Abstract
Man-Eater with Pennants, a rarely exhibited sculpture in Alexander Calder’s oeuvre, was commissioned by The Museum of Modern Art (MoMA) and installed in 1945. To exhibit the large standing mobile in Alexander Calder: Modern from the Start (2021), the derelict sculpture had to be remediated; this initiated a collaborative investigation with conservation scientists, conservators, curators, and the Calder Foundation into the original paint colors hidden beneath layers of repaint. XRF analysis was carried out to elucidate the paints’ composition, followed by sampling for analysis to assess the paint stratigraphy and binders. Scrapings were analyzed by µ-FTIR and Raman spectroscopies; cross sections were examined with optical microscopy and analyzed with SEM-EDS. Analysis differentiated between the original paints, which contain Prussian blue, parachlor red, chrome yellow, and the many layers of overpaint, which contain titanium white, molybdate orange, a variety of β-Naphthol reds, red lead, and ultramarine. A model for Man-Eater, Mobile with 14 Flags, is also part of the museum’s collection, and was first considered as a point of reference for the original colors. Similar analysis, however, indicates that the maquette was painted after the Man-Eater was first installed, therefore is not representative of the original colors. In addition to investigating an early primary palette for Calder’s outdoor sculptures, this study helped develop the plan for the restoration of the original color scheme of Man-Eater.
1. Introduction
Alexander Calder (1898–1976), one of America’s best-known sculptors, is renowned for developing two new idioms in modern art: mobiles, which hang from the ceiling and whose shapes move in response to air currents, and stabiles, large scale, stationary abstract sculpture, characterized by simple forms executed in sheet metal. Born into a family of artists, Calder showed facility in handling metals from a young age [1]. Although he trained as an engineer at the Stevens Institute of Technology in New Jersey and held several engineering jobs after graduation, at the age of 25, he committed himself as an artist, relocated to New York, and took art classes. In 1926, he moved to Paris where he mingled with established artists and writers of the time, including figures such as Picasso, Miró, Léger, and Duchamp. During a visit to Mondrian’s studio in 1930, Calder saw, tacked to the wall, colored cardboard rectangles that Mondrian used to work out compositions [2]. Calder proposed that the rectangles could be made “to oscillate in different directions, and at different amplitudes.” The visit proved to be the “shock that started things,” as he wrote later [1].
In 1931, Calder began constructing compositions of metal wire and wood and made them move via inherent kinetics, activating their motion with motors and other means [2], and then later allowing free form movement with the wind. The moving sculptures were dubbed “Mobiles” by artist and friend Marcel Duchamp. These sculptures of “detached bodies floating in space” embodied Calder’s fascination with the dynamism of the universe, which he sought to describe through a bold palette of primary and secondary colors [2]. Calder also created abstract stationary constructions for which Jean Arp then coined the term “Stabiles” to differentiate them from the moving sculptures.
Man-Eater with Pennants, abbreviated as Man-Eater in this paper, (Figure 1) was proposed by Alfred Barr and commissioned by the Board of Trustees for The Abby Aldrich Rockefeller Sculpture Garden in 1944. A maquette, Mobile with 14 Flags (Figure 2), was shown to museum leadership for approval before the final work was fabricated. Man-Eater was Calder’s largest mobile sculpture to date, towering more than 9 m with a wingspan of more than 14 m. Seven painted metal pennants perch atop long vertical rods; heavy flat black plates counterbalance them at the bottom. The wind- or human-propelled elements bounce, sway, and rotate around a central post. The maquette is nearly identical in form to the final expression of the massive Man-Eater with Pennants. Naturally, a full-scale sculpture designed for outdoor installation must be structurally different from a table-top model. For example, on the actual mobile, two of the uppermost polygonal shapes have oval-shaped cutouts, whereas the maquette does not. The main horizontal crossbar on the mobile is heavily reinforced to bear the weight of the vertical rods and the pennants, but on the maquette, all the wires of the structure are the same delicate gauge. However, the shapes and colors of the pennants are the same on both the maquette and the Man-Eater; they are black, red, blue, and yellow. In addition, the upright post is a crucial grounding aspect for the large-scale sculpture, whereas the moving parts of the maquette sit on a triangular base, allowing table-top display.

Figure 1.
Alexander Calder. Man-Eater with Pennants, 1945. Painted steel rods and sheet iron 14′ (425 cm) × approx. 30′ (915 cm) in diameter. Purchase. © 2021 Calder Foundation, New York/Artists Rights Society (ARS), New York All reproductions of this work are excluded from the CC: BY License.

Figure 2.
Alexander Calder. Mobile with 14 Flags (maquette for Man-Eater with Pennants), 1945. Painted steel 53″ (134.6 cm) × approximately 50″ (127 cm) diameter. Gift of the artist. © 2021 Calder Foundation, New York/Artists Rights Society (ARS), New York All reproductions of this work are excluded from the CC: BY License.
Man-Eater with Pennants was installed in MoMA’s garden from 1945–1949. From the outset, the merits and deficiencies of the mobile were debated within the museum. The curator Dorothy Miller expressed concern about the lack of movement due to the weight of the structure and the absence of ball bearings [3]. Philip Goodwin, Head of the Department of Architecture and Design, had deep reservations about the ironwork, which he called “clumsy,” the “far from pleasing” colored forms, and the faulty engineering [4]. Of the opposing opinion, Curator James Johnson Sweeney thought the Man-Eater was one of Calder’s most successful ventures in this field [5]. In addition, fears about the public injuring themselves on the mobile began right away; in the interest of public safety, a small fence was set around it. Sweeney, a fan of the sculpture, strongly advocated for their removal. “I would like to suggest in my last will and testament that the Calder mobile should move. I would recommend that the chains be taken off the monster” [6]. After four years on view, the mobile was taken off view, ostensibly because reconstruction of the garden space was underway. Man-Eater traveled to outdoor venues in London and Houston in the 1950s. After its last showing at MoMA from 1969–1970, it was housed in storage, where it has been since.
As for the maquette, with its purpose fulfilled, it was put away in storage with other Calder sculpture proposals. There it languished for ten years until a storage reorganization and clean-up effort was undertaken in 1959. Internal records called it an extended loan and it was returned to Calder at that time. The Registrar’s carefully observed description specifies that the model was made of “unpainted iron” and also notes surface accretions and defects. Ten years later, the artist gifted the maquette to the museum. Photographs from that acquisition suggest that at some time while it was in Calder’s possession between 1959 and 1969 it must have received its color coat. Because the maquette was painted sometime after the Man-Eater installation, the color scheme on the model cannot be considered a trial for the full-scale iteration; it is the exact opposite: the larger was the example for the smaller.
For MoMA’s 2021 exhibition, Alexander Calder: Modern from the Start, the exciting work of reviving this forgotten sculpture began with conservation, curatorial, and registrar teams reviewing the documentation in MoMA’s files and examining the work in storage. The sculpture had been repainted several times in its history. However, the only record of a repainting campaign is correspondence between the artist and curator Dorothy Canning Miller in 1948, where she describes the colors as not resembling the original paint applied by Calder even though the museum used the Ronan Japan Color paints preferred by the artist [7]. After its last exhibition between 1969 and 1970, extensive paint loss and rusting due to outdoor exposure went unaddressed. In addition, the metal rods were likely bent by painters holding them down to repaint them, or during handling [8]. This project provided a unique opportunity to study the painting history and the original colors of the large mobile and the associated maquette.
While the origins of Calder’s affinity for a palette of primary colors are explored in the art historical literature, research in the scientific literature on Calder’s paint choices is limited. A recent publication has investigated the red, black, and white paints used on a motorized work from 1932, including extensive overpainting from past treatments [9]. The work outlined here contributes to the discussion on the evolution of Calder’s color choices and subsequent attempts by The Museum of Modern Art and others to determine the hues of the early primary palette. To our knowledge, this is the first study to delve into Calder’s yellows and blues. As part of planning the restoration treatment for Man-Eater, the conservation team took cross section samples of the paint layers and mounted them for microscopy. These “slices of time” open a window into the history of the painting campaigns.
2. Materials and Methods
Scientific analysis was undertaken to better understand the pigments and binders used in Man-Eater and the associated maquette. Both invasive and non-invasive techniques were employed, with the latter involving both scrapings and cross sections. Initial analysis with portable X-Ray Fluorescence (p-XRF) yielded information about possible pigments and extenders as well as the composition of the metals. However, in the case of Man-Eater, the extensive overpainting, coupled with the lack of historical narrative about said campaigns necessitated micro-invasive sampling for more fingerprint spectroscopic techniques. Samples for analysis by micro-Fourier transform infra-red (µ-FTIR) and Raman spectroscopies were taken from areas that could be representative of different layers. While µ-FTIR also gave some indication of the binder used in the paint formulation, the pigments in each layer of the cross section were conclusively identified by Raman spectroscopy in addition to scanning electron microscopy (SEM), coupled with electron dispersive spectroscopy (EDS). Cross sections from the maquette showed a single campaign of colored paint, which makes the results more definitive than those taken from Man-Eater, especially as it relates to binder analysis by µ-FTIR.
The complementary nature of these methods made for a rich visualization and interpretation of the original colors of Man-Eater. These results helped determine the final colors to be used in the restoration campaign. The following is a description of the techniques used.
XRF analysis was performed with a Bruker Tracer III-SDD handheld XRF instrument with a Rh excitation source and silicon drift detector, with a 5 mm diameter approximate spot size. The instrument was operated at 40 kV and 3 μA, and spectra were acquired for 120 s. Additional XRF was performed on the maquette using a Bruker Tracer 5i at 40 kV and 4.5 μA and spectra were acquired for 120 s. All the spectra were examined with the Bruker Artax 8.0 software.
Micro-µ-FTIR (µ-FTIR) analysis was carried out in transmission mode using a Nicolet iS50-µ-FTIR coupled with a Thermo Nicolet Continuum infrared microscope equipped with an MCT detector. Spectra were collected in the 4000–600 cm−1 range with a 4 cm−1 resolution and 128 scans and using the Thermo Scientific OMNIC 9.12 software package. Spectra were examined using the Spectral Search and Multicomponent Search tools available in the Thermo Scientific OMNIC Specta 2.0 software and IRUG spectral databases [10].
Raman spectra were collected using a Renishaw In-via Raman system, equipped with a 785 nm diode laser at powers between 0.3 to 3 mW, a 1200 lines/mm grating, and a Leica confocal microscope with a 50× LWD or 100× objective. Final spectra represent an average of five acquisitions of 10 s. Raman spectra were evaluated Spectral Search and Multicomponent Search tools available in the Thermo Scientific OMNIC Specta 2.0 software and SOPRANO [11] and UCL [12]. spectral databases.
SEM-EDS was carried out under low vacuum and using a Hitachi TM3000 microscope (Tokyo, Japan) fitted with a Bruker Xflash MIN SVE and Quantax 70 software. Images were acquired using analysis mode at 15 kV and a four-segment backscatter electron (BSE) detector. The cross sections were not coated as imaging was carried out under low vacuum; remaining gas molecules in the chamber ionize the negative buildup up on the surface of uncoated, organic samples.
Optical Microscopy was carried out using a Leica DM IRM microscope using 5× and 10× objectives.
Cross sections were embedded in BioPlastic ®, [Aldon Corp., Avon, NY, USA] a blend of polyester and methacrylate monomers in a styrene solvent, trimmed with a jeweler’s saw, and dry polished with Micro-Mesh® [Micro-surface Finishing Products, Wilton, IA, USA] silicon carbide or aluminum oxide abrasives.
3. Results and Discussion
3.1. Metals
Based on XRF analysis, Man-Eater is made of a steel of containing manganese. Areas denuded of paint were severely rusted. In most cross sections, there are two metallic layers, a reddish layer followed by a silvery one, on top of which the first colored paint layer has been applied. SEM-EDS analysis confirmed what could be concluded visually from microscopy: a layer of rusted steel rich in iron coated with a silvery, flaky layer of aluminum. Aluminum paint functions as an anti-corrosion coat on top of a steel substrate. Due to its rapid oxidation when exposed to air, an aluminum flake paint can confer a high degree of corrosion protection on a steel substrate by forming a thin, transparent layer of aluminum oxide film. This impervious layer is self-adherent and reaches a maximum thickness of 100 Å [13]. The effect has been understood since the early 1900s and was popular in the 1920s and 1930s for automotive and structural parts. In line with Calder’s practice, the original color layer is applied directly atop the protective coating of aluminum [14]. The steel rust penetrated upwards through both the aluminum paint and the first colored paint layers in many cases, suggesting that the first repaint campaign was necessitated by surface paint loss due to rusting of the steel substrate.
The maquette’s metal structure is unusual because the colored flags are a different metal than the remaining black elements [15]. XRF analysis showed the colored pennants atop the vertical rods to be made of an aluminum alloy containing lead, iron, silicon, manganese, and zinc. This could suggest an effort to lighten the load on the vertical rods. The lower black pennants are an iron-based alloy whose weight serves to keep the rods vertical. The XRF spectrum of the rods showed an intense peak for copper in addition to iron. A closer examination of the dull brown patches on the black-painted rods shows that they appear to be copper-clad steel, which has application in the electric and automotive industries, for example [16,17]. This metal has been observed on rare occasions in other sculptures by Calder [15]. The rivets connecting the black rods to the colored aluminum pennants are made of a brass alloy of copper, zinc, arsenic, and possibly titanium.
3.2. Yellow Paint
The color of the sole yellow pennant in Man-Eater had bleached considerably, rendering it remarkably light in comparison with underlying layers visible in chips on the edges. The surface was also visibly scratched and scuffed but had the brush marks of hand application. Calder is known for hand painting many of his early painted outdoor works, and retaining that quality would have been paramount to any repainting campaign.
In the yellow cross section (Figure 3), nine individual layers of paint are clearly delineated, varying in shades from intense, to pale, to greenish. Chrome yellow (P.Y. 34; C.I. 77600), chemically a lead chromate (PbCrO4), was observed in the Raman spectra of the first eight layers. This was also confirmed by SEM-EDS (Figure 3), where Pb and Cr were recorded across those layers. Chrome yellow pigment was first synthesized in 1804 but only came into prominence when more abundant sources of chrome minerals were available [18]. Chemically, chrome yellow is available as a pure PbCrO4 or as solid solutions, with PbCrO4 and lead sulfate (PbCr1−xSxO4), in shades that range from yellow to orange (x < 0.1) to lemon-yellow (0.2 ≤ x ≤ 0.4) and pale yellow (x > 0.5) with increasing sulfate concentration (C.I. 77603 when coprecipitated with PbSO4) [19]. Partial replacement of the chromate in solid solutions brings about a reduction in tinctorial strength with increasing sulfate concentration but allows for the manufacture of yellows with a greenish hue. In terms of crystallography, PbCrO4 presents as monoclinic and PbSO4 as orthorhombic, and substitution of chromate ions by smaller sulfate ions leads to a compression of the monoclinic unit cell at low sulfate concentration and a change from monoclinic to orthorhombic when x exceeds 0.4 in a solid solution.
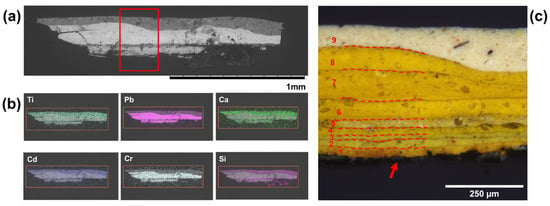
Figure 3.
Cross section imaging of a sample taken from the yellow pennant in Man-Eater. (a) BSE image of the cross section and (b) associated EDS mapping of relevant chemical elements, of particular note is the Cd and Ti in Layer 9 and Pb and Cr in the remaining layers. (c) Light microscope image at 20× magnification showing all 9 layers of paint and aluminum anticorrosive paint, indicated by an arrow. © 2021 MoMA, New York, NY, USA.
This phenomenon is also observed by Raman spectroscopy (Figure 4), where shifts to higher wavenumbers of some chromate bands indicate the presence of lead sulfate [19]. The ν1(CrO42−) symmetric stretching mode shifts to higher energy with increasing substitution of sulfate ions into the lattice. Discrete shifts between the spectra of the yellow layers point to at least two different yellows used in the overpainting. This is evident in Layers 1, 2, and 3 in the cross section, where ν1(CrO42−) is at 841 cm−1 in comparison with 839 cm−1 in the remaining layers. The ν4/ν2(CrO42−) bending multiplet is also influenced by sulfate substitution and cell compression; ν4(CrO42−) modes for pure chrome yellow are located at 400, 376, and 357 cm−1, while those at 336 and 323 cm−1 are attributable to ν2(CrO42−) modes. Further pointing to the presence of sulfate in the first three layers, the mode at 400 cm−1 is shifted to 403 cm−1 and that at 357 cm−1 to 360 cm−1. A band at 970 cm−1 attributed to a ν1(SO42−) mode [19]. The pair of Layers 2 and 3 cements the presence of a solid solution of chromates and sulfates, perhaps one that is still monoclinic with few sulfates. While this peak was not seen in layer 1 due to noise, it can be said with some certainty that a paint containing a PbCr1−xSxO4 pigment was the original color of the yellow pennant, one that was possibly lemony in hue at one time. Similarly, Layer 5 exhibits the presence of ν1(SO42−) from lead, barium, and calcium sulfates, based on Raman analysis (Figure 3). Layer 5 was also rich in silicates but contained less pigment material, as indicated by the elemental distribution observed in SEM-EDS. Layer 4 consists only of PbCrO4 and BaSO4, Similarly, the color and spectra of Layers 6, 7, and 8 consist of PbCrO4 and BaSO4 based on Raman analysis (Figure 3). They could have been applied successively in one repainting campaign with drying time between coats.
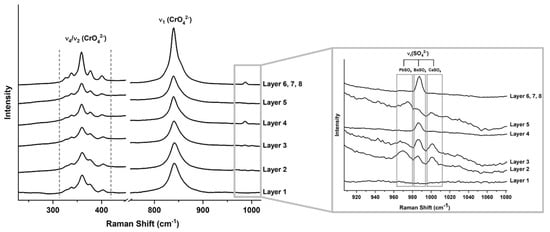
Figure 4.
Raman spectra of the first 8 layers in the cross section of a sample taken from the yellow pennant in Man-Eater. The slight shifts to higher wavenumbers in Layers 1, 2, 3, and 5 in both the ν1(CrO42−) stretching band and ν4/ν2(CrO42−) bending multiplet suggests the presence of a solid solution of lead chromate and lead sulfate (PbCr1−xSxO4). This is confirmed by the ν1(SO42−) stretching mode observed between 973 and 978 cm−1 in the inset.
Curiously, the Raman spectrum of Layer 9 in the cross section showed no signs of chrome yellow, only that of the tetragonal rutile form of titanium white (TiO2) with bands at 144 (B1g), 445 (Eg), and 610 (A1g) cm−1 [20]. The Raman spectrum exhibited a recently characterized luminescence emission pattern (1222, 1306, 1385, 1497, 1600, and 1686 cm−1; see Figure 5 and Figure 7) attributed to neodymium (Nd3+) ions substituting into the orthorhombic alkaline earth sulfates of titanium dioxide pigments, made through co-precipitation with barium sulfate (BaSO4) or calcium sulfate (CaSO4), where Nd3+ occurs naturally in ilmenite (FeTiO3), the source ore for Ti [21]. Observing this pattern can help with dating issues, as it was only detected in works dating from 1945–1977. These co-precipitated pigments of lower tinting strength were more prevalent in industrial paints, in particular oils and alkyds. However, the presence of both BaSO4 (988 cm−1) and CaSO4 (1017 cm−1) complicates the identification of the type of co-precipitate, where either could have been added mechanically to the pigment mixture. In turn, this makes it more difficult to establish the date of the final repainting campaign since BaSO4 and CaSO4 coprecipitates were phased out in the late 1940s and 1970s, respectively. Internal records show that, after the Salute to Calder exhibition closed in 1970, a repaint was considered but never executed [22]. The Nd3+ luminesce pattern in the Raman spectrum places a last repainting within the accepted range of 1945–1977.
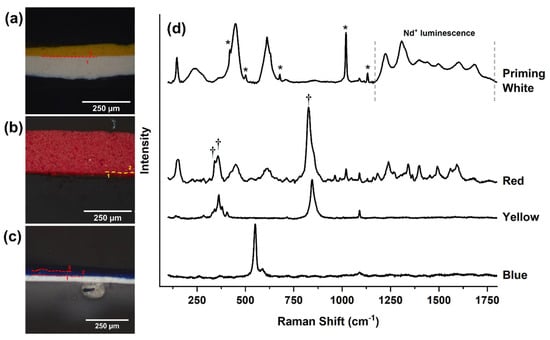
Figure 5.
Microscopy images of three cross sections (a–c) taken from the Mobile with 14 Flags, or the maquette, as referred to in the text. The Raman spectrum of each of these colors (d) shows the presence of ultramarine in the blue, chrome yellow in the yellow, and P.R. 4 and molybdate orange (†) in the red. The white priming layers observed in (a,c) contain titanium white in rutile form deposited on CaSO4 (*), which again shows a luminescence pattern (*) from a Nd3+ impurity. © 2021 MoMA, New York, NY, USA.
While no yellow pigment was detected via Raman spectroscopy in Layer 9, SEM-EDS (Figure 3) showed the top layer to contain cadmium unlike the remaining layers of paint, indicating the presence of the semiconductor pigment cadmium sulfide, known as cadmium yellow (P.Y. 37; C.I. 77199). Cd was also seen in the XRF spectra taken of the yellow pennant. SEM-EDS also showed this layer to be particularly rich in magnesium silicates and silicates that are used as fillers.
The presence of cadmium yellow could explain the pale appearance of the top yellow layer, as cadmium yellow is known to blanch with exposure to light, humidity, and environmental acid—a given for any outdoor sculpture. This exposure leads to the formation of cadmium sulfate (CdSO4), which can react further with carbon dioxide (CO2) to form cadmium carbonate (CdCO3) [23]. While these moieties were not identified directly, this drastic fading is characteristic of cadmium yellows, even under gallery conditions [24]. It was also found that CdS degrades most when illuminated with blue light, which is fully absorbed and generates the highest photocurrent electrochemically [25]. Consequently, the abundance of energetic ultraviolet (UV) and blue light from solar radiation can promote more rapid decay of CdS. Oddly, the reverse is also true: cadmium yellow has been shown to darken considerably when embedded in an alkyd resin [26,27]. While that was not seen here, it points to the photoactivity of cadmium sulfide. Additionally, TiO2 has a photocatalytic effect on some pigments when exposed to UV radiation [28], as when Man-Eater was installed outdoors. TiO2 also exhibits photocatalytic chalking, or degradation of the paint film that exposes pigment particles, and could have further hastened the blanching of Layer 9 [29]. Conversely, the dark color observed visually in Layer 1 can perhaps be attributed to the photo-induced reduction in chromate ions to Cr (III) compounds, which is driven by both visible and UV light [23] and can markedly affect those chromate yellows of the rhombic varieties [18]. Sulfur-rich orthorhombic yellows are more prone to browning, possibly due to the increased solubility of PbCrO4 and PbCr1−xSxO4 in this phase, making more chromate ions available for redox reactions [13].
In contrast, the paint of the yellow pennant (Figure 5) in the maquette is still vibrant yellow. A cross section from the maquette showed only two layers, a white priming layer followed by a yellow one. The yellow was similarly identified as a chrome yellow, one that is probably a solid solution of lead chromate and lead sulfate (PbCr1−xSxO4). As in Layers 1 through 3 in the cross section from Man-Eater, the symmetric ν1(CrO42−) stretch is broadened and shifted to 845 cm−1, and the ν4(CrO42−) bending modes to 404 and 364 cm−1, all of which are results of crystal compression. This paint is also characterized by a strong ν1(C–O) symmetric stretch at 1090 cm−1 and a weak in-plane bend ν4(C–O) at 717 cm−1, both characteristic of calcium carbonate (CaCO3). This confirms that the maquette and Man-Eater have two different yellow paints. Interestingly, the white priming layer also exhibited the same luminescence emission pattern attributed to Nd3+ ions in titanium-based whites. Both CaSO4 (1020 cm−1) and CaCO3 (1090 cm−1) are identified in this white, suggesting a different rutile-based paint pigment than in Man-Eater. The absence of BaSO4 is more diagnostic for dating and places the painting sometime between 1959, when it left the museum and 1969, when it came back, confirming internal registrar records.
3.3. Blue Paint
As with the yellow pennant in Man-Eater, the blue pennant had bleached significantly, presenting as a brittle, light blue layer of paint. In chipped areas, darker blue layers of paint were visible below the light blue layer with the most vibrant blue layers closest to the rusted metal. Interestingly, one face of the blue pennant was slightly more vivid in color, which prompted the analysis of two different cross sections. From this point onward, the lighter side will be referred to as Side A, and the darker as Side B. This could be a result of the static nature of this mobile as discussed in internal correspondence, allowing Side A to be more exposed to the sun than Side B.
Analysis of the cross section from Side A (Figure 6) showed a dramatic rusting of the steel, infiltrating through the silver layer and into the first layer of paint. As in the yellow cross section, SEM-EDS confirmed the steel rust and the aluminum flake paint. In total, 6 layers could be delineated on Side A, with colors ranging from midnight to baby blue.
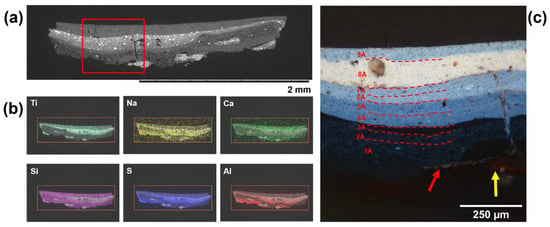
Figure 6.
Cross section imaging of a sample taken from Side A of the blue pennant in Man-Eater. (a) BSE image of the cross section and (b) associated EDS mapping of relevant chemical elements, of particular note are the Ti and Ca in Layers 8A and 9A, the distribution of Al, S, Na, and Si associated with ultramarine. (c) Light microscope image at 20× magnification showing all 9 layers of paint, aluminum anticorrosive paint (red arrow), and the rusting steel (yellow arrow). Image copyright 2021 MoMA, New York, NY, USA.
The analysis of the first two paint layers of the lighter side, 1A and 2A, indicated the use of Prussian blue (P.B. 27; C.I. 77510) as the only blue pigment. CaSO4 was also identified through Raman (1017 cm−1). Prussian blue (Feᴵᴵᴵ[Feᴵᴵ(CN)₆]₃−), was first synthesized accidentally by Dresbach in 1704 and became industrially produced by the nineteenth century [30]. It is the oldest synthetic coordination compound and has since found important use in the printing industry in addition to paints and artist materials. The dark blue color of Prussian blue is due to an intervalent transition between Feᴵᴵand Feᴵᴵᴵ through a coordinated cyano group (CN) where light in the orange-red region around 700 nm is absorbed [30]. The presence of Prussian blue was identified in the Raman spectrum (Figure 7) by the 1Ag ν(CN) stretching vibration at 2160 cm−1 and the Eg ν(CN) stretching vibration at 2090 cm−1 [31]. The spectrum also shows peaks for ν(Fe−C) stretching modes at 606 and 536 cm−1, σ(Fe−CN−Fe) bending modes at 376 and 278, and a σ(Fe−C−Fe) deformation at 189 cm−1. Perhaps most interesting is the shoulder at 2123 cm−1, which corresponds to a CN− stretch related to the 1Ag ν(CN) mode. This shoulder is most pronounced in the “soluble,” or colloidal varieties of Prussian blue, where association with a cationic species such a K+, NH4+, or Na+ maintains charge balance, as opposed to the insoluble form that relies on a higher concentration of Feᴵᴵᴵ [31,32]. Chemically, Prussian blue is prone to photoreduction and fading, the same quality that made the pigment valuable for cyanotypes, an early photographic method, and soluble varieties are far more sensitive to fading than the insoluble form [33]. The presence of extenders can also exacerbate photoinduced fading, and SEM-EDS showed this paint layer to be particularly rich in magnesium silicates and other silicates. This can explain the discoloration of Layers 1A and 2A and might have prompted repainting of the blue pennant with a darker blue Layer 3A, which was shown to contain ultramarine (P.B. 29; C.I. 77007) and a smaller amount of Prussian blue (Figure 7). The darker appearance of Layer 3A might correspond to the combination of two blues rather than a single color.
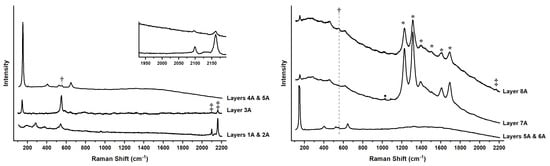
Figure 7.
Raman spectra of the pigments in all 8 layers from Side A of the blue pennant in Man-Eater. Ultramarine (†) is observed at 550 cm−1 in Layers 3A, 4A, 5A, 6A, and 8 A; Prussian blue (‡) at 2090 and 2160 cm−1 in Layers 1A, 2A, 3A, and 8A. Titanium white was observed in anatase form in layers 3A, 4A, 5A, and 6A, whereas the rutile form was observed in Layers 7A and 8A, in addition to a luminescence pattern (*) from a Nd3+ impurity attributed to titanium white deposited on CaSO4 (•).
By the 1940s, the use of synthetic ultramarine (3Na2O∙3Al2O3∙6SiO2∙2Na2S) was commonplace after its laboratory preparation in 1828 by Guimet in France and Gmelin in Germany [34]. The incorporation of a sulfur radical (S3−) into the sodalite crystal lattice of sodium, aluminum, silicon, and oxygen acts as a chromophore. The broad absorption of green-yellow-orange visible light of this radical, centered around 600 nm, gives the pigment its signature blue color. This energy corresponds to an electronic transition between two singly occupied molecular orbitals [35]. Ultramarine was identified through Raman spectroscopy (Figure 7) by the S3− radical symmetric stretch at 540 cm−1 [36]. Elemental analysis by XRF confirmed the presence of Al, Si, S, and K, whereas SEM-EDS showed the presence of the lighter Na. With a relatively low refractive index of 1.5, the opacity of the ultramarine is increased by the addition of white pigments, which is seen here with the addition of anatase (TiO2), seen in the Raman spectrum at 144 cm−1, and confirmed by the presence of Ti in SEM-EDS. The Raman spectrum of Layers 4A and 5A show a higher concentration of anatase resulting in a much lighter blue than Layer 3A; the two are similar in composition and could have been applied in two successive coats that contain ultramarine and anatase. Layers 6A and 7A are even lighter in color than the previous two, but they contain the same mixture of ultramarine and anatase and could also have been applied successively as part of one campaign. The white Layer 8A shows the Nd3+ luminescence pattern associated with rutile (TiO2) precipitated on CaSO4, which was also observed in Layer 9 of the yellow pennant. Finally, Layer 9A shows very small peaks for ultramarine and BaSO4 in addition to the dominant Nd3+ luminescence pattern of TiO2. Additionally, the detection of niobium (Nb) in the XRF spectra of the blue pennant further narrows down the type of rutile present. The presence of detectable amounts of this rare earth metal by XRF is indicative of the sulfate process for producing titanium white, where it remains after manufacture as an impurity from the ore [37]. As in the case of the pale-yellow Layer 9, TiO2 has been shown to have a catalytic effect on the degradation and fading of Prussian blue, which could explain some of the lighter blues observed in those layers containing the white pigment [28]. Interestingly, the color of the blue pennant was named “light gray” in internal correspondence from 1970, far from the original deep Prussian Blue [22].
Similar to Prussian blue, ultramarine is prone to photoinduced fading; it is also highly sensitive to acids, which are prevalent in urban settings [38]. Furthermore, it has been shown that ultramarine pigment dispersed in an alkyd binder accelerates the degradation of the paint film and results in a bleached and brittle film [26,27]. Consequently, the combination of chemical sensitivity and catalytic effect can explain the pale color of the blue pennant.
Side B (Figure 8) is darker in comparison to Side A, but it also exhibits fading. The same penetration of steel rust up through the aluminum and the first paint layers is again observed. Layers 1B and 2B contains Prussian blue and CaSO4; Layer 3B ultramarine, Prussian blue, and anatase; a thin Layer 4B followed by a thick Layer 5B contain ultramarine and anatase; a tan-colored Layer 6B with anatase; Layer 7B rutile precipitated on CaSO4; and Layer 8B ultramarine with rutile precipitated on CaSO4. After Layer 3B, there is a breakdown in the similarity between the two sides, as the lighter Layers 6A and 7A have no counterpart in the stratigraphy of Side B. The Raman spectrum of Layer 6B indicates it is composed of anatase, but the tan color can be attributed to the presence of Fe, possibly in the form of iron oxides, barites, and silicates, all detected by SEM-EDS. The purpose of this layer is unclear. Layers 7B and 8B have the same composition as Layers 7A and 8A were probably applied at the same time.
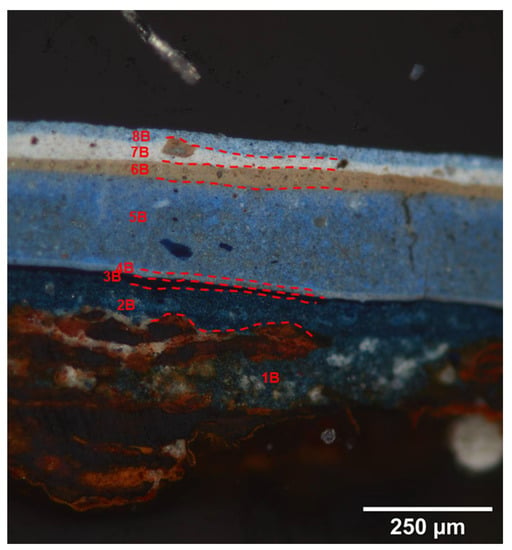
Figure 8.
Cross section imaging of a sample taken from Side B of the blue pennant in Man-Eater, showing layer 6B, which is unique to this face of the pennant. Raman analysis indicates this layer to be anatase-based, and SEM-EDS shows it to be rich in iron, which gives the layer its tan color. © 2021 MoMA, New York, NY, USA.
In contrast, the paint of the blue pennant on the maquette is still vivid, and a cross section showed three layers, a white priming layer followed by two blue ones of the same compositions. The blue was identified as ultramarine, which is typical of Calder’s mature palette [39]. This paint is also characterized by a ν1(C–O) symmetric stretch at 1090 cm−1 characteristic of CaCO3. This further confirms that the maquette and Man-Eater were painted at different times and with different blue paints. The white priming layer also exhibited the Nd3+ luminescence emission pattern attributed to Nd3+ ions in rutile, and both CaSO4 (1020 cm−1) and CaCO3 (1090 cm−1), all similar to the priming layer of the yellow pennant. These paints again do no match any of those found in the blue pennant of Man-Eater.
3.4. Red Paint
The extant red paint on the pennants in Man-Eater was the most vibrant of the three primary colors on the surface. Nevertheless, the paint was brittle and chipped; in some areas, the aluminum coating was visible, in others, the rusted metal was exposed. Multiple cross sections were taken from the five red pennants and all revealed an identical stratigraphy (Figure 9). Some of the red hues veered darker even to the naked eye, especially those closer to the iron surface save for Layer 1 (Figure 9). Similar to the yellow and blue, SEM-EDS confirmed the presence of a steel rust layer followed by an aluminum paint coating. Raman analysis (Figure 10) proved crucial in analyzing the red pigments, where every layer contained one or more β-naphthol organic red pigments. This class of pigments first became available at the turn of the 20th century by coupling a substituted aniline ring with β-naphthol [40]. It is worth noting that assigning a particular shade to β-naphthol organic red pigments can be challenging because the presence of fillers, particle size, and method of manufacture all affect their final color [13]. These pigments also range in photosensitivity, and for some, white reduction from a deep shade using diluents, such as titanium white, can make them prone to fading, possibly due to catalytic effects also observed on other organic reds [28]. These pigments are sensitive to solvents, acids, bases, and some even to water.
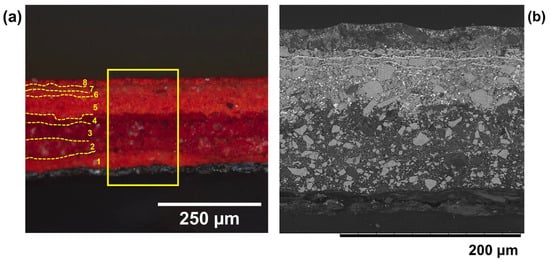
Figure 9.
Cross section imaging of a sample taken from one of the red pennants in Man-Eater. (a) Light microscope image at 20× magnification showing all 8 layers of paint and aluminum anticorrosive paint, and (b) BSE image of the cross section that shows the division between layers 5, 6, and 7 in particular. © 2021 MoMA, New York, NY, USA.
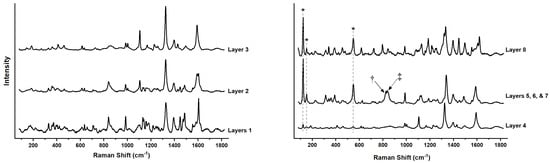
Figure 10.
Raman spectra of all 8 Layers of a cross section from one of the red pennants in Man-Eater. All layers contain one or more β-naphthol red and all peak positions are listed in the text. In addition, Layers 4, 5, 6, 7, and 8 contain red lead (*); and layers 5, 6, and 7 molybdate orange (†) at 825 cm−1 and chrome yellow (‡) at 839 cm−1.
In Layer 1, Parachlor Red (P.R. 6; C.I. 12090) was identified by Raman spectroscopy (163, 176, 219, 267, 331, 372, 394, 421, 465, 484, 533, 613, 635, 658, 710, 743, 763, 840, 861(sh), 985, 1091, 1103, 1132, 1140, 1158, 1180, 1218, 1243, 1265, 1291, 1326, 1392, 1447, 1474, 1485, 1555, 1565, 1584, 1605, 1623 (sh) cm−1). P.R. 6 was first synthesized in 1906 and was used until the late 1980s when it rapidly fell out of favor due to high solubility in organic solvents and mineral spirits as well as poor lightfastness [13]. Nevertheless, this pigment was used in air-drying alkyd systems, because deep shades without much white reduction are relatively lightfast [40]. In the context of Calder’s preference for matte paints, it is plausible that the original layer of paint was an alkyd one that contained P.R. 6. Chlorine Kα1 and Kα2 lines were obscured by Rh L-series lines and as such not discerned in XRF, and mapping with SEM-EDS only indicated that the paint was likely extended with silicates and/or magnesium silicates. Furthermore, the difficulty of detecting chlorine elementally is due to P.R. 6 having only one chlorine substituent. Layer 2 shows a mixture of P.R. 6 and Para Red (P.R. 1; C.I. 12070) in the Raman spectrum (185, 360, 410, 460, 1002, 1105, 1428, 1592 cm−1). The latter was the first synthetic organic red discovered in 1895 but has since lost its industrial significance. P.R. 1 has a dull brown hue, and is not fast against organic solvents, acids, bases, and even water; it is also prone to darken on exposure to light [40].
Layers 3 and 4 are similar in composition and could have been applied in two coats succession. They both also contain P.R. 1 according to their Raman spectra (185, 326, 360, 410, 461, 614, 632, 729, 986, 1002, 1105, 1167, 1201, 1228, 1257, 1324, 1396, 1428, 1496, 1592 cm−1), with SEM-EDS indicating the presence of a calcium-based filler in addition to silicates and/or magnesium silicates. However, Layer 4 also contains some red lead (Pb3O4) as evident by lattice bands at 121 (A1g), 151(E2g), and 547 (A1g) cm−1 [41] in addition to lead Mα and Mβ lines in SEM-EDS. Red lead (P.R. 105; C.I. 77518), one of the earliest pigments to be artificially made, has been used as a red pigment since as early as the 5th B.C. century in China [42]. Today, it is manufactured by heating lead oxides and is mostly used as a protective pigment to passivate iron and steel and inhibit corrosion [13]. The choice of a paint containing red lead might have seemed appropriate at the time of repainting because of evidence of rusting on the red pennants.
Layers 5, 6, and 7 are rather complex paint layers, with at least 4 pigments present, one organic and three inorganics, and could be three coats applied in one campaign. Layer 5 seems to be painted over a physically degraded Layer 4 since it fills several voids to form a jagged layer. The organic pigment, chlorinated para red (P.R. 4; C.I. 12085) is a positional isomer of P.R. 6 and has a yellowish red hue (313, 340, 358, 594, 624, 708, 719. 736, 769, 890, 986, 1096, 1117, 1125, 1188, 1296, 1337, 1396, 1451, 1487, 1555, 1587, 1617 cm−1). P.R. 4 has lost much of its commercial impact, as it is tinctorially weak and loses much of its light fastness in white reductions, while, in contrast, even full shades darken upon exposure to light [40]. The inorganics include red lead, chrome yellow—possibly the monoclinic form with few or no sulfates considering the position of the ν1(CrO42−) stretch at 839 cm−1—and molybdate orange (P.R. 104; C.I. 77605).
Molybdate orange is a solid solution of PbCrO4·PbMoO4·PbSO4, used mainly in paints, coatings, and colored plastics. It was first industrialized in 1934-35, and commercial pigments contain ~10% lead molybdate (PbMoO4) [38]. This pigment is often found in mixtures with chrome yellow to match the color of the orange basic lead chromate (PbCrO4·PbO), which is no longer of commercial importance. Molybdate orange is also found in mixtures with organic reds to give an extended color range and impart lightfastness and weather resistance onto a paint film [13]. Molybdenum was first identified in the paint stratigraphy through non-invasive XRF and confirmed by Raman spectroscopy with a ν1(CrO42−) symmetric stretch at 825 cm−1 presenting as a doublet with chrome yellow [43].
Layer 8 is a mixture of both red lead and toluidine red (P.R. 3; C.I. 12120). P.R. 3 is the most lightfast of the β-naphthol reds in deep shades and is manufactured and used on a large industrial scale. Primarily employed in air drying paints, it is the most important of the β-naphthol reds and was also used in printing inks, pastels, and watercolors [40]. Nevertheless, much as with the remaining β-naphthol reds, it suffered from a sensitivity to light and solvents and declined in popularity after the 1970s. SEM-EDS indicated Layer 8 to be extended with a calcium-based filler, silicates, and magnesium silicates. In comparison with Man-Eater, the red on the maquette appears to have been painted in two layers, a very thin preliminary one with a thicker coat of red on top. Unlike the blue and yellow pennants, the red paint was applied directly to the aluminum with no white layer. While Raman and SEM-EDS analysis indicated that they are similar in composition, the lower layer is visually more saturated in color. The pigments consisted of P.R. 4 and molybdate orange, the latter identified in the Raman spectrum by a ν1(CrO42−) stretch at 827 cm−1 and a ν1/ν2(CrO42−) bending multiplet at 341/359 cm−1. Mo was observed in the XRF spectrum using the Tracer 5i and confirms the presence of molybdate orange. The paint also included TiO2 in rutile form (449, 612 cm−1) and CaSO4 (1020 cm−1). The B1g mode at 144 is obscured due to a broad band at 155 ascribed to Pb–O lattice modes in lead chromate [44]. Similar to the rest of the primary colors on the maquette, the red pennant is different in shade and composition than in Man-Eater.
3.5. Black
The black paint from the lower pennants of Man-Eater showed two layers in the cross section, in addition to extensive rusting. The aluminum layer was not seen in the cross section but observed during treatment. This points to consistent surface preparation of the metal before applying any paint. Raman analysis of the layers proved difficult due to overwhelming fluorescence. Mapping with SEM-EDS was more fruitful here and confirmed XRF analysis, where Layer 1 was rich in Ca and P, which indicates the presence of bone black (P.Bk. 9; C.I. 77267). Bone black is defined as a carbonized product of collagen mixed with hydroxyapatite (Ca5(PO4)3), and the latter is the source of Ca and P in the SEM-EDS and XRF [45]. Layer 2 did not show the presence of these secondary constituents and can indicate a carbonaceous black not derived from bone matter. The black-painted shapes on the maquette also had only two layers. Fluorescence was not problematic in this instance, and Raman analysis of Layers 1 and 2 indicated the presence of a carbon-based black in both layers, with D and G bands at 1324 and 1597 cm−1, respectively [46]. The D band is indicative of disorder in the crystal structure of carbonaceous blacks, while the G band arises from C–C stretching [47]. While both layers are rich with large particles of Ca that were identified as CaCO3 by Raman spectroscopy (156, 238, 714, 1090 cm−1), only Layer 1 is rich in aluminosilicates based on SEM-EDS. Finally, Layer 2 interestingly showed the presence of Prussian blue by Raman spectroscopy with weak but readily identifiable 1Ag ν(CN) stretching vibration at 2160 cm−1 and the Eg ν(CN) stretching vibration at 2090 cm−1. Curiously, XRF spectra indicate that the rods were painted with a bone black, where hydroxyapatite was detected by the presence of phosphorous, unlike the pennants.
3.6. Paint Binder
Obtaining a sample of the original paint layer from Man-Eater proved difficult considering the many layers of overpaint. In all samples, the presence of both pigments and fillers obscured most peaks in the fingerprint region of the µ-FTIR spectrum, generally in the mid-IR from 1800 to 500 cm−1 [48]. Diagnostic C–O and C–C skeletal vibrations in the fingerprint region were obscured by the broad peaks of pigments such as ultramarine (1096 cm−1) and chrome yellow (851 and 819 cm−1) and fillers such as calcium sulfate (1620, 1425 (sh), 1148 (sh), 1120, 670 cm−1), calcium carbonate (1803, 1447, 1411, 879 cm−1), and barium sulfate (1186, 1124, 1074, 982 cm−1). All pigments and fillers seen in Raman analysis of the cross sections were confirmed by µ-FTIR but assigning them to a respective stratigraphy is not possible. However, samples from different representative areas were analyzed by transmission µ-FTIR in hopes of identifying any binding media from the now brittle paint.
A carbonyl (C=O) stretching band at 1734 cm−1 was detected in all µ-FTIR spectra from Man-Eater, with intensities varying from medium to very weak, exemplified here by a spectrum of a sample taken from the edge of the blue pennant (Figure 11). Detected across all spectra were C–H stretches at 2956 (sh), 2927, and 2855 cm−1. Blue was also identified in this sample by peaks at 2098 [ν(CN)] and 1415 cm−1, in addition to calcium sulfate (1620, 1425 (sh) 1148 (sh), 1120, 670 cm−1). An unidentified peak at 794 cm−1 could belong to a silicate, perhaps quartz, considering the presence of silicon in the EDS mapping. This composition matches the Raman composition of the first paint layer in the blue cross sections, with only Prussian blue. While it is difficult to make a conclusive assessment without more chromatographic techniques, the identified bands in these spectra point towards an alkyd resin paint [49]. Alkyd paints were manufactured for commercial use, such as household or industrial paints, and only Winsor & Newton continues to have a line of artist-grade alkyd paints [49,50]. Calder’s preference for these matte paints, especially those manufactured by Ronan, is well documented [8,9,14,39]. Most often, alkyd paints are polyester resin-based, made from combining polyhydric alcohol with a polybasic acid, to which monobasic drying oils are added, lending elasticity to an otherwise hard resin film. Here, the C=O band points to the polyester resin, as the fingerprint region is not particularly diagnostic for the drying oil; furthermore, oil C=O peaks are comparatively lower in absorption [49]. Conversely, the C–H bands probably belong to the drying oil component in alkyd paints, which can vary in concentration depending on the desired finish and drying time, among other factors [50]. Samples taken from the surface of the maquette are more representative of the binder in comparison with Man-Eater considering the absence of many layers of overpaint. The µ-FTIR spectra of the samples from the maquette showed the same C=O and C–H absorption bands as that of Man-Eater, indicating a possible alkyd binding medium as well.
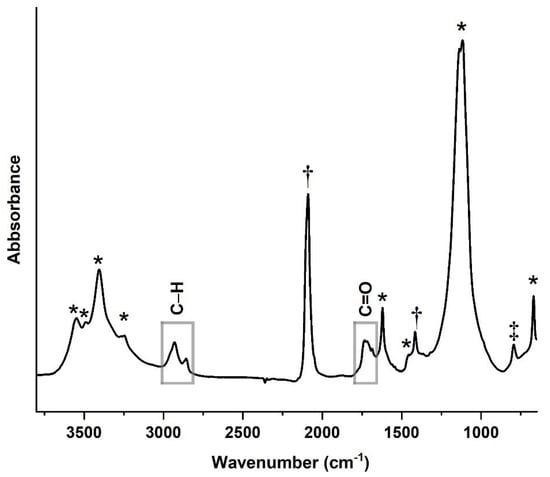
Figure 11.
A μ-FTIR spectrum acquired from a sample taken from the edge of the blue pennant in Man-Eater. The presence of Prussian blue as was detected in Raman spectroscopy in Layers 1A and 1B (†), and not ultramarine, possibly indicates this sample as original. A carbonyl (C=O) stretching band at 1734 cm−1 and C–H stretches at 2956 (sh), 2927, and 2855 cm−1 point towards an alkyd binder, but any remaining diagnostic peaks are obscured by the presence of CaSO4 (*) and a silicate (‡).
4. Conclusions
For MoMA’s 2021 exhibition, Alexander Calder: Modern from the Start, the possibility arose for reviving Man-Eater with Pennants, a forgotten sculpture by the artist. The sculpture had been repainted several times early in its history; however, after its last exhibition in 1969–1970, extensive paint loss and rusting, due to outdoor exposure, were not addressed. As part of planning the restoration treatment for Man-Eater, cross sections and samples were taken for further analysis by Raman spectroscopy, SEM-EDS, and XRF to determine the pigments present and aid in the identification of the artist’s original color choices. The original paint layers were identified as the ones closest to an aluminum anti-corrosive layer or those penetrated by drastic rusting of the metal. Through these analyses, we can establish an early primary palette for Calder: a darker blue based on Prussian blue, a brown-red based on P.R. 6, and a lemon yellow based on a sulfate-containing chrome yellow. Among the many layers of overpaint, the ones last applied can be dated to no later than the 1970’s due to the presence of the Nd3+ rich titanium white, which seems plausible considering that the work has not been exhibited since 1970. Analysis of the associated maquette confirmed the later painting date as described in internal records based on the presence of the Nd3+ rich titanium white as a completely different palette consisting of ultramarine blue, a chrome yellow, and the yellowish-red P.R. 4 in combination with molybdate orange. Considering that the maquette was painted between 1959 and 1969 and does not appear to have been repainted since, the colors do not match those of the original paints in Man-Eater. To our knowledge, this is the first analysis of his early primary palette in the literature and presents a unique path forward for other treatments of works of Calder from this period held at other institutions and private collections worldwide.
As part of the treatment undertaken by Monumenta Art Conservation and Finishing LLC, the aged paint layers and surface rust were removed before applying an anti-corrosion primer, followed by epoxy paint base coats. The analysis of Man-Eater and the maquette helped determine the tonality of yellow, blue, and red used in the restoration campaign. Additionally, it was important to note the presence of toxic pigments, such as red lead, from a safety perspective. Most significantly, understanding the differences in pigment composition between the maquette and final sculpture prevented the use of inaccurate colors, as was done in other instances in the past [14]. The final color coats were applied by spray and brush. Now, half a century after its last exhibition, Man-Eater with Pennants is newly conserved and installed, ready to delight and charm visitors to MoMA’s sculpture garden.
Author Contributions
A.H. carried out some sampling in addition to Raman Spectroscopy, µ-FTIR, SEM-EDS, microscopy, XRF data interpretation, and drafting this manuscript. M.R. carried out sampling for cross sections in addition to XRF. spectroscopy and digital microscopy. L.Z. carried out art historical research, mined MoMA’s internal records, and drafted sections of the manuscript. A.M. carried out XRF interpretation and revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on request.
Acknowledgments
The authors are grateful to our partners in the restoration of Man-Eater with Pennants: Cara Manes, Associate Curator in the Department of Painting and Sculpture, The Museum of Modern Art, Abigail Mack and Ellen Rand at Monumenta Art Conservation and Finishing LLC, and Alexander S. C. Rower, President of the Calder Foundation, New York. We are also grateful for instrumental access by the Conservation Center at the Institute of Fine Arts, New York University. Finally, this project was possible with generous support from the Bank of America.
Conflicts of Interest
The opinions: findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the Museum of Modern Art. The authors declare no competing financial interest.
References and Notes
- Toll, S.I. My Way: Calder in Paris. Sewanee Rev. 2010, 118, 589–602. [Google Scholar] [CrossRef]
- Morris, G.L.K.; De Kooning, W.; Calder, A.; Glarner, F.; Motherwell, R.; Davis, S. What Abstract Art Means to Me. Bull. Mus. Mod. Art. 1951, 18, 15. [Google Scholar] [CrossRef]
- Canning Miller, D. Letter to Sweeney, J.J., then curator at The Museum of Modern Art, New York. The Museum of Modern Art, Department of Painting and Sculpture files. 1945.
- Goodwin, P. Letter to Sweeney, J.J., then curator at The Museum of Modern Art, New York. The Museum of Modern Art, Department of Painting and Sculpture files. 1945.
- Sweeney, J.J. Letter to Goodwin, P., then curator at The Museum of Modern Art, New York. The Museum of Modern Art, Department of Painting and Sculpture files. 1945.
- Sweeney, J.J. Letter to Canning Miller, D., then curator at The Museum of Modern Art, New York. The Museum of Modern Art, Department of Painting and Sculpture files. 1945.
- Canning Miller, D. Letter to Calder, A., The Museum of Modern Art, Department of Painting and Sculpture files. 1945.
- Calder, A. Internal museum correspondence with the artist. undated.
- Pozzi, F.; Arslanoglu, J.; Nagy, E. Alexander Calder’s Half-Circle, Quarter-Circle, and Sphere (1932): A complex history of re-painting unraveled. Herit. Sci. 2020, 8, 79. [Google Scholar] [CrossRef]
- Price, B.A.; Pretzel, B.; Lomax, S. (Eds.) Infrared and Raman Users Group Spectral Database, 2007 ed.; IRUG: Philadelphia, PA, USA, 2009; Volume 1–2. [Google Scholar]
- Fremout, W.; Saverwyns, S. Identification of synthetic organic pigments: The role of a comprehensive digital Raman spectral library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Bell, I.M.; Clark, R.J.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (pre- ≈ 1850 AD). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Lewis, P.A. (Ed.) Pigment Handbook, 2nd ed.; Properties and Economics; Wiley-Interscience: New York, NY, USA, 1988; Volume 1. [Google Scholar]
- Lodge, R.G.; Lodge, E.W. Notes for a History of “Calder Red” Color and Its Paints in the United States in Relation to the Re-coatings of Alexander Calder’s Flamingo (1973) and La Grande Vitesse (1969) and Other Calder Stabiles. 32.
- Zycherman, L.; Correspondence with the Calder Foundation, New York, NY, USA. Personal communication, 2021.
- Telephony. Teleph. Am. Teleph. J. 1911, 60, 12.
- Liu, B.; Wei, J.; Yang, M.; Yin, F.; Xu, K. Effect of heat treatment on the mechanical properties of copper clad steel plates. Vacuum 2018, 154, 250–258. [Google Scholar] [CrossRef]
- Kuhn, H.; Curran, M. Chrome Yellow and Other Chromate Pigments. In Artists Pigments Handbook: Their History and Characteristics; Feller, R.L., Ed.; Cambridge University Press: New York, NY, USA, 1987; Volume 1. [Google Scholar]
- Monico, L.; Janssens, K.; Hendriks, E.; Brunetti, B.G.; Miliani, C. Raman study of different crystalline forms of PbCrO4 and PbCr1−xSxO4 solid solutions for the noninvasive identification of chrome yellows in paintings: A focus on works by Vin-cent van Gogh. J. Raman Spectrosc. 2014, 45, 1034–1045. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N. Raman spectra of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Rogge, C.E.; Arslanoglu, J. Luminescence of coprecipitated titanium white pigments: Implications for dating modern art. Sci. Adv. 2019, 5, eaav0679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memo, N.J. to Houlihan, P., Conservator. The Museum of Modern Art, Department of Painting and Sculpture files. 1970.
- Miliani, C.; Monico, L.; Melo, M.J.; Fantacci, S.; Angelin, E.M.; Romani, A.; Janssens, K. Photochemistry of Artists’ Dyes and Pigments: To-wards Better Understanding and Prevention of Colour Change in Works of Art. Angew. Chem. Int. Ed. 2018, 57, 7324–7334. [Google Scholar] [CrossRef] [PubMed]
- Mass, J.L.; Opila, R.; Buckley, B.; Cotte, M.; Church, J.; Mehta, A. The photodegradation of cadmium yellow paints in Henri Ma-tisse’s Le Bonheur de vivre (1905–1906). Appl. Phys. A 2013, 111, 59–68. [Google Scholar] [CrossRef]
- Anaf, W.; Trashin, S.; Schalm, O.; van Dorp, D.; Janssens, K.; De Wael, K. Electrochemical Photodegradation Study of Semiconduc-tor Pigments: Influence of Environmental Parameters. Anal Chem. Am. Chem. Soc. 2014, 86, 9742–9748. [Google Scholar]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Pagnin, L.; Calvini, R.; Wiesinger, R.; Weber, J.; Schreiner, M. Photodegradation Kinetics of Alkyd Paints: The Influence of Vary-ing Amounts of Inorganic Pigments on the Stability of the Synthetic Binder. Front. Mater. 2020, 7, 423. [Google Scholar] [CrossRef]
- Van Driel, B.A. Titanium White, Friend or Foe?: Understanding and Predicting Photocatalytic Degradation of Modern Oil Paintings. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2018. [Google Scholar]
- van Driel, B.A.; Wezendonk, T.A.; van den Berg, K.J.; Kooyman, P.J.; Gascon, J.; Dik, J. Determination of early warning signs for photocatalytic degradation of titanium white oil paints by means of surface analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 172, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrie, B. Prussian Blue. In Artists Pigments Handbook: Their History and Characteristics; West FitzHugh, E., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3. [Google Scholar]
- Moretti, G.; Gervais, C. Raman spectroscopy of the photosensitive pigment Prussian blue. J. Raman Spectrosc. 2018, 49, 1198–1204. [Google Scholar] [CrossRef]
- Amat, A.; Rosi, F.; Miliani, C.; Sassi, P.; Paolantoni, M.; Fantacci, S. A combined theoretical and experimental investigation of the electronic and vibrational properties of red lead pigment. J. Cult. Herit. 2020, 46, 374–381. [Google Scholar] [CrossRef]
- Kirby, K.; Saunders, D. Fading and Colour Change of Prussian Blue: Methods and Manufacture and the Influence of Extenders. Natl. Gallery Tech. Bull. 2004, 25, 73–91. [Google Scholar]
- Plesters, J. Ultramarine Blue, Natural and Artificial. In Artists Pigments Handbook: Their History and Characteristics; Roy, A., Ed.; Cambridge University Press: New York, NY, USA, 1993; Volume 2. [Google Scholar]
- Rejmak, P. Structural, Optical, and Magnetic Properties of Ultramarine Pigments: A DFT Insight. J. Phys. Chem. C 2018, 12, 29338–29349. [Google Scholar] [CrossRef]
- Osticioli, I.; Mendes, N.; Nevin, A.; Gil, F.; Becucci, M.; Castellucci, E. Analysis of natural and artificial ultramarine blue pigments using laser induced breakdown and pulsed Raman spectroscopy, statistical analysis and light microscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laver, M. Titanium Dioxide Whites. In Artists Pigments Handbook: Their History and Characteristics; FitzHugh, E.W., Ed.; National Gallery of Art: Washington, DC, USA, 1997; Volume 3. [Google Scholar]
- Buxbaum, G.; Pfaff, G. (Eds.) Industrial Inorganic Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Schmelzer, P. The Problem of Paint. Sightlines. 2011. Available online: https://walkerart.org/magazine/the-problem-of-paint (accessed on 19 May 2021).
- Herbst, W.; Hunger, K.; Wilker, G. Industrial Organic Pigments: Production, Properties, Applications, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Burgio, L.; Clark, R.J.H.; Firth, S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analysts 2001, 126, 222–227. [Google Scholar] [CrossRef] [PubMed]
- FitzHugh, E.W. Red Lead and Minium. In Artists Pigments Handbook: Their History and Characteristics; Feller, R.L., Ed.; Cambridge University Press: New York, NY, USA, 1987; Volume 1. [Google Scholar]
- Chua, L.; Hoevel, C.; Smith, G.D. Characterization of Haku Maki prints from the “Poem” series using light-based techniques. Herit. Sci. 2016, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, R.W.T. The Raman spectrum of crocoite. Miner. Mag. 1971, 38, 249–250. [Google Scholar] [CrossRef]
- van Loon, A.; Boon, J.J. Characterization of the deterioration of bone black in the 17th century Oranjezaal paintings using electron-microscopic and micro-spectroscopic imaging techniques. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 1601–1609. [Google Scholar] [CrossRef]
- Daly, N.S.; Sullivan, M.; Lee, L.; Trentelman, K. Multivariate analysis of Raman spectra of carbonaceous black drawing media for the in situ identification of historic artist materials. J. Raman Spectrosc. 2018, 49, 1497–1506. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Halac, E.B.; Reinoso, M.; Di Liscia, E.J.; Maier, M.S. Micro-Raman spectroscopy of carbon-based black pigments. J. Raman Spectrosc. 2012, 43, 1671–1675. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Learner, T.; Institute, G.C. Analysis of Modern Paints; Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Standeven, H.A.L. House Paints, 1900–1960, History and Use; Getty Conservation Institute: Los Angeles, CA, USA, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).