Abstract
Sodium-ion batteries (SIBs) have demonstrated noticeable development since the 2010s, being complementary to the lithium-ion technology in predominantly large-scale application niches. The projected SIB market growth will inevitably lead to the generation of tons of spent cells, posing a notorious issue for proper battery lifecycle management, which requires both the establishment of a regulatory framework and development of technologies for recovery of valuable elements from battery waste. While lithium-ion batteries are mainly based on layered oxides and lithium iron phosphate chemistries, the variety of sodium-ion batteries is much more diverse, extended by a number of other polyanionic families (crystal types), such as NASICON (Na3V2(PO4)3), Na3V2(PO4)2F3−yOy, (0 ≤ y ≤ 2), KTiOPO4-type AVPO4X (A—alkali metal cation, X = O, F) and β-NaVP2O7, with all of them relying on vanadium and phosphorous—critical elements in a myriad of industrial processes and technologies. Overall, the greater chemical complexity of these vanadium-containing phosphate materials highlights the need for designing specific recycling approaches based on distinctive features of vanadium and phosphorus solution chemistry, fine-tuned for the particular electrodes used. In this paper, an overview of recycling methods is presented with a focus on emerging chemistries for SIBs.
1. Introduction
The development of energy harvesting and storage technologies is an integral part of changes under way in many sectors of the economy and everyday life, spanning from portable electronics and electric vehicles to local power supplies and renewable electricity generation [1,2,3,4]. Currently, lithium-ion batteries (LIBs) are one of the key enablers for efficient energy management. However, the rapidly growing LIB production faces a number of issues, such as unevenly distributed Li resources, long and intermittent supply chains, negative socio-economic aspects of raw materials mining, and environmental concerns [5,6,7]. This in turn has stimulated research on complementary metal-ion battery (MIB) architectures, with notable progress achieved in recent decades [8,9]. Among them, sodium-ion batteries (SIBs) are considered as reasonable candidates for several niche applications, for example, at large-scale stationary storage facilities [10,11,12,13]. The expected growth of the SIB market will unavoidably bring about the generation of tons of spent batteries demanding proper recycling and utilization. This can be achieved through realization of a closed-loop cycle economy that requires the development of regulatory frameworks and specific technologies. This statement is strongly supported by recent decisions made by several countries, as covered in the following reviews [14,15].
The sodium-ion battery architecture is very close to that of lithium-ion batteries [12]. The most distinguishing differences entail the usage of a wider variety of active electrode materials as well as an Al current collector on the anode side instead of the Cu in LIBs, resulting in the capacity for safe discharge and storage at 0 V. The obvious similarities to LIBs (electrolyte design, salt chemistries in general, material of separator, etc.) allows for the transfer of almost all methods of cell pretreatment and separation of conductive additives, binder and electrochemically active compounds (so-called “black mass”) from the already established recycling technologies. These technological aspects are areas of particular interest and are covered in a review by R. Sommerville et al. [16]. Another fundamental issue—the lack or absence of a detailed and unified battery labeling system—has also attracted battery research community attention and was articulated by Bao et al. recently [17].
In general, the choice of a battery recycling approach is hugely dominated by the positive electrode (cathode) chemistry. While for LIBs, cathodes mainly rely on two chemistries—layered oxides of lithium and transition metals (TM) and lithium iron phosphate (LiFePO4, LFP) and its derivatives, the playground for sodium-ion batteries is noticeably larger (Figure 1). Several representatives of the following crystal types, namely (Na3V2(PO4)2F3−yOy, 0 ≤ y ≤ 2), NASICON (Na3V2(PO4)3), KTiOPO4 (AVPO4X, A—alkali metal cation, X = O, F) and KAlP2O7 (β-NaVP2O7), demonstrate attractive electrochemical properties, and some have already been commercialized [18,19,20,21,22,23]. Interestingly, all of the abovementioned formulas rely on vanadium—a critical component in many sectors of industry [24,25]. Lately, several reports have been devoted to the recycling of used vanadium catalysts or steel industry slags [25]. However, poor attention was paid to phosphate-containing or phosphate- and fluoride-containing systems [26,27,28], and even less attention to the life cycle of vanadium-based battery electrode materials [29].
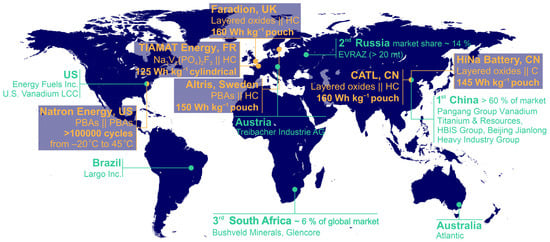
Figure 1.
Key vanadium producers and SIB R&D according to [30,31,32,33,34]. Vanadium market data are given for 2021. PBAs—Prussian blue analogues, HC—hard carbon, C—carbon.
In this paper, we provide an overview of processing methods for used MIBs and reported approaches for the treatment of vanadium-containing phosphate ores (phosphorites/phosphate rocks) and related mixtures, including slags. The chemistry of vanadium is discussed from the perspective of designing recycling approaches for sodium-vanadium-based oxo- and fluoride phosphate electrode materials for SIBs. This paper is organized as follows. In the first chapter, state-of-the-art recycling techniques are reviewed. The second part is devoted to the current status of vanadium production and consumption. Further, selected vanadium-based electrode materials for SIBs are compared, and a discussion of vanadium–phosphorus solution chemistry is presented. In the last part, phosphorus-containing wastewater treatment methods are highlighted.
2. Overview of Metal-Ion Battery Recycling Methods
Inevitable performance degradation during operation limits the lifetime of any MIB, thus determining the necessity of developing recycling methods for used batteries. The degradation processes can be divided into two groups: physical and chemical. Chemical degradation is driven by irreversible structural transformations, which lead to capacity loss, and/or by partial dissolution of metal cations and various reactions that occur on the electrodes’ surface coupled with electrolyte decomposition [5,35,36,37]. Physical (or mechanical) degradation is associated with defect formation and stress accumulation, resulting in particles cracking and detaching, which mainly occur due to the crystal lattice breathing as a result of mobile ion insertion and removal during cycling [38]. In many cases (overcharge, physical damage, etc.), the battery cannot be reused; thus, full disassembly with subsequent recycling should be applied. The initial stage of recycling processing usually includes careful sorting, pre-discharge and, if necessary, partially or fully automated disassembly [17]. The classification of battery recycling methods will follow and is schematically shown in Figure 2.
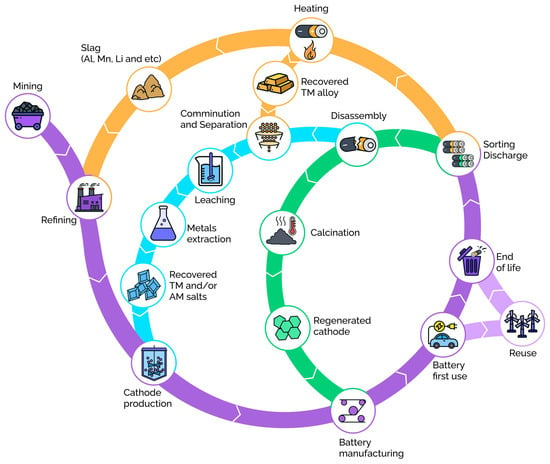
Figure 2.
Schemes of direct (green), pyrometallurgical (orange), and hydrometallurgical (light blue) recycling methods for recovery of active materials in MIBs. Violet depicts initial electrode material synthesis from raw resources, battery assembly and use. TM = transition metal, AM = alkali metal.
(1) Pyrometallurgical methods were first widely implemented in the industry. The approach implies multi-stage heating. At low temperatures (<200 °C), the electrolyte evaporates, and the polymer binder melts. Further heating at higher temperatures is necessary to burn the battery case and other component polymer-containing fractions. The required temperature, as a rule, depends on the specific technology and composition of the batteries being processed, but can reach 1500 °C. The products of the pyrometallurgical process are metal alloy, slag and gases. The resulting flux is leached to obtain pure metal compounds, and the slag is separated. Normally, the slag contains a certain amount of lithium, but a significant proportion of it is lost with vapors and evolved gases. The search for methods to reduce lithium losses in the pyrolysis process is a separate technological problem in the field of metallurgy and chemical technology. The disadvantages of this approach include the low quality of the extracted materials, high energy costs, as well as high capital expenditures due to the need to capture, clean up and utilize harmful gases [5,39].
(2) Hydrometallurgical approaches are implemented based on reactions in solutions and dissolution of electrode materials with a subsequent extraction in the form of pure salts, oxides or hydroxides [39,40,41]. Materials previously separated from other parts of the battery are most often leached by acidic aqueous solutions, with sulfuric acid and hydrogen peroxide being the most common combination of inorganic reagents used [40]. Also of practical interest are organic acids, which are large-scale products of the chemical industry and whose anions can act as complexing agents (for example, oxalic, citric, malic, tartaric, succinic, ascorbic acids) [40,42]. At the next stage, metal salts are isolated either through selective precipitation by adjusting the pH value of the solution, or by extraction using organic solvents. The unconditional advantages of hydrometallurgy include the conversion of metal compounds into a soluble form convenient for further use, as well as lower temperatures required for the processing procedure, which reduces its energy consumption. Hydrometallurgical production is characterized by a higher degree of extraction if proper processing and sorting of raw materials have been performed. However, it requires using high-concentration leaching agents and leads to the formation of large amounts of waste solutions; all of these features increase the number of technological units.
(3) Direct recycling consists in the regeneration of the used electrode (usually cathode) material without introducing a significant deviation from its chemical composition. The used MIBs are sorted and further disassembled into individual components. At the next stage of production, the electrode mass is separated from the aluminum tape and calcined to restore the crystal structure of materials suitable for use in storage devices. Heat treatment allows one to decrease the number of defects in the crystals and to remove organic residues that form during prolonged cycling and negatively affect the electrochemical performance. This method is the most advantageous in terms of lithium recovery efficiency. The main drawbacks of this approach are the technical complexity and complicated time-consuming processes, which increase production costs. In addition, this group of methods can also include the steps of disassembling the used batteries, mechanically cleaning possible foreign inclusions, and filling with a fresh portion of the electrolyte [43].
The vast majority of works related to the processing of MIB materials are aimed at studying the process of leaching and selective extraction of Li, Co, Ni and Mn compounds. Typical objects of study are widely used layered oxides (including Li-NMC, Li-NCA) [5,44], oxides with a spinel structure (LiMn2O4, LiMn1.5Ni0.5O4) [5,14,40], as well as triphylite-structured phosphates (LiFePO4) [17,45]. To date, the enterprises of industrial groups have implemented a joint approach for the processing of batteries, combining pyrometallurgical and hydrometallurgical methods. The application of pyrometallurgical methods is primarily due to the lack of proper labeling (see above), as well as significant differences in battery sizes and form factors. A small proportion (a few percent) of used batteries are subjected to “direct recycling” (or regeneration), meaning they are manually disassembled in an inert environment, filled with a fresh portion of the electrolyte, and subsequently sealed [14]. The latest developments in the direct recycling methods are more of a technical problem at the intersection of chemistry, chemical technology, and engineering; their investigation is of particular interest for some niche applications.
3. Vanadium: Occurrence in Nature, Industrial Production and Toxicity
Vanadium is generally a rather widespread element in the Earth’s crust [46] ranking 5th among the metals of the d-block [47,48]). Notably, its abundance is higher than that of Ni and Co combined, which are critical metals of modern industry, twice as large in comparison with Cu, and slightly exceeds that of Cr. In this regard, vanadium provides an attractive basis for the design of functional materials including battery electrodes (Table 1).

Table 1.
Abundances (10–4 wt. %) of chemical elements used in batteries in the upper continental crust, according to [46] and [47] respectively.
Figure 3 shows elements that will play a key role in the manufacture of SIBs with vanadium-based cathodes: Na, V, P, F (cathode design), Al (current collectors), Fe (battery casing). All of them are widespread in the Earth’s crust, and much more evenly distributed in comparison with lithium resources, which gives the sodium-ion technology a certain advantage. In addition to these elements, carbon will be an important constituent of SIB electrodes, being most likely produced from biomass [49,50].
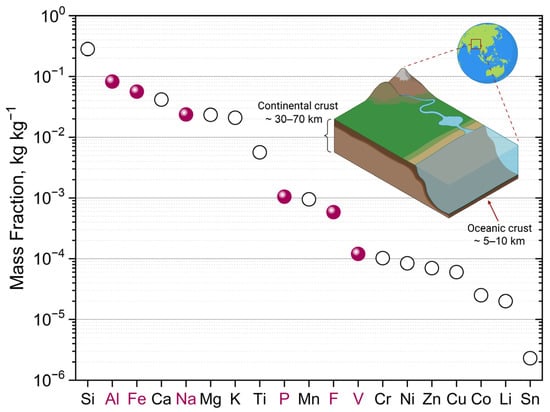
Figure 3.
Elemental abundance in the Earth’s upper continental crust. Adapted from [59] with permission. Key elements, used for the production of phosphate-based electrode materials for sodium-ion-batteries are highlighted in color. Copyright (2014) American Chemical Society. Created with BioRender.com (accessed on 6 June 2023).
Vanadium occurs naturally in a variety of minerals (around 90), such as patronite (VS4), vanadinite (Pb5(VO4)3Cl), carnonite (K2(UO2)2(VO4)2·3H2O), descloizite ((Pb,Zn)2(OH)VO4), etc. [51]. It was also found in oil as a by-product [52,53]. However, vanadium is predominantly mined from titanomagnetite ores, where it isomorphically replaces iron due to the proximity of their ionic radii (64.5 pm for Fe3+ (high-spin state) and 64 pm for V3+, coordination number 6) [54,55]. Oil ash [56], spent catalysts [57] and vanadium sludge [58] can also be useful sources for extracting vanadium. In nature, vanadium mostly occurs in the V5+ or V4+ oxidation states as various vanadates or vanadyl-containing compounds, respectively. Furthermore, V3+ or V2+ are also known in both the solid and solution states.
The thermodynamic data for vanadium species in solution were recently summarized by Shaheen et al.; the ESHE-pH diagram is given in Figure 4 [60]. Analysis of this plot allows one to estimate the stability of a specific state of the element as a function of pH. For example, this ESHE-pH diagram provides a quick understanding of the formation of insoluble V3+ compounds in a moderately alkaline environment, while in a strongly alkaline solution, HVO42− (which is soluble) is the most thermodynamically probable anion. Non-single component systems are more complex, however, if the elements are separated—an example of such a method follows in the section below. The ESHE-pH diagram might be a useful tool for the research in battery recycling.
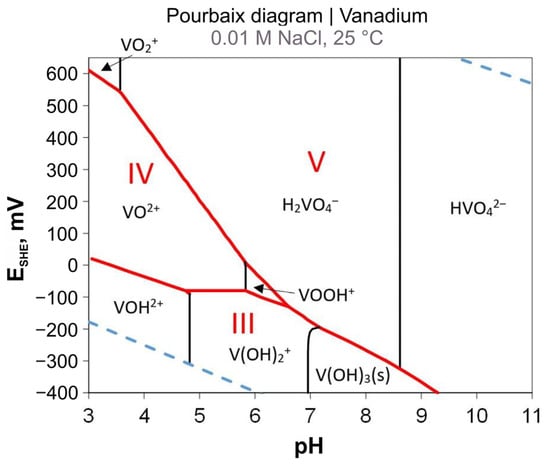
Figure 4.
ESHE–pH diagram of vanadium species in water at a total vanadium concentration of 1 µmol L−1, in a background electrolyte of 0.01 M NaCl at 25 °C. The roman numerals III, IV, and V indicate the predominance fields of V3+, V4+ and V5+, respectively. Red lines designate the transition between oxidation states, and the blue dashed lines delimit the stability window for water. Reproduced with permission from [60]. Copyright (2019) Elsevier.
The global vanadium ore market was estimated to be valued at $2.78 billion in 2022 [61]. The biggest three vanadium producers are China, Russia and South Africa. China (predominantly Pangang Group Vanadium Titanium & Resources) accounts for more than 60% of metal supplies. In Russia, the main producer is EVRAZ Vanadium Tula JSC [62,63], and in South Africa, they are Bushveld Minerals and Glencore [25]. The Brazilian company Largo Inc. is also an important market player, being a supplier of high-purity metallic vanadium (powder and flakes) [34]. Vanadium production is an industrial process, still characterized by a strong ecological footprint, that causes soil, water and air contamination and/or the formation of technologically dictated landfills mainly consisting of Ti-containing gypsum slags [24,64,65,66].
Most vanadium (about 92% by mass in 2021) is used in the production of construction steels (including ferrovanadium), with China being the main consumer. A small percentage is consumed in catalyst production, and a certain amount is reserved for vanadium redox flow batteries and for academic purposes [24,25].
The degree of vanadium compound toxicity is still an object of discussion. It significantly depends on the solubility, as well as morphology and the particle’s size. For example, a negative effect on metabolic processes in the body was observed in rats when given vanadium in feed and water [67]. A number of studies demonstrated a non-negligible impact of vanadium on people’s health; one case study, for example, was the town of Chusovoi, where a vanadium plant is located [68]. The maximum allowable concentration (MPC) of vanadium in water in the Russian Federation is 0.1 mg L−1. For Europe, a similar range was reported [68]. In the USA, the amount of vanadium in drinking water is subject to monitoring (it is listed on Candidate List 4, CCL4) [69].
Due to very limited data, it is currently difficult to accurately estimate the scale of the global vanadium (in the area of batteries) or SIB markets. This is due to the fact that most SIB production lines are expected to start manufacturing cells in 2023–2024 and reach full capacity even later. Nevertheless, it is worth mentioning that the forecast provided by CIC energiGUNE (with data by Deloitte) predicts that the growth of market demand for sodium-ion batteries will reach 270 GWh in 2025 [70].
4. Sodium-Vanadium Oxo- and Polyanions: Prospective Electrode Materials for SIBs
From a commercial point of view, vanadium oxo-(phosphates and pyrophosphates) and polyanions (fluoride phosphates) are extremely interesting due to the high working potential caused by the inductive effect of anionic groups, outstanding structural and thermal stability, as well as small variation in unit cell volume during operation of the cathode material. Na3V2(PO4)3 NASICON (in nature, represented as a member of the kosnarite mineral family [71]) has been known since the end of the 1980s [72,73], and its substituted derivatives are one of the most popular objects of study in the battery materials scientific community. Many efforts have been dedicated to achieving a reversible deinsertion of 3 Na+ per formula unit (f.u.). Interestingly, full deinsertion of 3 Na+ per f.u. through the chemical route has been reported only once [73], and to date, that result has not been reproduced [59]. Stable cycling performance was demonstrated for 2 Na+ per f.u., resulting in 118 mAh g−1 of specific capacity with a flat operating voltage profile at ~3.4 V vs. Na+/Na (gravimetric energy density ~400 Wh kg−1) (Figure 5). Vanadium substitution is claimed as a promising strategy for enhancing the electrochemical performance of members of this class [74,75,76].
A number of other oxoanions were investigated as electrode materials during the 2010s and 2020s. Promising results were achieved for β-NaVP2O7, which belongs to the KAlP2O7 crystal type [77,78]. β-NaVP2O7 delivers 104 mAh g−1 (0.1C charge/discharge current rate) at an average potential of 3.9 V vs. Na+/Na (energy density ca. 406 Wh kg−1, Figure 5) coupled with small volume changes (around 0.5%) [77,79].
The Na-V-P-O-F system has been widely investigated by many research groups since the beginning of the 2000s. To date, three different crystal types represented by a number of solid-solutions (O-to-F ratio and/or series of compounds, where another cation is substituted for V) have been found.
Na3V2(PO4)2F3−yOy (0 ≤ y ≤ 2) are characterized by attractive gravimetric energy density values of about 500 Wh kg−1 for the Na3V2(PO4)2F3 end-member (128 mAh g−1 for 2Na+ cycling, two plateaus at 3.7 and 4.2 V vs. Na+/Na, Figure 5) [80,81,82]. The fluorine-to-oxygen ratio directly affects the electrochemical properties of the corresponding fluoride-phosphate, and currently, the end-member attracts practical interest mostly. Tiamat—a spinoff of the RS2E network in France—assembled the first Na3V2(PO4)2F3||HC 18650 cell prototype (energy density of 90 Wh kg−1) for high-power application a few years ago. So far, energy density of 125 Wh kg−1 has been achieved, and at least 4000 cycles at 80 % capacity retention have been reported [31] (Figure 1). Furthermore, an E-Scooter was powered by Tiamat (18650 Na-ion cells) recently [83].
NaVPO4F is another representative of the Na-V-P-O-F system. An insufficiently thorough chemical and structural analysis of the first-ever synthesized “NaVPO4F” [84] led to some confusion in the battery society for almost two decades regarding the existence of representatives with such “NaVPO4F” stoichiometry. Since then, questionable tetragonal (SG: I4/mmm) and monoclinic (SG: C2/c) forms with the ascribed “NaVPO4F” composition have long been discussed in the literature as promising cathode materials for SIBs [85,86]. However, as shown later from the perspectives of synthesis, crystal structure and electrochemical properties, the claimed forms simply represent tetragonal Na3V2(PO4)2F3 and monoclinic Na3V2(PO4)3 phases. For instance, a direct annealing of VPO4 with a source of fluorine and sodium (normally, NaF) in an inert atmosphere with strict control of synthesis conditions in order to avoid loss of fluorine, which was suggested as a synthesis route for the abovementioned questionable “NaVPO4F” forms, typically leads to stabilization of the Na3V2(PO4)2F3 composition (wrongly considered as a tetragonal “NaVPO4F”). Further heating at elevated temperatures induces its decomposition, coupled with crystallization of Na3V2(PO4)3 (incorrectly perceived as a monoclinic “NaVPO4F”) due to elimination of volatile VF3 [86].
In fact, the NaVPO4F composition can be stabilized only in two structural types: tavorite and potassium titanyl phosphate (KTiOPO4 or KTP). Due to the structural features, tavorite-NaVPO4F does not demonstrate any practically interesting capacities, thus remaining almost electrochemically inactive against Na [87,88,89]. In contrast, the KTP-structured NaVPO4F (or k-NaVPO4F), reveals attractive characteristics. It is synthesized through an ion exchange reaction between a sodium-salt and NH4VPO4F precursor [90]. KTP-NaVPO4F shows 136 mAh·g−1 discharge capacity at a 0.1 C rate, with an average operating potential of about 4.0 V vs. Na+/Na (gravimetric energy density of ca. 540 Wh·kg−1, Figure 5a,b) and is characterized by impressive high-rate performance (123 mAh g−1 discharge capacity at 40 C current rate in half-cell). More importantly, unlike its polyanionic competitors, whose theoretical energy density limits are nearly reached, KTP-NaVPO4F still has significant room for improvement [20,90,91]. In the sections below, the analysis of the closest case studies to these chemistries will be provided; here, it is worth mentioning that the synthesis of the materials normally includes several solution-based stages, and the recycling approaches also will be formulated on the basis of the solution chemistry of the corresponding elements.
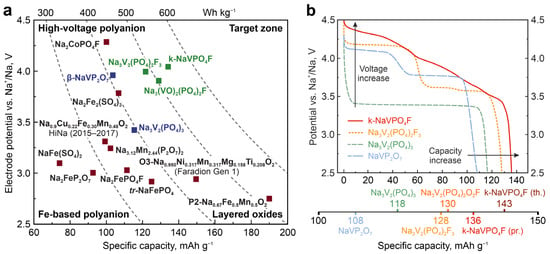
Figure 5.
(a) Selected Na-ion cathode materials. Phosphates and fluoride-phosphates are depicted with blue and green, respectively. Data for HiNa layered oxide are provided according to Chayambuka et al. [83]; data for Faradion Gen 1 oxide are provided according to Rudola et al. [12]. (b) Potential vs. specific capacity plots for Na3V2(PO4)3, Na3V2(PO4)2F3, β-NaVP2O7 and k-NaVPO4F per one-electron V4+/V3+ transition from experimental data. Adapted with permission from [90]. CC BY 4.0.
5. Hydrometallurgical Processing of Vanadium Compounds and Phosphates
5.1. Case 1: Direct Recycling of NASICON-Based Cell
To the best of our knowledge, only one report (case 1) was devoted to the formulation of a recycling approach toward vanadium-based electrodes of SIBs. In the pioneering work of Tiefeng Liu and colleagues [29], a design of a so-called “fully recyclable rechargeable sodium-ion battery” was proposed (Figure 6a,b). Two cases were considered: the first one is a half-cell vs. metallic sodium, and the second one is a symmetric cell. The authors used Na3V2(PO4)3 as a positive electrode material. The cell was dismantled after operation manually. In the first case, all components made of Al were dissolved in basic media (the basic medium was provided by metallic Na dissolution), while the Na3V2(PO4)3 was separated and treated at 550 °C for 4 h in air to remove the carbon coating, conductive additive, and polymer binder. In the second case, all components made of Al were dissolved by immersing in the NaOH solution. The total recycling efficiency was claimed to be as high as 98% (Figure 6a). From a formal point of view, the proposed utilization scheme can be technically assigned to the group of direct recycling methods—manual disassembly with the use of a coin hydraulic crimper was demonstrated as one unit of the process. It seems that Na3V2(PO4)3 was assumed by the authors to be a highly stable and robust electrode material that is not affected by noticeable degradation in a chemical or physical way. If the battery is damaged, the state-of-charge is difficult to calculate precisely, and pretreatment of the active material before high-temperature annealing becomes a very complex task. At the same time, the authors state that oxidation of vanadium was observed (Figure 8 of supporting information in [29]). The further analysis of XRD patterns (refinement based on powder X-ray diffraction data of initial and treated electrode) would be useful for better understanding of the material evolution after the aforementioned manipulations; heat treatment in air may lead to the formation of impurities or induce partial decomposition (Figure 6b). Finally, the use of metallic sodium as anode is currently valid for laboratory research mainly, since it is still extremely hazardous to utilize in a device or vehicle. In general, this approach can be applied conceptually to cells that are not dramatically damaged and to electrodes that do not degrade strongly.
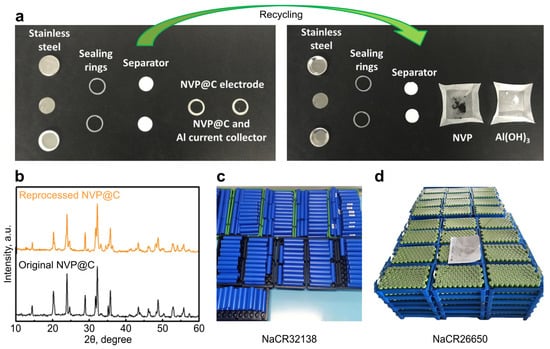
Figure 6.
(a) Optical images of the original and recycled components of an asymmetric bipolar SIB cell with Na3V2(PO4)3/C cathode and metallic Na anode. (b) XRD pattern of initial and reprocessed Na3V2(PO4)3/C composites. (c) View of HiNa Battery Technology Co., Ltd. NaCR32138 batteries. (d) View of HiNa Battery Technology Co., Ltd. NaCR26650 batteries. Adapted (a,b) from Liu et al. [29]. CC BY 4.0. Digital photos of HiNa Battery Technology Co., Ltd. Cells are provided at the company’s website (https://www.hinabattery.com/en/ (accessed on 15 April 2023)).
Technology-wise, commercial SIBs will be implemented in blocks and modules of various types, which can significantly complicate the direct recycling procedure (Figure 6c,d). Compared to laboratory studies, additional technological steps will be required. However, these can be most likely borrowed from the lithium-ion battery recycling technology. Moreover, it makes sense for manufacturers of SIBs to learn from the mistakes of the lithium-ion technology and try to unify cell form factors and geometries, which will greatly facilitate recycling processes.
5.2. Case 2: Acidic Leaching of LiFePO4
The closest case study (case 2) relevant to the design of phosphate-containing vanadium compound recycling concerns the treatment of triphylite-structured LiFePO4 (further, LiFePO4 or tr-LiMPO4). All groups of recycling methods were studied in general for tr-LiMPO4, starting from the early 2000s [45]. In the context of the current market and existing technologies, pyrometallurgical methods are of no interest for the phosphate-based electrode materials. In contrast hydrometallurgical methods are reasonable from an economic point of view. In the following section, the LiFePO4 case study will be reviewed, and valid leaching strategies for the above-discussed V compounds will be discussed.
Hydrometallurgical methods of LiFePO4 treatment can be divided into two subgroups. The first one deals with Li extraction only [45]. The second one is based on complete dissolution of this phosphate with subsequent precipitation of Li, Fe and P in the form of salts. This method was successfully realized by a few companies, including a battery recycling pioneer—Recupyl (France) [40,92]. The dissolution reaction occurs according to the following equation:
2LiFePO4(s) + H2SO4(aq) + H2O2(aq) → 2FePO4(s) + Li2SO4(aq) + 2H2O
In the original patent by F. Tedjar and J.-C. Foudraz, sulfuric acid was utilized [92]. A number of other inorganic acids were reported during the last two decades; the most relevant choices for industry are hydrochloric (HCl) and nitric (HNO3) acids [40,44]. Oxalic (H2C2O4) and citric (C6H8O7) acids were employed as metal leaching agents during recent decades, as well [42]. It is worth mentioning that the reaction should occur at elevated temperatures (e.g., 80 °C). Technological schemes in patents typically include units that deal with iron (another metal cation, e.g., Mn2+) and phosphorus; however, only lithium extraction demonstrates economical interest in the case of LiFePO4. This statement is relevant for other lithium phosphates that can be found on the market.
As soon as both the abundance (Table 1, Figure 2) and market availability of sodium compounds are noticeably higher than those for lithium, the first subgroup of methods will become non-relevant for sodium-vanadium compounds. In light of those facts, only complete dissolution (second subgroup in this notation) may be valid.
5.3. Case 3: Mining Industry: Vanadium as a By-Product
Another case study (case 3) closely relevant to electrode materials has to do with vanadium leaching from ores (uranium ores, phosphorites, bauxites) [93,94,95,96]. From a chemical point of view, vanadium-rich phosphorites (ore used for production of phosphates) are quite similar to the objects of discussion in this paper. Typically, the raw material contains vanadium compounds in mixed oxidation states. Two approaches have been realized in both scientific and industrial communities for the extraction of V4+ or V5+ species. For example, in the technology proposed by a factory in Colorado (Cotter Corporation, Canon city, Colorado, USA, in the 1980s [95]), all residual VO2+ cations are oxidized to VO2+ cations, and a tertiary amine (not specified originally) is used to extract V5+:
2VO2+ + H2O2 → 2VO2+ + 2H+
VO3− + R3NH+ = R3NHVO3
As was claimed by the authors, an excess of hydrogen peroxide should be avoided in this reaction (Equations (1) and (2)); this suggests that formation of vanadium peroxo complexes negatively affects the vanadium extraction yield [96]. Some features of vanadium peroxocomplex chemistry are discussed separately at the end of this section.
5.4. Case 4: Study of Vanadium-Phosphorus Separation from Slag
Detailed research (case 4) devoted to the investigation of phosphorus-rich vanadium converter slag (in this context, the converter slag is a mixture, composed of many phases), that forms as a by-product in this industry [24,66]) was published recently [27]. The authors provided an analysis of different forms of vanadium, phosphorus and their mixed species (heteropolyanions) in solution and proposed three schemes for selective vanadium or phosphorus leaching that are based on the interplay between the pH value and choice of an extraction agent. The key findings by Zhang et al. are summarized in Figure 7 [27].
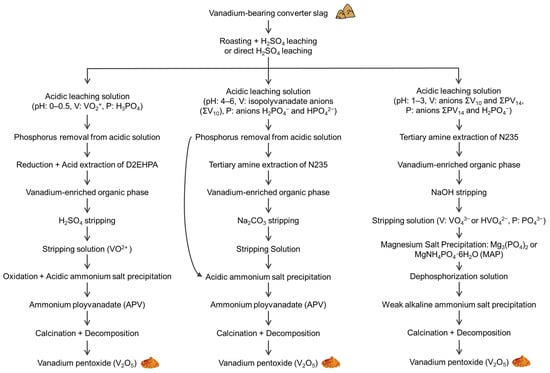
Figure 7.
Scheme of selective V and P leaching from P-rich vanadium-bearing converter slag proposed in [27]. ΣV10 refers to the isopolyvanadate anions, and ΣPV14 refers to the P-V heteropolyacid anions. Adapted with permission from Zhang et al. [27]. Copyright (2019) Elsevier.
All told, the selective extraction of V5+- or V4+-based species is an area of particular interest [97]. Extraction agents such as N235 (widely available commercial tertiary amine, CAS 68814-95-9), D2EHPA (Di-(2-ethylhexyl)phosphoric acid), PRIMENE 81R (CAS 68955-53-3), and ALAMINE 336 (CAS 1116-76-3) were reported for this purpose, and research in this area is still in progress [98,99]. Methods from the area of catalysis, metallurgy and the mining industry represent a viable basis for designing V-based electrode material treatment processes.
5.5. Leaching of Vanadium-Phosphates: Identifying the Strategy
The next paragraphs are organized as follows. Firstly, two scenarios of the initial leaching of compounds are proposed (examples are considered for Na3V2(PO4)3; the features specific for fluoride-phosphates, e.g., Na3V2(PO4)2F3, are discussed separately). Secondly, aspects of vanadium solution chemistry are presented and described. Finally, methods of processing phosphate-containing wastewater are given. It is assumed that all preprocessing manipulations, including black mass pretreatment, are provided in the context of technologies that are already implemented in industry. Of note, both the initial substance and the resulting compound can be contaminated by a residual conductive additive. Their influence on human and animal health is still an open question [100].
In the first scenario, a vanadium compound should be dissolved in a non-oxidizing acid (in order to avoid vanadium oxidation); thus, HCl seems the most reasonable choice among inorganic agents. The reaction in strongly acidic media can be described by the following equation:
Na3V2(PO4)3 + 9HCl = 2V3+ + 9Cl− + 3Na+ + 3H3PO4
Since V3+ is a strong reductant, the reaction should be conducted free of oxidizing agents; that includes initially degassing the acid solution of O2. However, heating and/or the use of complexing agents might be required to improve dissolution kinetics. An important feature of such a reaction to be mentioned is the possible formation of mixed vanadium-phosphorus species, i.e., heteropolyanions (for example, H5PV14O424−, H4PV14O425− and H3PV14O426−)—they were overviewed in the case study (case 4) of the phosphorus-containing vanadium bearing slag [27]. The key peculiarity to be highlighted is that the pH and concentration interplay allows for the separation of vanadium and phosphorus compounds with rather high efficiency. It seems that the aforementioned technological scheme for phosphorites (case 3) implies that vanadium-phosphorus heteropolyanion formation is a rather minor process.
In the second step, vanadium can be precipitated by adjusting the pH (bases, (NH4)2S or NH3 solution). According to the literature data, this precipitate is described by the VOOH formula [48,84]. In fact, V3+ species are easily oxidized and readily undergo transformation to VO(OH)2 in the presence of an oxidant, for example, O2 dissolved in water [93,94,96]. If V3+ is managed properly, it can be annealed, resulting in V2O3 (heat treatment in inert media) or in V2O5 (heat treatment in the presence of O2) [84]. Additionally, a stoichiometric mixture of V(III) and V(V) can be used for the synthesis of VO2 through the coproportionation reaction [101]. All vanadium oxides are valuable industrial products that can be further utilized for the synthesis of any vanadium-based materials [25]. Stabilization of V3+ in the form of an adduct with tetrahydrofuran (VCl3·3THF), a cyanide complex (K3[V(CN)6]), or other vanadium complexes such as carboxylates should not be neglected. For example, the first compound from this list is commercially available, stable at ambient conditions and soluble both in water and in organic solvents [48]. The proposed scheme is summarized in Figure 8.
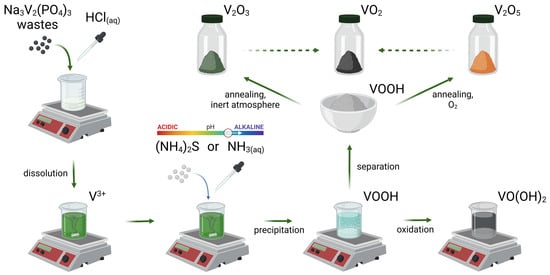
Figure 8.
Pathways of the possible recycling process for vanadium-based electrode materials (on Na3V2(PO4)3 example). Created with BioRender.com (accessed on 6 June 2023).
The second scenario for V compounds relies on a reaction with H2O2 in the presence of an acid. The original Recupyl scheme (Equation (1)) for LiFePO4 includes hydrogen peroxide, H2O2, acting as an oxidizing agent for Fe2+ cations, allowing one to shift the equilibrium to the reaction products [93,94,96]. In the case of vanadium, the oxidant leads to the transformation of V3+ to VO2+ with subsequent formation of V5+ species (the reaction scheme is given for strongly acidic media, pH < 2):
V3+ + H2O2 = [VO2]+ + 2H+
Noteworthily, VO2+ is stable only under strongly acidic conditions. With the increase in pH, it hydrolyzes, forming oxy-anions that tend to polymerize and form protonated polyanions ([HyV2xO5x+2]y−4) [97]. Stabilization of the vanadium (4+) cation is a particular task that should be solved by optimizing the amount of the oxidizing agent, temperature and reaction time. The so-called vanadyl (VO2+) cation can be stabilized by complexation with widespread ligands generated by citric acid, oxalic acids, etc. [102,103,104].
In the presence of an H2O2 excess in the reaction mixture, the formation of V5+ peroxo complexes is possible:
V3+ + 2H2O2 + 2H2O = [VO(O-O)(H2O)3]+ + 2H+
The monoperoxo complex [VO(O-O)(H2O)3]+ cation is stable in a strong acidic medium (pH < 2) and characterized by frank red color. In the pH range 2–8, a yellow vanadium diperoxo complex [VO(O-O)2H2O]− is in equilibrium with monoperoxo ones (Table 2). It can be separated by a number of routes. One of them is precipitation of specific compounds such as ammonium oxodiperoxoamminvanadate NH4[VO(O-O)2(NH3)]. Another is stabilization of [VO(O-O)(H2O)3]+ by organic molecules (Figure 9). The chemistry and synthesis of vanadium peroxocomplexes have been widely investigated, and some of them are irreplaceable in specific niche applications, for example, as specific reagents for the epoxide group introduction into organic molecules (Figure 9) [96,105]. The complexes’ stoichiometries are summarized in Table 2.

Table 2.
Forms of vanadium peroxo complexes in solution in various pH ranges. Adapted from [48] based on literature data [96,105].
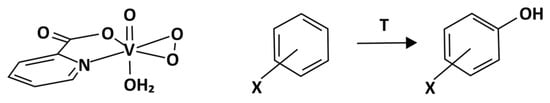
Figure 9.
An example of a V peroxocomplex structure and its application to benzene and substituted benzene hydroxylation reactions [105]. Reproduced with permission from [105]. Copyright (1996) Wiley.
The similarity between these phosphates hints at almost the same reaction scheme for NaVP2O7 (in inert atmosphere) dissolution in acidic media:
NaVP2O7 + H2O + 4H+ = Na+ + V3+ + 2H3PO4
Na3V2(PO4)2F3 + 6HCl = 2V3+ + 6Cl− + 3Na+ + 2H3PO4 + 3F−
If an oxidizing agent is added, formation of peroxocomplexes will be triggered, as was indicated earlier (Equation (6), Table 2). However, it should be noted that fluoride ions have to be removed from wastewater due to their toxicity [106]. Additionally, some experimental difficulties, related to the reaction of fluoride anions with the material from which the reactor is made, might occur. Further complex analysis of Na/V/P/O/F-containing solutions is necessary to obtain a better understanding of technological prospects. It is worth mentioning that the treatment of the residual mixture (solution containing phosphate, fluoride, or other anions) does not demonstrate any economic benefit currently—similarly to the LiMPO4 and related Li-transition metal phosphates. Nevertheless, processing of phosphorus-fluorine-containing wastewater is an important environmental problem that is becoming a necessary stage of sewage management. It can be provided using already established industrial methods; for example, chemical fluoride anion removal is mainly based on the formation of insoluble salts, such as CaF2 (Ca(OH)2 + 2F– = CaF2↓ +2OH–) [106]. Finally, one should note that purification of recycling products, such as V2O5, is a specific task relevant for studies in the chemical technology area. According to the literature data, the typical purity of V2O5 (or any other source of vanadium used in electrode material synthesis) is at least 98% [20,21,36,75,90]. Further growth of electrode materials production will shed light on this technological aspect and allow drawing a solid conclusion regarding the necessity of the development of purification technologies.
5.6. Management of Phosphorus-Containing Sewage
The technological units for phosphate-containing wastes are well-developed now and widely implemented for water purification [107,108,109,110]. Modern technologies used in urban sewage treatment plants include chemical, physico-chemical and biochemical units. The following reactions are the basis for a group of chemical methods.
Aluminum salts (Al3+) are typical coagulants that easily form an AlPO4 precipitate:
Al2(SO4)3·nH2O + 6HCO3− = 2Al(OH)3↓ + 3SO42− + nH2O + 6CO2↑
Al2(SO4)3·nH2O + 2PO43− = 2AlPO4↓ + 3SO42− + nH2O
Similarly, Fe3+ salts can be used:
Fe3+ + PO43− = FePO4↓
If Ca2+ is used, calcium carbonate or oxyapatite is precipitated:
Ca(OH)2 + HCO3− = CaCO3↓ + H2O + OH−
5Ca2+ + 4OH− + 3HPO42− = Ca5(OH)(PO4)3↓ + 3H2O
In the presence of NH4+, the reaction below occurs (possible both for Mg2+, Ca2+):
NH4+ + Mg2+ + PO43− + nH2O = NH4MgPO4·nH2O↓
3Mg2+ + 2PO43− = Mg3(PO4)2↓
This set of reactions does not cover all the complexities of wastewater treatment. Other technologies, including clay- and zeolite-based methods and ion-exchange resins, are also employed nowadays [106,111].
6. Summary and Outlook
The direct recycling approach for SIBs seems to have room for further improvement; additionally, the attention of the scientific and industrial communities in this direction will be affected by many factors, including the availability of, and market prices for, vanadium compounds. The hydrometallurgy of used triphylite-structured electrodes is now widely developed, and several technological units can be easily transferred to sodium-vanadium phosphates, while further optimization and fine-tuning with regard to vanadium and phosphorus solution chemistries will be required to achieve high efficiency. For instance, the formation of vanadium-phosphorus heteropolyanions may be a noticeable obstacle that should be paid particular attention. Furthermore, the ability of vanadium to form peroxocomplexes can be both an advantage and disadvantage at the same time. However, this feature provides vast opportunities for developing new, selective and refined methods of vanadium extraction. Contrarily, the design of organic compounds with lower toxicity and fewer costs, mainly amines, for vanadium leaching and optimization of the vanadium extraction processes presents additional, prospective directions for R&D.
This review covers only selected aspects of vanadium and phosphorus chemistry that are relevant for the development of a closed-loop life cycle economy and production of SIBs. Many other methods, based on some new or already known findings—such as liquid–liquid extraction, solid-phase extraction with resins for functionalized silica adsorbents, or sequential extraction, which are widely used in the soil sciences and in analytical chemistry—can be a viable basis for further recycling design [60,112].
In general, Na-ion battery chemistries have displayed notable progress in the last 10 years. Upon commercial deployment and further mass production, tons of spent battery wastes might penetrate into the environment, posing ecological risks unless properly recycled. To mitigate the potential ecological impact, a unified regulatory framework for all MIBs should be formulated and implemented, and specific chemical approaches for recycling battery materials and recovering critical components should be developed. Since vanadium-based electrode materials are of interest to Na-ion technology, in this review, we discussed the recycling techniques that are currently relevant or that might be adapted for vanadium- and phosphate-containing electrode materials, including those with fluorine, illustrated and elaborated in several case studies, and considered key strategies for creating and designing recycling technologies for batteries in the context of existing scientific and industrial reports.
Author Contributions
Conceptualization, A.S.S. and S.S.F.; validation, A.S.S., S.S.F. and A.V.I.; writing—original draft preparation, A.S.S. and A.V.I.; writing—review and editing, A.S.S., A.V.I. and S.S.F. All authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Russian Science Foundation (#17-73-30006).
Acknowledgments
The authors are grateful to Alexandra Ivannikova for her help with creating the scheme of MIB recycling methods (Figure 2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meyer, D.C.; Leisegang, T.; Zschornak, M.; Stöcker, H. Electrochemical Storage Materials: From Crystallography to Manufacturing Technology; De Gruyter: Berlin, Germany, 2018; ISBN 978-3-11-049398-6. [Google Scholar]
- Arutyunov, V.S.; Lisichkin, G.V. Energy resources of the 21st century: Problems and forecasts. Can renewable energy sources replace fossil fuels? Russ. Chem. Rev. 2017, 86, 777. [Google Scholar] [CrossRef]
- Arutyunov, V. Is it Possible to Stabilize the Earth Climate by Transition to Renewable Energy? Eurasian Chem.-Technol. J. 2021, 23, 67–75. [Google Scholar] [CrossRef]
- Antipov, E.V.; Abakumov, A.M.; Drozhzhin, O.A.; Pogozhev, D.V. Lithium-Ion Electrochemical Energy Storage: The Current State, Problems, and Development Trends in Russia. Therm. Eng. 2019, 66, 219–224. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Fröhlich, P.; Lorenz, T.; Martin, G.; Brett, B.; Bertau, M. Valuable Metals-Recovery Processes, Current Trends, and Recycling Strategies. Angew. Chem. Int. Ed. 2016, 56, 2544–2580. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Tarascon, J.-M. Na-ion versus Li-ion Batteries: Complementarity Rather than Competitiveness. Joule 2020, 4, 1616–1620. [Google Scholar] [CrossRef]
- Hasa, I.; Mariyappan, S.; Saurel, D.; Adelhelm, P.; Koposov, A.Y.; Masquelier, C.; Croguennec, L.; Casas-Cabanas, M. Challenges of today for Na-based batteries of the future: From materials to cell metrics. J. Power Sources 2021, 482, 228872. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of high energy density sodium-ion batteries: Faradion’s journey and outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Komaba, S. Sodium-driven Rechargeable Batteries: An Effort towards Future Energy Storage. Chem. Lett. 2020, 49, 1507–1516. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Bird, R.; Baum, Z.J.; Yu, X.; Ma, J. The Regulatory Environment for Lithium-Ion Battery Recycling. ACS Energy Lett. 2022, 7, 736–740. [Google Scholar] [CrossRef]
- Sommerville, R.; Stewart, J.-S.; Goodship, V.; Rowson, N.; Kendrick, E. A review of physical processes used in the safe recycling of lithium ion batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Whittingham, M.S.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Khasanova, N.R.; Drozhzhin, O.A.; Yakubovich, O.V.; Antipov, E.V. Mineral inspired electrode materials for metal-ion batteries. In Comprehensive Inorganic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Samarin, A.S.; Trussov, I.A.; Fedotov, S.S. Electrode Materials for Reversible Sodium Ions de/Intercalation. In Comprehensive Inorganic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Fedotov, S.S.; Samarin, A.S.; Antipov, E.V. KTiOPO4-structured electrode materials for metal-ion batteries: A review. J. Power Sources 2020, 480, 228840. [Google Scholar] [CrossRef]
- Fedotov, S.S.; Samarin, A.S.; Nikitina, V.A.; Stevenson, K.J.; Abakumov, A.M.; Antipov, E.V. α-VPO4: A Novel Many Monovalent Ion Intercalation Anode Material for Metal-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 12431–12440. [Google Scholar] [CrossRef]
- Fedotov, S.S.; Khasanova, N.R.; Samarin, A.S.; Drozhzhin, O.A.; Batuk, D.; Karakulina, O.M.; Hadermann, J.; Abakumov, A.M.; Antipov, E.V. AVPO4F (A = Li, K): A 4 V Cathode Material for High-Power Rechargeable Batteries. Chem. Mater. 2016, 28, 411–415. [Google Scholar] [CrossRef]
- Nikitina, V.A.; Fedotov, S.S.; Vassiliev, S.Y.; Samarin, A.S.; Khasanova, N.R.; Antipov, E.V. Transport and Kinetic Aspects of Alkali Metal Ions Intercalation into AVPO4F Framework. J. Electrochem. Soc. 2017, 164, A6373–A6380. [Google Scholar] [CrossRef]
- Moskalyk, R.; Alfantazi, A. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Petranikova, M.; Tkaczyk, A.; Bartl, A.; Amato, A.; Lapkovskis, V.; Tunsu, C. Vanadium sustainability in the context of innovative recycling and sourcing development. Waste Manag. 2020, 113, 521–544. [Google Scholar] [CrossRef]
- Shalavina, E.L.; Tarasenko, V.; Tyurekhodzhaeva, T.S.; Zazubin, A.I. Patent, of Vanadium-Bearing Solution from Phosphorus Purification. Available online: https://yandex.ru/patents/doc/SU206089A1_19671202 (accessed on 6 June 2023). (In Russian).
- Zhang, W.; Zhang, T.; Lv, G.; Cao, X.; Zhu, H. Thermodynamic study on the V(V)-P(V)-H2O system in acidic leaching solution of vanadium-bearing converter slag. Sep. Purif. Technol. 2019, 218, 164–172. [Google Scholar] [CrossRef]
- Tarabrin, G.K.; Biryukova, V.A.; Rabinovich, E.M.; Chekalin, V.V.; Merzlyakov, N.E.; Kuz’michev, S.E.; Tartakovskij, I.M.; Frolov, A.T.; Tarabrina, V.P.; Chutchikov, V.N. Method of Vanadium Exctration. 1998. Available online: https://yandex.ru/patents/doc/RU2102511C1_19980120 (accessed on 6 June 2023). (In Russian).
- Liu, T.; Zhang, Y.; Chen, C.; Lin, Z.; Zhang, S.; Lu, J. Sustainability-inspired cell design for a fully recyclable sodium ion battery. Nat. Commun. 2019, 10, 1965. [Google Scholar] [CrossRef]
- Liu, S.; Xue, W.; Wang, L. Extraction of the Rare Element Vanadium from Vanadium-Containing Materials by Chlorination Method: A Critical Review. Metals 2021, 11, 1301. [Google Scholar] [CrossRef]
- Tan, K.; Ren, Y. Practical Application of Room Temperature Na-Ion Batteries. In Sodium-Ion Batteries: Materials, Characterization, and Technology; Wiley: Hoboken, NJ, USA, 2022; ISBN 978-3-527-82576-9. [Google Scholar]
- Rudola, A.; Sayers, R.; Wright, C.J.; Barker, J. Opportunities for moderate-range electric vehicles using sustainable sodium-ion batteries. Nat. Energy 2023, 8, 215–218. [Google Scholar] [CrossRef]
- Bushveld Minerals. Available online: https://www.bushveldminerals.com/ (accessed on 15 April 2023).
- Largo Inc. Available online: https://www.largoinc.com/ (accessed on 15 April 2023).
- Li, L.; Zhang, N.; Su, Y.; Zhao, J.; Song, Z.; Qian, D.; Wu, H.; Tahir, M.; Saeed, A.; Ding, S. Fluorine Dissolution-Induced Capacity Degradation for Fluorophosphate-Based Cathode Materials. ACS Appl. Mater. Interfaces 2021, 13, 23787–23793. [Google Scholar] [CrossRef]
- Desai, P.; Forero-Saboya, J.; Meunier, V.; Rousse, G.; Deschamps, M.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. Mastering the synergy between Na3V2(PO4)2F3 electrode and electrolyte: A must for Na-ion cells. Energy Storage Mater. 2023, 57, 102–117. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Wang, C.-M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Beaudet, A.; Larouche, F.; Amouzegar, K.; Bouchard, P.; Zaghib, K. Key Challenges and Opportunities for Recycling Electric Vehicle Battery Materials. Sustainability 2020, 12, 5837. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- How Batteries Are Recycled in Russia. Available online: https://www.rbc.ru/ (accessed on 15 March 2021). (In Russian).
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Slater, P.; Abbott, A.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Dutta, S.; Alessi, D.S.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C. Recycling of lithium iron phosphate batteries: Status, technologies, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 163, 112515. [Google Scholar] [CrossRef]
- Yaroshevsky, A.A. Abundances of chemical elements in the Earth’s crust. Geochem. Int. 2005, 44, 48–55. [Google Scholar] [CrossRef]
- Carmichael, R.S. Practical Handbook of Physical Properties of Rocks and Minerals; CRC Press: Boca Raton, FL, USA, 1989; ISBN 978-0-203-71096-8. [Google Scholar]
- Tret’yakov, Y.D.; Martynenko, L.I.; Grigoriev, A.N.; Tsyvadze, A.Y. Neorganicheskaya Himiya; V 3 Tomah; Akademiya: Chennai, India, 2004; ISBN 5-7695-1437-X. (In Russian) [Google Scholar]
- Lakienko, G.P.; Bobyleva, Z.V.; Apostolova, M.O.; Sultanova, Y.V.; Dyakonov, A.K.; Zakharkin, M.V.; Sobolev, N.A.; Alekseeva, A.M.; Drozhzhin, O.A.; Abakumov, A.M.; et al. Sosnowskyi Hogweed-Based Hard Carbons for Sodium-Ion Batteries. Batteries 2022, 8, 131. [Google Scholar] [CrossRef]
- Bobyleva, Z.V.; Drozhzhin, O.A.; Alekseeva, A.M.; Dosaev, K.A.; Peters, G.S.; Lakienko, G.P.; Perfilyeva, T.I.; Sobolev, N.A.; Maslakov, K.I.; Savilov, S.V.; et al. Caramelization as a Key Stage for the Preparation of Monolithic Hard Carbon with Advanced Performance in Sodium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 181–190. [Google Scholar] [CrossRef]
- Yakovleva, E.V. Gidrotermal’nyj Sintez v Boro-Fosfatnyh Sistemah, Soderzhashchih Vanadij i Shchelochnye Kationy, i Issledovanie Produktov Kristallizacii. 2008. Available online: http://cryst.geol.msu.ru/literature/kurs/2008_03_yakovleva.pdf (accessed on 6 June 2023).
- Yashchenko, I.G. Heavy oil of Russia. Izv. TPU 2012, 105–111. Available online: https://earchive.tpu.ru/handle/11683/4325 (accessed on 6 June 2023). (In Russian).
- Navarro, R.; Guzman, J.; Saucedo, I.; Revilla, J.; Guibal, E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Manag. 2007, 27, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Results for the transition metals and quantification of the factors underlying bond-length variation in inorganic solids. IUCrJ 2020, 7, 581–629. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Goncharov, K.V.; Kashekov, D.Y.; Sadykhov, G.B.; Olyunina, T.V. Processing of fuel oil ash from thermal power plant with extraction of vanadium and nickel. Non-Ferr. Met. 2020, 1, 3–7. [Google Scholar] [CrossRef]
- Pak, J.-J.; Kim, D.-H.; Paek, M.-K.; Kim, Y.-D. Ferroalloy Production from Spent Petroleum Catalysts by Reductive Smelting and Selective Oxidation Processes. In REWAS2019; Springer: Cham, Switzerland, 2019; pp. 167–175. [Google Scholar] [CrossRef]
- Kologrieva, U.; Volkov, A.; Zinoveev, D.; Krasnyanskaya, I.; Stulov, P.; Wainstein, D. Investigation of Vanadium-Containing Sludge Oxidation Roasting Process for Vanadium Extraction. Metals 2021, 11, 100. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Alessi, D.S.; Tack, F.M.; Ok, Y.S.; Kim, K.-H.; Gustafsson, J.P.; Sparks, D.L.; Rinklebe, J. Redox chemistry of vanadium in soils and sediments: Interactions with colloidal materials, mobilization, speciation, and relevant environmental implications-A review. Adv. Colloid Interface Sci. 2019, 265, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Vanadium Ore Global Market Report 2023; Research and Markets: Dublin, Ireland, 2023. [Google Scholar]
- Volkov, A.I. Status and perspective of heavy metal use in Russia. ROIN 2020, 11–18. (In Russian) [Google Scholar]
- EVRAZ JSC. Annual Report 2021 EVRAZ JSC. 2021. Available online: https://www.evraz.com/en/investors/reports-and-results/annual-reports/ (accessed on 16 April 2023).
- Savinkova, S.A. Influence of vanadium compound on people health in Tula. Chem. Lett. 2016, 30–35. (In Russian) [Google Scholar]
- Vatolin, N.A.; Halezov, B.D.; Krasheninin, A.G.; Bornovolokov, A.S. Complex Eco-Friendly Technology of Mn-Bearing Vanadium Slags for the Extraction of Vanadium Pentaozide with Higher Purity; Conference Report. 2012, pp. 8–12. Available online: http://www.semikonf.ru/upload/iblock/9ca/Sbornik_Prioritetnye_napravleniya.pdf#page=8 (accessed on 6 June 2023). (In Russian).
- Goncharov, K.V. Odnostadijnyj Process Pryamogo Polucheniya Zheleza i Titanovanadievogo Shlaka iz Titanomagnetitovyh Koncentratov i Gidrometallurgicheskoe Izvlechenie Vanadiya iz Shlaka. Ph.D. Thesis, Baikov Institute RAS, Moscow, Russia, 2015. (In Russian). [Google Scholar]
- Tripathi, D.; Mani, V.; Pal, R.P. Vanadium in Biosphere and Its Role in Biological Processes. Biol. Trace Element Res. 2018, 186, 52–67. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N. Standards for the contents of heavy metals and metalloids in soils. Eurasian Soil Sci. 2012, 45, 321–328. [Google Scholar] [CrossRef]
- Contaminant Candidate List 4-CCL 4. Available online: https://www.epa.gov/ccl/contaminant-candidate-list-4-ccl-4-0 (accessed on 15 April 2023).
- The Sodium-ion Battery Boom: The Perfect (and Sustainable) Complement to Lithium-Ion Batteries. Available online: https://cicenergigune.com/en/blog (accessed on 19 July 2022).
- Yakubovich, O.; Khasanova, N.; Antipov, E. Mineral-Inspired Materials: Synthetic Phosphate Analogues for Battery Applications. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- D’Yvoire, F.; Pintard-Scrépel, M.; Bretey, E.; de la Rochère, M. Phase transitions and ionic conduction in 3D skeleton phosphates A3M2(PO4)3: A = Li, Na, Ag, K; M = Cr, Fe. Solid State Ion. 1983, 9–10, 851–857. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Rangan, K.K. Vanadium Phosphate (V2(PO4)3): A Novel NASICON-Type Vanadium Phosphate Synthesized by Oxidative Deintercalation of Sodium from Sodium Vanadium Phosphate (Na3V2(PO4)3)). Chem. Mater. 1992, 4, 745–747. [Google Scholar] [CrossRef]
- Samigullin, R.R.; Zakharkin, M.V.; Drozhzhin, O.A.; Antipov, E.V. Thermal Stability of NASICON-Type Na3V2(PO4)3 and Na4VMn(PO4)3 as Cathode Materials for Sodium-ion Batteries. Energies 2023, 16, 3051. [Google Scholar] [CrossRef]
- Zakharkin, M.V.; Drozhzhin, O.A.; Ryazantsev, S.V.; Chernyshov, D.; Kirsanova, M.A.; Mikheev, I.V.; Pazhetnov, E.M.; Antipov, E.V.; Stevenson, K.J. Electrochemical properties and evolution of the phase transformation behavior in the NASICON-type Na3+xMnxV2−x(PO4)3 (0 ≤ x ≤ 1) cathodes for Na-ion batteries. J. Power Sources 2020, 470, 228231. [Google Scholar] [CrossRef]
- Zakharkin, M.V.; Drozhzhin, O.A.; Tereshchenko, I.V.; Chernyshov, D.; Abakumov, A.M.; Antipov, E.V.; Stevenson, K.J. Enhancing Na+ Extraction Limit through High Voltage Activation of the NASICON-Type Na4MnV(PO4)3 Cathode. Appl. Energy Mater. 2018, 1, 5842–5846. [Google Scholar] [CrossRef]
- Drozhzhin, O.A.; Tertov, I.V.; Alekseeva, A.M.; Aksyonov, D.A.; Stevenson, K.J.; Abakumov, A.M.; Antipov, E.V. β-NaVP2O7 as a Superior Electrode Material for Na-Ion Batteries. Chem. Mater. 2019, 31, 7463–7469. [Google Scholar] [CrossRef]
- Tertov, I.V.; Drozhzhin, O.A.; Alekseeva, A.M.; Kirsanova, M.A.; Mironov, A.V.; Abakumov, A.M.; Antipov, E.V. β-LiVP2O7 as a positive electrode material for Li-ion batteries. Electrochim. Acta 2021, 389, 138759. [Google Scholar] [CrossRef]
- Alekseeva, A.M.; Tertov, I.V.; Mironov, A.V.; Mikheev, I.V.; Drozhzhin, O.A.; Zharikova, E.V.; Rozova, M.G.; Antipov, E.V. Exploring Route for Pyrophosphate-based Electrode Materials: Interplay between Synthesis and Structure. Z. Für Anorg. Und Allg. Chem. 2020, 646, 1260–1266. [Google Scholar] [CrossRef]
- Semykina, D.O.; Sharafutdinov, M.R.; Kosova, N.V. Understanding of the Mechanism and Kinetics of the Fast Solid-State Reaction between NaF and VPO4 to Form Na3V2(PO4)2F3. Inorg. Chem. 2022, 61, 10023–10035. [Google Scholar] [CrossRef]
- Broux, T.; Fauth, F.; Hall, N.; Chatillon, Y.; Bianchini, M.; Bamine, T.; Leriche, J.; Suard, E.; Carlier, D.; Reynier, Y.; et al. High Rate Performance for Carbon-Coated Na3V2(PO4)2F3 in Na-Ion Batteries. Small Methods 2019, 3, 1800215. [Google Scholar] [CrossRef]
- Boivin, E.; Chotard, J.-N.; Masquelier, C.; Croguennec, L. Towards Reversible High-Voltage Multi-Electron Reactions in Alkali-Ion Batteries Using Vanadium Phosphate Positive Electrode Materials. Molecules 2021, 26, 1428. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-Ion Batteries toward Na-Ion Chemistries: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Barker, J.; Saidi, M.Y.; Swoyer, J.L. A Sodium-Ion Cell Based on the Fluorophosphate Compound NaVPO4F. Electrochem. Solid-State Lett. 2003, 6, A1–A4. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Y.; Tang, F.; Yao, Y.; Lv, X.; Liu, L.; Xu, C.; Feng, Y.; Rui, X.; Yu, Y. Vanadium fluorophosphates: Advanced cathode materials for next-generation secondary batteries. Mater. Horiz. 2023, 10, 1901–1923. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Sun, X.; Chang, R.; Zhang, Y.; Zhang, X.; Li, J. Fluorophosphates from Solid-State Synthesis and Electrochemical Ion Exchange: NaVPO4F or Na3V2(PO4)2F3 ? Adv. Energy Mater. 2018, 8, 1801064. [Google Scholar] [CrossRef]
- Boivin, E.; Chotard, J.-N.; Bamine, T.; Carlier, D.; Serras, P.; Palomares, V.; Rojo, T.; Iadecola, A.; Dupont, L.; Bourgeois, L.; et al. Vanadyl-type defects in Tavorite-like NaVPO4F: From the average long range structure to local environments. J. Mater. Chem. A 2017, 5, 25044–25055. [Google Scholar] [CrossRef]
- Kosova, N.; Semykina, D. Mechanochemically assisted solid-state synthesis of sodium vanadium fluorophosphates. Solid State Ion. 2019, 343, 115119. [Google Scholar] [CrossRef]
- Semykina, D.O.; Yakovlev, I.V.; Lapina, O.B.; Kabanov, A.A.; Kosova, N.V. Crystal structure and migration paths of alkaline ions in NaVPO4F. Phys. Chem. Chem. Phys. 2020, 22, 15876–15884. [Google Scholar] [CrossRef]
- Shraer, S.D.; Luchinin, N.D.; Trussov, I.A.; Aksyonov, D.A.; Morozov, A.V.; Ryazantsev, S.V.; Iarchuk, A.R.; Morozova, P.A.; Nikitina, V.A.; Stevenson, K.J.; et al. Development of vanadium-based polyanion positive electrode active materials for high-voltage sodium-based batteries. Nat. Commun. 2022, 13, 4097. [Google Scholar] [CrossRef] [PubMed]
- Dembitskiy, A.D.; Aksyonov, D.A.; Abakumov, A.M.; Fedotov, S.S. NH+-based frameworks as a platform for designing electrodes and solid electrolytes for Na-ion batteries: A screening approach. Solid State Ion. 2022, 374, 115810. [Google Scholar] [CrossRef]
- Tedjar, F.; Foudraz, J.C. Method for the Mixed Recycling of Lithium-Based Anode Batteries and Cells. U.S. Patent No. 7,820,317, 23 August 2007. [Google Scholar]
- Lesher, D.; Brown, R.A.; Norris, R.D. The Use of Hydrogen Peroxide in the Recovery of By-Product Vanadium at Cotter Corportation Canon City Plant. In Proceedings of the 11th Annual Meeting AIME, Dallas, TX, USA, 14–18 February 1982. [Google Scholar]
- Norris, R.D.; Brown, R.A. Separation and Recovery of By-Product and Secondary Metals with Hydrogen Peroxide Chemicals. In Proceedings of the 11th Annual Meeting AIME, Dallas, TX, USA, 14–18 February 1982. [Google Scholar]
- Briggs, S.J. Vanadium—A brief review of world production and trade 1976–1980. Met. Technol. 1982, 10, 150–158. [Google Scholar] [CrossRef]
- Vol’nov, I. Peroxo Complexes of Vanadium, Niobium and Tantalum; Nauka: Moscow, Russia, 1987. (In Russian) [Google Scholar]
- Lozano, L.; Godınez, C. Comparative study of solvent extraction of vanadium from sulphate solutions by primene 81R and alamine 336. Miner. Eng. 2003, 16, 291–294. [Google Scholar] [CrossRef]
- An, Y.; Ma, B.; Li, X.; Chen, Y.; Wang, C.; Wang, B.; Gao, M.; Feng, G. A review on the roasting-assisted leaching and recovery of V from vanadium slag. Process. Saf. Environ. Prot. 2023, 173, 263–276. [Google Scholar] [CrossRef]
- Moskalyuk, E.V.; Blokhin, A.A.; Murashkin, Y.V.; Mikhaylenko, M.A. Adsorption recovery of vanadium from sulphate solutions with complex composition. Tsvetnye Met. 2017, 41–46. [Google Scholar] [CrossRef]
- Predtechenskiy, M.R.; Khasin, A.A.; Bezrodny, A.E.; Bobrenok, O.F.; Dubov, D.Y.; Muradyan, V.E.; Saik, V.O.; Smirnov, S.N. New perspectives in SWCNT applications: Tuball SWCNTs. Part 1. Tuball by itself—All you need to know about it. Carbon Trends 2022, 8, 100175. [Google Scholar] [CrossRef]
- Mamakhel, A.; Gjørup, F.H.; Kløve, M.; Borup, K.; Iversen, B.B. Synthesis of Phase-Pure Thermochromic VO2 (M1). Inorg. Chem. 2022, 61, 8760–8766. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Makarevich, O.N.; Boytsova, O.V.; Tsymbarenko, D.M.; Eliseev, A.A.; Amelichev, V.A.; Makarevich, A.M. Citrate-assisted hydrothermal synthesis of vanadium dioxide textured films with metal-insulator transition and infrared thermochromic properties. Ceram. Int. 2020, 46, 19919–19927. [Google Scholar] [CrossRef]
- Makarevich, A.M.; Makarevich, O.; Ivanov, A.; Sharovarov, D.; Eliseev, A.A.; Amelichev, V.; Boytsova, O.; Gorodetsky, A.; Navarro-Cia, M.; Kaul, A. Hydrothermal epitaxy growth of self-organized vanadium dioxide 3D structures with metal–insulator transition and THz transmission switch properties. CrystEngComm 2020, 22, 2612–2620. [Google Scholar] [CrossRef]
- Tsaramyrsi, M.; Kaliva, M.; Salifoglou, A.; Raptopoulou, C.P.; Terzis, A.; Tangoulis, V.; Giapintzakis, J. Vanadium(IV)−Citrate Complex Interconversions in Aqueous Solutions. A pH-Dependent Synthetic, Structural, Spectroscopic, and Magnetic Study. Inorg. Chem. 2001, 40, 5772–5779. [Google Scholar] [CrossRef] [PubMed]
- Conte, V.; Di Furia, F.; Moro, S. The Versatile Chemistry of Peroxo Complexes of Vanadium, Molybdenum and Tungsten as Oxidants of Organic Compounds. J. Phys. Org. Chem. 1996, 9, 329–336. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Denzanov, G.A.; Tapekhin, A.; Kozlova, Z.A. Method of Sodium Phosphate Synthesis. S.U. Patent No. 1,133,230, 1985. Available online: https://i.moscow/patents/su1133230a1_19850107 (accessed on 6 June 2023). (In Russian).
- Vlasov, P.P.; Garkun, V.K.; Golubkova, V.N.; Sarkitz, I.A.; Gaidabura, I.P.; Binstein, A.B.; Laktionov, V.N.; Ovechkin, V.F. Method of Sodium Phosphate Synthesis. S.U. Patent No. 1,177,270, 1985. Available online: https://yandex.ru/patents/doc/SU1177270A1_19850907 (accessed on 6 June 2023). (In Russian).
- Pozin, M.E. Technology of Mineral Salts (Fertilizers, Pesticides, Industrial Salts, Oxides and Acids); Khimiya: Leningrad, Russia, 1974. [Google Scholar]
- Ambrosova, G.T.; Matyshenko, E.N.; Sineeva, N.V. Dephosphating Sites of Urban Wastewater and the Effect of Phosphorus Removal by Reagents. Water Ecol. Probl. Solut. 2017, 13–25. (In Russian) [Google Scholar] [CrossRef]
- Kumar, P.S.; Suganya, S.; Srinivas, S.; Priyadharshini, S.; Karthika, M.; Sri, R.K.; Swetha, V.; Naushad, M.; Lichtfouse, E. Treatment of fluoride-contaminated water. A review. Environ. Chem. Lett. 2019, 17, 1707–1726. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Gao, X.; Zuo, R.; Song, L.; Jin, C.; Wang, J.; Teng, Y. Vanadium: A Review of Different Extraction Methods to Evaluate Bioavailability and Speciation. Minerals 2022, 12, 642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).