Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Visible Light Micro-Reflectance Spectroscopy Analysis
2.3. Raman and SERS Analysis
3. Results
3.1. Visible Light Micro-Reflectance Spectroscopy Analysis
3.2. Raman and SERS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahapedia. Available online: https://www.sahapedia.org/ (accessed on 29 June 2021).
- Autiero, S. Danzando Nella Luce. Il Teatro Delle Ombre Nell’India Meridionale. In Il Principe e la sua Ombra; National Museum of Oriental Art “G. Tucci”: Roma, Italy, 2013; p. 41. [Google Scholar]
- Chandra Sekhar, A. A Selected Crafts of Andhra Pradesh. In Census of India 1961; The Government Press: New Delhi, India, 1961. [Google Scholar]
- Sarma, D.; Homen, A. Storytelling and Puppet Traditions of India; Indira Gandhi National Centre for the Arts: New Delhi, India, 2010. [Google Scholar]
- Sorensen, N. Tolu Bommalu Kattu: Shadow Theater. J. South Asian Nat. His. 1975, 10, 1–19. [Google Scholar]
- Collins, M.; Buckely, M.; Grundy, H.; Thomas Oates, J.; Wilson, J.; van Doorn, N. ZooMS: The Collagen Barcode and Fingerprints. Spectrosc. Eur. 2010, 22, 6–10. [Google Scholar]
- Campo, G.; Bagan, R.; Oriols, N. Identificaciò de Fibres: Suports Tèxtils de Pintures; Genelaitat de Catalunya, Departament de Cultura i Mitjans de Comunicació: Barcelona, Spain, 2009. [Google Scholar]
- Ollendorf, A.L.; Mulholland, S.C.; Rapp, G.J. Phytolith Analysis as a Means of Plant Identification: Arundo Donax and Phragmites Communis. Ann. Bot. 1988, 61, 209–214. [Google Scholar] [CrossRef]
- Cosentino, A. FORS Spectral Database of Historical Pigments in Different Binders. E Conserv. J. 2015, 54–65. [Google Scholar] [CrossRef]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Idone, A.; Gulmini, M.; Picollo, M.; Ricciardi, P.; Delaney, J.K. Characterisation of Colourants on Illuminated Manuscripts by Portable Fibre Optic UV-Visible-NIR Reflectance Spectrophotometry. Anal. Methods 2014, 6, 1488–1500. [Google Scholar] [CrossRef]
- Montagner, C.; Bacci, M.; Bracci, S.; Freeman, R.; Picollo, M. Library of UV-Vis-NIR Reflectance Spectra of Modern Organic Dyes from Historic Pattern-Card Coloured Papers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1669–1680. [Google Scholar] [CrossRef]
- Bacci, M.; Picollo, M.; Trumpy, G.; Tsukada, M.; Kunzelman, D. Non-Invasive Identification of White Pigments on 20Th-Century Oil Paintings by Using Fiber Optic Reflectance Spectroscopy. J. Am. Inst. Conserv. 2007, 46, 27–37. [Google Scholar] [CrossRef]
- Pozzi, F.; Leona, M. Surface-Enhanced Raman Spectroscopy in Art and Archaeology. J. Raman Spectrosc. 2016, 47, 67–77. [Google Scholar] [CrossRef]
- Cesaratto, A.; Leona, M.; Pozzi, F. Recent Advances on the Analysis of Polychrome Works of Art: SERS of Synthetic Colorants and Their Mixtures with Natural Dyes. Front. Chem. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Leona, M.; Vo-Dinh, K.C.; Yan, F.; Wabuyele, M.B.; Vo-Dinh, T. Application of Surface-Enhanced Raman Scattering (SERS) for the Identification of Anthraquinone Dyes Used in Works of Art. J. Raman Spectrosc. 2006, 37, 520–527. [Google Scholar] [CrossRef]
- Candela, R.G.; Lombardi, L.; Ciccola, A.; Serafini, I.; Bianco, A.; Postorino, P.; Pellegrino, L.; Bruno, M. Deepening inside the Pictorial Layers of Etruscan Sarcophagus of Hasti Afunei: An Innovative Micro-Sampling Technique for Raman/SERS Analyses. Molecules 2019, 24, 3403. [Google Scholar] [CrossRef]
- Ciccola, A.; Serafini, I.; Ripanti, F.; Vincenti, F.; Coletti, F.; Bianco, A.; Fasolato, C.; Montesano, C.; Galli, M.; Curini, R.; et al. Dyes from the Ashes: Discovering and Characterizing Natural Dyes from Mineralized Textiles. Molecules 2020, 25, 1417. [Google Scholar] [CrossRef]
- Germinario, G.; Ciccola, A.; Serafini, I.; Ruggiero, L.; Sbroscia, M.; Vincenti, F.; Fasolato, C.; Curini, R.; Ioele, M.; Postorino, P.; et al. Gel Substrates and Ammonia-EDTA Extraction Solution: A New Non-Destructive Combined Appraoch Useful for the Identification of Anthraquinone Dyes from Wool Textiles. Microchem. J. 2020, 155, 104780. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Guineau, B.; Guichard, V. Identification des colorants organiques naturels par microspectrometrie Raman de resonance et par effet Raman exalte de surface (SERS). In ICOM Committee for Conservation, 8th Triennial Meeting, Sydney, Australia, 6–11 September 1987: Preprints; ICOM: Paris, France, 1987; pp. 659–666. [Google Scholar]
- Leona, M.; Londero, P.S.; Lombardi, J.R. 10 Years of Surface-Enhanced Raman Spectroscopy in Art and Archaeology. Microsc. Microanal. 2014, 20 (Suppl. 3), 2006–2007. [Google Scholar] [CrossRef][Green Version]
- Serafini, I.; Ciccola, A. Nanotechnologies and Nanomaterials: An Overview for Cultural Heritage. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Lazzara, G., Fakhrulli, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–380. [Google Scholar]
- Bruni, S.; De Luca, E.; Guglielmi, V.; Pozzi, F. Identification of Natural Dyes on Laboratory-Dyed Wool and Ancient Wool, Silk, and Cotton Fibers Using Attenuated Total Reflection (ATR) Fourier Transform Infrared (FT-IR) Spectroscopy and Fourier Transform Raman Spectroscopy. Appl. Spectrosc. 2011, 65, 1017–1023. [Google Scholar] [CrossRef]
- Bruni, S.; Guglielmi, V.; Pozzi, F.; Mercuri, A.M. Surface-Enhanced Raman Spectroscopy (SERS) on Silver Colloids for the Identification of Ancient Textile Dyes. Part II: Pomegranate and Sumac. J. Raman Spectrosc. 2011, 42, 465–473. [Google Scholar] [CrossRef]
- Bruni, S.; Guglielmi, V.; Pozzi, F. Historical Organic Dyes: A Surface-Enhanced Raman Scattering (SERS) Spectral Database on Ag Lee-Meisel Colloids Aggregated by NaClO4. J. Raman Spectrosc. 2011, 42, 1267–1281. [Google Scholar] [CrossRef]
- Bruni, S.; Guglielmi, V.; Pozzi, F. Surface-Enhanced Raman Spectroscopy (SERS) on Silver Colloids for the Identification of Ancient Textile Dyes: Tyrian Purple and Madder. J. Raman Spectrosc. 2010, 41, 175–180. [Google Scholar] [CrossRef]
- Leona, M. Microanalysis of Organic Pigments and Glazes in Polychrome Works of Art by Surface-Enhanced Resonance Raman Scattering. Proc. Natl. Acad. Sci. USA 2009, 106, 14757–14762. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.S.; Feng, Z. Simple Preparation Method for Silver SERS Substrate by Reduction of AgNO3 on Copper Foil. Appl. Spectrosc. 2002, 56, 300–305. [Google Scholar] [CrossRef]
- Platania, E.; Lofrumento, C.; Lottini, E.; Azzaro, E.; Ricci, M.; Becucci, M. Tailored Micro-Extraction Method for Raman/SERS Detection of Indigoids in Ancient Textiles. Anal. Bioanal. Chem. 2015, 407, 6505–6514. [Google Scholar] [CrossRef]
- Platania, E.; Lombardi, J.R.; Leona, M.; Shibayama, N.; Lofrumento, C.; Ricci, M.; Becucci, M.; Castellucci, E. Suitability of Ag-Agar Gel for the Microextraction of Organic Dyes on Different Substrates: The Case Study of Wool, Silk, Printed Cotton and a Panel Painting Mock-Up. J. Raman Spectrosc. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Ricci, M.; Lofrumento, C.; Castellucci, E.; Becucci, M. Microanalysis of Organic Pigments in Ancient Textiles by Surface-Enhanced Raman Scattering on Agar Gel Matrices. J. Spectrosc. 2016, 2016. [Google Scholar] [CrossRef]
- Lofrumento, C.; Ricci, M.; Platania, E.; Becucci, M.; Castellucci, E. SERS Detection of Red Organic Dyes in Ag-Agar Gel. J. Raman Spectrosc. 2013, 44, 47–54. [Google Scholar] [CrossRef]
- Leona, M.; Decuzzi, P.; Kubic, T.A.; Gates, G.; Lombardi, J.R. Nondestructive Identification of Natural and Synthetic Organic Colorants in Works of Art by Surface Enhanced Raman Scattering. Anal. Chem. 2011, 83, 3990–3993. [Google Scholar] [CrossRef]
- Doherty, B.; Brunetti, B.G.; Sgamellotti, A.; Miliani, C.A. Detachable SERS Active Cellulose Film: A Minimally Invasive Approach to the Study of Painting Lakes. J. Raman Spectrosc. 2011, 42, 1932–1938. [Google Scholar] [CrossRef]
- Pozzi, F.; Van Den Berg, K.J.; Fiedler, I.; Casadio, F.A. Systematic Analysis of Red Lake Pigments in French Impressionist and Post-Impressionist Paintings by Surface-Enhanced Raman Spectroscopy (SERS). J. Raman Spectrosc. 2014, 45, 1119–1126. [Google Scholar] [CrossRef]
- Calà, E.; Benzi, M.; Gosetti, F.; Zanin, A.; Gulmini, M.; Idone, A.; Serafini, I.; Ciccola, A.; Curini, R.; Whitworth, I.; et al. Towards the Identification of the Lichen Species in Historical Orchil Dyes by HPLC-MS/MS. Microchem. J. 2019, 150, 104140. [Google Scholar] [CrossRef]
- Castro, R.; Pozzi, F.; Leona, M.; Melo, M.J. Combining SERS and Microspectrofluorimetry with Historically Accurate Reconstructions for the Characterization of Lac Dye Paints in Medieval Manuscript Illuminations. J. Raman Spectrosc. 2014, 45, 1172–1179. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Reagan, D.A.; Lombardi, J.R.; Leona, M. TLC-SERS of Mauve, the First Synthetic Dye. J. Raman Spectrosc. 2014, 45, 1147–1152. [Google Scholar] [CrossRef]
- Ciccola, A.; Tozzi, L.; Romani, M.; Serafini, I.; Ripanti, F.; Curini, R.; Vitucci, F.; Cestelli Guidi, M.; Postorino, P. Lucio Fontana and the Light: Spectroscopic Analysis of the Artist’s Collection at the National Gallery of Modern and Contemporary Art. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 1–8. [Google Scholar] [CrossRef]
- Pérez-Arantegui, J.; Rupérez, D.; Almazán, D.; Díez-de-Pinos, N. Colours and Pigments in Late Ukiyo-e Art Works: A Preliminary Non-Invasive Study of Japanese Woodblock Prints to Interpret Hyperspectral Images Using in-Situ Point-by-Point Diffuse Reflectance Spectroscopy. Microchem. J. 2018, 139, 94–109. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Sessa, C.; Weiss, R.; Niessner, R.; Ivleva, N.P.; Stege, H. Towards a Surface Enhanced Raman Scattering (SERS) Spectra Database for Synthetic Organic Colourants in Cultural Heritage. The Effect of Using Different Metal Substrates on the Spectra. Microchem. J. 2018, 138, 209–225. [Google Scholar] [CrossRef]
- Centeno, S.A.; Hale, C.; Carò, F.; Cesaratto, A.; Shibayama, N.; Delaney, J.; Dooley, K.; van der Snickt, G.; Janssens, K.; Stein, S.A. Van Gogh’s Irises and Roses: The Contribution of Chemical Analyses and Imaging to the Assessment of Color Changes in the Red Lake Pigments. Herit. Sci. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Schneider, S.; Brehm, G.; Freunscht, P. Comparison of Surface-Enhanced Raman and Hyper-Raman Spectra of the Triphenylmethane Dyes Crystal Violet and Malachite Green. Phys. Status Solidi 1995, 189, 37–42. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Chenal, C.; Birke, R.L.; Lombardi, J.R. DFT, SERS, and Single-Molecule SERS of Crystal Violet. J. Phys. Chem. C 2008, 112, 20295–20300. [Google Scholar] [CrossRef]
- Gühlke, M.; Heiner, Z.; Kneipp, J. Surface-Enhanced Hyper-Raman and Raman Hyperspectral Mapping. Phys. Chem. Chem. Phys. 2016, 18, 14228–14233. [Google Scholar] [CrossRef]
- Greeneltch, N.G.; Davis, A.S.; Valley, N.A.; Casadio, F.; Schatz, G.C.; Van Duyne, R.P.; Shah, N.C. Near-Infrared Surface-Enhanced Raman Spectroscopy (NIR-SERS) for the Identification of Eosin Y: Theoretical Calculations and Evaluation of Two Different Nanoplasmonic Substrates. J. Phys. Chem. A 2012, 116, 11863–11869. [Google Scholar] [CrossRef]
- Narayanan, V.A.; Stokes, D.L.; Vo-Dinh, T. Vibrational Sprectral Analysis of Eosin Y and Erytheosin B—Intensity Studies for Quantiative Detection by Dyes. J. Raman Spectrosc. 1994, 24, 415–422. [Google Scholar] [CrossRef]
- Michaels, A.M.; Nirmal, M.; Brus, L.E. Surface Enhanced Raman Spectroscopy of Individual Rhodamine 6G Molecules on Large Ag Nanocrystals. J. Am. Chem. Soc. 1999, 121, 9932–9939. [Google Scholar] [CrossRef]
- Sun, C.H.; Wang, M.L.; Feng, Q.; Liu, W.; Xu, C.X. Surface-Enhanced Raman Scattering (SERS) Study on Rhodamine B Adsorbed on Different Substrates. Russ. J. Phys. Chem. A 2015, 89, 291–296. [Google Scholar] [CrossRef]
- Zhao, Y.; Yamaguchi, Y.; Liu, C.; Li, M.; Dou, X. Rapid and Quantitative Detection of Trace Sudan Black B in Dyed Black Rice by Surface-Enhanced Raman Spectroscopy (SERS). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 216, 202–206. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Ng, K.; Gueissaz, L.; Leona, M.; Lombardi, J.R. Raman and SERS Characterization of Solvent Dyes: An Example of Shoe Polish Analysis. Dye. Pigment. 2017, 137, 539–552. [Google Scholar] [CrossRef]
- Whitney, A.V.; Van Duyne, R.P.; Casadio, F. An Innovative Surface-Enhanced Raman Spectroscopy (SERS) Method for the Identification of Six Historical Red Lakes and Dyestuffs. J. Raman Spectrosc. 2006, 37, 993–1002. [Google Scholar] [CrossRef]
- Cesaratto, A.; Lombardi, J.R.; Leona, M. Tracking Photo-Degradation of Triarylmethane Dyes with Surface-Enhanced Raman Spectroscopy. J. Raman Spectrosc. 2017, 48, 418–424. [Google Scholar] [CrossRef]
- Geiman, I.; Leona, M.; Lombardi, J.R. Application of Raman Spectroscopy and Surface-Enhanced Raman Scattering to the Analysis of Synthetic Dyes Found in Ballpoint Pen Inks. J. Forensic Sci. 2009, 54, 947–952. [Google Scholar] [CrossRef]
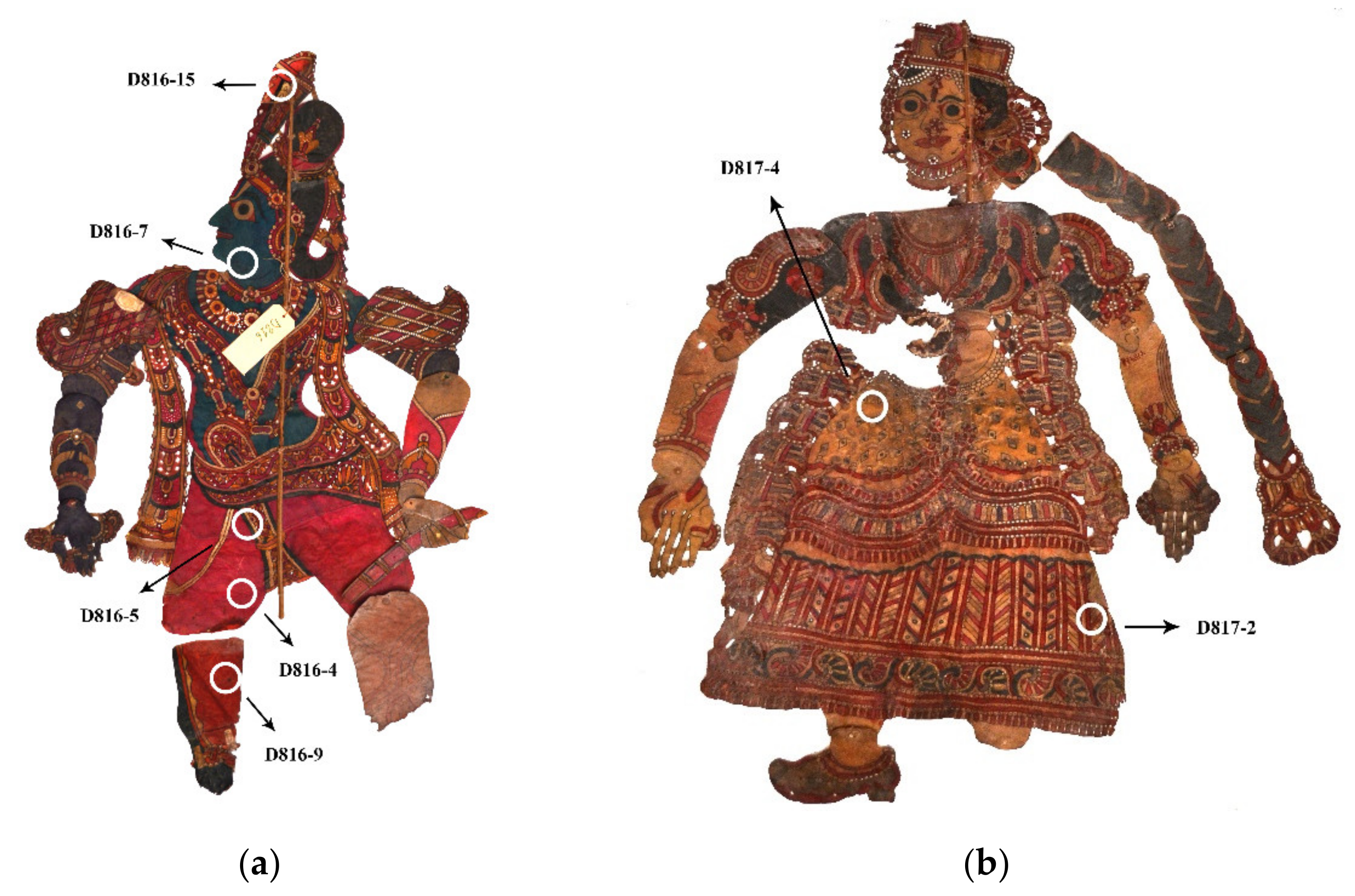


| Sample | Color of the Sampled Area | Appearance of Sample on the Stubbon | Apparent Absorption Bands (nm) | SERS Peaks (cm−1) | Dye Assignment |
|---|---|---|---|---|---|
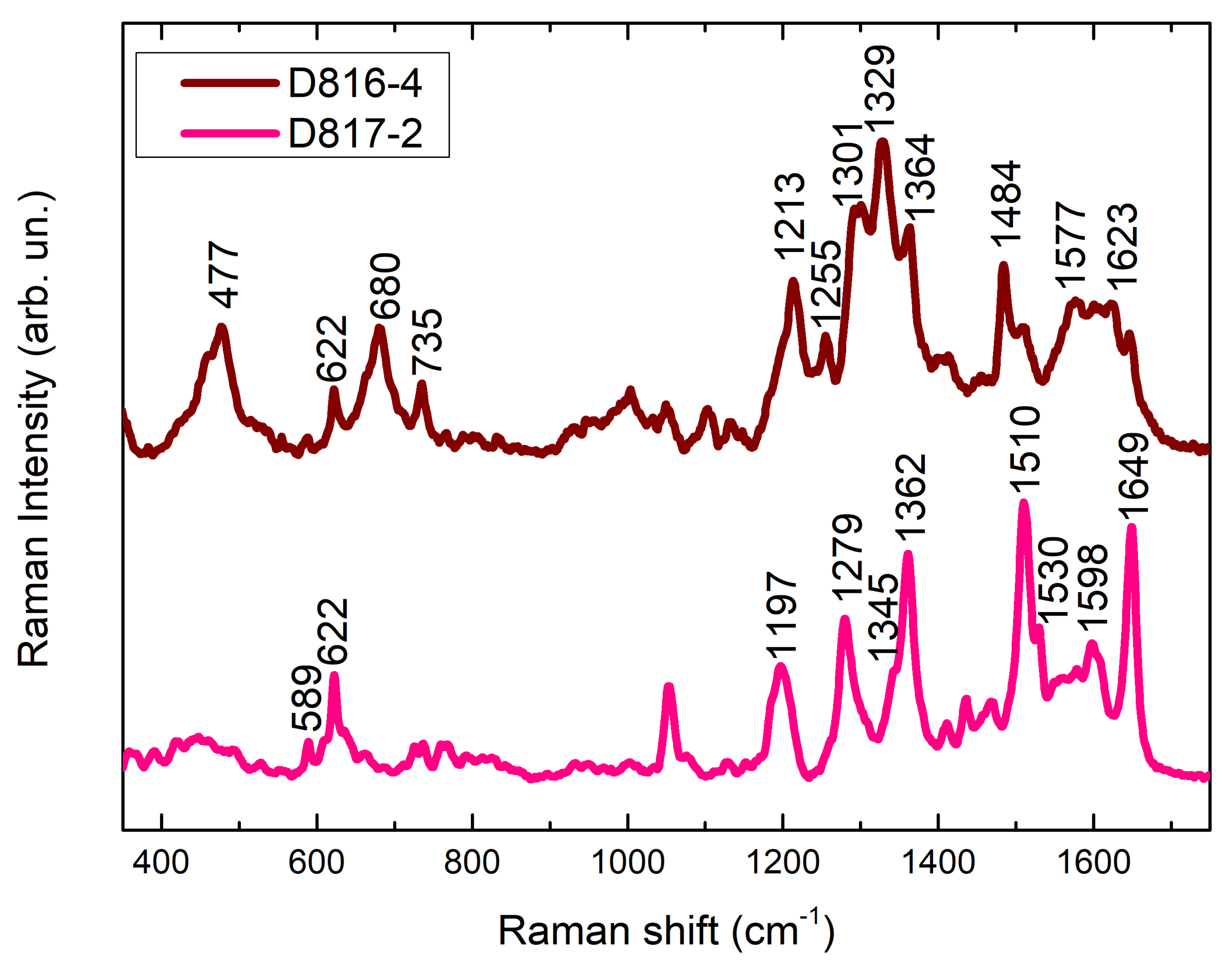
| D816-4 | Pinkish red | Red stain | 501, 550 | 622, 680, 735, 1364, 1436, 1509, 1565, 1582, 1601, 1646 (Rhodamine B); 637, 714, 1329, 1452, 1577, 1623 (Eosin Y) | Rhodamine B, Eosin Y |
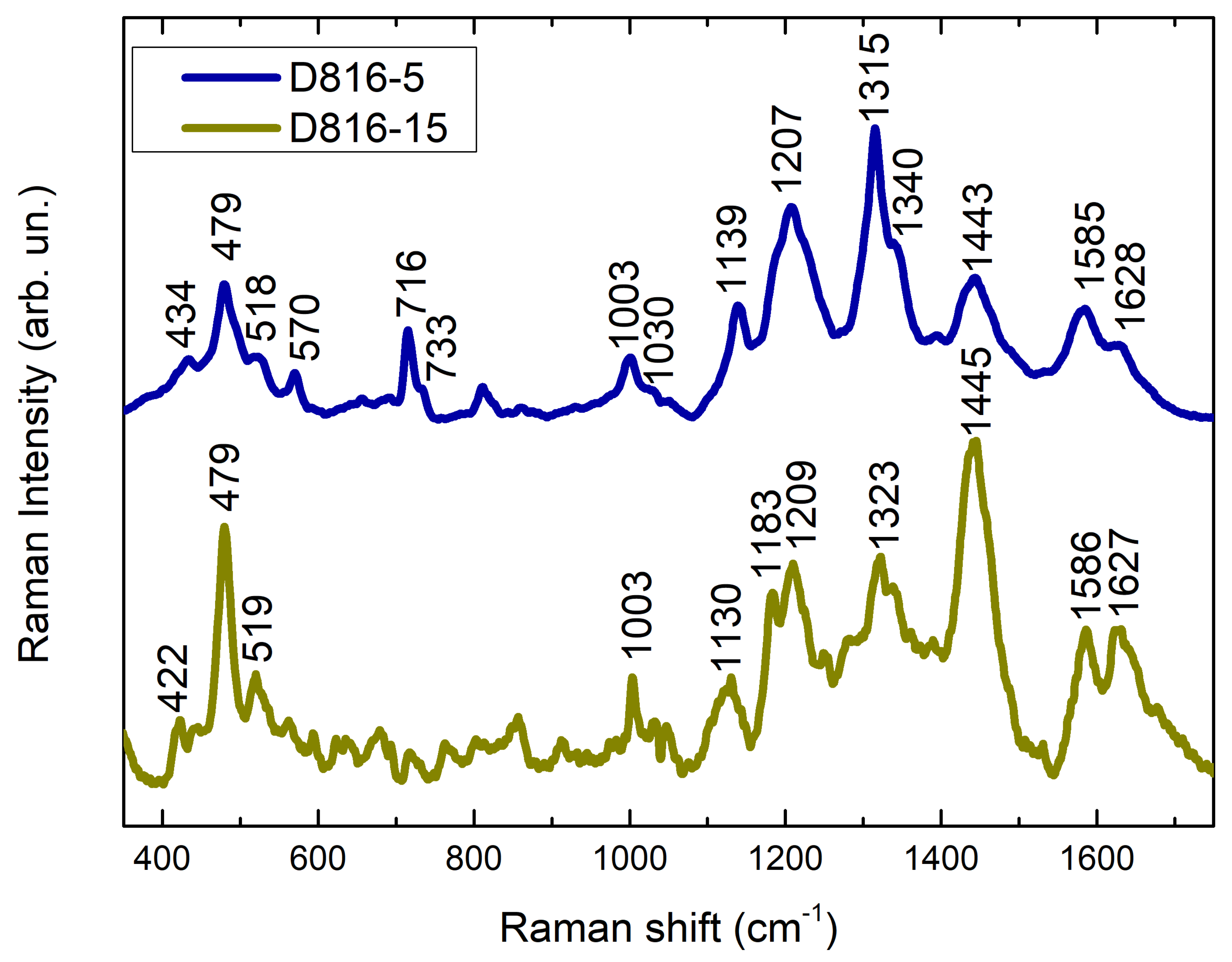
| D816-5 | Black | Black spot | No main feature | See Discussion | Mixture of dyes (?) |
| D816-7 | Blue-green | Black-greenish particles | 640 | 1221, 1364, 1399, 1429 (Malachite Green); 732, 761, 1476, 1530 (Crystal or Methyl Violet); further intense peaks are present but common to both the dyes (see Discussion) | Malachite Green, Crystal (or Methyl) Violet |
| D816-9 | Pinkish red | Red stain | 510, 550 | 479, 1184, 1623 | Eosin Y (?) |
| D816-15 | Black | Black spot | No main feature | See Discussion | Mixture of dyes (?) |
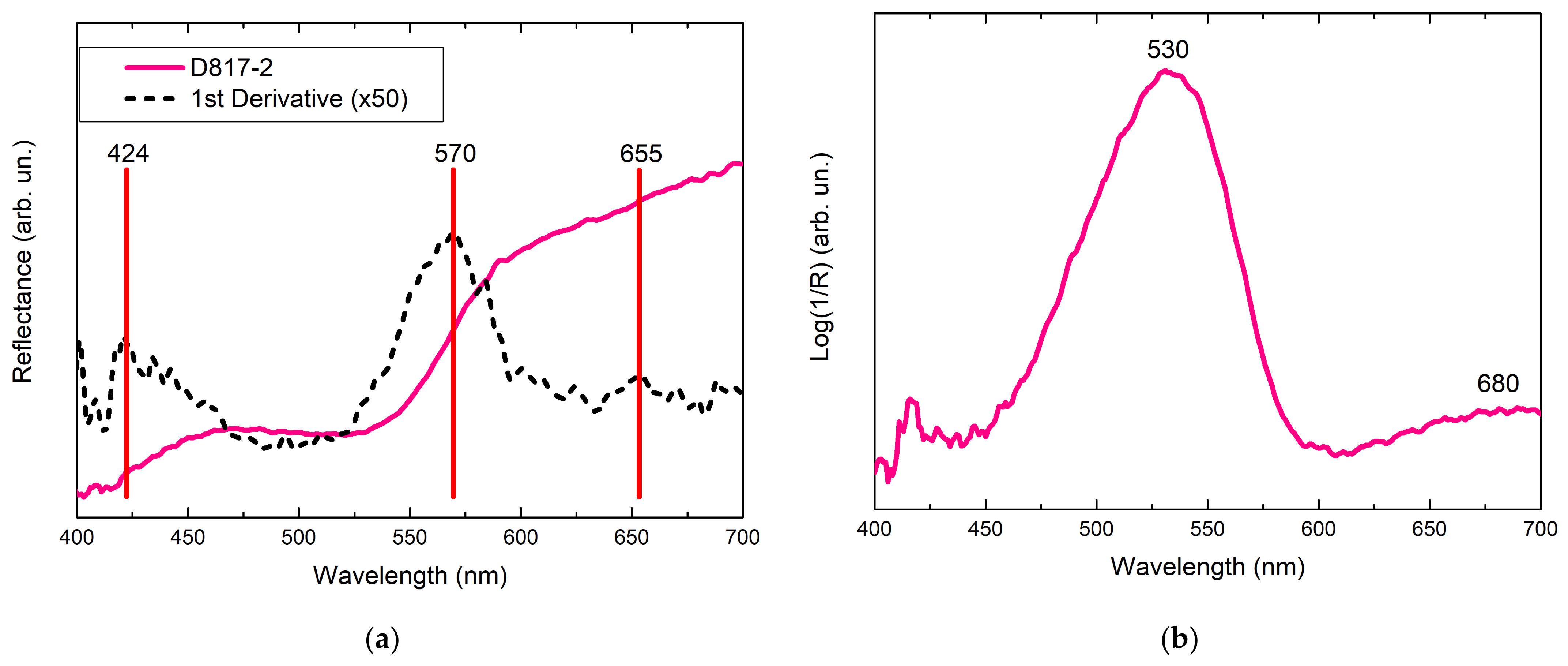
| D817-2 | Red | Red stain | 490 (shoulder), 530 | 622, 768, 1129, 1185 1197, 1279, 1345, 1362, 1437, 1510, 1530, 1578, 1598, 1649 | Rhodamine B |
| D817-4 | Yellow with black decorations | Pale orange with some black particles | 506, 540 (Mono-azo dye) | 423, 443, 530, 570, 732, 760, 802, 914, 944, 1181, 1298, 1376, 1444, 1473, 1533, 1589, 1621 (Crystal or Methyl Violet) | Mono-azo dye (?), Crystel (or Mehyl) Violet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccola, A.; Serafini, I.; D'Agostino, G.; Giambra, B.; Bosi, A.; Ripanti, F.; Nucara, A.; Postorino, P.; Curini, R.; Bruno, M. Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach. Heritage 2021, 4, 1807-1820. https://doi.org/10.3390/heritage4030101
Ciccola A, Serafini I, D'Agostino G, Giambra B, Bosi A, Ripanti F, Nucara A, Postorino P, Curini R, Bruno M. Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach. Heritage. 2021; 4(3):1807-1820. https://doi.org/10.3390/heritage4030101
Chicago/Turabian StyleCiccola, Alessandro, Ilaria Serafini, Giulia D'Agostino, Belinda Giambra, Adele Bosi, Francesca Ripanti, Alessandro Nucara, Paolo Postorino, Roberta Curini, and Maurizio Bruno. 2021. "Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach" Heritage 4, no. 3: 1807-1820. https://doi.org/10.3390/heritage4030101
APA StyleCiccola, A., Serafini, I., D'Agostino, G., Giambra, B., Bosi, A., Ripanti, F., Nucara, A., Postorino, P., Curini, R., & Bruno, M. (2021). Dyes of a Shadow Theatre: Investigating Tholu Bommalu Indian Puppets through a Highly Sensitive Multi-Spectroscopic Approach. Heritage, 4(3), 1807-1820. https://doi.org/10.3390/heritage4030101









