, A.; Jimenez De Haro, M.C.; Perez-Rodriguez, J.L.; Franquelo, M.L.; Herrera, L.K.; Justo, A. Determination of pigments and binders in pompeian wall paintings using synchrotron radiation—High-resolution X-ray powder diffraction and conventional spectroscopy—Chromatography. Archaeometry 2010, 52, 286–307. [Google Scholar] [CrossRef]
- Corti, C.; Rampazzi, L.; Visoná, P. Hellenistic mortar and plaster from Contrada Mella near Oppido Mamertina (Calabria, Italy). Int. J. Conserv. Sci. 2016, 7, 57–70. [Google Scholar]
- Garofano, I.; Perez-Rodriguez, J.L.; Robador, M.D.; Duran, A. An innovative combination of non-invasive UV–Visible-FORS, XRD and XRF techniques to study Roman wall paintings from Seville, Spain. J. Cult. Herit. 2016, 22, 1028–1039. [Google Scholar] [CrossRef]


| Sample | Images | Colors | Identified Pigments | Analytical Techniques |
|---|---|---|---|---|
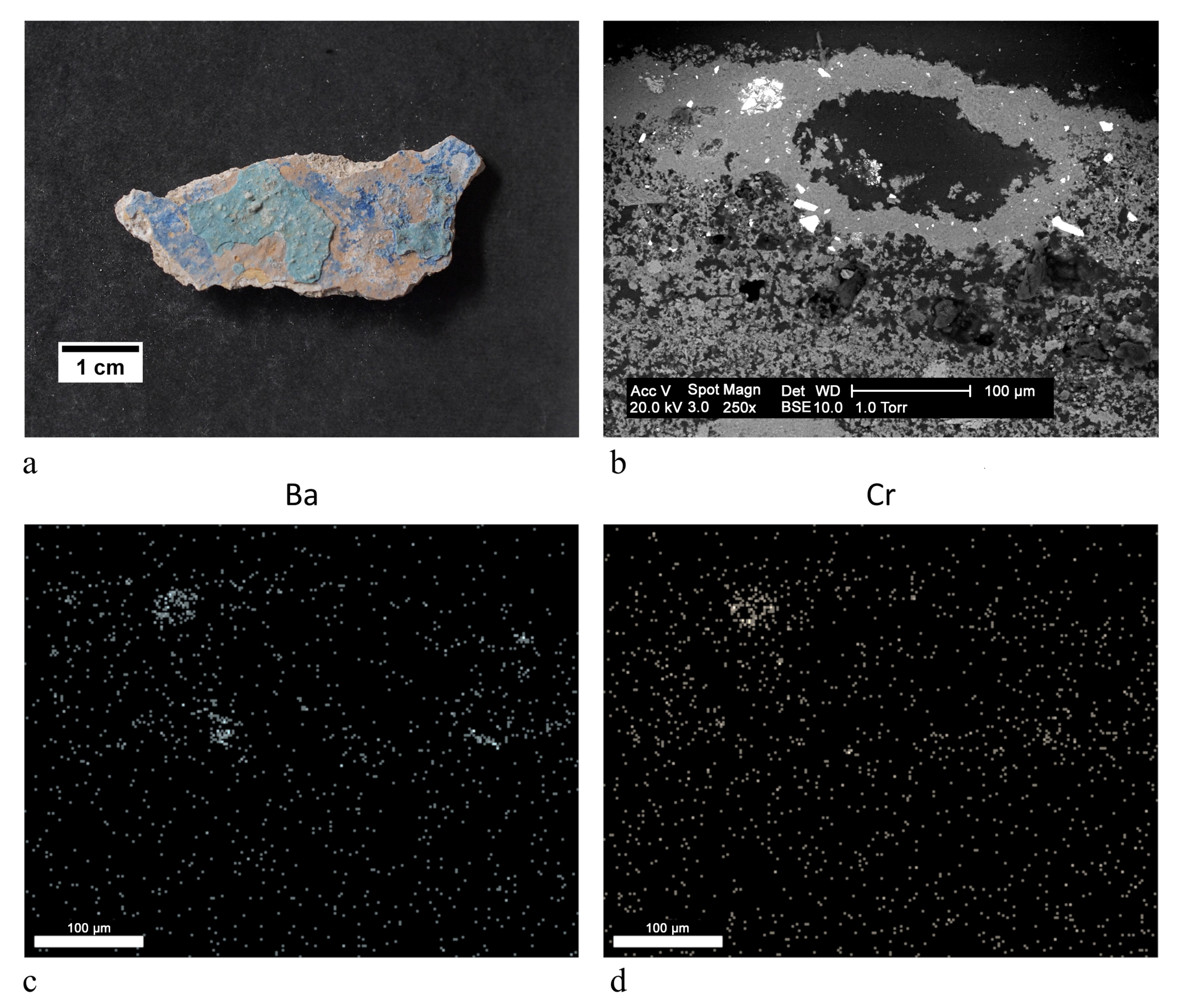
| ST1 |  | aqua green | blanc fixe (BaSO4), clinochlore (Mg4Fe2Al(Si3Al) O10(OH)8), ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, XRD, SEM-EDX |
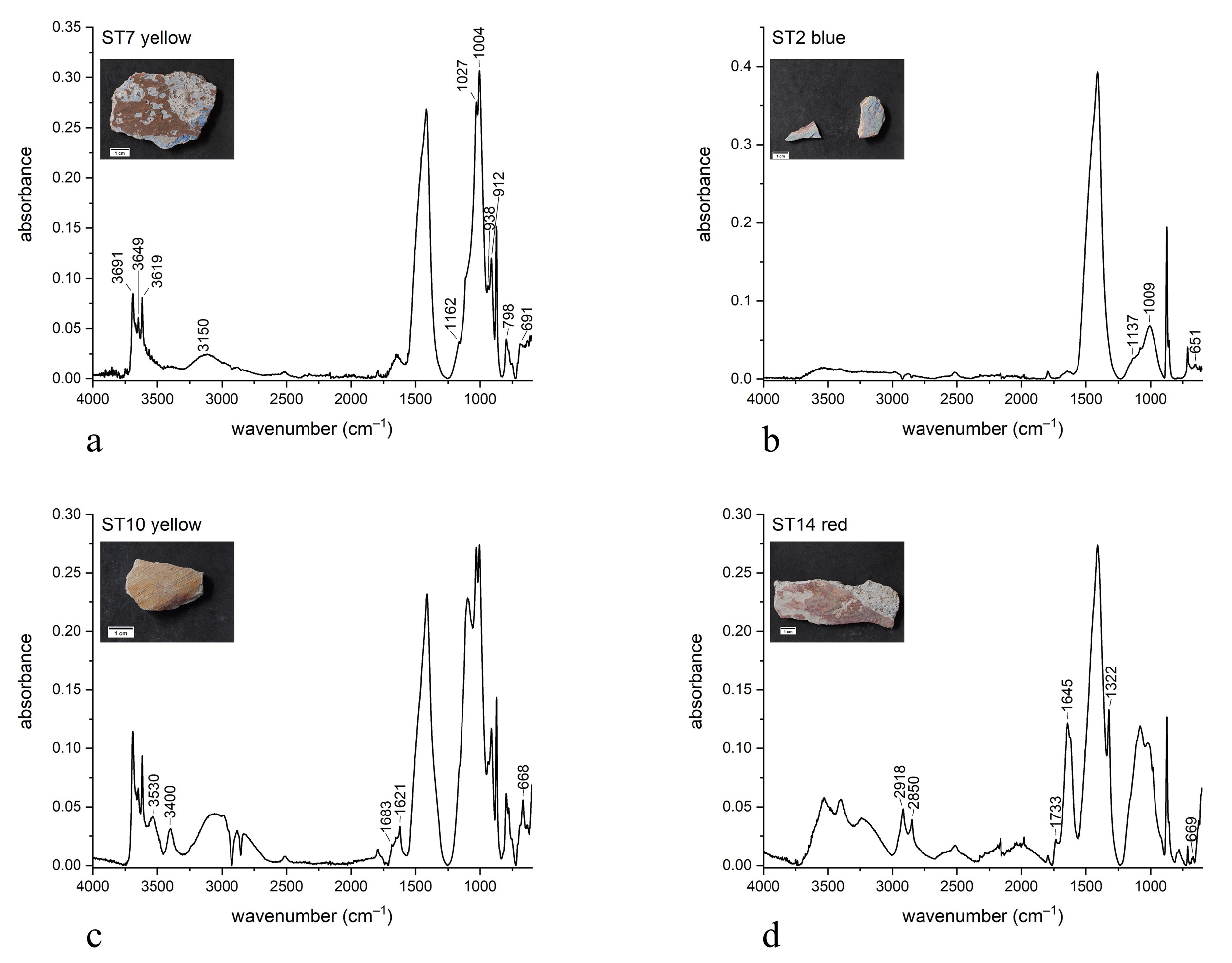
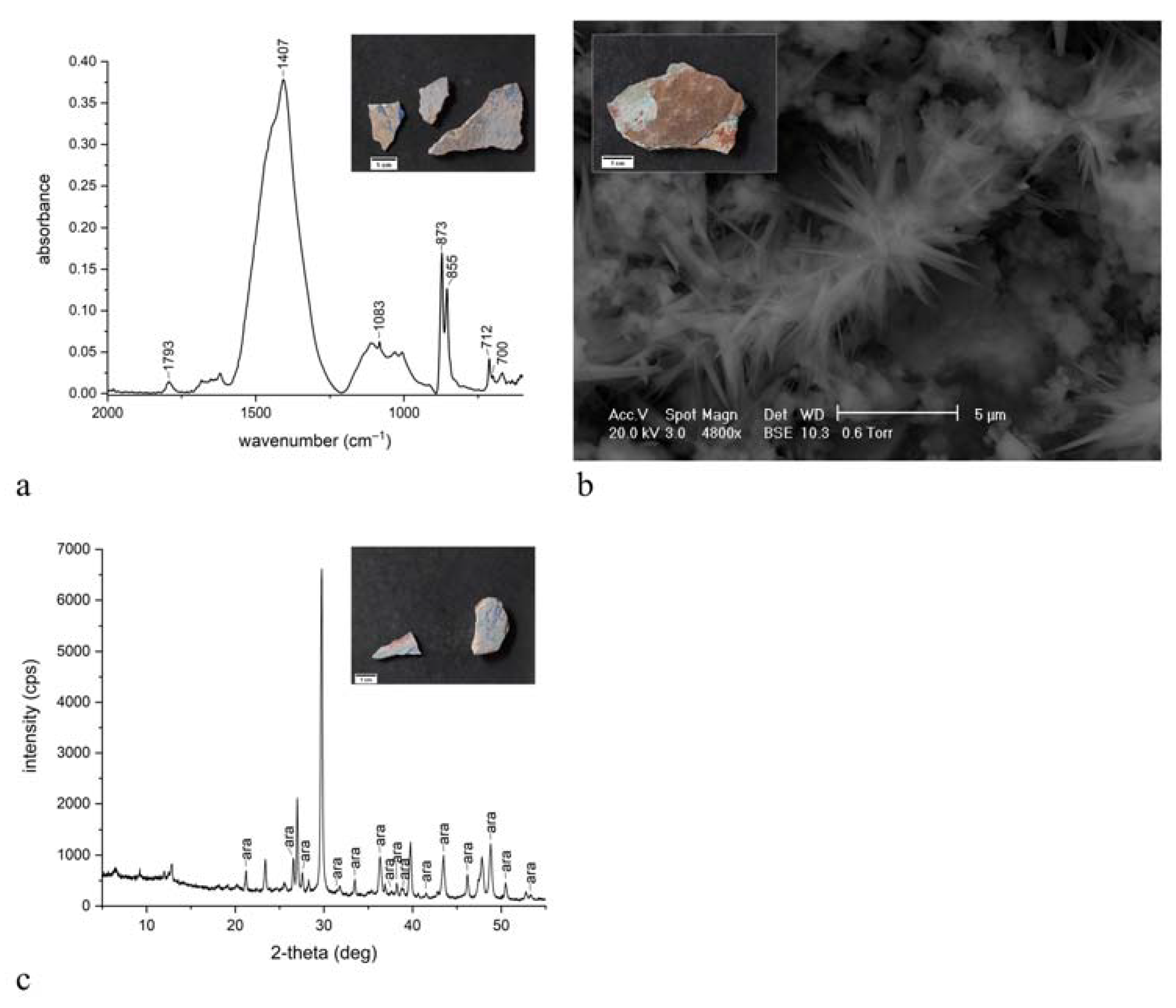
| ST2 |  | aqua green | blanc fixe (BaSO4), ultramarine blue (Na8Al6Si6O24S4) | FTIR-ATR, XRD, SEM-EDX, PCS |
| blue | ultramarine blue (Na8Al6Si6O24S4) | |||
| red | red ochre (Fe2O3, clay) | |||
| white | blanc fixe (BaSO4) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| yellow | yellow ochre (FeOOH, clay) | |||
| ST3 |  | aqua green | ultramarine blue (Na8Al6Si6O24S4), ultramarine yellow (BaCrO4) | FTIR-ATR, SEM-EDX, PCS |
| blue | ultramarine blue (Na8Al6Si6O24S4) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| yellow | yellow ochre (FeOOH, clay) | |||
| ST4 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | FTIR-ATR, XRD, SEM-EDX |
| ST5 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, XRD, SEM-EDX, PCS |
| red | red ochre (Fe2O3, clay) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| ST6 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | FTIR-ATR, XRD, SEM-EDX |
| red | red ochre (Fe2O3, clay) | |||
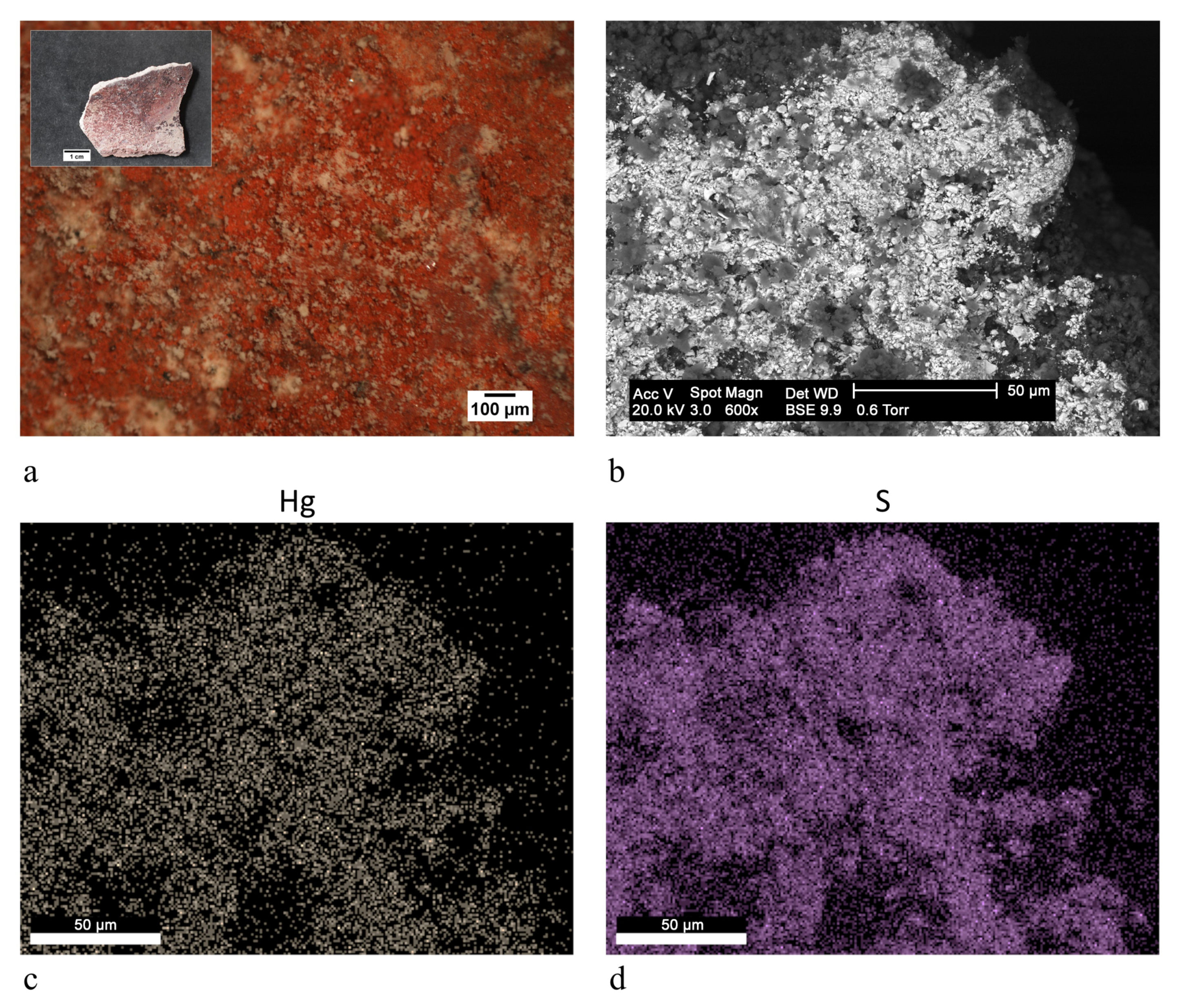
| yellow | cinnabar/vermilion (HgS), yellow ochre (FeOOH, clay) | |||
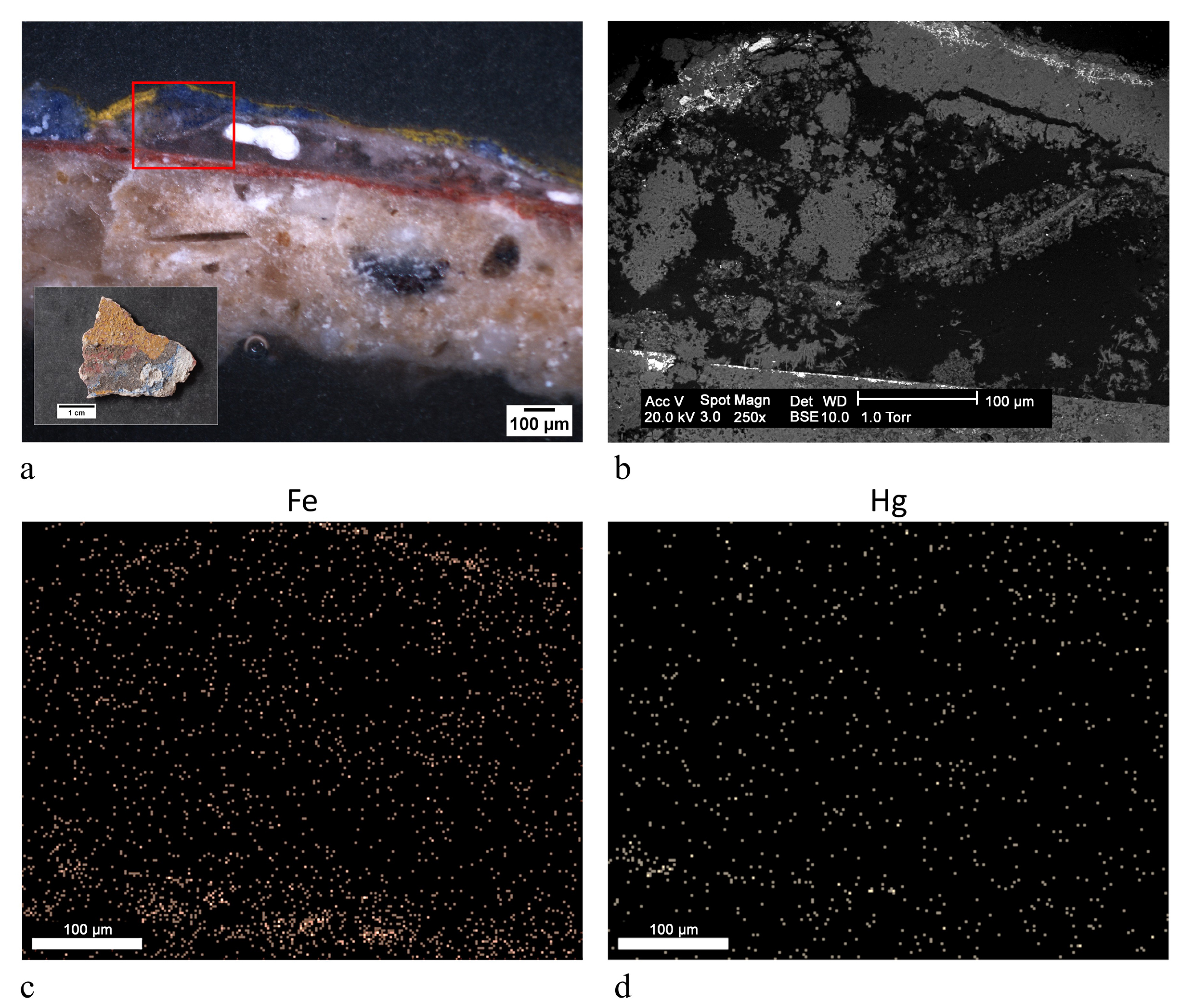
| ST7 |  | black | red ochre (Fe2O3, clay) | OM, FTIR-ATR, SEM-EDX |
| blue | blanc fixe (BaSO4), ultramarine blue (Na8Al6Si6O24S4) | |||
| light blue | blanc fixe (BaSO4), ultramarine blue (Na8Al6Si6O24S4), white lead (2PbCO3 Pb(OH)2) | |||
| grey | manganese black (MnO2), yellow ochre (FeOOH, clay) | |||
| dark ochre | manganese black (MnO2), yellow ochre (FeOOH, clay) | |||
| yellow | yellow ochre (FeOOH, clay) | |||
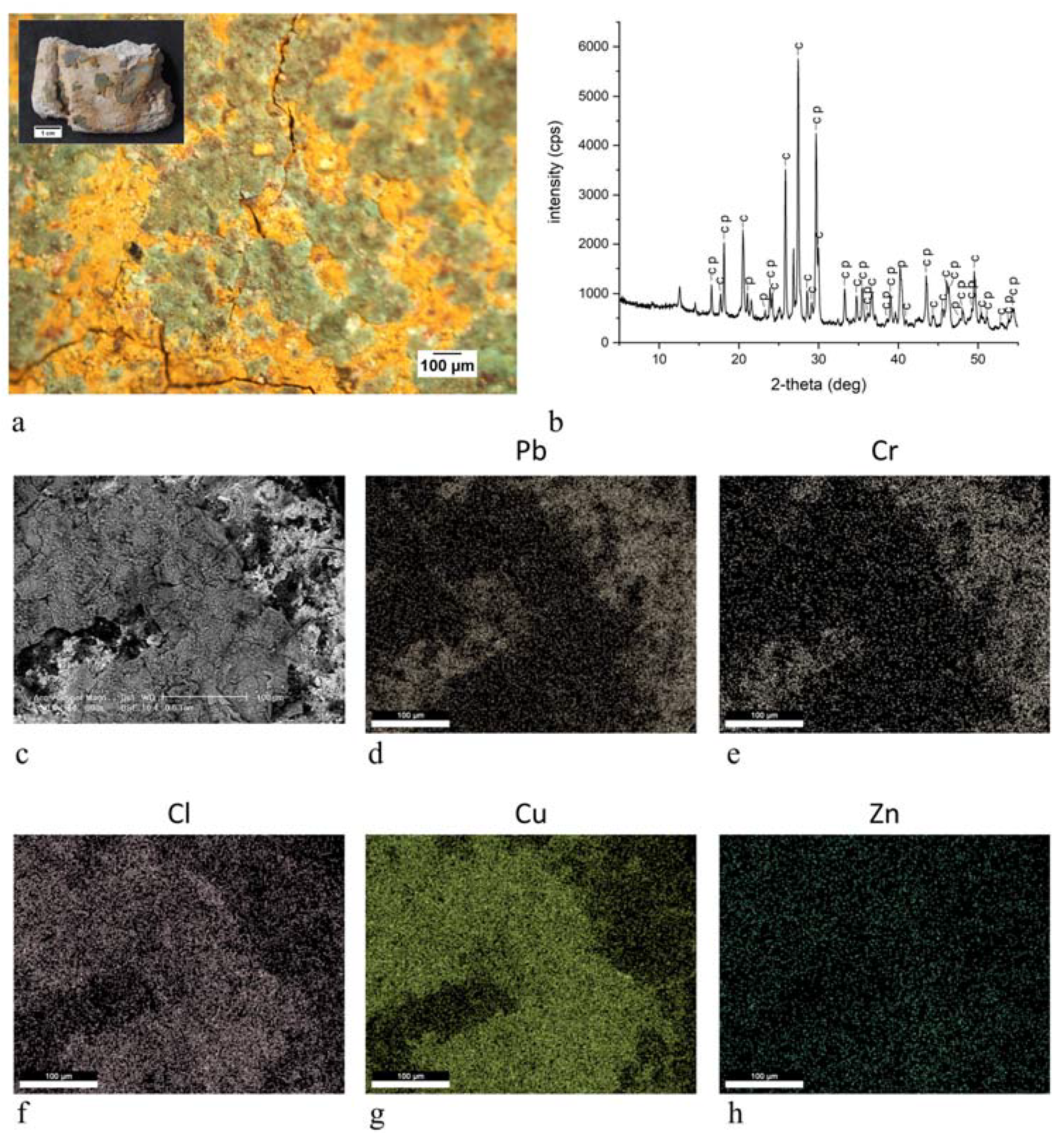
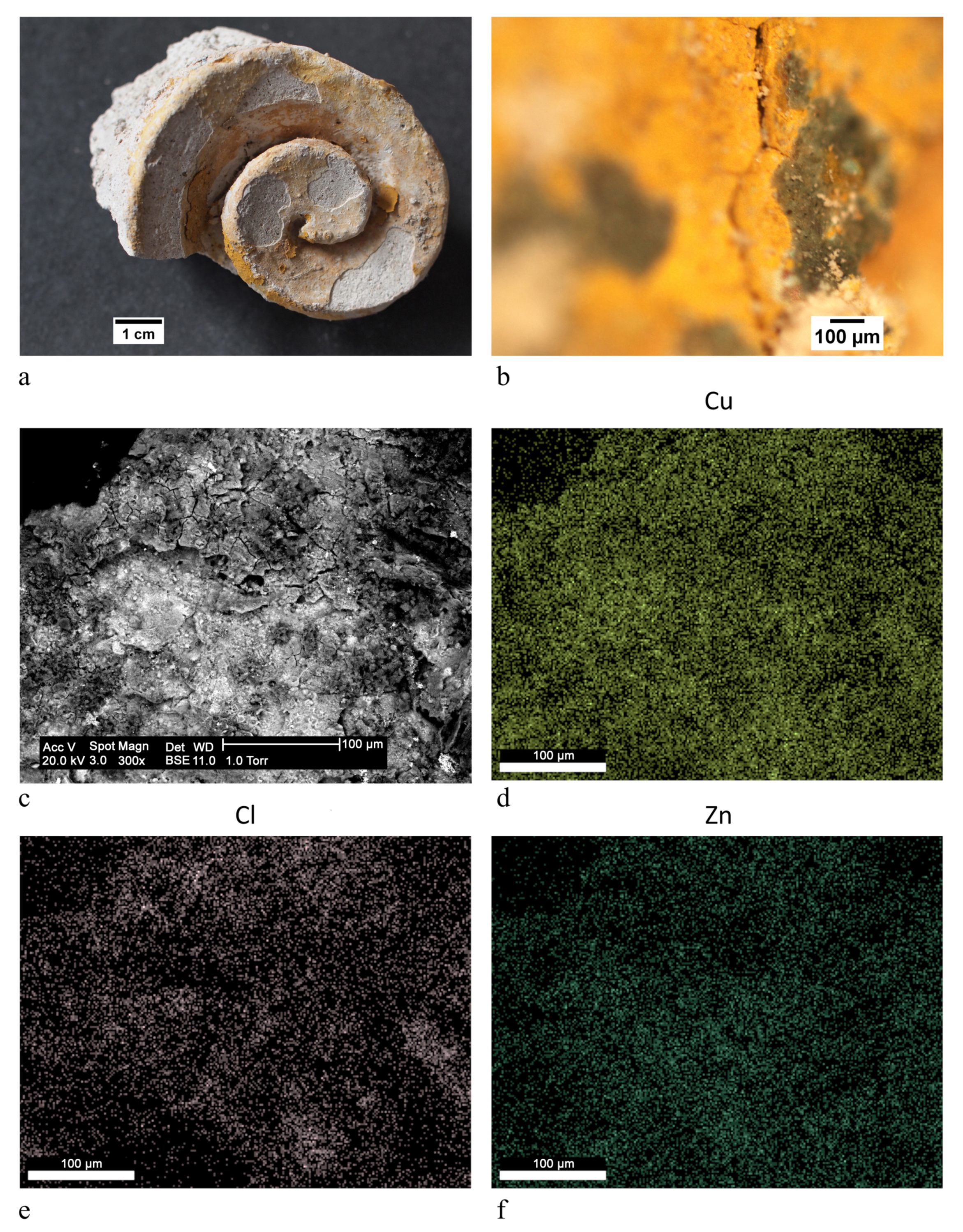
| ST8 |  | green | Brunswick green ((Cu,Zn)2(OH)3Cl) | OM, FTIR-ATR, XRD, SEM-EDX |
| orange | yellow ochre (FeOOH, clay) | |||
| yellow | chrome yellow (PbCrO4) | |||
| ST9 |  | ochre | yellow ochre (FeOOH, clay) | FTIR-ATR, SEM-EDX |
| ST10 |  | red | red ochre (Fe2O3, clay) | OM, FTIR-ATR, SEM-EDX |
| yellow | yellow ochre (FeOOH, clay) | |||
| ST11 |  | green | Brunswick green ((Cu,Zn)2(OH)3Cl) | OM, FTIR-ATR, XRD, SEM-EDX |
| yellow | chrome yellow (PbCrO4), yellow ochre (FeOOH, clay) | |||
| ST12 |  | red | cinnabar/vermilion (HgS) | FTIR-ATR, SEM-EDX |
| yellow | chrome yellow (PbCrO4), yellow ochre (FeOOH, clay) | |||
| ST13 |  | red | cinnabar/vermilion (HgS), yellow ochre (FeOOH, clay) | FTIR-ATR, SEM-EDX |
| yellow | chrome yellow (PbCrO4), ultramarine yellow (BaCrO4) | |||
| ST14 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | FTIR-ATR, SEM-EDX, PCS |
| red | cinnabar/vermilion (HgS), red ochre (Fe2O3, clay) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| yellow | chrome yellow (PbCrO4), yellow ochre (FeOOH, clay) | |||
| ST15 |  | red | cinnabar/vermilion (HgS), red ochre (Fe2O3, clay) | OM, FTIR-ATR, SEM-EDX |
| light red | blanc fixe (BaSO4), red ochre (Fe2O3, clay) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| yellow | chrome yellow (PbCrO4), yellow ochre (FeOOH, clay) | |||
| ST16 |  | light blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, SEM-EDX |
| red | cinnabar/vermilion (HgS), red ochre (Fe2O3, clay) | |||
| light red | red ochre (Fe2O3, clay) | |||
| yellow | chrome yellow (PbCrO4) | |||
| ST17 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, SEM-EDX, PCS |
| dark red | red ochre (Fe2O3, clay) | |||
| light red | red ochre (Fe2O3, clay) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| ST18 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, XRD, SEM-EDX, PCS |
| light blue | ultramarine blue (Na8Al6Si6O24S4) | |||
| red | red ochre (Fe2O3, clay) | |||
| yellow | yellow ochre (FeOOH, clay) | |||
| ST19 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, SEM-EDX, PCS |
| light blue | azurite (2CuCO3 Cu(OH)2 | |||
| dark ochre | yellow ochre (FeOOH, clay) | |||
| red | red ochre (Fe2O3, clay) | |||
| ST20 |  | blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, SEM-EDX, PCS |
| red | cinnabar/vermilion (HgS), red ochre (Fe2O3, clay) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| yellow | chrome yellow (PbCrO4), yellow ochre (FeOOH, clay) | |||
| ST21 |  | light blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, SEM-EDX |
| red | red ochre (Fe2O3, clay) | |||
| white | bianco di Sangiovanni (CaCO3) | |||
| yellow | yellow ochre (FeOOH, clay) | |||
| ST22 |  | light blue | ultramarine blue (Na8Al6Si6O24S4) | OM, FTIR-ATR, SEM-EDX |
| red | red ochre (Fe2O3, clay) | |||
| yellow | yellow ochre (FeOOH, clay) | |||
| ST23 |  | red | yellow ochre (FeOOH, clay) | OM, FTIR-ATR, SEM-EDX |
| yellow | red ochre (Fe2O3, clay) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampazzi, L.; Corti, C.; Geminiani, L.; Recchia, S. Unexpected Findings in 16th Century Wall Paintings: Identification of Aragonite and Unusual Pigments. Heritage 2021, 4, 2431-2448. https://doi.org/10.3390/heritage4030137
Rampazzi L, Corti C, Geminiani L, Recchia S. Unexpected Findings in 16th Century Wall Paintings: Identification of Aragonite and Unusual Pigments. Heritage. 2021; 4(3):2431-2448. https://doi.org/10.3390/heritage4030137
Chicago/Turabian StyleRampazzi, Laura, Cristina Corti, Ludovico Geminiani, and Sandro Recchia. 2021. "Unexpected Findings in 16th Century Wall Paintings: Identification of Aragonite and Unusual Pigments" Heritage 4, no. 3: 2431-2448. https://doi.org/10.3390/heritage4030137
APA StyleRampazzi, L., Corti, C., Geminiani, L., & Recchia, S. (2021). Unexpected Findings in 16th Century Wall Paintings: Identification of Aragonite and Unusual Pigments. Heritage, 4(3), 2431-2448. https://doi.org/10.3390/heritage4030137






