New Insights into Synthetic Copper Greens: The Search for Specific Signatures by Raman and Infrared Spectroscopy for Their Characterization in Medieval Artworks
Abstract
:1. Introduction
1.1. Medieval Copper Greens
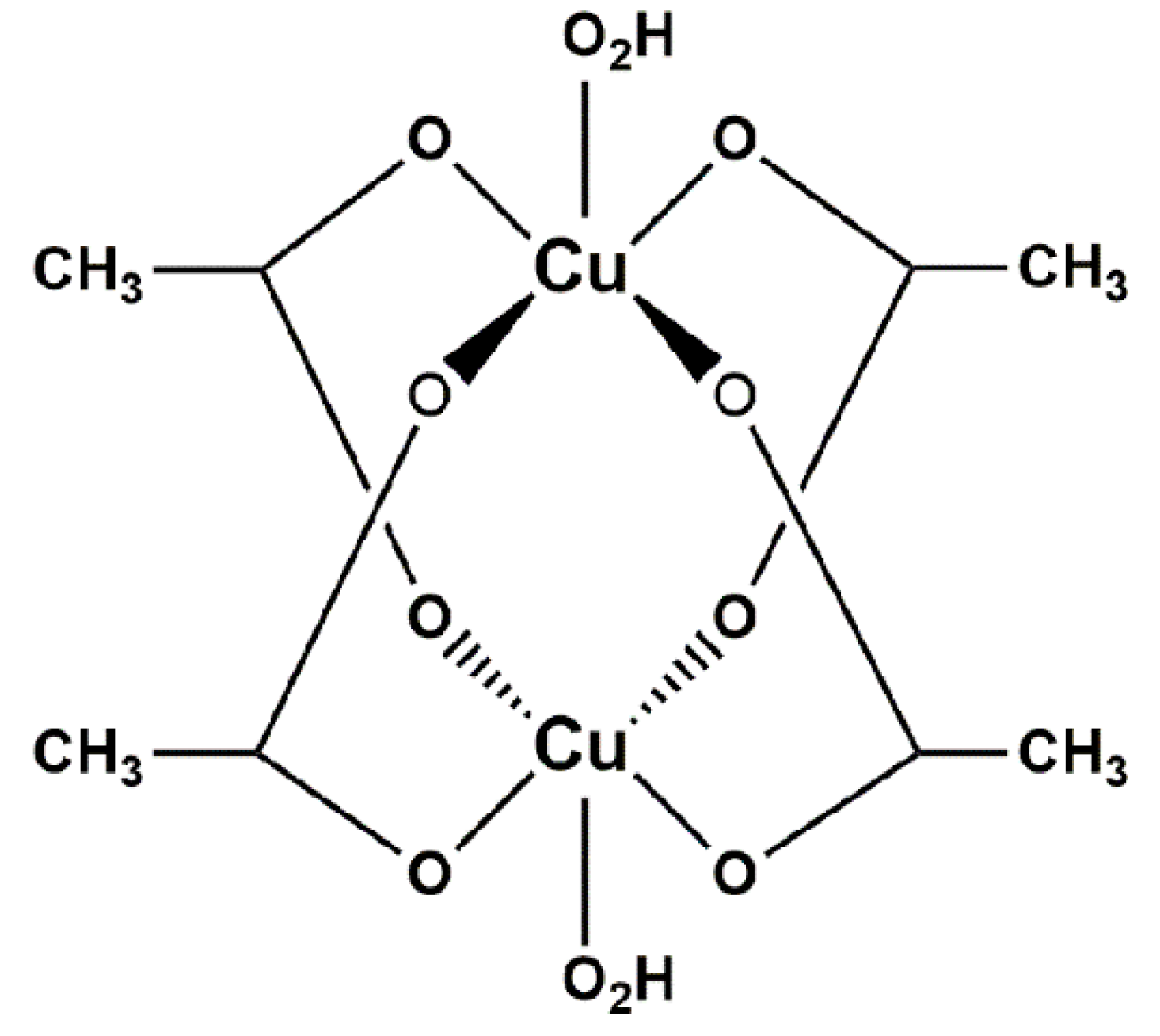
1.2. The Synthesis of Cu(CH3COO)2·H2O Using Copper in the Presence of Acetic Acid
| 4Cu + O2 → 2Cu2O | (1) redox reaction |
| Cu2O + 4CH3COOH → 2Cu(CH3COO) 2·H2O | (2) redox reaction (O2 as oxidant) and ligand exchange |
1.3. Characterizing Verdigris and Other Copper Patinas by Raman Spectroscopy
1.4. Characterizing Verdigris and Other Copper Patinas by Infrared Spectroscopy
2. Experimental
2.1. Materials
2.1.1. Reference Materials
2.1.2. Ingredients
2.2. Preparation of Medieval Copper Greens
2.3. Equipment and Characterization Methods
2.3.1. Raman Microscopy (μRaman)
2.3.2. Fourier Transform Infrared Microspectroscopy (μFTIR)
2.3.3. Micro-Energy Dispersive X-ray Fluorescence (μ-EDXRF)
2.3.4. X-Ray Diffraction (XRD)
3. Results and Discussion
3.1. Characterization of the Synthesized Pigments
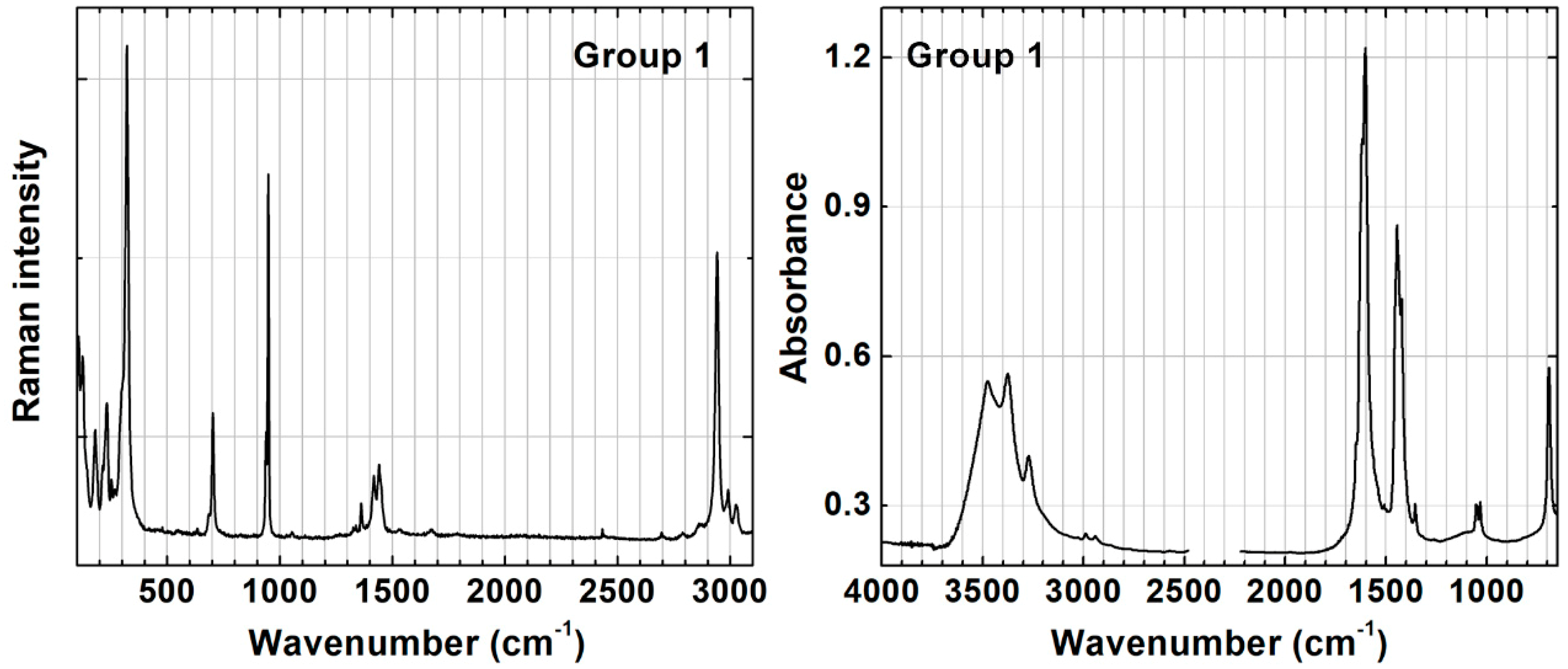
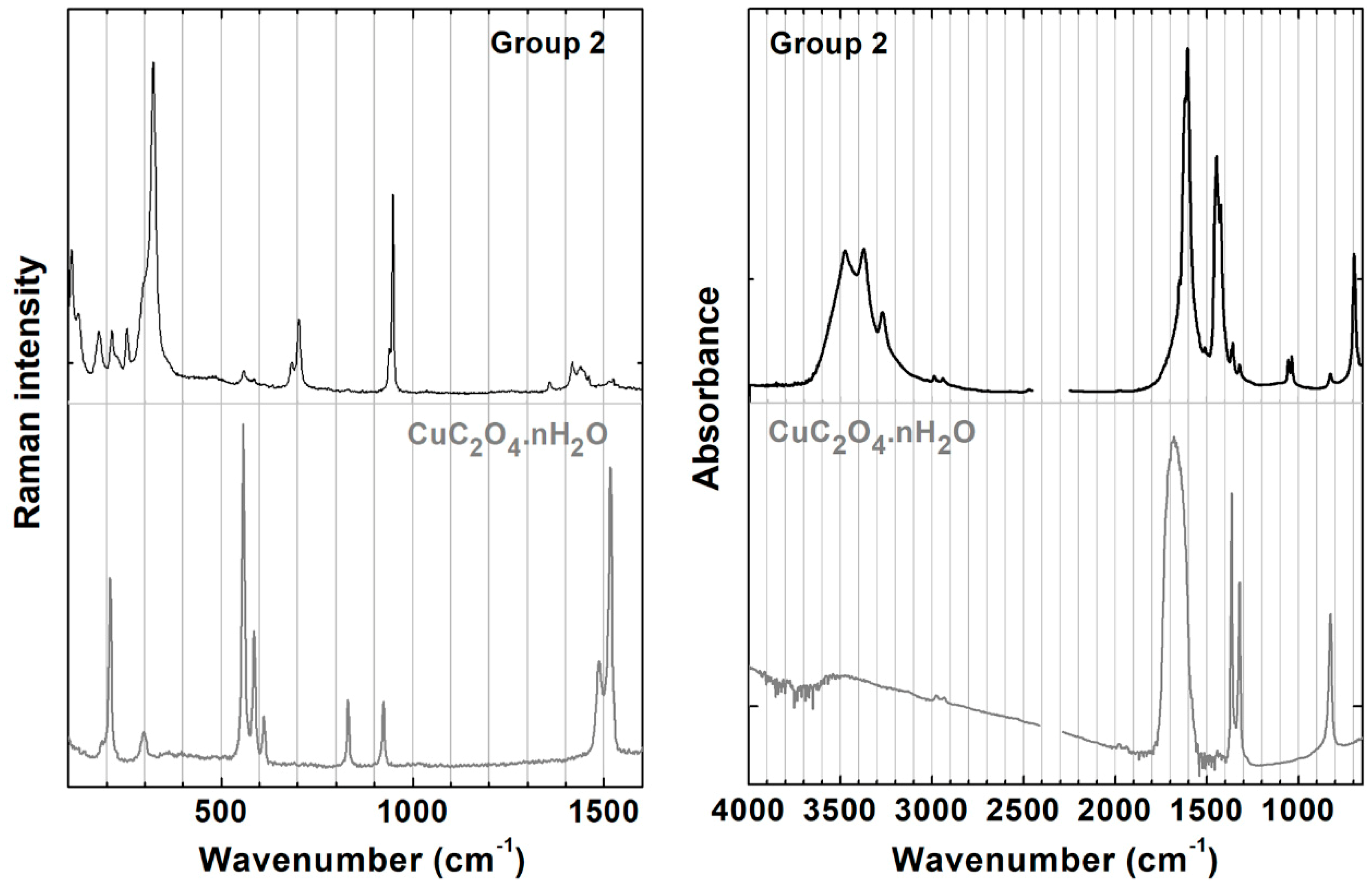
3.2. Detailed Characterization of Groups 1 and 2
3.3. Detailed Characterization of Group 3
3.4. Oxalates as Degradation Products or Markers of Specific Recipes?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kühn, H. Artists’ Pigments: A Handbook of Their History and Characteristics; Ashok, R., Ed.; National Gallery of Art: Washington, DC, USA; Oxford University Press: Oxford, UK, 1993; Volume 2, pp. 131–158.
- Ball, P. Bright Earth E Art and the Invention of Color; Farrar, Straus and Giroux: New York, NY, USA, 2001. [Google Scholar]
- Miguel, C.; Claro, A.; Melo, M.J.; Lopes, J.A. Sources and Serendipity: Testimonies of Artists’ Practice; Hermens, E., Townsend, J.H., Eds.; Archetype: London, UK, 2009; pp. 33–38. [Google Scholar]
- San Andrés, M.; Sancho, N.; Santos, S.; De La Roja, J.M. Fatto D’archimia: Los Pigmentos Artificiales en las Técnicas Pictóricas; Diego, C., Fominaya, M.D., Muiña, I., Eds.; Ministerio de Educación, Cultura y Deporte, Subdirección General de Documentación y Publicaciones: Madrid, Spain, 2012; pp. 197–233.
- San Andreés, M.; De La Roja, J.M.; Santos, S.; Sancho, N. Fatto d’archimia: Los pigmentos artificiales en las técnicas pictóricas; Diego, C., Fominaya, M.D., Muiña, I., Eds.; Ministerio de Educación, Cultura y Deporte, Subdirección General de Documentación y Publicaciones: Madrid, Spain, 2012; pp. 235–257.
- Berg, K.J.V.D.; Hommes, M.H.V.E.; Groen, K.M.; Boon, J.J.; Berrie, B.H. Art et chimie, la couleur: Actes du Congrès; Paris, 16–18 September 1998; Goupy, J., Mohen, J.-P., Eds.; CNRS: Paris, France, 2000; pp. 18–21. [Google Scholar]
- Ricciardi, P.; Pallipurath, A.; Rose, K. ‘It’s not easy being green’: A spectroscopic study of green pigments used in illuminated manuscripts. Anal. Methods 2013, 5, 3819–3824. [Google Scholar] [CrossRef]
- Gilbert, B.; Denoël, S.; Weber, G.; Allart, D. Analysis of green copper pigments in illuminated manuscripts by micro-Raman spectroscopy. Analyst 2003, 10, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.R. Os Livros de Horas (séc. XV) na colecção do Palácio Nacional de Mafra: Estudo e conservação. Master’s Thesis, NOVA University Lisbon, Lisbon, Portugal, 2012. [Google Scholar]
- Salvadó, N.; Pradell, T.; Pantos, E.; Papiz, M.Z.; Molera, J.; Seco, M.; Vendrell-Saz, M.J. Identification of copper-based green pigments in Jaume Huguet’s Gothic altarpieces by Fourier transform infrared microspectroscopy and synchrotron radiation X-ray diffraction. Synchrotron Rad. 2002, 9, 215–222. [Google Scholar] [CrossRef]
- Salvadó, N.; Butí, S.; Cotte, M.; Cinque, G.; Pradell, T. Shades of green in 15th century paintings: Combined microanalysis of the materials using synchrotron radiation XRD, FTIR and XRF. Appl. Phys. A 2013, 111, 47–57. [Google Scholar] [CrossRef]
- Knipe, P.; Eremin, K.; Walton, M.; Babini, A.; Rayner, G. Materials and techniques of Islamic manuscripts. Herit. Sci. 2018, 6, 55. [Google Scholar] [CrossRef]
- Naumova, M.M.; Pisareva, S.A.; Nechiporenko, G.O. Green copper pigments of old Russian frescoes. Stud. Conserv. 1990, 35, 81–88. [Google Scholar]
- Naumova, M.M.; Pisareva, S.A. A note on the use of blue and green copper compounds in paintings. Stud. Conserv. 1994, 39, 277–283. [Google Scholar]
- Hofmann, C.; Hartl, A.; Ahn, K.; Druceikaite, K.; Henniges, U.; Potthast, A. Stabilization of Verdigris. J. Pap. Conserv. 2016, 17, 88–99. [Google Scholar] [CrossRef]
- Kolar, J.; Strlič, M. Iron–gall Inks: On Manufacture, Characterization, Degradation and Stabilization; National and University Library: Ljubljana, Slovenia, 2006; p. 25. [Google Scholar]
- Ahn, K.; Hofmann, C.; Horsky, M.; Potthast, A. How copper corrosion can be retarded—New ways investigating a chronic problem for cellulose in paper. Carbohydr. Polym. 2015, 134, 136–143. [Google Scholar] [CrossRef]
- Hofmann, C.; Hartl, A.; Ahn, K.; Faerber, I.; Henniges, U.; Potthast, A. Studies on the Conservation of Verdigris on Paper. Restaurator 2015, 36, 147–182. [Google Scholar] [CrossRef]
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- López-Delgado, A.; Díaz, E.C.; Rull, J.M.B.; López, F.A. A Laboratory Study of the Effect of Acetic Acid Vapor on Atmospheric Copper Corrosion. J. Electrochem. Soc. 1998, 145, 4140–4147. [Google Scholar] [CrossRef]
- López-Delgado, A.; Díaz, E.C.; Rull, J.M.B.; López, F.A. A comparative study on copper corrosion originated by formic and acetic acid vapours. J. Mater. Sci. 2001, 36, 5203–5211. [Google Scholar] [CrossRef]
- Leygraf, C.; Hedberg, J.; Qiu, P.; Gil, H.; Henriquez, J.; Johnson, C.M. 2007 W.R. Whitney Award Lecture: Molecular in Situ Studies of Atmospheric Corrosion. Corrosion 2007, 63, 715–721. [Google Scholar] [CrossRef]
- Chang, T.; Wallinder, I.O.; De la Fuente, D.; Chico, B.; Morcillo, M.; Welter, J.-M.; Leygraf, C. Analysis of Historic Copper Patinas. Influence of Inclusions on Patina Uniformity. Materials 2017, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Martens, W.N.; Kloprogge, J.T.; Williams, P.A. Raman spectroscopy of the basic copper chloride minerals atacamite and paratacamite: Implications for the study of copper, brass and bronze objects of archaeological significance. J. Raman Spectrosc. 2002, 33, 801–806. [Google Scholar] [CrossRef]
- Drozdzewski, P.; Brozyna, A. Metal isotope and density functional study of the tetracarboxylatodicopper(II) core vibrations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, T.D.; Clark, R.J.H.; Scott, A. Study by Raman microscopy of nine variants of the green-blue pigment verdigris. J. Raman Spectrosc. 2006, 37, 223–229. [Google Scholar] [CrossRef]
- Musumeci, A.; Frost, R.L. A spectroscopic and thermoanalytical study of the mineral hoganite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 67, 48–57. [Google Scholar] [CrossRef]
- San Andrés, M.; Roja, J.M.; Baonza, V.G.; Sancho, N. Verdigris pigment: A mixture of compounds. Input from Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1468–1476. [Google Scholar] [CrossRef]
- Coccato, A.; Bersani, D.; Coudray, A.; Sanyova, J.; Moens, L.; Vandenabeele, P. Raman spectroscopy of green minerals and reaction products with an application in Cultural Heritage research. J. Raman Spectrosc. 2016, 47, 1429–1443. [Google Scholar] [CrossRef]
- Frost, R.L.; Williams, P.A.; Martens, W.; Leverett, P.; Kloprogge, J.T. Raman spectroscopy of basic copper (II) and some complex copper (II) sulfate minerals: Implications for hydrogen bonding. Am. Mineral. 2004, 89, 1130–1137. [Google Scholar] [CrossRef]
- Hayez, V.; Costa, V.; Guillaume, J.; Terryn, H.; Hubin, A. Micro Raman spectroscopy used for the study of corrosion products on copper alloys: Study of the chemical composition of artificial patinas used for restoration purposes. Analyst 2005, 130, 550–556. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, M.C.; Palacios, D.; Coggiola, L.; Baran, E.J. Vibrational and electronic spectra of synthetic moolooite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.; Sarmiento, A.; Martínez-Arkarazo, I.; Madariaga, J.M.; Fernánde, L.A. Green Copper Pigments Biodegradation in Cultural Heritage: From Malachite to Moolooite, Thermodynamic Modeling, X-ray Fluorescence, and Raman Evidence. Anal. Chim. 2008, 80, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Nevin, A.; Melia, J.L.; Osticioli, I.; Gautier, G.; Colombini, M.P. The identification of copper oxalates in a 16th century Cypriot exterior wall painting using micro FTIR, micro Raman spectroscopy and Gas Chromatography-Mass Spectrometry. J. Cult. Herit. 2008, 9, 154–161. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Theory and Applications in Inorganic Chemistry, 6th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2009. [Google Scholar]
- Buti, D.; Rosi, F.; Brunetti, B.G.; Miliani, C. In-situ identification of copper-based green pigments on paintings and manuscripts by reflection FTIR. Anal. Bioanal. Chem. 2013, 405, 2699–2711. [Google Scholar] [CrossRef]
- Monico, L.; Rosi, F.; Miliani, C.; Daveri, A.; Brunetti, B.G. Non-invasive identification of metal-oxalate complexes on polychrome artwork surfaces by reflection mid-infrared spectroscopy. Spectrochim. Acta A 2013, 116, 270–280. [Google Scholar] [CrossRef]
- Švarcová, S.; Hradil, D.; Hradilová, J.; Kočí, E.; Bezdička, P. Micro-analytical evidence of origin and degradation of copper pigments found in Bohemian Gothic murals. Anal. Bioanal. Chem. 2009, 395, 2037–2050. [Google Scholar] [CrossRef]
- Lluveras, A.; Boularand, S.; Andreotti, A.; Vendrell-Saz, M. Degradation of azurite in mural paintings: Distribution of copper carbonate, chlorides and oxalates by SRFTIR. Appl. Phys. A 2010, 99, 363–375. [Google Scholar] [CrossRef]
- Castro, P.; Huber, M.E. Marine Biology, 4th ed.; McGraw-Hill: Boston, MA, USA, 2003. [Google Scholar]
- Theophilus. On Divers Arts: The Foremost Medieval Treatise on Painting, Glassmaking, and Metalwork; Translated, Introduced and Annotated by John, G. Hawthorne e; Cyril Stanley Smith: Dover, UK; New York, NY, USA, 1979; pp. 35, 36, 41. [Google Scholar]
- Strolovitch, D.L. Old Portuguese in Hebrew Script: Convention, Contact, and Convivência. Ph.D. Thesis, Department of Linguistics, Cornell University, Cornell, WI, USA, 2005; pp. 40, 141. [Google Scholar]
- Clarke, M. Mediaeval Painters’ Materials and Techniques: The Montpellier Liber Diversarum arcium; Archetype: London, UK, 2011; p. 112. [Google Scholar]
- Merrifield, M.P. Medieval and Renaissance Treatises on the Arts of Painting: Original Texts with English Translations; Dover: Mineola, NY, USA, 2013; pp. 273, 706. [Google Scholar]
- Bojar, H.-P.; Walter, F.; Baumgartner, J.; Farber, G. Ammineite, CuCl2(NH3)2, a New Species Containing an Ammine Complex: Mineral Data and Crystal Structure. Can. Mineral. 2010, 48, 1359–1371. [Google Scholar] [CrossRef]
- Silva, C.E.; Silva, L.P.; Edwards, H.G.M.; Oliveira, L.F.C. Diffuse reflection FTIR spectral database of dyes and pigments. Anal. Bioanal. Chem. 2006, 386, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Schrader, I.; Wittig, L.; Richter, K.; Vieker, H.; Beyer, A.; Gölzhäuser, A.; Hartwig, A.; Swiderek, P. Formation and structure of copper(II) oxalate layers on carboxy-terminated self-assembled monolayers. Langmuir 2014, 30, 11945–11954. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014. [Google Scholar]
- Frost, R.L.; Yang, J.; Ding, Z. Raman and FTIR spectroscopy of natural oxalates: Implications for the evidence of life on Mars. Chin. Sci. Bull. 2003, 48, 1844–1852. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farwell, D.W.; Rose, S.J.; Smith, D.N. Vibrational spectra of copper (II) oxalate dihydrate, CuC2O4·2H2O, and dipotassium bis-oxalato copper (II) tetrahydrate, K2Cu(C2O4)2·4H2O. J. Mol. Struct. 1991, 249, 233–243. [Google Scholar] [CrossRef]
- Clarke, R.M.; Williams, I.R. Moolooite, a naturally occurring hydrated copper oxalate from Western Australia. Mineral. Mag. 1986, 50, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Martens, W.N.; Frost, R.L.; Williams, P.A. Raman and infrared spectroscopic study of the basic copper chloride minerals—Implications for the study of the copper and brass corrosion and “bronze disease”. Neues Jahrbuch für Mineralogie-Abhandlungen 2003, 178, 197–215. [Google Scholar] [CrossRef]
- Jambor, J.L.; Dutrizac, J.E.; Roberts, A.C.; Grice, J.D.; Szymanski, J.T. Clinoatacamite, a new polymorph of Cu2(OH)3Cl, and its relationship to paratacamite and ‘anarakite’. Can. Mineral. 1996, 34, 61–72. [Google Scholar]
- Braithwaite, R.S.W.; Mereiter, K.; Paar, W.H.; Clark, A.M. Herbertsmithite, Cu3Zn(OH)6Cl2, a new species, and the definition of paratacamite. Mineral. Mag. 2004, 68, 527–539. [Google Scholar] [CrossRef]
- Liu, X.D.; Meng, D.D.; Zheng, X.G.; Hagihala, M.; Guo, Q.X. Mid-IR and Raman Spectral Properties of Clinoatacamite-Structure Basic Copper Chlorides. Adv. Mater. Res. 2010, 146–147, 1202–1205. [Google Scholar] [CrossRef]
- Švarcová, S.; Čermáková, Z.; Hradilová, J.; Bezdička, P.; Hradil, D. Non-destructive micro-analytical differentiation of copper pigments in paint layers of works of art using laboratory-based techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 514–525. [Google Scholar] [CrossRef]
- Banik, G. Discoloration of Green Copper Pigments in Manuscripts and Works of Graphic Art. Restaurator 1989, 10, 61–73. [Google Scholar] [CrossRef]
- Scott, D.A.; Taniguchi, Y.; Koseto, E. The verisimilitude of verdigris: A review of the copper carboxylates. Rev. Conserv. 2001, 2, 73–91. [Google Scholar]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; Getty Conservation Institute: Los Angeles, CA, USA, 2002. [Google Scholar]
- Cariati, F.; Rampazzi, L.; Toniolo, L.; Pozzi, A. Calcium Oxalate Films on Stone Surfaces: Experimental Assessment of the Chemical Formation. Stud. Conserv. 2000, 45, 180–188. [Google Scholar]
- Salvadó, N.; Butí, S.; Nicholson, J.; Emerich, H.; Labrador, A.; Pradell, T. Identification of reaction compounds in micrometric layers from gothic paintings using combined SR-XRD and SR-FTIR. Talanta 2009, 79, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Devine, K.G.; Matveeva, L.N.; Powell, D.H. The missing organic molecules on Mars. Proc. Natl. Acad. Sci. USA 2000, 97, 2425–2430. [Google Scholar] [CrossRef] [Green Version]
- Otero, V.; Vilarigues, M.; Carlyle, L.; Cotte, M.; De Nolf, W.; Melo, M.J. A little key to oxalate formation in oil paints: Protective patina or chemical reactor? Photochem. Photobiol. Sci. 2018, 17, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Miguel, C. Le vert et le Rouge: A Study on the Materials, Techniques and Meaning of the Green and Red Colours in Medieval Portuguese Illuminations. Ph.D. Dissertation, NOVA University Lisbon, Lisbon, Portugal, 2012. [Google Scholar]
- Melo, M.J.; Araújo, R.; Castro, R.; Casanova, C. Colour degradation in medieval manuscripts. Microchem. J. 2016, 124, 837–844. [Google Scholar] [CrossRef]
- Bernard, M.C.; Joiret, S. Understanding corrosion of ancient metals for the conservation of cultural heritage. Electrochim. Acta 2009, 54, 5199–5205. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Maucuer, M.; Meynard, P. On-site Raman and XRF analysis of Japanese/Chinese bronze/brass patina—The search forspecific Raman signatures. J. Raman Spectrosc. 2012, 43, 799–808. [Google Scholar] [CrossRef]
- Ropret, P.; Kosec, T. Raman investigation of artificial patinason recent bronze—Part I: Climaticchamber exposure. J. Raman Spectrosc. 2012, 43, 1578–1586. [Google Scholar] [CrossRef]
- Bongiorno, V.; Campodonico, S.; Caffara, R.; Piccardo, P.; Carnasciali, M.M. Micro-Raman spectroscopy for the characterization of artistic patinas produced on copper-based alloys. J. Raman Spectrosc. 2012, 43, 1617–1622. [Google Scholar] [CrossRef]
- Clarke, M. Middle English Recipes for Painters, Stainers, Scribes and Illuminators; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Dinnebier, R.E.; Fischer, A.; Eggert, G.; Runčevski, T.; Wahlberg, N. X-ray Powder Diffraction in Conservation Science: Towards Routine Crystal Structure Determination of Corrosion Products on Heritage Art Objects. J. Vis. Exp. 2016, 112, 54109. [Google Scholar] [CrossRef] [PubMed]
- Bette, S.; Kremer, R.K.; Eggert, G.; Dinnebier, R.E. On verdigris, part II: Synthesis of the 2-1-5 phase, Cu3(CH3COO)4(OH)2•5H2O, by long-term crystallisation from aqueous solution at room temperature. Dalton Trans. 2018, 47, 8209–8220. [Google Scholar] [CrossRef] [PubMed]
| 1 | In a recipe using brass in an oak box, we also detected the presence of zinc acetate, in agreement with the literature [17]. |
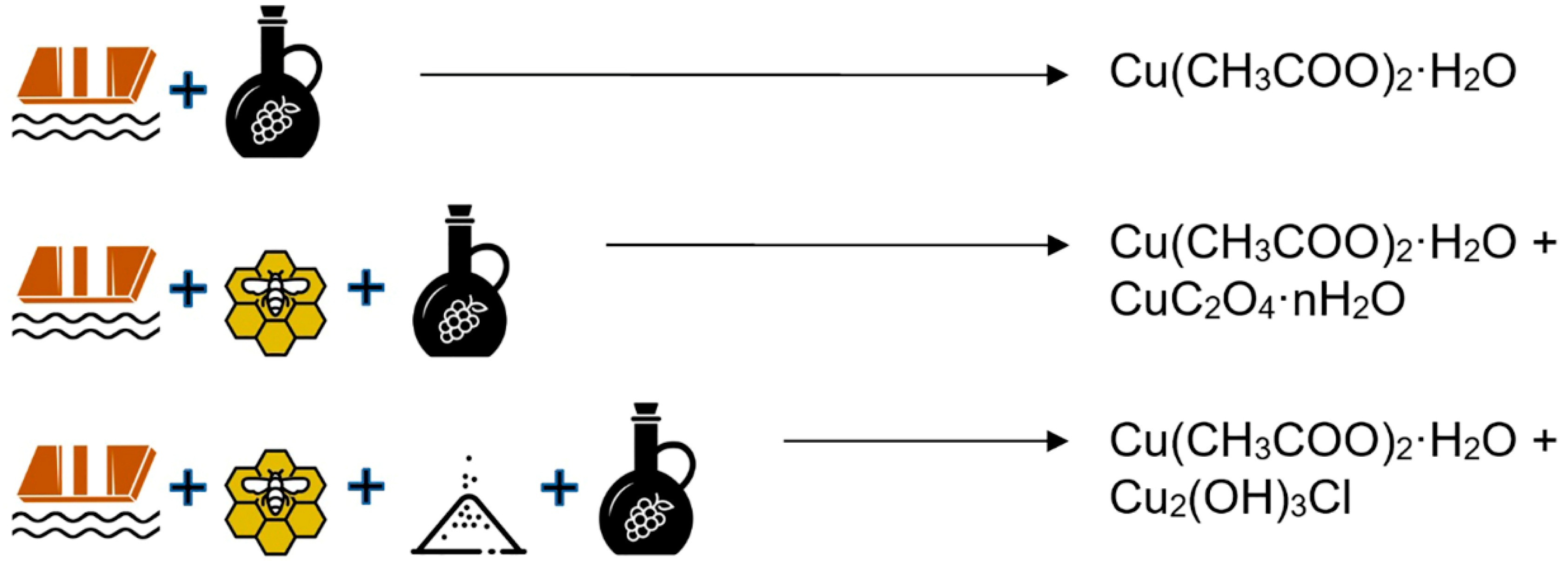

| Reaction Vessel | Group 1 | Group 2 § | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| Cu(CH3COO)2·H2O | Cu(CH3COO)2·H2O CuC2O4·nH2O | Cu(CH3COO)2·H2O Cu2(OH)3Cl CuCl | miscellaneous | no products | ||
| glass vessel |  | 6 | 6 | 1 | 2 | 11 |
| 13.6% | 13.6% | 2.3% | 4.5% | 25% | ||
| oak box |  | 7 | 3 | 5 | 1 | 2 |
| 15.9% | 6.8% | 11.4% | 2.3% | 4.5% |
| Copper Acetate | Copper Oxalate | Basic Copper Chloride | Copper(II) Oxide | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu(CH3COO)2·H2O | CuC2O4·nH2O | Cu2(OH)3Cl | Cu2O | ||||||||
| 231 | m | ν(CuO) | 209 | m | ν(CuO) | 119 | w | 218 | vs | ν(CuO) | |
| 322 | vs | 556 | vs | 147 | w | δ(OCuO) | 418 | w | |||
| 703 | m | δ(OCO) | 585, 610 | sh | 513 | w | ν(CuO) | 630 | w | ||
| 948 | s | ν(CC) | 831 | m | δ(OCO) | 911 | w | δ(CuOH) | |||
| 1418 | sh | δ(CH3) | 924 | m | ν(CC) | 3329 | w | ν(OH) | |||
| 1440 | m | ν(COO−) | 1486 | sh | ν(CO) | 3350 | m | ||||
| 2941 | s | ν(CH) | 1518 | s | 3435 | vs | |||||
| 2989, 3024 | sh | 1615 | w | ||||||||
| Copper Acetate | Copper Oxalate | Basic Copper Chloride | ||||||
|---|---|---|---|---|---|---|---|---|
| Cu(CH3COO)2·H2O | CuC2O4·nH2O | Cu2(OH)3Cl | ||||||
| 629 | m | δ(COO−) | 824 | m | ν(CC) | 849 | w | |
| 692 | m | δ(COO−) | 1320 | s | νs(COO−)/ | 894 | w | |
| 1033 | w | δ(CH3) | 1364 | s | δ(OCO) | 949 | w | δ(OH) |
| 1052 | w | δ(CH3) | 1677 | vs | νa(COO−) | 986 | w | |
| 1445 | s | δ(CH3) | 1655 | w | ||||
| 1602 | vs | ν(COO−) | 3341 | vs | ν(OH) | |||
| 3475, 3375, 3272 | m | ν(OH) | 3443 | sh | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buse, J.; Otero, V.; Melo, M.J. New Insights into Synthetic Copper Greens: The Search for Specific Signatures by Raman and Infrared Spectroscopy for Their Characterization in Medieval Artworks. Heritage 2019, 2, 1614-1629. https://doi.org/10.3390/heritage2020099
Buse J, Otero V, Melo MJ. New Insights into Synthetic Copper Greens: The Search for Specific Signatures by Raman and Infrared Spectroscopy for Their Characterization in Medieval Artworks. Heritage. 2019; 2(2):1614-1629. https://doi.org/10.3390/heritage2020099
Chicago/Turabian StyleBuse, Juliana, Vanessa Otero, and Maria J. Melo. 2019. "New Insights into Synthetic Copper Greens: The Search for Specific Signatures by Raman and Infrared Spectroscopy for Their Characterization in Medieval Artworks" Heritage 2, no. 2: 1614-1629. https://doi.org/10.3390/heritage2020099
APA StyleBuse, J., Otero, V., & Melo, M. J. (2019). New Insights into Synthetic Copper Greens: The Search for Specific Signatures by Raman and Infrared Spectroscopy for Their Characterization in Medieval Artworks. Heritage, 2(2), 1614-1629. https://doi.org/10.3390/heritage2020099





