Oxygen Depletion Testing of Metals
Abstract
:1. Introduction
- Research into preventive conservation;
- A screening technique for archaeological iron and copper alloy stability;
- Research into interventive conservation (not researched in this work);
- A detection method for accelerated corrosion tests, such as the Oddy test.
1.1. Research into Preventive Conservation
- Temperature compensation;
- Angle of measurement—the angle between the analysor fibre and sensor can affect the measured value; much less impact is seen on phase shift type measurements than absolute intensity measurements;
- Distance from the probe head to the sensor can affect the measured value;
- 80% maximum RH, without further calibration;
- Air pressure;
- Moving the containers appears to affect the measured value for some minutes;
- Light sensitivity of the sensors;
- Cleaning—the packaging industry practice of adhering the sensor to the inside of the glass vessel is unsuitable when cleaning of the container is required between heritage conservation experiments.
1.2. A Screening Technique for Archaeological Iron and Copper Alloy
1.3. Research into Interventive Conservation
1.4. A Detection Method for Accelerated Corrosion Tests, Such as the Oddy Test
2. Materials and Methods
2.1. Samples
2.2. Experimental Methods
3. Results
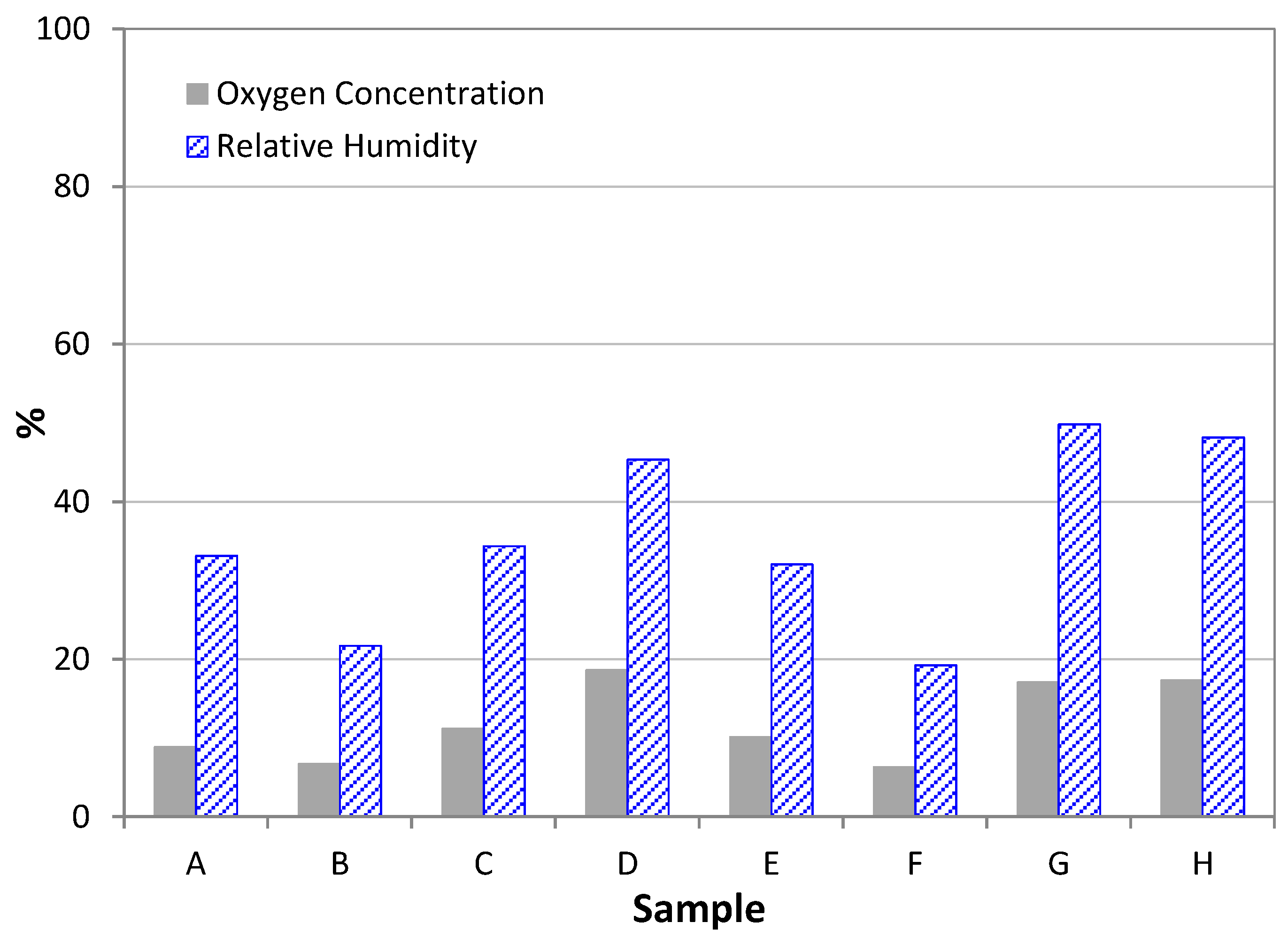
3.1. Research into Preventive Conservation
3.2. A Screening Technique for Archaeological Iron and Copper Alloy
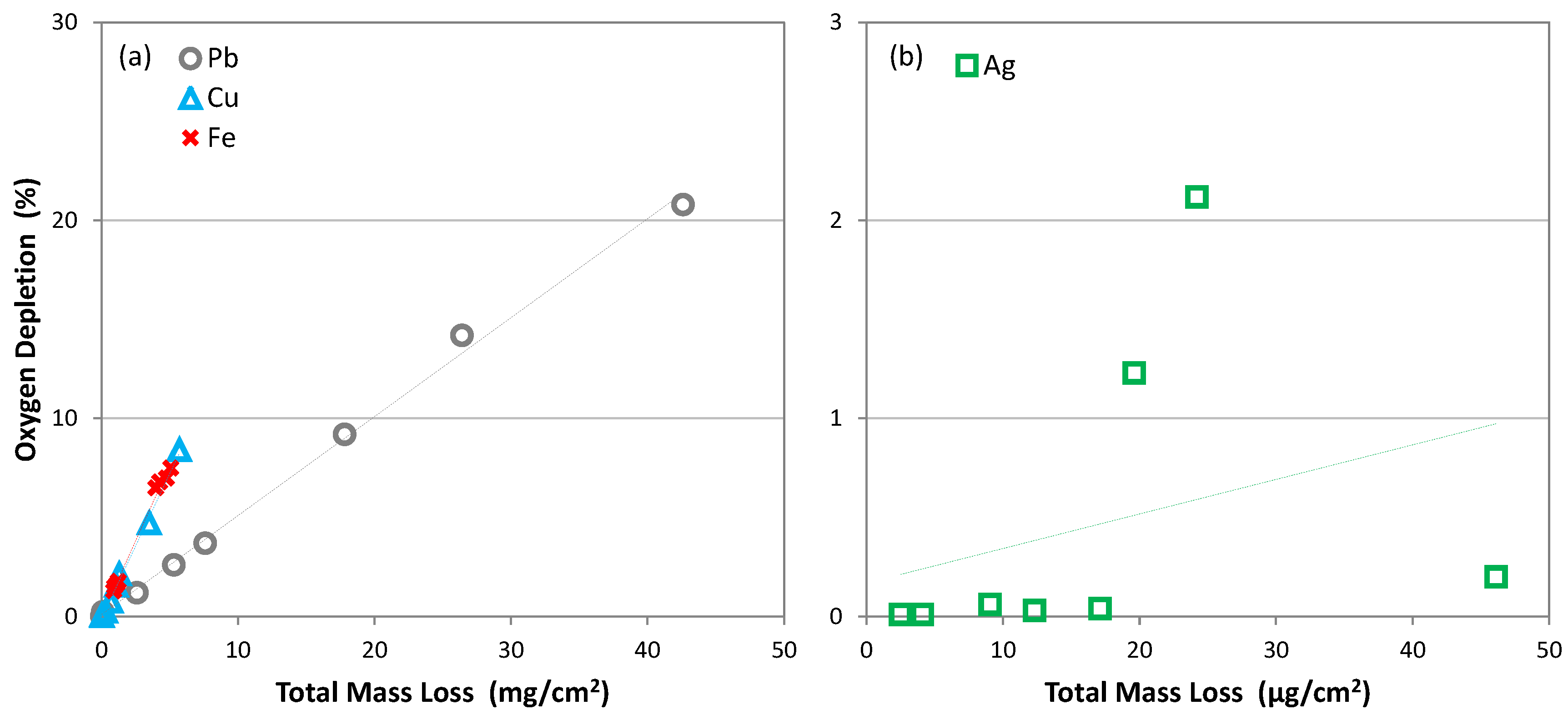
3.3. A Detection Method for Accelerated Corrosion Tests, Such as the Oddy Test
4. Discussion and Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- ISO 9223 Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. Available online: https://www.iso.org/standard/53499.html (accessed on 6 August 2021).
- Thickett, D.; Lambarth, S.; Wyeth, P. Determining the Stability and Durability of Archaeological Materials. In Proceedings of the 9th International Conference on Nondestructive Testing (NDT) of Art, Jerusalem, Israel, 25–30 May 2008; Available online: www.ndt.net/search/docs.php3?MainSource=65 (accessed on 6 August 2021).
- Matthiesen, H. A Novel Method to Determine Oxidation Rates of Heritage Materials In Vitro and In Situ. Stud. Conserv. 2007, 52, 271–280. [Google Scholar] [CrossRef]
- Thickett, D. Post Excavation Changes and Preventive Conservation of Archaeological Iron. Ph.D. Thesis, University of London, London, UK, 2012. Available online: https://www.english-heritage.org.uk/siteassets/home/learn/conservation/collections-advice--guidance/thickettthesisfinalversion.pdf (accessed on 6 August 2021).
- Matthiesen, H.; Stemann-Petersen, K.S. A Fast and Non-destructive Method to Document and Quantify the Efficiency of Metals Conservation. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, Scotland, UK, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; Historic Scotland: Edinburgh, UK, 2013; pp. 175–180. [Google Scholar]
- Thickett, D. Critical Relative Humidity Levels and Carbonyl Pollution Concentrations for Archaeological Copper Alloys. In Proceedings of the Metal 2016: Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; International Council of Museums Committee for Conservation and Indira Ghandi National Centre for the Arts: New Delhi, India, 2016; 2016, pp. 180–187. [Google Scholar]
- Watkinson, D.; Emmerson, N.; Seifert, J. Matching Display Relative Humidity to Corrosion Rate: Quantitative Evidence for Marine Cast Iron Cannon Balls. In Proceedings of the Metal 2016: Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; International Council of Museums Committee for Conservation and Indira Ghandi National Centre for the Arts: New Delhi, India, 2016; pp. 195–202. [Google Scholar]
- Watkinson, D.E.; Rimmer, M.B.; Emmerson, N.J. The Influence of Relative Humidity and Intrinsic Chloride on Post-excavation Corrosion Rates of Archaeological Wrought Iron. Stud. Conserv. 2019, 64, 456–471. [Google Scholar] [CrossRef]
- Thickett, D. The Formation and Transformation of Akaganéite. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, Scotland, UK, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; Historic Scotland: Edinburgh, UK, 2013; pp. 103–110. [Google Scholar]
- Rimmer, M.; Watkinson, D.; Wang, Q. The Impact of Chloride Desalination on the Corrosion Rate of Archaeological Iron. Stud. Conserv. 2013, 58, 326–337. [Google Scholar] [CrossRef]
- Watkinson, D.; Rimmer, M. Quantifying Effectiveness of Chloride Desalination Treatments for Archaeological Iron Using Oxygen Measurement. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, Scotland, UK, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; Historic Scotland: Edinburgh, UK, 2013; pp. 95–102. [Google Scholar]
- Schmutzler, B.; Revay, Z.; Stieghorst, C. Desalination of Archaeological Iron Objects: Comparing the Effectiveness of Sodium Hydroxide Treatments. In Proceedings of the Metal 2019: Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; International Council of Museums Committee for Conservation and Haute Ecole Arc Conservation-Restauration (HE-Arc CR): Neuchâtel, Switzerland, 2019; pp. 250–256. [Google Scholar]
- Emmerson, N.; Watkinson, D. Preparing Historic Wrought Iron for Protective Coatings: Quantitative Assessment to Produce Evidence-based Protocols. In Proceedings of the Metal 2013: Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, Scotland, UK, 16–20 September 2013; Hyslop, E., Gonzalez, V., Troalen, L., Wilson, L., Eds.; Historic Scotland: Edinburgh, UK, 2013; pp. 119–128. [Google Scholar]
- Lawson, A.J. Assessment of the Performance of Three Clear Coatings for Use in Heritage Conservation by an Oxygen Consumption Technique. Ph.D. Thesis, University of Cardiff, Cardiff, UK, 2016. Available online: https://orca.cardiff.ac.uk/97644/1/2017lawsonajphd.pdf (accessed on 6 August 2021).
- Thickett, D.; Fletcher, P.; Calver, A.; Lambarth, S. The Effect of Air Tightness on RH Buffering and Control. In Proceedings of the Museum Microclimates, Copenhagen, Denmark, 19–23 November 2007; Padfield, T., Borchersen, K., Eds.; The National Museum of Denmark: Copenhagen, Denmark, 2007; pp. 245–251, ISBN 978-87-7602-080-4. Available online: https://conservationphysics.org/mm/ (accessed on 6 August 2021).
- Robinet, L.; Thickett, D. A New Methodology for Accelerated Corrosion Testing. Stud. Conserv. 2003, 48, 263–268. [Google Scholar] [CrossRef]
- Green, L.R.; Thickett, D. Interlaboratory Comparison of the Oddy Test. In Conservation Science in the U.K.: Preprints of the Meeting Held in Glasgow, UK, May 1993; Tennent, N.H., Ed.; James & James Science Publishers Ltd.: London, UK, 1993; pp. 111–116. [Google Scholar]
- Chiantore, O.; Riedo, C.; Poli, T.; Cotrufo, G.; Hohenstatt, P. Risk Assessment and Preservative Measures for Volatile Organic Compounds in Museum Showcases. Stud. Conserv. 2018, 63 (Suppl. 1), 58–63. [Google Scholar] [CrossRef]
- Eggert, G.; Kuiter, R.; Korenberg, C.; Ziegler, J.; Bette, S.; Stelzner, J. Metal Conservation, Cellulose Nitrate and the Oddy Test. In Proceedings of the Metal 2019: Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; International Council of Museums Committee for Conservation and Haute Ecole Arc Conservation-Restauration (HE-Arc CR): Neuchâtel, Switzerland, 2019; pp. 125–131. [Google Scholar]
- Heine, H.; Jeberien, A. Oddy Test Reloaded: Standardized Test Equipment and Evaluation Methods for Accelerated Corrosion Testing. Stud. Conserv. 2018, 63 (Suppl. 1), 362–365. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; An, Z.; Chen, J.; Wu, L.; Zhou, X. An Improved Oddy Test Using Metal Films. Stud. Conserv. 2011, 56, 138–153. [Google Scholar] [CrossRef]
- van Iperen, J.; van Keulen, H.; Keune, K.; Abdulah, K.; van Langh, R. Crystalline Deposits in New Display Cases at the Rijksmuseum: Characterisation and Origin. Stud. Conserv. 2021, 66, 253–271. [Google Scholar] [CrossRef]
- ISO 16000-1 Indoor Air—Part 1: General Aspects of Sampling Strategy. 2004. Available online: https://www.iso.org/obp/ui/#iso:std:iso:16000:-1:ed-1:v1:en (accessed on 6 August 2021).
- Milner, C.S.; Dalton, N.N. Glycerol; Reinhold Publishing Corporation: New York, NY, USA, 1953; p. 269. [Google Scholar]
- Celina, M.C.; Quintana, A. Oxygen Diffusivity and Permeation Through Polymers at Elevated Temperature. Polymer 2018, 150, 326–342. [Google Scholar] [CrossRef]
- Costa, V.; Dubus, M. Impact of the Environmental Conditions on the Conservation of Metal Artifacts: An Evaluation Using Electrochemical Techniques. In Proceedings of the Museum Microclimates, Copenhagen, Denmark, 19–23 November 2007; Padfield, T., Borchersen, K., Eds.; National Museum of Denmark: Copenhagen, Denmark, 2007; pp. 63–66. Available online: https://conservationphysics.org/mm/ (accessed on 6 August 2021).
- Capelo, S.; Homem, P.M.; Cavalheiro, J.; Fonseca, I.T.E. Linear sweep voltammetry: A Cheap and Powerful Technique for the Identification of the Silver Tarnish Layer Constituents. J. Solid State Electrochem. 2013, 17, 223–234. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM G1-03(2017)e1 Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2017; Available online: http://www.astm.org/cgi-bin/resolver.cgi?G1-03(2017)e1 (accessed on 6 August 2021). [CrossRef]
- Thickett, D.; Odlyha, M. Assessment of Dry Storage Microenvironments for Archaeological Iron. In The Conservation of Archaeological Materials—Current Trends and Future Directions; Williams, E., Peachey, C., Eds.; Archaeopress: London, UK, 2010; pp. 187–199. [Google Scholar]
- Rimmer, M.B.; (University of Cardiff, Cardiff, UK). Personal communication. 2008. [Google Scholar]
- Seifert, J.; (University of Cardiff, Cardiff, UK). Personal communication, 2019.

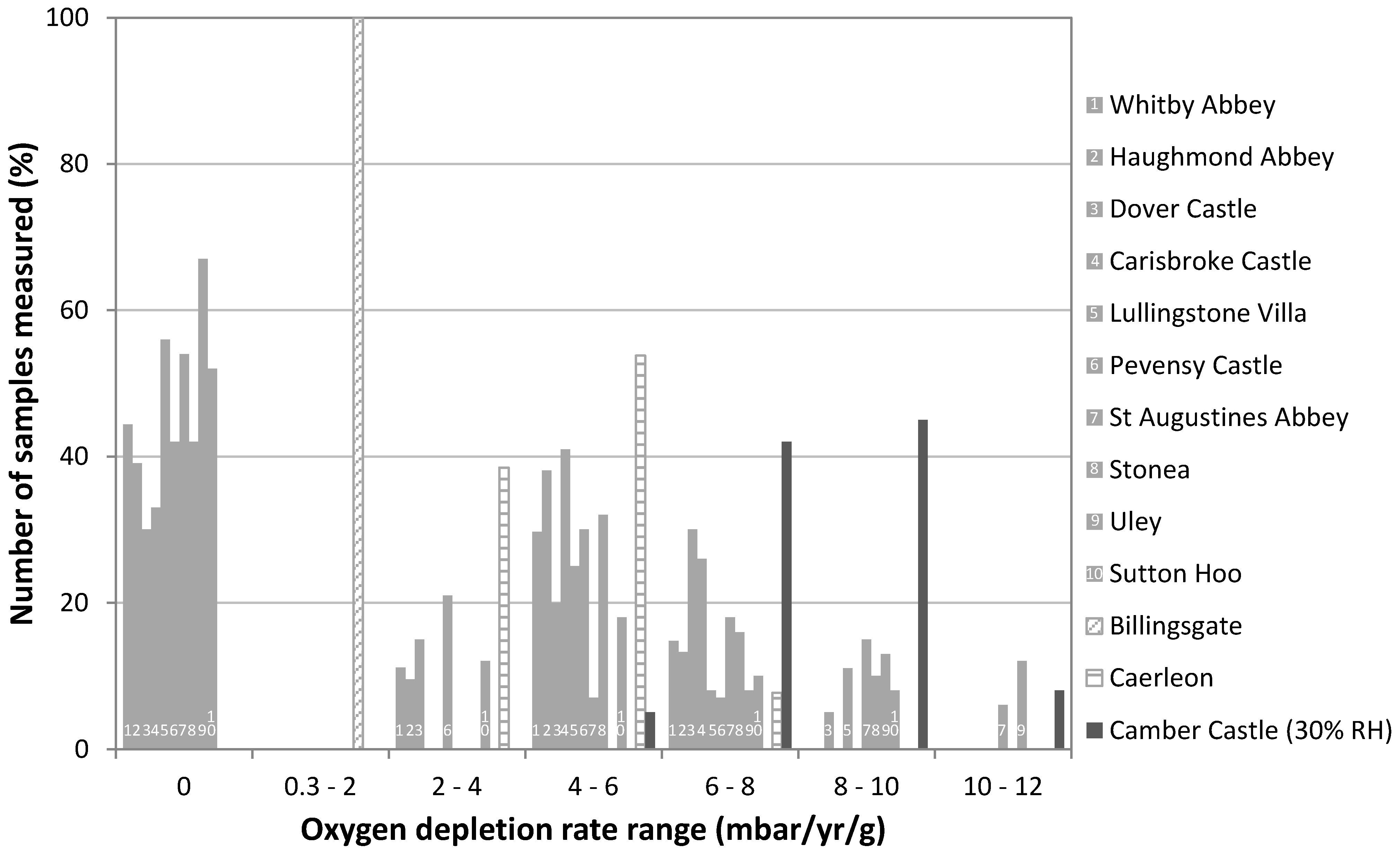



| Location (Code) | Object Description | Number of Objects Tested | Test Method 1 | Storage Conditions |
|---|---|---|---|---|
| Billingsgate | Nails | 27 | as [8] | unreported, data taken from Watkinson et al. [8] |
| Caerleon (CPF) | Nails | 30 | as [8] | unreported, data taken from Watkinson et al. [8] |
| Camber Castle (Cam) | Cannon balls, musket fittings, swords, daggers | 41 | (ii) | dry silica gel |
| Carisbrooke Castle | Lock, chainmail, nails, knives, spear heads, arrowheads, helmets | 31 | (iii) | dry silica gel |
| Dover Castle | Horseshoes, keys, arrowheads, nails | 27 | (iii) | dry silica gel |
| Haughmond Abbey | Buckles, pins | 15 | (iii) | dry silica gel |
| Lullingstone Villa | Pin, knife, lock, nails | 21 | (iii) | dry silica gel |
| Pevensey Castle | Horseshoes, keys, arrowheads, nails, staff terminal | 12 | (iii) | dry silica gel |
| St Augustine’s Abbey | Keys, nails, brackets, pins | 32 | (iii) | dry silica gel |
| Stonea (Stn) | Hook, daggers, spearheads, arrowheads, nails | 32 | (i) | uncontrolled conditions |
| Sutton Hoo (SH) | Daggers, spearheads, arrowheads, nails | 29 | (i) | dry silica gel |
| Uley | Daggers, spearheads, arrowheads, nails | 21 | (i) | uncontrolled conditions |
| Whitby Abbey | Knives, pins, bar, bracket | 23 | (iii) | dry silica gel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thickett, D. Oxygen Depletion Testing of Metals. Heritage 2021, 4, 2377-2389. https://doi.org/10.3390/heritage4030134
Thickett D. Oxygen Depletion Testing of Metals. Heritage. 2021; 4(3):2377-2389. https://doi.org/10.3390/heritage4030134
Chicago/Turabian StyleThickett, David. 2021. "Oxygen Depletion Testing of Metals" Heritage 4, no. 3: 2377-2389. https://doi.org/10.3390/heritage4030134
APA StyleThickett, D. (2021). Oxygen Depletion Testing of Metals. Heritage, 4(3), 2377-2389. https://doi.org/10.3390/heritage4030134






