GC/MS Characterization of Beeswax, Protein, Gum, Resin, and Oil in Romano-Egyptian Paintings
Abstract
1. Introduction
1.1. Project Description
1.2. History of Mummy Portraits
1.3. Treatment and Interventions
1.4. Beeswax Paint and Modified Wax
1.5. Tempera Paint
2. Materials and Methods
2.1. Butyl Prep: Beeswax and Soap Analysis
- 1)
- To make the Butyl Prep reagent, add 400 µL of Meth Prep reagent into a 2 mL autosampler vial and evaporate under nitrogen to dryness at 50 °C. Add 50 µL of toluene, evaporate to dryness, then add 100 µL of t-butanol. Mix contents until the salt is in solution, add 600 µL of chloroform, then mix until the solution is clear.
- 2)
- Add between 20 and 50 µL of the Butyl Prep reagent to the paint sample in a high recovery autosampler vial, cover with a crimp top lid, and heat the vial for one hour at 60 °C in an oven.
- 3)
- An Agilent 6890N/5973 inert GC/MS system was used for analysis with a Zebron™ ZB-5HT column (30 M x 0.25 mm x 0.10 µm) for separation. Helium gas 1.2 mL/min; splitless injection liner without glass wool at 300 °C; transfer line 320 °C. Oven program: 80 °C (2 min), 10 °C/min to 210 °C; 20°C/min to 360 °C (6 min); 40 °C/min to 380 °C (2 min). Total run time was 31 min in SCAN mode (50–550 m/z). Fatty acid standards were used for calibration.
2.2. Meth Prep: Oil Analysis
- 1)
- Prepare reagent by diluting (1:2) Meth Prep in toluene.
- 2)
- Gently evaporate the Butyl Prep from the micro vials without heat, or allow the solution to evaporate at room temperature.
- 3)
- Add between 20 and 50 µL of Meth Prep reagent to the vial, cap, and heat at 60 °C for 1 hour. Inject into GC/MS with the same final volume and instrumental parameters for Butyl Prep.
2.3. Amino Acid Analysis: Proteins (egg, animal glue, casein)
- 1)
- Evaporate Meth Prep solution in the sample micro vial with nitrogen at 50 °C. Add 100 µL of 6N HCL, cover with a crimp top lid, and heat at 105 °C for 24 h (or 4 h at 145 °C). Then follow the published amino acid protocol for sample preparation.
- 2)
- An INNOWAX column (25 M × 0.2 mm × 0.2µm) was used for separation. Helium gas 1 mL/min, splitless injection at 240 °C; transfer line 240 °C. Oven program: 70 °C for 1 min; 20 °C/min to 250°C; isothermal for 3.5 min. The total run time was 12 min. SIM mode was used. The identification of proteins by GC/MS is accomplished by comparing the amino acids (building blocks of proteins) of each sample to those of standard reference materials using the method of correlation coefficients. Amino acid standards were used for calibration.
2.4. Monosaccharide Analysis: Plant Gums (Acacia sp. gums, monosaccharides, starch, and honey)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. Enzyme-linked Immunosorbent Assay (ELISA)
2.7. ESCAPE Data Evaluation
2.8. Reference Materials
- 1)
- An ancient beeswax writing tablet (6-20403), Roman Empire (27 BC–395 AD). 14 cm × 18 cm × 0.5 cm. Arthur S. Hunt and Bernard P. Grenfell. Roman tomb, Tebtunis, Faiyum region. Phoebe Hearst Museum
- 2)
- 19th C Beeswax, black color, Boston Museum of Fine Arts
- 3)
- Fresh Beeswax from GCI reference collection
- 4)
- Mummy portraits and panel paintings are described in Table 1
3. Results
3.1. Summary
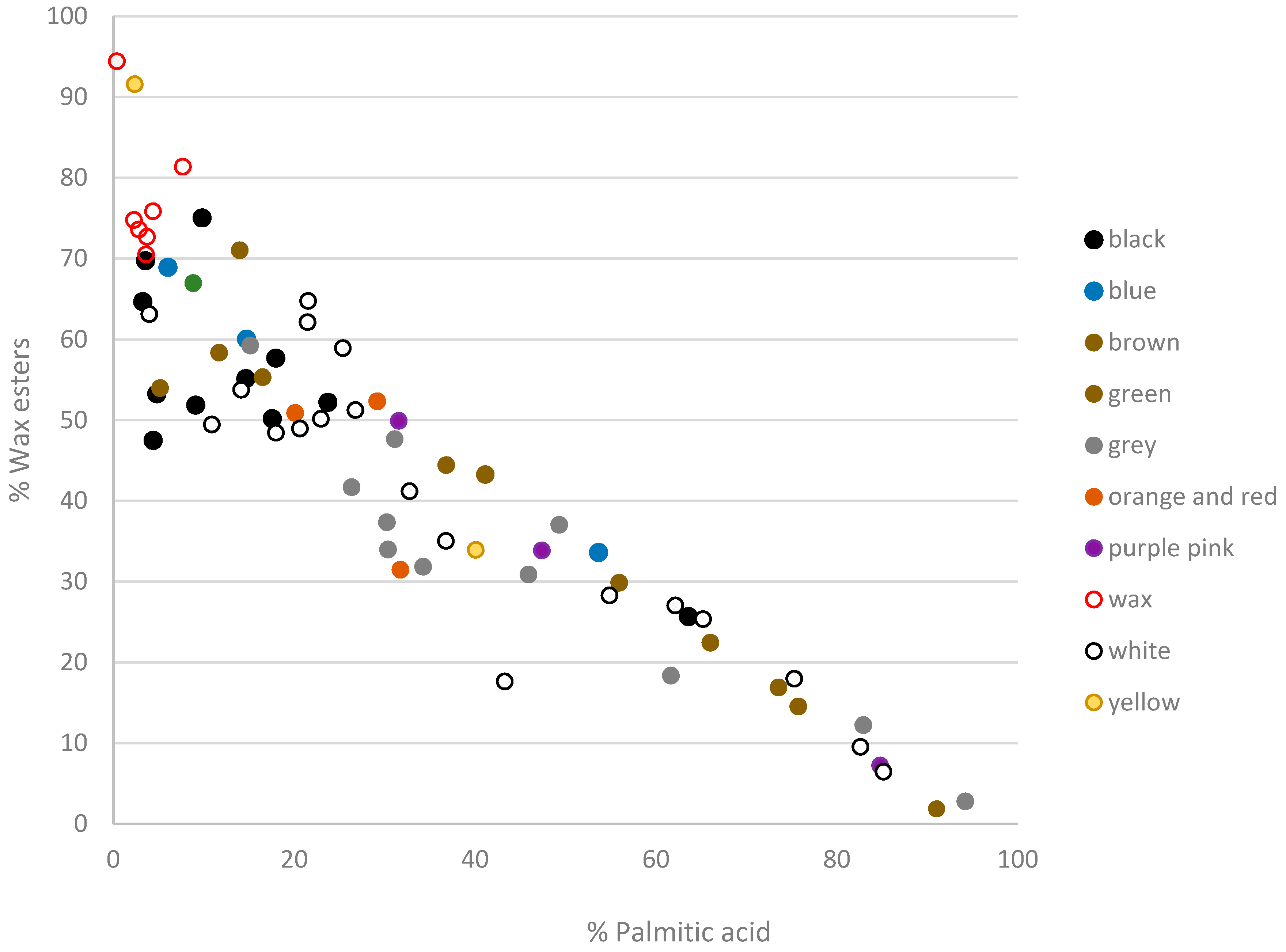
3.2. Beeswax Portraits
3.3. FTIR: Beeswax and Metal Soaps
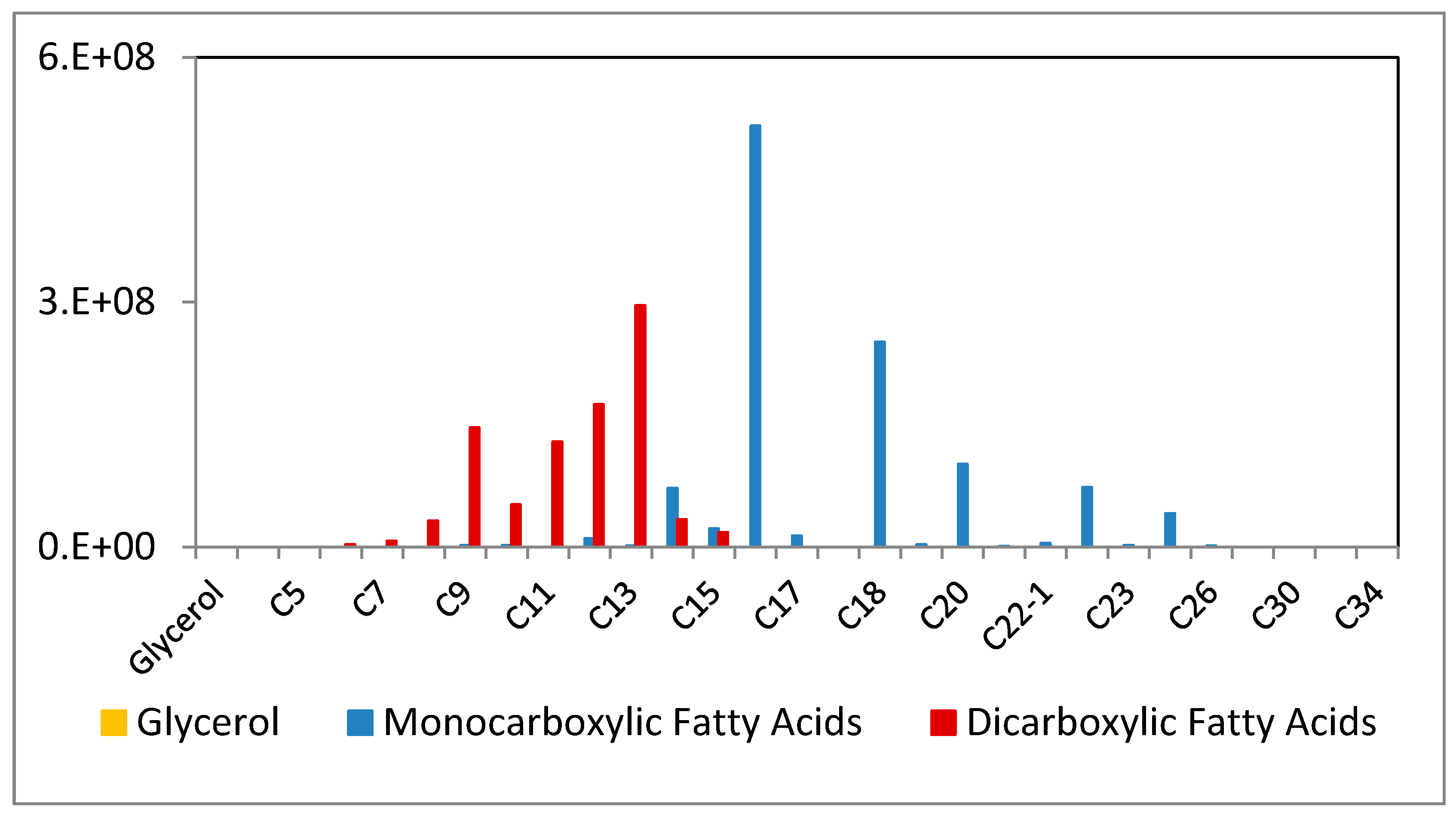
3.4. Meth Prep: Oil Analysis
3.5. Location of Oxidized Oil in Beeswax Paint Samples
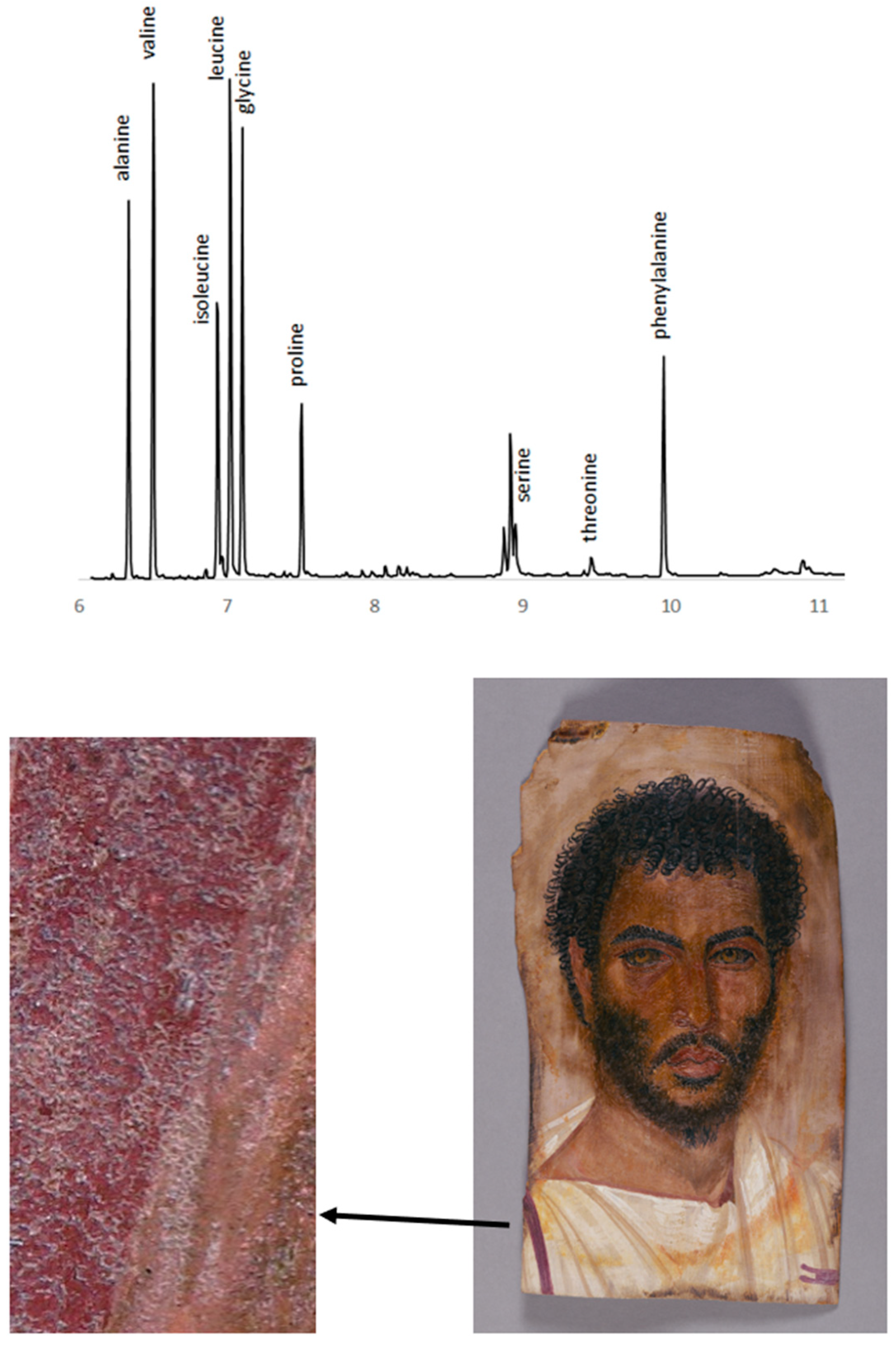
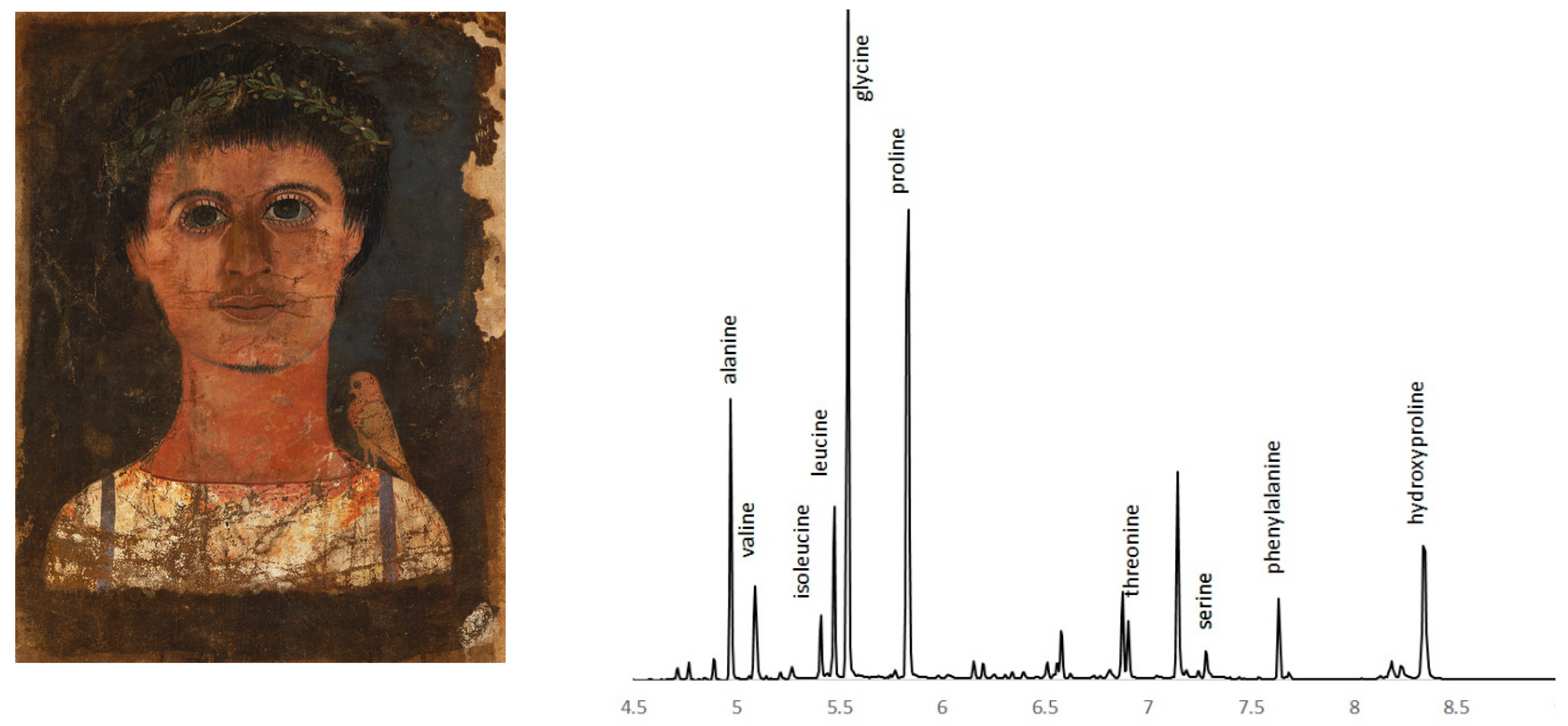
3.6. Amino Acid Analysis
3.7. Amino acid, ELISA, and Peptide Analysis: Egg Protein
3.8. Mixtures of Organic Materials
3.9. Monosaccharide Analysis: Plant Gums
3.10. Sampling Strategies
4. Discussion
4.1. Beeswax Characterization
4.2. Oxidized Oil
4.3. Oil and Beeswax: Evidence for Additive or Restoration Treatment?
4.4. The Indicators for Modified Beeswax
4.5. Animal Glue Tempera and Protein
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Borg, B.E.; Most, G.W. The Face of the Elite. Arion 2000, 8, 63–96. [Google Scholar]
- Walker, S. Ancient Faces: Mummy Portraits from Roman; Egypt; Taylor & Francis: Didcot, UK, 2000. [Google Scholar]
- Freccero, A. Fayum Portraits: Documentation and Scientific Analyses of Mummy Portraits Belonging to Nationalmuseum in Stockholm; Acta Universitatis Gothoburgensis: Gothenburg, Sweden, 2000. [Google Scholar]
- Doxiadis, E. The Mysterious Fayum Portraits: Faces from Ancient Egypt; Harry, N., Ed.; Abrams: New York, NY, USA, 1995. [Google Scholar]
- Picton, J.; Quirke, S.; Roberts, P.C. LIVING Images: Egyptian Funerary Portraits in the Petrie Museum; Routledge: Abingdon, UK, 2018. [Google Scholar]
- Petrie, W.M.F. Hawara, Biahmu, and Arsinoe; Field & Tuer: London, UK, 1889. [Google Scholar]
- Borg, B. The Dead as a Guest at Table? Continuity and Change in the Egyptian Cult of the Dead. 1997. Available online: https://core.ac.uk/download/pdf/35120463.pdf (accessed on 1 March 2019).
- Petrie, W.M.F. Seventy Years in Archaeology; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Thompson, D.L. Mummy Portraits in the J. Paul Getty Museum; Getty Publications: Los Angeles, CA, USA, 1982. [Google Scholar]
- Bagh, T. Archival photo of Petrie’s exhibition at University College London in 1911 showing the mummies and portraits from Hawara. In Finds from W.M.F Petrie’s Excavations in Egypt on the Nu Carlsberg Glyptotek; ISD LLC: Bristol, CT, USA, 2011; Volume 9. [Google Scholar]
- Cotte, M.; Checroun, E.; Susini, J.; Dumas, P.; Tchoreloff, P.; Besnard, M.; Walter, P. Kinetics of oil saponification by lead salts in ancient preparations of pharmaceutical lead plasters and painting lead mediums. Talanta 2006, 70, 1136–1142. [Google Scholar] [CrossRef]
- White, R. The application of gas-chromatography to the identification of waxes. Stud. Conserv. 1978, 23, 57–68. [Google Scholar] [CrossRef]
- Kühn, H. Detection and Identification of Waxes, Including Punic Wax, by Infra-red Spectrography. Stud. Conserv. 1960, 5, 71–81. [Google Scholar] [CrossRef]
- Regert, M.; Colinart, S.; Degrand, L.; Decavallas, O. Chemical Alteration and Use of Beeswax Through Time: Accelerated Ageing Tests and Analysis of Archaeological Samples from Various Environmental Contexts. Archaeometry 2003, 43, 549–569. [Google Scholar] [CrossRef]
- Stacey, R.J. The composition of some Roman medicines: Evidence for Pliny’s Punic wax? Anal. Bioanal. Chem. 2011, 401, 1749. [Google Scholar] [CrossRef]
- Stacey, R.J.; Dyer, J.; Mussell, C.; Lluveras-Tenorio, A.; Colombini, M.P.; Duce, C.; La Nasa, J.; Cantisani, E.; Prati, S.; Sciutto, G.; et al. Ancient encaustic: An experimental exploration of technology, ageing behaviour and approaches to analytical investigation. Microchem. J. 2018, 138, 472–487. [Google Scholar] [CrossRef]
- Leone, R.; Breuil, C. Filamentous fungi can degrade aspen steryl esters and waxes. Int. Biodeterior. Biodegrad. 1998, 41, 133–137. [Google Scholar] [CrossRef]
- Hunter, G.W. Laboratory preparation of cold cream to show saponification and emulsification. J. Chem. Educ. 1944, 21, 175. [Google Scholar] [CrossRef]
- Agoston, G.A. Acrylics, oils and encaustic: Experiences and opinions of an artist-chemical engineer. Leonardo 1971, 4, 211–219. [Google Scholar] [CrossRef]
- Vereshchagin, A.G. Occurrence of Lipophilic Micellae Formed by Fatty Alcohols and Fatty-Acid Sodium Salts in Jojoba-Wax Aqueous Hydrolyzate. Available online: http://www.dr-baumann.ca/science/Chemical%20composition%20of%20Jojba%20Oil.pdf (accessed on 1 March 2019).
- Salvant, J.; Williams, J.; Ganio, M.; Casadio, F.; Daher, C.; Sutherland, K.; Monico, L.; Vanmeert, F.; De Meyer, S.; Janssens, K.; et al. A Roman Egyptian Painting Workshop: Technical Investigation of the Portraits from Tebtunis, Egypt. Archaeometry 2017, 60, 815–833. [Google Scholar] [CrossRef]
- Henderson, J.; Morkot, R.; Peltenburg, E.; Quirke, S.; Serpico, M.; Tait, J.; White, R. Ancient Egyptian Materials and Technology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Sack, S.P.; Tahk, F.C.; Peters, T. A technical examination of an ancient Egyptian painting on canvas. Stud. Conserv. 1981, 26, 15–23. [Google Scholar] [CrossRef]
- Mathews, T.F.; Muller, N.E. The Dawn of Christian Art in Panel Paintings and Icons; J. Paul Getty Museum: Los Angeles, CA, USA, 2017. [Google Scholar]
- Mazurek, J.; Svoboda, M.; Maish, J.; Kawahara, K.; Fukakusa, S.; Nakazawa, T.; Taniguchi, Y. Characterization of Binding Media in Egyptian Romano Portraits Using Enzyme-Linked Immunosorbant Assay and Mass Spectrometry. e-Preserv Sci. 2014, 11, 76–83. [Google Scholar]
- Lluveras-Tenorio, A.; Mazurek, J.; Restivo, A.; Colombini, M.P.; Bonaduce, I. The Development of a New Analytical Model for the Identification of Saccharide Binders in Paint Samples. PLoS ONE 2012, 7, e49383. [Google Scholar] [CrossRef] [PubMed]
- Granzotto, C.; Arslanoglu, J. Revealing the binding medium of a Roman Egyptian painted mummy shroud. J. Cult. Herit. 2017, 27, 170–174. [Google Scholar] [CrossRef]
- Van Kuijk, F.; Thomas, D.; Konopelski, J.; Dratz, E. Transesterification of phospholipids or triglycerides to fatty acid benzyl esters with simultaneous methylation of free fatty acids for gas-liquid chromatographic analysis. J. Lipid Res. 1986, 27, 452–456. [Google Scholar]
- Drechsel, D.; Dettmer, K.; Engewald, W. Studies of thermally assisted hydrolysis and methylation-GC-MS of fatty acids and triglycerides using different reagents and injection systems. Chromatographia 2003, 57, 283–289. [Google Scholar] [CrossRef]
- Sutherland, K. Derivatisation using m-(trifluoromethyl) phenyltrimethylammonium hydroxide of organic materials in artworks for analysis by gas chromatography–mass spectrometry: Unusual reaction products with alcohols. J. Chromatogr. A 2007, 1149, 30–37. [Google Scholar] [CrossRef]
- Schilling, M.R.; Khanjian, H.P.; Souza, L.A. Gas chromatographic analysis of amino acids as ethyl chloroformate derivatives. Part 1, Composition of proteins associated with art objects and monuments. J. Am. Inst. Conserv. 1996, 35, 45–59. [Google Scholar] [CrossRef]
- Schilling, M.R.; Khanjian, H.P. Gas chromatographic analysis of amino acids as ethyl chloroformate derivatives. Part 2, Effects of pigments and accelerated aging on the identification of proteinaceous binding media. J. Am. Inst. Conserv. 1996, 35, 123–144. [Google Scholar] [CrossRef]
- Lluveras-Tenorio, A.; Mazurek, J.; Restivo, A.; Colombini, M.P.; Bonaduce, I. Analysis of plant gums and saccharide materials in paint samples: Comparison of GC-MS analytical procedures and databases. Chem. Cent. J. 2012, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, J.; Heginbotham, A.; Schilling, M.; Chiari, G. Antibody assay to characterize binding media in paint. ICOM Comm. Conserv. 2008, 2, 678–685. [Google Scholar]
- Schilling, M.R.; Heginbotham, A.; van Keulen, H.; Szelewski, M. Beyond the basics: A systematic approach for comprehensive analysis of organic materials in Asian lacquers. Stud. Conserv. 2016, 61, 3–27. [Google Scholar] [CrossRef]
- Cartwright, C.R.; Bierbrier, M. Egyptian mummy portraits: Examining the woodworkers’ craft. In Portraits and Masks. Burial Customs in Roman Egypt; Taylor & Francis: Didcot, UK, 1997; pp. 106–111. [Google Scholar]
- Cartwright, C.; Spaabaek, L.R.; Svoboda, M. Portrait mummies from Roman Egypt: Ongoing collaborative research on wood identification. Br. Mus. Tech. Res. Bull. 2011, 5, 49–58. [Google Scholar]
- Sutherland, K.S.R.C.; Pozzi, F. Forthcoming publication: Challenges in the Characterization and Categorization of Binding Media in Mummy Portraits” in Romano-Egyptian Funerary Paintings: Emerging Research from the APPEAR Project. In Proceedings of the Getty publication of APPEAR conference, Los Angeles, CA, USA; 2018. [Google Scholar]
- Ramer, B. The Technology, Examination and Conservation of the Fayum Portraits in the Petrie Museum. Stud. Conserv. 1979, 24, 1–13. [Google Scholar] [CrossRef]
- Mills, J.S.; White, R. The gas-chromatographic examination of paint media. Part II. Some examples of medium identification in paintings by fatty acid analysis. Stud. Conserv. 1972, 17, 721–728. [Google Scholar] [CrossRef]
- Kirby, D. Peptide Mass Fingerprint (PMF) of Egg on JPGM Portraits. 2019; Unpublished data. [Google Scholar]
- Kirby, D.; Khandekar, N.; Arslanoglu, J.; Sutherland, K. Protein identification in artworks by peptide mass fingerprinting. In Proceedings of the Preprints of ICOMCC, 16th Triennial Conference, Lisbon, Portugal, 19–23 September 2011. [Google Scholar]
- Franck, P.; Garnery, L.; Loiseau, A.; Oldroyd, B.P.; Hepburn, H.R.; Solignac, M.; Cornuet, J.M. Genetic diversity of the honeybee in Africa: Microsatellite and mitochondrial data. Heredity 2001, 86, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Aichholz, R.; Lorbeer, E. Investigation of combwax of honeybees with high-temperature gas chromatography and high-temperature gas chromatography–chemical ionization mass spectrometry: I. High-temperature gas chromatography. J. Chromatogr. A 1999, 855, 601–615. [Google Scholar] [CrossRef]
- Aichholz, R.; Lorbeer, E. Investigation of combwax of honeybees with high-temperature gas chromatography and high-temperature gas chromatography–chemical ionization mass spectrometry: II: High-temperature gas chromatography–chemical ionization mass spectrometry. J. Chromatogr. A 2000, 883, 75–88. [Google Scholar] [CrossRef]
- Jiménez, J.; Bernal, J.; Aumente, S.; del Nozal, M.J.; Martín, M.T.; Bernal, J., Jr. Quality assurance of commercial beeswax: Part, I. Gas chromatography–electron impact ionization mass spectrometry of hydrocarbons and monoesters. J. Chromatogr. A 2004, 1024, 147–154. [Google Scholar] [CrossRef]
- Evershed, R.P.; Heron, C.; Goad, J. Analysis of organic residues of archaeological origin by high-temperature gas chromatography and gas chromatography-mass spectrometry. Analyst 1990, 115, 1339–1342. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F.; Ribechini, E. Organic mass spectrometry in archaeology: Evidence for Brassicaceae seed oil in Egyptian ceramic lamps. J. Mass Spectrom. 2005, 40, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Freccero, A. Encausto and Ganosis: Beeswax as Paint and Coating during the Roman Era and its Applicability in Modern Art, Craft and Conservation; Acta Universitatis Gothoburgensis: Gothenburg, Sweden, 2002. [Google Scholar]
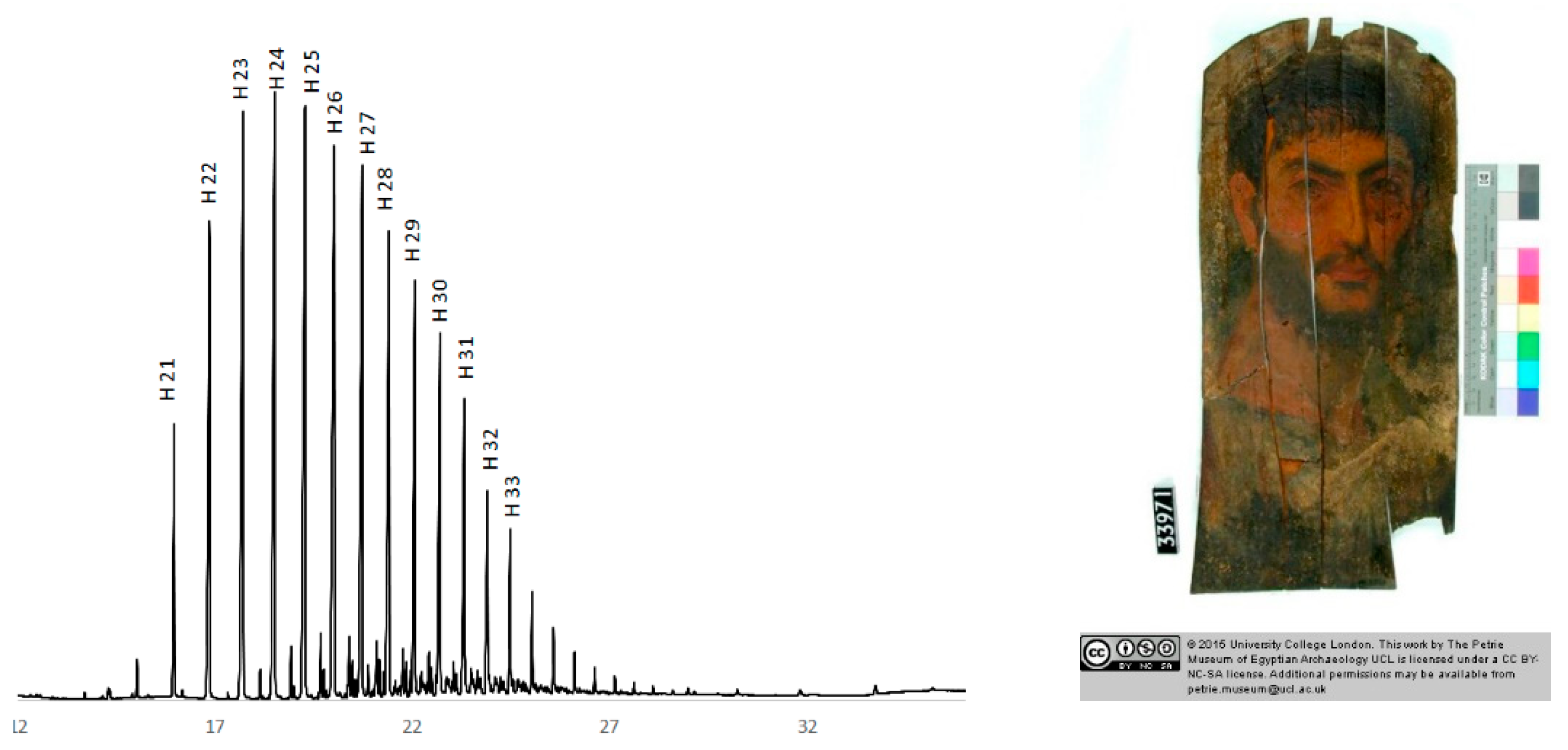
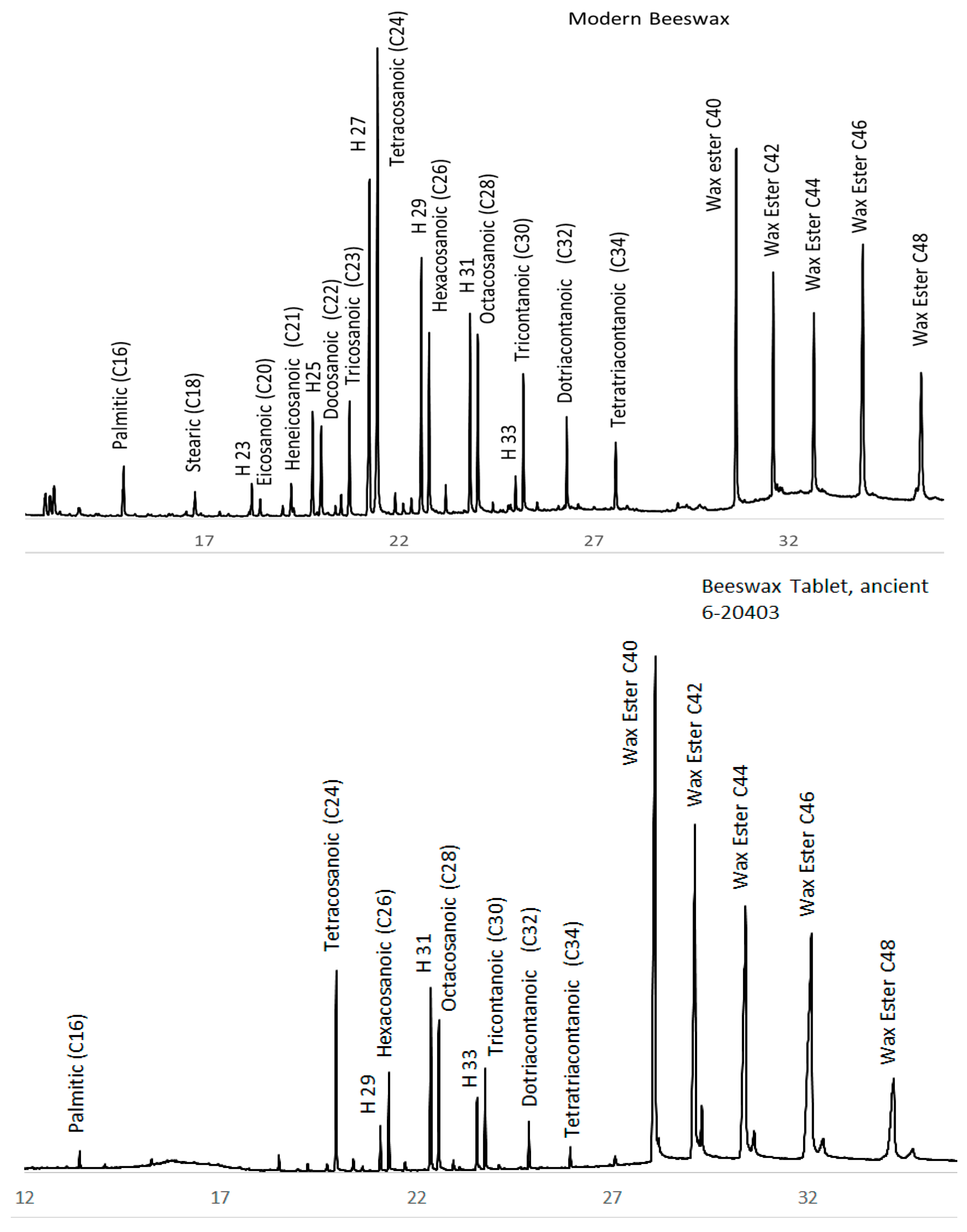
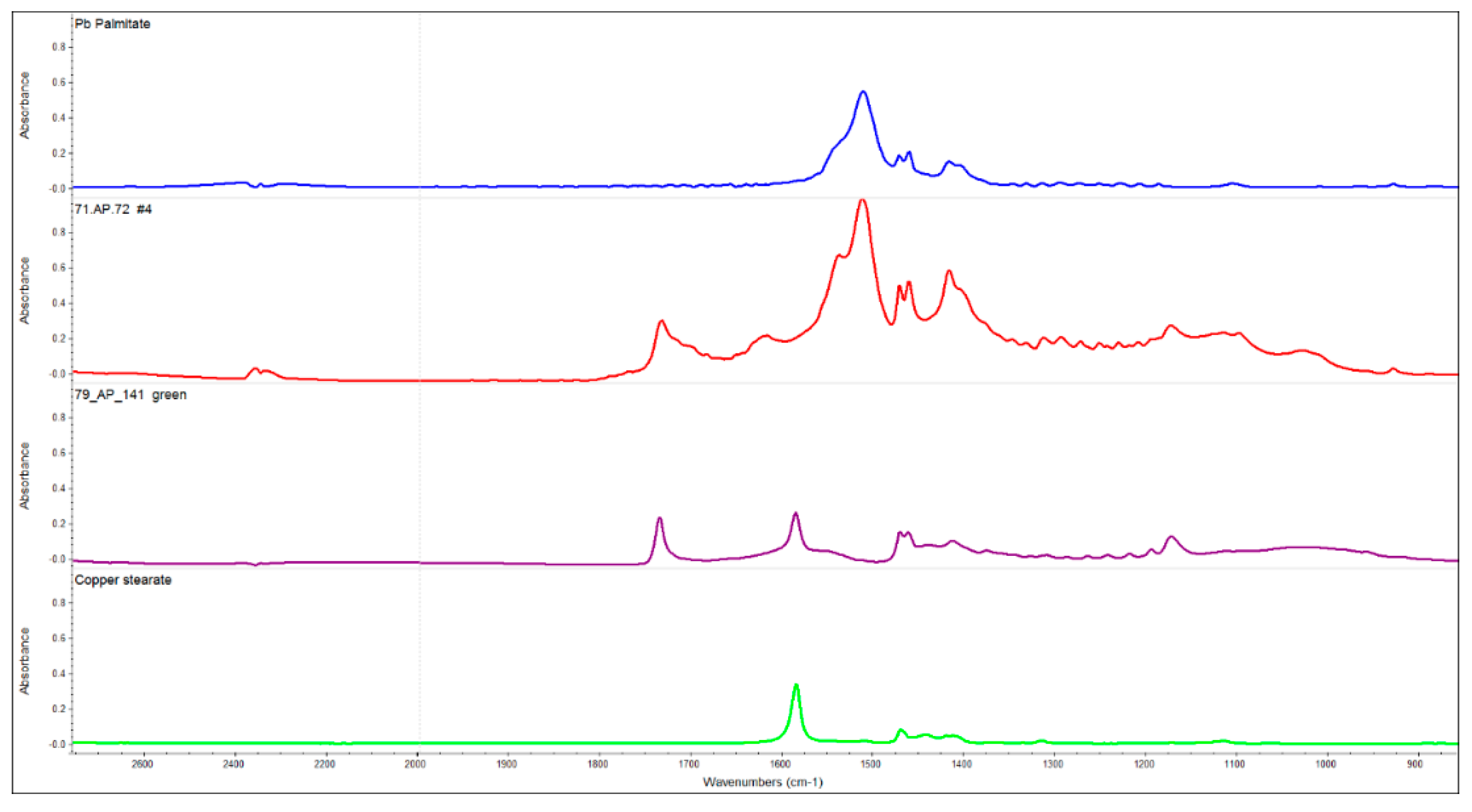

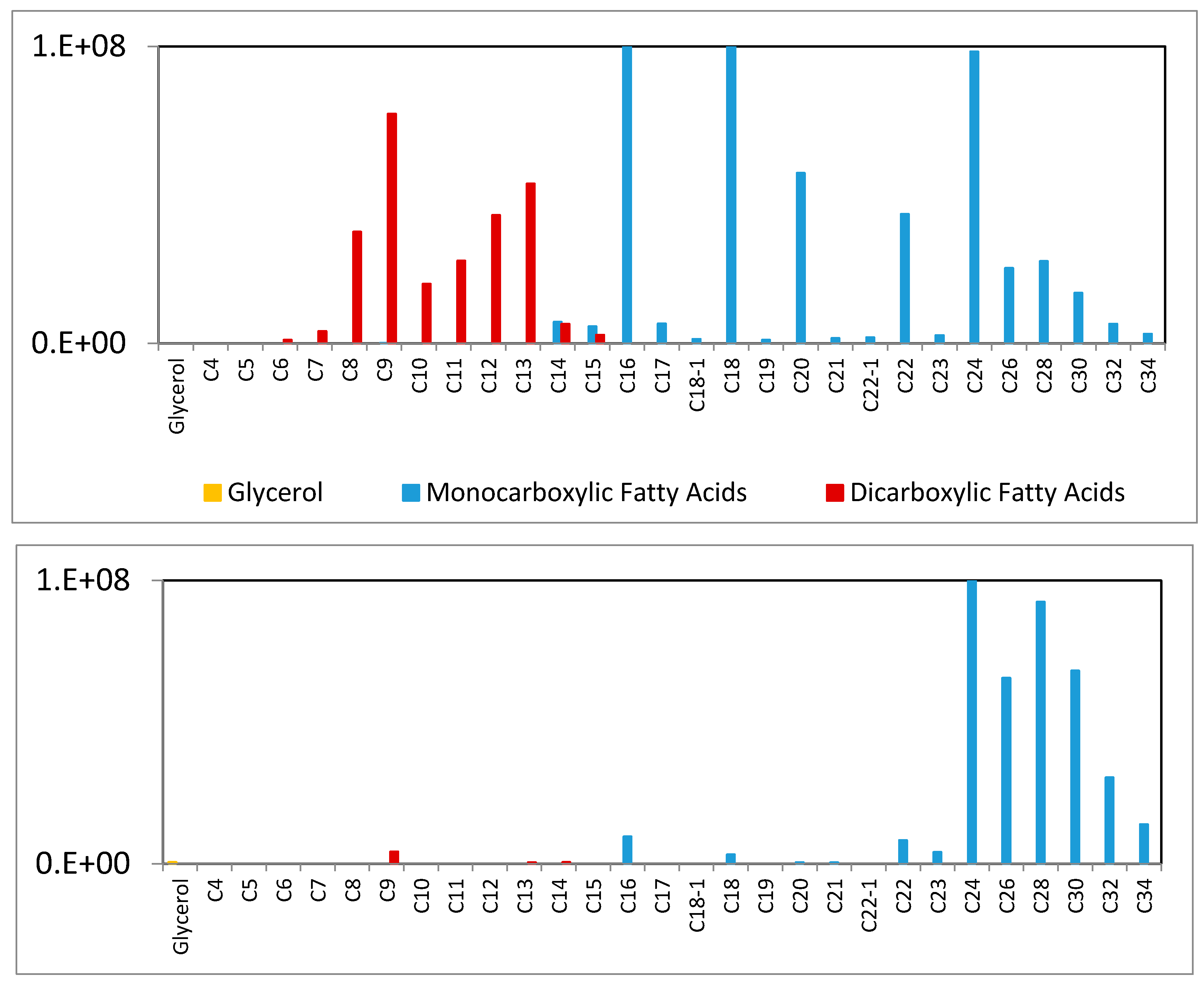

| Accession | Source | Date A.D. | Wood (MM) | Technique |
|---|---|---|---|---|
| J Paul Getty Museum | ||||
| 71.AP.72 | (Hawara) | 100–125 | Ω Tileu (2) | Beeswax, heavy, impasto |
| 73.AP.91 | (Hawara) | 75–100 | Ω Tileu (2) | Beeswax, lean, cracking, glue 1 |
| 73.AP.94 | -- | 140–160 | Ω Tileu (2) | Beeswax, flat, lean, restored |
| 74.AP.11 | (er-Rubayat) | 150–170 | Δ Tileu (2) | Beeswax, heavy, (egg coating) |
| 74.AP.20 | -- | 180–200 | Π Fsyc (3) | Glue tempera, flat, (egg coating) |
| 74.AP.21 | (er-Rubayat) | 100–200 | Π Fsyc (16) | Glue tempera, flat, (egg coating) |
| 74.AP.22 | (er-Rubayat) | 100–200 | Π Fsyc (16) | Glue tempera, flat, (egg coating) |
| 75.AP.87 | -- | 150–250 | Linen | Glue tempera, flat |
| 78.AP.262 | -- | 150–200 | Π Tileu (1.4) | Beeswax, impasto, bubbles in wax |
| 79.AP.129 | (er-Rubayat)/Graf | 175–200 | Π Clib (11) | Glue tempera, flat, lean |
| 79.AP.141 | -- | 220–235 | Π Clib (11) | Beeswax, lean, impasto, cracking glue ground |
| 79.AP.142 | (er-Rubayat) | 220–250 | Δ Clib (11) | Glue & gum tempera, flat, heavy |
| 79.AP.219 | -- | 220–250 | Linen | Glue tempera |
| 81.AP.29 | (er-Rubayat)/Graf | 170–200 | Δ Fsyc (11) | Glue tempera |
| 81.AP.42 | -- | 100–110 | Ω Tileu (1.7) | Beeswax, heavy, impasto |
| 91.AP.6 | (el-Hibbeh) | 50–100 | Ω Tileu | Glue tempera, flat, (egg coating) |
| Ny Carlsberg Glyptotek | ||||
| AEIN680 | (er-Rubayat)Graf | 100–125 | Ω Tileu (2) | Beeswax, lean, impasto, glue 1 |
| AEIN681 | (er-Rubayat)Graf | 125–150 | Ω Tileu (1) | Beeswax, lean, impasto, glue 1, (egg coating) |
| AEIN682 | (er-Rubayat)Graf | 140–160 | Δ Tileu (2) | Beeswax, impasto, white bloom, glue 1 |
| AEIN683 | (er-Rubayat)Graf | 125–150 | Ω Tileu (1.5) | Beeswax, impasto |
| AEIN684 | (er-Rubayat)Graf | 140–200 | Δ Tileu (1) | Beeswax, glue 1, (egg coating) |
| AEIN685 | -- | -- | Π panel | Gum tempera, flat |
| AEIN686 | -- | -- | Π (Fsyc) (12) | Glue tempera, flat |
| AEIN1425 | Hawara, Petrie | 25–75 | Linen | Beeswax, lean, heat 2 |
| AEIN1426 | Hawara, Petrie | 100–125 | Ω Tileu (1.5) | Beeswax, impasto, (paraffin) |
| AEIN1473 | Hawara, Petrie | 100–150 | Ω Tileu (2.5) | Beeswax, impasto heat 2, (paraffin) |
| National Museum Demark | ||||
| AS 3891 | er-Rubayat | 150–200 | Δ Tileu (1.5) | Beeswax, impasto |
| AS 3892 | (er-Rubayat) | 150–200 | Ω Tileu (1.5) | Beeswax, impasto |
| AS 8940 | er-Rubayat | 100–125 | Δ Fsyc (11) | Glue tempera, flat |
| Santa Barbara Museum of Art | ||||
| VL.2015.5 | -- | 3–4th C | Δ panel (6) | Glue tempera, flat |
| Museum of Fine Art Houston | ||||
| CA5879 | (Sheikh) | 150–200 | Linen | Tempera 7, flat |
| 1984-45DJ | -- | 200–250 | Π panel (10) | Beeswax, impasto, (shellac) |
| 1970 001DJ | -- | Modern | Δ pinus (2) | Linseed oil, impasto |
| CA5878 | -- | Modern | Π fagus (1) | Linseed oil, flat |
| CA7124 | (er-Rubayat)Graf | 150–200 | Δ Fsyc (10) | Beeswax, flat |
| CA7013 | -- | 150–200 | Δ Tileu (1.4) | Beeswax, impasto |
| Museum of Fine Art Houston | ||||
| TR:184-2013 | -- | 1–200 | Ω Tileu (2.4) | Beeswax, impasto |
| 2009.16 | -- | 1–100 | Ω Tileu (2.5) | Beeswax, impasto |
| Los Angeles County Museum | ||||
| M.71.73.62 | Graf | 3–4th C | Δ panel (6) | Glue tempera, flat |
| Walters Art Museum | ||||
| 32.4 | Antinoöpolis | 130–300 | Beech (6) 3,4 | Beeswax, (paraffin) |
| 32.6 | (er-Rubayat) | 117–138 | Δ Tileu (2) 4 | Beeswax, saliva cleaning |
| Ashmolean | ||||
| AN188.342 | -- | -- | Ω Tileu (1.5) | Beeswax and glue tempera |
| Cantor Arts Center | ||||
| JLS.22225 | -- | 180–235 | Δ panel (2.4) | Beeswax |
| JLS.22226 | -- | -- | Δ panel | Glue tempera (oil, mastic, paraffin) |
| Yale University Art Gallery | ||||
| 1935.551 | Europos, Hopkins | 256 | Round | Glue tempera (beeswax, paraffin) |
| Phoebe Hearst Museum | ||||
| 5-2327 | Kerke, Graf | 300 | Fsyc (3) 5 | Glue tempera, flat |
| 6-21377 | Tebtunis, Hunt | 96–192 | Δ Fsyc (12) | Beeswax, impasto, heavy |
| 6-21384 | Tebtunis, Hunt | 1–395 | Π Ziziphus | Glue tempera, flat, lean |
| 6-21385 | Tebtunis, Hunt | 1–395 | Π Ziziphus | Glue tempera, flat, lean |
| 6-21386 | Tebtunis, Hunt | 1–395 | Π Ziziphus | Glue tempera, flat, lean |
| 6-21387 | Tebtunis, Hunt | 1–395 | Π Cedar | Protein tempera7, flat, lean |
| Petrie Museum | ||||
| UC14768 | Kafr Ammar, Petrie | 160–180 | Fir 6 | Glue tempera |
| UC19607 | Hawara, Petrie | 100–120 | Δ Tileu (4) | Beeswax, (paraffin) |
| UC19608 | Hawara, Petrie | 70–100 | Δ Tileu (2) | Beeswax |
| UC19610 | Hawara, Petrie | 140–160 | Δ Tileu (2) | Beeswax |
| UC19612 | Hawara, Petrie | 160–170 | Δ Tileu (2) | Beeswax |
| UC30081 | Hawara, Petrie | 100–120 | Δ Tileu (2) | Beeswax, (paraffin) |
| UC33971 | Hawara, Petrie | 100–130 | Δ Tileu (4) | Beeswax, (paraffin) |
| Cleveland Museum of Art | ||||
| 1971.135 | -- | 138–192 | linen | Beeswax, (paraffin) |
| 1971.136 | -- | 138–192 | linen | Beeswax, (paraffin) |
| ID | Location | P | WE | Di | FA | Other |
|---|---|---|---|---|---|---|
| 71.AP.72 Beeswax | ||||||
| white | tunic bot PR | 46 | 16 | 1 | 36 | protein, monos |
| white | tunic bot PL | 52 | 11 | 5 | 32 | protein, monos, pine |
| 73.AP.91 Beeswax | ||||||
| grey | BG, C PL | 76 | 2 | 5 | 18 | |
| purple | shoulder PL | 69 | 5 | 0.5 | 26 | pine |
| 73.AP.94 Beeswax | ||||||
| white | BG, C PR | 9 | 19 | - | 72 | pine |
| 74.AP.11 Beeswax with egg coating | ||||||
| flesh | shoulder, PR | 50 | 16 | 3 | 32 | protein, pine |
| grey | BG, top PR | 37 | 13 | 11 | 39 | protein, pine, brassica |
| flesh | cheek, PL | 51 | 0.5 | 15 | 34 | pine, brassica |
| 75.AP.262 Beeswax | ||||||
| white | BG, top, PR | 17 | 28 | 3 | 52 | |
| white | tunic, PL | 29 | 22 | 10 | 39 | pine |
| 79.AP.141 Beeswax on plant gum black ground | ||||||
| white | tunic, PR | 24 | 7 | 0.4 | 68 | pine |
| green | bot, C | 11 | 30 | 2 | 56 | protein |
| 81.AP.42 Beeswax | ||||||
| flesh | shoulder, PL | 31 | 8 | 0.2 | 61 | pine |
| red | edge, bot PR | 19 | 41 | 3 | 36 | pine |
| AEIN 680 Beeswax | ||||||
| yellow | clavi, bot L | 30 | 15 | 1 | 53 | pine |
| grey | BG, C top | 19 | 25 | 1 | 55 | |
| black | hair, C top | 7 | 30 | 0.5 | 63 | glue |
| AEIN 681 Beeswax | ||||||
| black | hair, PL | 2 | 41 | 1 | 56 | pine |
| flesh | neck, PR | 9 | 35 | 2 | 55 | glue, pine |
| grey | BG, C top | 12 | 37 | 4 | 48 | glue, pine |
| AEIN 682 Beeswax | ||||||
| flesh | chest, PR | 41 | 19 | 4 | 36 | glue |
| black | hair, TL | 16 | 37 | 3 | 45 | protein |
| AEIN 683 Beeswax | ||||||
| yellow | jewelry, C | 2 | 80 | 1 | 16 | skin |
| black | hair, edge PR | 13 | 29 | 2 | 57 | |
| flesh | forehead, C | 13 | 31 | 6 | 50 | |
| AEIN 684 Beeswax with egg coating | ||||||
| black | eyelid, PL | 8 | 54 | 3 | 35 | protein |
| beige | edge, top PR | 31 | 29 | 3 | 37 | protein, pine |
| AEIN 1425 Beeswax | ||||||
| flesh | neck, textile | 26 | 27 | 2 | 45 | protein, pine |
| flesh | chin, PL | 55 | 9 | 4 | 33 | pine |
| grey | BG, bot PL | 33 | 24 | 6 | 37 | pine |
| AEIN 1426 Beeswax | ||||||
| grey | BG, top PL | 33 | 24 | 6 | 37 | pine |
| black | shoulder, PR | 4 | 33 | 0.4 | 63 | |
| white | tunic, top PL | 13 | 28 | 3 | 57 | protein |
| AEIN 1473 Beeswax | ||||||
| grey | BG, C top | 31 | 20 | 2 | 47 | glue |
| black | hair, on crack | 19 | 41 | 2 | 39 | glue |
| white | edge, bot PR | 33 | 20 | 1 | 46 | glue |
| AS 3891 Beeswax | ||||||
| green | dress | 7 | 47 | 1 | 45 | protein |
| grey | grey | 12 | 41 | 1 | 45 | glue |
| AS 3892 Beeswax | ||||||
| black | hair | 10 | 34 | 4 | 52 | protein, pine |
| white | dress | 3 | 41 | 1 | 55 | skin, pine |
| TR:184-2013 Beeswax | ||||||
| flesh | chest, PR | 22 | 19 | 2 | 57 | glue |
| 2009.16 Beeswax | ||||||
| brown | eye, PL | 3 | 31 | 1 | 64 | monos |
| black | hair, C | 3 | 26 | 2 | 70 | glue, pine |
| CA 7124 Beeswax | ||||||
| grey | edge, top PR | 23 | 19 | 4 | 55 | glue, monos |
| CA 7013 Beeswax | ||||||
| flesh | chest | 40 | 11 | 5 | 44 | monos, pine |
| 32.4 Beeswax | ||||||
| black | edge, top PL | 34 | 17 | 6 | 43 | glue, pine, brassica |
| white | tunic, C | 22 | 23 | 19 | 37 | pine |
| 32.6 Beeswax | ||||||
| black | hair | 4 | 56 | 0 | 40 | glue |
| white | BG | 13 | 30 | 0.1 | 57 | pine |
| JLS22225 Beeswax | ||||||
| grey | BG | 19 | 20 | 12 | 49 | pine |
| orange | tunic | 23 | 18 | 3 | 56 | pine |
| 6-21377 Beeswax | ||||||
| white | tunic | 20 | 43 | 2 | 35 | |
| grey | BG | 13 | 52 | 1 | 34 | protein |
| flesh | 26 | 30 | 5 | 40 | ||
| UC 19607 Beeswax | ||||||
| white | BG, C PL | 8 | 34 | - | 58 | glue |
| UC 19608 Beeswax | ||||||
| brown | BG, top C | 43 | 9 | 18 | 31 | glue, pine, brassica |
| UC 19610 Beeswax | ||||||
| grey | BG, C PL | 64 | 4 | 7 | 25 | pine |
| UC 19612 Beeswax | ||||||
| white | BG, PL | 24 | 39 | 2 | 35 | |
| UC 30081 Beeswax | ||||||
| white | BG, PR | 19 | 5 | - | 77 | |
| UC 33971 Beeswax | ||||||
| brown | BG, edge, PR | 37 | 21 | - | 42 | glue |
| References Beeswax | ||||||
| 6-20403 | tablet | 0.4 | 80 | 0.2 | 20 | |
| GCI | beeswax | 3 | 47 | 0.5 | 48 | |
| ID | Location | P | WE | Di | FA |
|---|---|---|---|---|---|
| 91.AP.6 Beeswax | |||||
| red | top | 3 | 25 | 15 | 57 |
| middle | 15 | 25 | 13 | 47 | |
| bottom | 19 | 18 | 21 | 41 | |
| 81.AP.42 Beeswax | |||||
| red | top | 28 | 6 | 5 | 61 |
| bottom | 29 | 5 | 6 | 60 | |
| AEIN 1426 Beeswax | |||||
| white | top | 7 | 32 | 7 | 53 |
| bottom | 12 | 33 | 3 | 52 | |
| Sample | ALA | VAL | ILE | LEU | GLY | PRO | OHP |
|---|---|---|---|---|---|---|---|
| Egg | 23 | 16 | 14 | 21 | 19 | 7 | 0 |
| Human, skin | 16 | 11 | 10 | 22 | 34 | 6 | 0 |
| Isinglass, AG | 18 | 3 | 2 | 4 | 47 | 15 | 10 |
| Collagen, AG | 16 | 3 | 2 | 4 | 47 | 17 | 12 |
| Mummy Skin | 20 | 7 | 5 | 10 | 34 | 16 | 7 |
| Saliva | 11 | 8 | 5 | 12 | 27 | 35 | 0.7 |
| Casein | 11 | 17 | 13 | 22 | 9 | 29 | 0 |
| Egyptian Fungus | 22 | 11 | 7 | 13 | 38 | 8 | 2 |
| Sample and Location | ala | val | ile | leu | gly | pro | ohp | C. Coef. | % Protein [ELISA (OD)] | |
|---|---|---|---|---|---|---|---|---|---|---|
| 71.AP.72 Beeswax | ||||||||||
| white | tunic bot PR | 15 | 9 | 8 | 15 | 37 | 9 | 8 | protein | 0.9% |
| white | tunic bot PL | 12 | 10 | 7 | 18 | 36 | 15 | 3 | protein | 0.6% |
| resin | ear PR | 13 | 7 | 5 | 14 | 53 | 8 | 0.9 | protein | 0.1% |
| 74.AP.11 Beeswax with egg coating | ||||||||||
| grey | BG, top PL | 17 | 13 | 10 | 22 | 29 | 8 | 1 | skin, 0.99 | 1.3% |
| coating | neck, PR | 23 | 15 | 9 | 24 | 19 | 10 | 0 | egg, 0.98 | high [egg (0.8)] |
| skin | shoulder, PR | 21 | 12 | 10 | 19 | 30 | 8 | 0 | skin, 0.96 | 0.2% |
| 79.AP.141 Beeswax on plant gum black ground | ||||||||||
| wood | edge, C PR | 14 | 13 | 9 | 20 | 32 | 12 | 1 | skin, 0.97 | low |
| green | C bot | 18 | 16 | 15 | 9 | 29 | 13 | 1 | protein | low |
| 81.AP.42 Beeswax | ||||||||||
| wood | shoulder, PL | 19 | 11 | 6 | 16 | 28 | 13 | 7 | protein | low |
| flesh | shoulder, PL | 14 | 5 | 3 | 8 | 44 | 14 | 13 | glue, 0.99 | low [egg (0.5)] |
| AEIN 680 Beeswax | ||||||||||
| black | hair, C top | 18 | 7 | 4 | 10 | 46 | 13 | 2 | glue, 0.96 | low |
| AEIN 681 Beeswax with egg coating | ||||||||||
| flesh | neck, PR | 12 | 4 | 4 | 8 | 49 | 13 | 10 | glue, 0.98 | 6% |
| grey | BG, C top | 18 | 10 | 7 | 14 | 43 | 8 | 0 | glue, 0.97 | med |
| coating | unknown | 25 | 15 | 8 | 16 | 27 | 8 | 1 | egg, 0.94 | med |
| AEIN 682 Beeswax | ||||||||||
| flesh | chest, PR | 19 | 5 | 3 | 6 | 53 | 14 | 0 | glue, 0.97 | 0.4% |
| black | hair, TL | 17 | 10 | 6 | 14 | 40 | 8 | 5 | protein | low |
| AEIN 683 Beeswax | ||||||||||
| yellow | jewelry, C | 11 | 10 | 7 | 16 | 48 | 7 | 1 | skin, 0.93 | low |
| AEIN 684 Beeswax with egg coating | ||||||||||
| black | eyelid, PL | 22 | 10 | 7 | 16 | 36 | 9 | 1 | protein | low |
| beige | edge, top PR | 27 | 12 | 7 | 17 | 28 | 8 | 1 | protein | low |
| coating | nose, PL | 24 | 14 | 9 | 24 | 20 | 8 | 0 | egg, 0.96 | high |
| AEIN 1425 Beeswax | ||||||||||
| flesh | neck, textile | 18 | 9 | 6 | 13 | 34 | 17 | 3 | protein | 0.3% |
| AEIN 1426 Beeswax | ||||||||||
| white | tunic, top PL | 17 | 8 | 5 | 16 | 41 | 14 | 0 | protein | high |
| AEIN 1473 Beeswax | ||||||||||
| grey | BG, C top | 21 | 10 | 0 | 13 | 39 | 11 | 6 | glue, 0.93 | 0.03% |
| black | hair | 20 | 9 | 5 | 11 | 43 | 8 | 4 | glue, 0.93 | 0.5% |
| white | bot PR | 21 | 8 | 4 | 9 | 47 | 9 | 3 | glue, 0.95 | 0.2% |
| AS 3891 Beeswax | ||||||||||
| green | dress | 17 | 9 | 6 | 16 | 36 | 13 | 3 | protein | med |
| grey | BG | 16 | 4 | 2 | 6 | 36 | 20 | 18 | glue, 0.97 | high |
| AS 3892 Beeswax | ||||||||||
| resin | unknown | 22 | 8 | 4 | 10 | 47 | 7 | 2 | glue, 0.92 | med |
| black | hair | 17 | 8 | 5 | 13 | 47 | 8 | 2 | protein | med |
| white | dress | 21 | 12 | 8 | 17 | 33 | 9 | 1 | skin, 0.95 | med |
| TR:184-2013 Beeswax | ||||||||||
| flesh | chest, PR | 16 | 3 | 3 | 4 | 48 | 14 | 12 | glue, 0.99 | med |
| 2009.16 Beeswax | ||||||||||
| black | hair, C | 17 | 7 | 6 | 13 | 47 | 7 | 4 | glue, 0.92 | low |
| CA 7124 Beeswax | ||||||||||
| grey | top PR | 13 | 4 | 3 | 6 | 49 | 13 | 11 | glue, 0.99 | 0.4% |
| 32.4 Beeswax | ||||||||||
| black | top PL | 20 | 6 | 4 | 8 | 54 | 7 | 2 | glue, 0.95 | med |
| red | resin | 20 | 6 | 4 | 10 | 25 | 11 | 23 | protein | vhigh |
| black | resin | 13 | 9 | 8 | 14 | 46 | 8 | 2 | protein | low |
| 32.6 Beeswax | ||||||||||
| black | hair | 14 | 7 | 5 | 8 | 49 | 13 | 5 | glue, 0.97 | low |
| resin | wrapping | 14 | 5 | 4 | 7 | 54 | 10 | 6 | glue, 0.97 | med |
| black | BG, edge bot | 14 | 5 | 4 | 9 | 44 | 16 | 9 | glue, 0.98 | med |
| 6-21377 Beeswax | ||||||||||
| grey | BG | 19 | 9 | 6 | 15 | 36 | 11 | 5 | protein | 1% |
| UC19607 Beeswax | ||||||||||
| white | BG, C PL | 16 | 6 | 4 | 10 | 40 | 13 | 11 | glue, 0.99 | med |
| UC19608 Beeswax | ||||||||||
| brown | BG, top C | 17 | 4 | 2 | 6 | 50 | 11 | 11 | glue, 0.99 | med |
| UC19612 Beeswax | ||||||||||
| white | BG, C PL | 16 | 5 | 3 | 9 | 46 | 11 | 10 | glue, 0.99 | low |
| UC33971 Beeswax | ||||||||||
| brown | BG, edge, PR | 15 | 6 | 5 | 15 | 44 | 10 | 5 | glue, 0.93 | med |
| 74.AP.20 Glue tempera with egg coating | ||||||||||
| red | mace, PL | 19 | 8 | 5 | 12 | 38 | 13 | 5 | glue, 0.96 | 3% [egg (0.3) glue (0.3)] |
| grey | tunic, PR | 17 | 5 | 3 | 8 | 44 | 15 | 8 | glue, 0.99 | 2% |
| white | tunic, PR | 19 | 7 | 4 | 11 | 40 | 12 | 7 | glue, 0.97 | 1% |
| 74.AP.21 Glue tempera with egg coating | ||||||||||
| black | tunic, PL | 20 | 8 | 5 | 10 | 39 | 12 | 7 | glue, 0.97 | 5% [egg (0.4) glue (0.4)] |
| 74.AP.22 Glue tempera with egg coating | ||||||||||
| yellow | top, PL | 18 | 6 | 4 | 9 | 42 | 13 | 9 | glue, 0.99 | 3% [egg (0.24)] |
| 75.AP.87 Glue tempera | ||||||||||
| white | tunic, bot PL | 15 | 4 | 2 | 6 | 44 | 16 | 14 | glue, 0.99 | med |
| black | BG, center PR | 17 | 7 | 4 | 10 | 38 | 15 | 10 | glue, 0.99 | low [glue (0.4)] |
| Flesh | cheek, PL | 18 | 4 | 2 | 5 | 46 | 13 | 11 | glue, 0.99 | med |
| 79.AP.129 Glue tempera | ||||||||||
| grey | BG, C top | 16 | 6 | 4 | 9 | 46 | 11 | 8 | glue, 0.98 | med |
| white | tunic, bot PR | 15 | 6 | 4 | 10 | 49 | 10 | 6 | glue, 0.96 | med |
| pink | bot PR | 18 | 3 | 2 | 5 | 53 | 10 | 8 | glue, 0.99 | high |
| 79.AP.142 Glue tempera | ||||||||||
| flesh | hand, bot PL | 17 | 5 | 3 | 7 | 43 | 15 | 10 | glue, 0.99 | 2% |
| grey | BG, top PR | 18 | 5 | 3 | 7 | 46 | 14 | 8 | glue, 0.99 | 1% |
| blue | clavi, bot PL | 19 | 6 | 4 | 9 | 40 | 13 | 9 | glue, 0.99 | 1% |
| 79.AP.129 Glue tempera | ||||||||||
| grey | BG, C top | 16 | 6 | 4 | 9 | 46 | 11 | 8 | glue, 0.98 | med |
| white | tunic, bot C | 15 | 6 | 4 | 10 | 49 | 10 | 6 | glue, 0.96 | med |
| pink | clavi, bot PL | 18 | 3 | 2 | 5 | 53 | 10 | 8 | glue, 0.99 | low |
| 81.AP.29 Glue tempera | ||||||||||
| grey | side, bot PL | 18 | 4 | 2 | 5 | 55 | 8 | 8 | glue, 0.99 | 0.8% |
| 91.AP.6 Glue tempera | ||||||||||
| black | hair, top PR | 14 | 9 | 7 | 16 | 35 | 18 | 1 | protein | 2% [egg (0.7) glue (0.4)] |
| flesh | neck, bot PR | 12 | 5 | 4 | 7 | 42 | 15 | 15 | glue, 0.99 | 0.6% |
| white | ground | 15 | 7 | 5 | 13 | 45 | 9 | 7 | glue, 0.94 | 3% |
| AS 8940 Glue tempera | ||||||||||
| flesh | 18 | 9 | 6 | 18 | 33 | 12 | 3 | skin, 0.94 | high | |
| black | hair | 19 | 8 | 5 | 13 | 40 | 14 | 3 | glue, 0.93 | high |
| AEIN 685 Gum tempera with glue ground | ||||||||||
| white | ground | 16 | 6 | 1 | 4 | 44 | 18 | 10 | glue, 0.99 | high |
| AEIN 684 Glue tempera | ||||||||||
| red | dress | 21 | 4 | 1 | 3 | 47 | 15 | 10 | glue, 0.99 | high |
| VL.2015.5 Glue tempera | ||||||||||
| dk.grey | edge, top PL | 15 | 8 | 5 | 9 | 43 | 12 | 8 | glue, 0.98 | 1% |
| lt.grey | edge, C bot | 15 | 8 | 5 | 11 | 41 | 12 | 7 | glue, 0.97 | 0.7% |
| M.71.73.62 Glue tempera | ||||||||||
| red | clavi | 16 | 5 | 3 | 7 | 46 | 12 | 11 | glue, 0.99 | med |
| JLS.22226 Glue tempera | ||||||||||
| flesh | eye | 18 | 7 | 4 | 7 | 46 | 10 | 7 | glue, 0.98 | med |
| grey | BG | 17 | 4 | 2 | 4 | 51 | 11 | 10 | glue, 0.99 | med |
| 1935.551 Glue tempera | ||||||||||
| blue | BG | 14 | 3 | 2 | 7 | 50 | 10 | 13 | glue, 0.98 | high |
| red | BG | 14 | 4 | 3 | 8 | 48 | 10 | 13 | glue, 0.97 | low |
| 5-2327 Glue tempera | ||||||||||
| grey | BG, C PR | 20 | 9 | 6 | 10 | 37 | 10 | 8 | glue, 0.96 | high |
| red | tunic | 9 | 4 | 4 | 9 | 42 | 12 | 19 | glue, 0.95 | high |
| 6-21384 Glue tempera | ||||||||||
| red | sleeve, PL | 14 | 4 | 3 | 6 | 51 | 20 | 11 | glue, 0.98 | 5% |
| white | sleeve, PL | 12 | 3 | 3 | 6 | 47 | 11 | 16 | glue, 0.98 | high |
| 6-21385 Glue tempera | ||||||||||
| white | nimbus, PR | 18 | 9 | 7 | 12 | 34 | 12 | 8 | glue, 0.97 | 2% |
| 6-21386 Glue tempera | ||||||||||
| blue | tunic, PL | 20 | 9 | 6 | 12 | 35 | 12 | 6 | glue, 0.95 | high |
| wood | tunic, PL | 17 | 12 | 9 | 16 | 26 | 15 | 6 | protein | high |
| 6-21387 Tempera | ||||||||||
| green | bot PR | 11 | 8 | 9 | 17 | 32 | 17 | 6 | protein | med |
| wood | bot PR | 12 | 8 | 9 | 15 | 37 | 16 | 4 | protein | med |
| UC 14768 Glue tempera | ||||||||||
| white | tunic, C | 16 | 4 | 3 | 7 | 49 | 13 | 9 | glue, 0.99 | high |
| grey | BG, PL | 16 | 4 | 2 | 6 | 47 | 14 | 11 | glue, 0.99 | high |
| ID | Location | rha | fuc | ara | xyl | man | glu | gal | % monos | Match |
|---|---|---|---|---|---|---|---|---|---|---|
| 71.AP.72 Beeswax | ||||||||||
| white | Bot P.R. | 1 | - | 5 | 14 | 7 | 62 | 12 | 0.3% | None |
| white | Bot P.L. | 0.5 | 0.1 | 2 | 9 | 4 | 80 | 4 | 0.3% | None |
| resin | ear P.R. | 0.6 | 0.2 | 4 | 8 | 4 | 77 | 6 | 0.1% | None |
| 75.AP.87 Glue tempera | ||||||||||
| white | tunic, bot., PL | - | - | - | - | - | 96 | 4 | med | none |
| black | BG, C PR | 0.2 | - | 2 | 0.7 | 0.9 | 93 | 3 | vhigh | none |
| 79.AP.141 Beeswax on plant gum black ground | ||||||||||
| white | tunic, PR | |||||||||
| black | BG edge, PR | - | - | 68 | 6 | - | - | 26 | med | Acacia1 |
| wood | edge, C. PR | - | - | 13 | 87 | - | - | - | low | wood |
| 71.AP.142 Glue and gum tempera | ||||||||||
| flesh | hand, bot. PL | 0.1 | - | 3 | 1 | - | 92 | 4 | 20% | starch |
| grey | BG, top PR | 0.2 | - | 20 | 2 | - | 68 | 10 | 12% | gum |
| blue | clavi, bot PL | 0.4 | - | 4 | 2 | - | 86 | 7 | High | starch |
| 71.AP.142 Glue and gum tempera | ||||||||||
| Grey | side, B PL | - | - | 11 | 57 | - | 23 | 8 | 0.1% | wood |
| 81.AP.42 Beeswax | ||||||||||
| linen | shoulder, PL | 1 | 0.2 | 0.5 | 65 | 1 | 29 | 3 | vhigh | wood |
| 91.AP.6 Glue tempera | ||||||||||
| flesh | neck, bot PR | 3 | 0.2 | 1 | 53 | 7 | 13 | 20 | high | wood |
| AEIN 685 Gum tempera | ||||||||||
| green | - | - | 47 | - | - | 9 | 44 | high | Acacia1 | |
| 2009.16 Beeswax | ||||||||||
| brown | eye, PL | - | - | 6 | 11 | - | 41 | 31 | low | none |
| CA 7124 Beeswax | ||||||||||
| grey | edge, top PR | - | - | 14 | 18 | - | 43 | 25 | low | none |
| CA 7013 Beeswax | ||||||||||
| flesh | chest | - | - | - | 45 | - | 39 | 17 | low | none |
| 32.4 Beeswax | ||||||||||
| black | resin | 4 | - | 11 | 29 | - | 4 | 52 | vhigh | none |
| AN1888.342 Beeswax | ||||||||||
| black | BG, edge bot | - | - | 12 | 88 | - | - | - | high | wood |
| wood | BG, edge bot | - | - | 6 | 94 | - | - | - | high | wood |
| 6-21385 Glue tempera | ||||||||||
| white | nimbus, PR | - | - | - | 13 | - | 77 | 20 | low | starch |
| 6-21386 Glue tempera | ||||||||||
| wood | tunic, PL | - | - | - | 2 | - | 97 | 1 | high | wood |
| 6-21387 Glue tempera | ||||||||||
| wood | bot, PR | - | - | - | - | - | 99 | - | vhigh | wood |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, J.; Svoboda, M.; Schilling, M. GC/MS Characterization of Beeswax, Protein, Gum, Resin, and Oil in Romano-Egyptian Paintings. Heritage 2019, 2, 1960-1985. https://doi.org/10.3390/heritage2030119
Mazurek J, Svoboda M, Schilling M. GC/MS Characterization of Beeswax, Protein, Gum, Resin, and Oil in Romano-Egyptian Paintings. Heritage. 2019; 2(3):1960-1985. https://doi.org/10.3390/heritage2030119
Chicago/Turabian StyleMazurek, Joy, Marie Svoboda, and Michael Schilling. 2019. "GC/MS Characterization of Beeswax, Protein, Gum, Resin, and Oil in Romano-Egyptian Paintings" Heritage 2, no. 3: 1960-1985. https://doi.org/10.3390/heritage2030119
APA StyleMazurek, J., Svoboda, M., & Schilling, M. (2019). GC/MS Characterization of Beeswax, Protein, Gum, Resin, and Oil in Romano-Egyptian Paintings. Heritage, 2(3), 1960-1985. https://doi.org/10.3390/heritage2030119





