Kolbe Electrolysis for the Conversion of Carboxylic Acids to Valuable Products—A Process Design Study
Abstract
:1. Introduction
2. Methodology
2.1. Thermophysical Data Survey
2.2. Process Development
2.2.1. Evaluations in Aspen Plus
2.2.2. Quantification of Process Efficiency
3. Results
3.1. Properties of Pure Fatty Acids
3.2. Solubility of Fatty Acids and Their Products
3.3. Basic Process Design
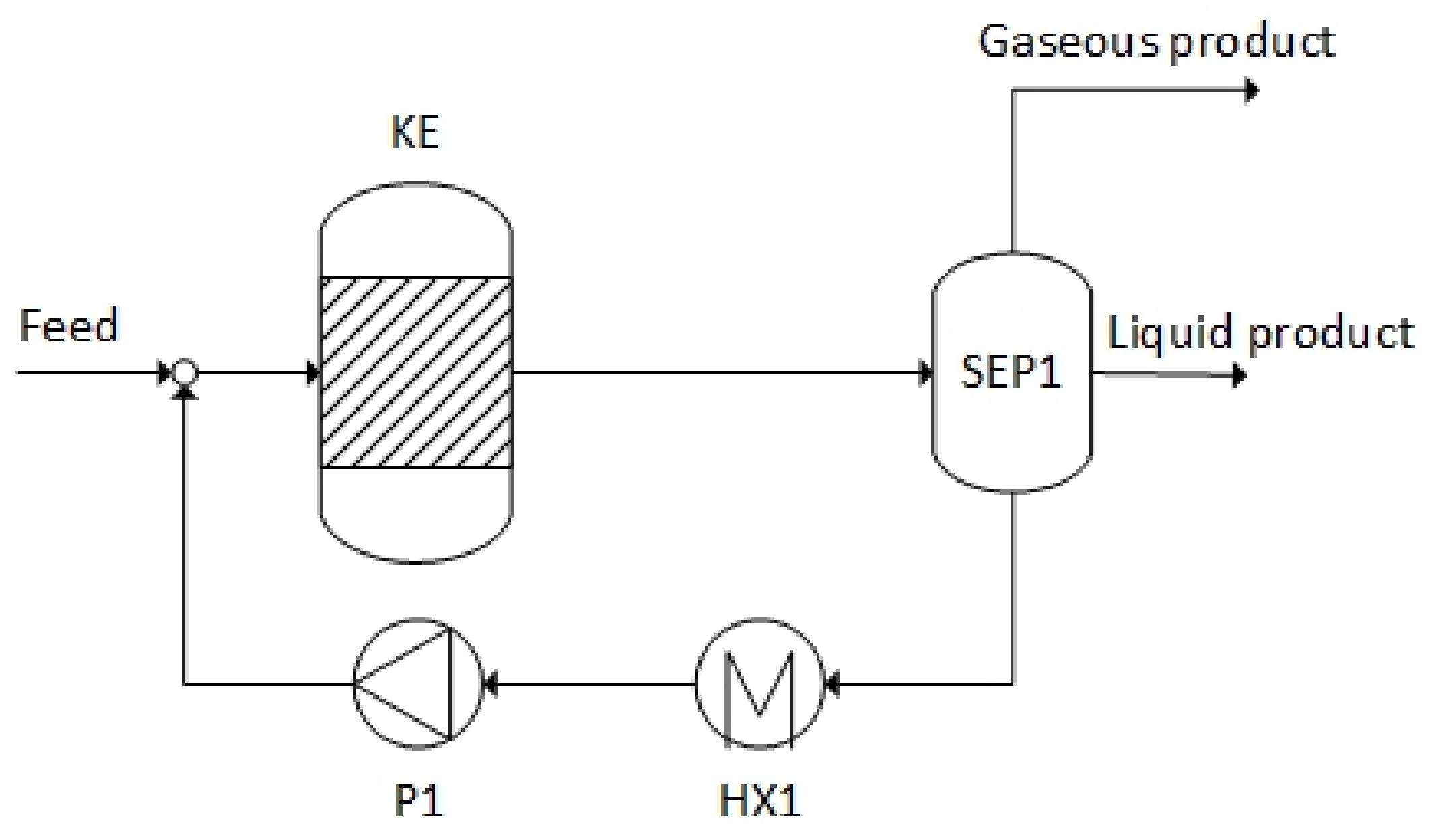
3.3.1. Acetic Acid as Feedstock
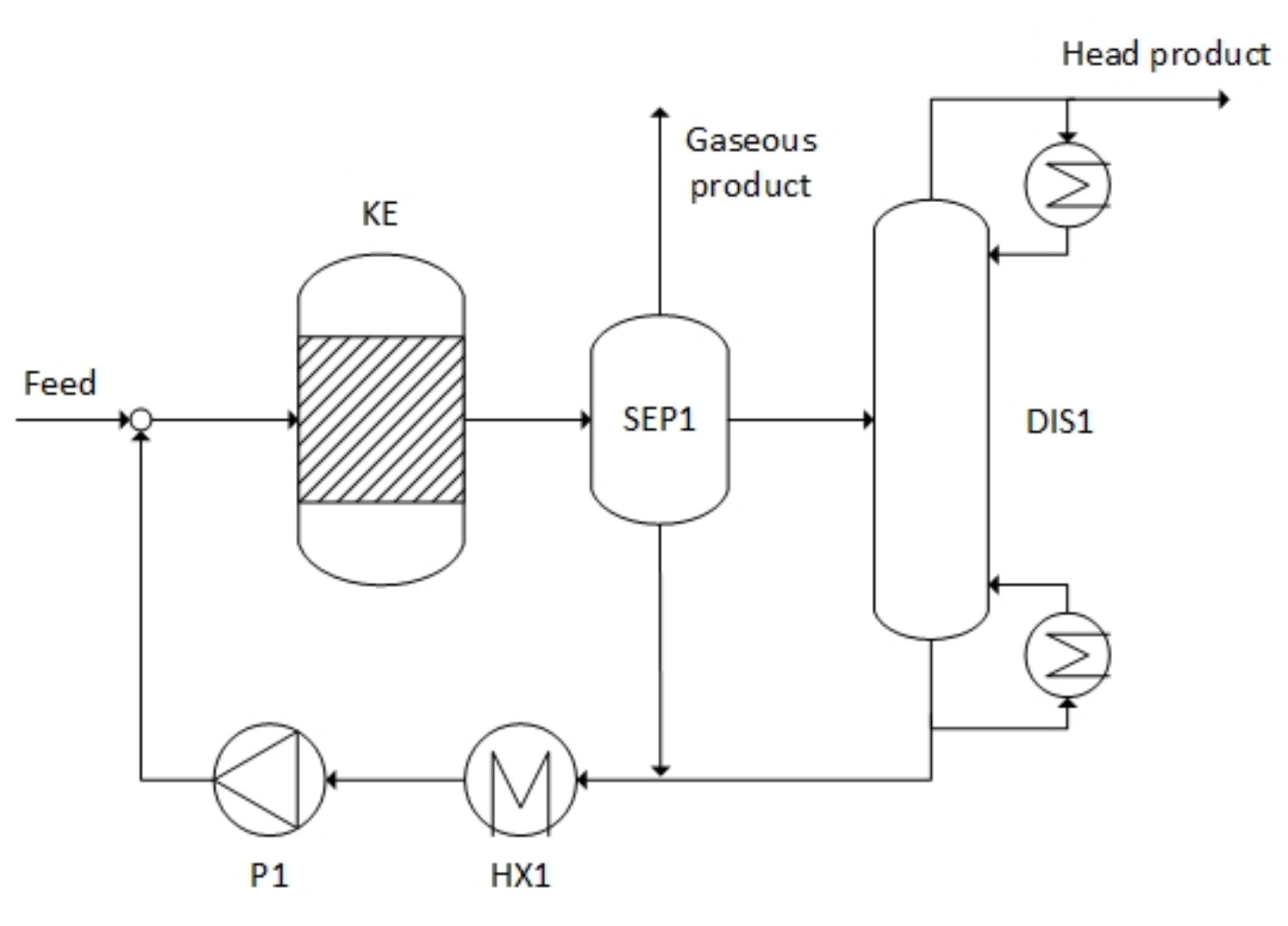
3.3.2. Valeric Acid as Feedstock
3.3.3. Lauric Acid as Feedstock
3.4. Comparision of the Scenarios
3.5. General Aspects of Process Design
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Compound | Compound | Compound | |||
|---|---|---|---|---|---|
| kJ/mol | kJ/mol | kJ/mol | |||
| −285.8 | |||||
| −393.5 | |||||
| 0.0 | |||||
| −457.1 | −541.3 | −738.6 | |||
| −83.8 | −250.2 | −603.6 | |||
| −74.5 | −328.6 | −506.5 | |||
| −614.0 | −886.8 [a] |
| Producttitle | Reaction Equation | |
|---|---|---|
| Processing of acetic acid | kJ/mol | |
| Kolbe product: ethane | −21.7 | |
| Side product: methane | 10.9 | |
| Processing of valeric acid | ||
| Kolbe product: n-octane | 22.6 | |
| Side product: 1-butanol | 104.9 | |
| Side product: butyl valerate | 37.5 | |
| Processing of lauric acid | ||
| Kolbe product: docosane | 43.3 | |
| Side product: 1-undecanol | 43.3 | |
| Side product: undecyl laurate | 98.5 |
Appendix B
| Unit | Scenario Acetic Acid | Scenario Valeric Acid | Scenario Lauric Acid | |
|---|---|---|---|---|
| kJ/mol | 901.5 | 2855.3 | 7413.1 | |
| kJ/mol | 21.7 | 22.6 | 43.3 | |
| kJ/mol | 2412.1 | 2412.1 | 2412.1 | |
| % | 27.9 | 54.6 | 75.9 |
Appendix C

References
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.L.; Engelbrecht, F.; et al. Impacts of 1.5 °C Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Fraunhofer ISE. Net Installed Electricity Generation Capacity in Germany in 2018. Available online: https://energy-charts.info/charts/installed_power/chart.htm?l=de&c=DE&stacking=stacked_percent (accessed on 16 December 2020).
- Burger, B. Net Public Electricity Generation in Germany in 2018. Available online: https://www.ise.fraunhofer.de/content/dam/ise/en/documents/News/Stromerzeugung_2018_2_en.pdf (accessed on 16 December 2020).
- Kolbe, H. Zersetzung der Valeriansäure durch den elektrischen Strom. Ann. Chem. Pharm. 1848, 64, 339–341. [Google Scholar] [CrossRef]
- Palkovits, S.; Palkovits, R. The Role of Electrochemistry in Future Dynamic Bio–Refineries: A Focus on (Non-)Kolbe Electrolysis. Chem. Ing. Tech. 2019, 91, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.-J. Recent contributions of kolbe electrolysis to organic synthesis. In Electrochemistry IV; Steckhan, E., Ed.; Springer: Berlin, Germany, 1990; pp. 91–151. ISBN 3-540-51461-9. [Google Scholar]
- Urban, C.; Harnisch, F. Deterioration of Aqueous n- Octanoate Electrolysis with Electrolytic Conductivity Collapse Caused by the Formation of n -Octanoic Acid/ n -Octanoate Agglomerates. Chem. Electro. Chem. 2017, 4, 1378–1389. [Google Scholar] [CrossRef]
- Schäfer, H.J. Recent synthetic applications of the Kolbe electrolysis. Chem. Phys. Lipids 1979, 24, 321–333. [Google Scholar] [CrossRef]
- Ahad, N.; de Klerk, A. Fischer–Tropsch acid water processing by Kolbe electrolysis. Fuel 2018, 211, 415–419. [Google Scholar] [CrossRef]
- dos Santos, T.R.; Harnisch, F.; Nilges, P.; Schröder, U. Electrochemistry for biofuel generation: Transformation of fatty acids and triglycerides to diesel-like olefin/ether mixtures and olefins. ChemSusChem 2015, 8, 886–893. [Google Scholar] [CrossRef]
- Meyers, J.; Mensah, J.B.; Holzhäuser, F.J.; Omari, A.; Blesken, C.C.; Tiso, T.; Palkovits, S.; Blank, L.M.; Pischinger, S.; Palkovits, R. Electrochemical conversion of a bio-derivable hydroxy acid to a drop-in oxygenate diesel fuel. Energy Environ. Sci. 2019, 12, 2406–2411. [Google Scholar] [CrossRef]
- Holzhäuser, F.J.; Creusen, G.; Moos, G.; Dahmen, M.; König, A.; Artz, J.; Palkovits, S.; Palkovits, R. Electrochemical cross-coupling of biogenic di-acids for sustainable fuel production. Green Chem. 2019, 21, 2334–2344. [Google Scholar] [CrossRef] [Green Version]
- Nilges, P.; dos Santos, T.R.; Harnisch, F.; Schröder, U. Electrochemistry for biofuel generation: Electrochemical conversion of levulinic acid to octane. Energy Environ. Sci. 2012, 5, 5231–5235. [Google Scholar] [CrossRef]
- Urban, C.; Xu, J.; Sträuber, H.; dos Santos Dantas, T.R.; Mühlenberg, J.; Härtig, C.; Angenent, L.T.; Harnisch, F. Production of drop-in fuels from biomass at high selectivity by combined microbial and electrochemical conversion. Energy Environ. Sci. 2017, 10, 2231–2244. [Google Scholar] [CrossRef]
- de Kruijff, G.H.M.; Waldvogel, S.R. Electrochemical Synthesis of Aryl Methoxymethyl Ethers. Chem. Electro. Chem. 2019, 6, 4180–4183. [Google Scholar] [CrossRef]
- Wu, L.; Mascal, M.; Farmer, T.J.; Arnaud, S.P.; Wong Chang, M.-A. Electrochemical Coupling of Biomass-Derived Acids: New C8 Platforms for Renewable Polymers and Fuels. ChemSusChem 2017, 10, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Wu, J. Electrochemical conversion of palmitic acid via Kolbe electrolysis for synthesis of n-triacontane. J. Electroanal. Chem. 2018, 822, 73–80. [Google Scholar] [CrossRef]
- Ho, C.K.; McAuley, K.B.; Peppley, B.A. Biolubricants through renewable hydrocarbons: A perspective for new opportunities. Renew. Sustain. Energy Rev. 2019, 113, 109261. [Google Scholar] [CrossRef]
- Stang, C.; Harnisch, F. The Dilemma of Supporting Electrolytes for Electroorganic Synthesis: A Case Study on Kolbe Electrolysis. ChemSusChem 2016, 9, 50–60. [Google Scholar] [CrossRef]
- Ziogas, A.; Hofmann, C.; Baranyai, S.; Löb, P.; Kolb, G. Novel Flexible Electrochemical Microreactor and its Validation by Three Model Electrosyntheses. Chem. Ing. Tech. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ziogas, A.; Pennemann, H.; Kolb, G. Electrochemical Synthesis of Tailor-Made Hydrocarbons from Organic Solvent Free Aqueous Fatty Acid Mixtures in a Micro Flow Reactor. Electrocatalysis 2020, 11, 432–442. [Google Scholar] [CrossRef]
- Holzhäuser, F.J.; Mensah, J.B.; Palkovits, R. (Non-)Kolbe electrolysis in biomass valorization—A discussion of potential applications. Green Chem. 2020, 22, 286–301. [Google Scholar] [CrossRef] [Green Version]
- Kooijman, H.; Taylor, R. ChemSep v8.01 Pure Component Data. 2018. Available online: http://www.chemsep.org/ (accessed on 16 December 2020).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Dunn, R.O. A Comprehensive Evaluation of the Melting Points of Fatty Acids and Esters Determined by Differential Scanning Calorimetry. J. Am. Oil Chem Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Klopman, G.; Wang, S.; Balthasar, D.M. Estimation of aqueous solubility of organic molecules by the group contribution approach. Application to the study of biodegradation. J. Chem. Inf. Comput. Sci. 1992, 32, 474–482. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘green’ chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Bell, G.H. Solubilities of normal aliphatic acids, alcohols and alkanes in water. Chem. Phys. Lipids 1973, 10, 1–10. [Google Scholar] [CrossRef]
- Bond, A.D. On the crystal structures and melting point alternation of the n-alkyl carboxylic acids. New J. Chem. 2004, 28, 104–114. [Google Scholar] [CrossRef]
- Ogino, K.; Ichikawa, Y. The Solubilities and Kraft Points of Fatty Acid Soaps of Odd Carbon Numbers. BCSJ 1976, 49, 2683–2686. [Google Scholar] [CrossRef] [Green Version]
- McBain, J.W.; Sierichs, W.C. The solubility of sodium and potassium soaps and the phase diagrams of aqueous potassium soaps. J. Am. Oil Chem. Soc. 1948, 25, 221–225. [Google Scholar] [CrossRef]
- Quast, K. The use of zeta potential to investigate the p K a of saturated fatty acids. Adv. Powder Technol. 2016, 27, 207–214. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef]
- Soontravanich, S.; Lopez, H.E.; Scamehorn, J.F.; Sabatini, D.A.; Scheuing, D.R. Dissolution Study of Salt of Long Chain Fatty Acids (Soap Scum) in Surfactant Solutions. Part I: Equilibrium Dissolution. J. Surfactants Deterg. 2010, 13, 367–372. [Google Scholar] [CrossRef]
- Quast, K. Flotation of hematite using C6–C18 saturated fatty acids. Miner. Eng. 2006, 19, 582–597. [Google Scholar] [CrossRef]
- Lucassen, J. Hydrolysis and Precipitates in Carboxylate Soap Solutions. J. Phys. Chem. 1966, 70, 1824–1830. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Danov, K.D.; Pishmanova, C.I.; Kralchevska, S.D.; Christov, N.C.; Ananthapadmanabhan, K.P.; Lips, A. Effect of the precipitation of neutral-soap, acid-soap, and alkanoic acid crystallites on the bulk pH and surface tension of soap solutions. Langmuir 2007, 23, 3538–3553. [Google Scholar] [CrossRef] [PubMed]
- Moroi, Y.; Matuura, R. Relationship between Solubility and Micellization of Surfactants; Micelle Temperature Range (MTR) Instead of Krafft Point. BCSJ 1988, 61, 333–339. [Google Scholar] [CrossRef] [Green Version]
- De Mul, M.N.G.; Davis, H.T.; Evans, D.F.; Bhave, A.V.; Wagner, J.R. Solution Phase Behavior and Solid Phase Structure of Long-Chain Sodium Soap Mixtures. Langmuir 2000, 16, 8276–8284. [Google Scholar] [CrossRef]
- Klein, R.; Kellermeier, M.; Drechsler, M.; Touraud, D.; Kunz, W. Solubilisation of stearic acid by the organic base choline hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 129–134. [Google Scholar] [CrossRef]
- Klein, R.; Touraud, D.; Kunz, W. Choline carboxylate surfactants: Biocompatible and highly soluble in water. Green Chem. 2008, 10, 433. [Google Scholar] [CrossRef]
- Wolfrum, S. Long chain soaps and alkyl sulfates in aqueous solutions at room temperature. Ph.D. Thesis, Universität Regensburg, Regensbug, Germany, 24 October 2018. [Google Scholar]
- Lin, B.; McCormick, A.V.; Davis, H.T.; Strey, R. Solubility of sodium soaps in aqueous salt solutions. J. Colloid Interface Sci. 2005, 291, 543–549. [Google Scholar] [CrossRef]
- Umemura, J.; Mantsch, H.H.; Cameron, D.G. Micelle formation in aqueous n-alkanoate solutions: A fourier transform infrared study. J. Colloid Interface Sci. 1981, 83, 558–568. [Google Scholar] [CrossRef]
- Mukerjee, P.; Mysels, K. Critical Micelle Concentrations of Aqueous Surfactant Systems. 1971. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiptfHFh-TtAhUbIIgKHTW2BtEQFjABegQIAhAC&url=https%3A%2F%2Fnvlpubs.nist.gov%2Fnistpubs%2FLegacy%2FNSRDS%2Fnbsnsrds36.pdf&usg=AOvVaw2AxOwGeIA2pma58QErkHey (accessed on 16 December 2020).
- Dickinson, T.; Wynne-Jonnes, W.F.K. Mechanism of Kolbe’s electrosynthesis. Part 1—Anode potential phenomena. Trans. Faraday Soc. 1962, 382–387. [Google Scholar] [CrossRef]
- Grote, K.-H.; Bender, B.; Gohlich, D. Dubbel Taschenbuch für den Maschinenbau; Grote, K.-H., Bender, B., Göhlich, D., Eds.; Springer: Berlin, Germany, 2018; ISBN 978-3-662-54804-2. [Google Scholar]
- Feinstein, M.E.; Rosano, H.L. Influence of micelles on titrations of aqueous sodium and potassium soap solutions. J. Phys. Chem. 1969, 73, 601–607. [Google Scholar] [CrossRef]
- Saleeb, F.Z.; Cante, C.J.; Streckfus, T.K.; Frost, J.R.; Rosano, H.L. Surface pH and stability of oil-water emulsions derived from laurate solutions. J. Am. Oil Chem Soc. 1975, 52, 208–212. [Google Scholar] [CrossRef]
- dos Santos, T.R.; Nilges, P.; Sauter, W.; Harnisch, F.; Schröder, U. Electrochemistry for the generation of renewable chemicals: Electrochemical conversion of levulinic acid. RSC Adv. 2015, 5, 26634–26643. [Google Scholar] [CrossRef]
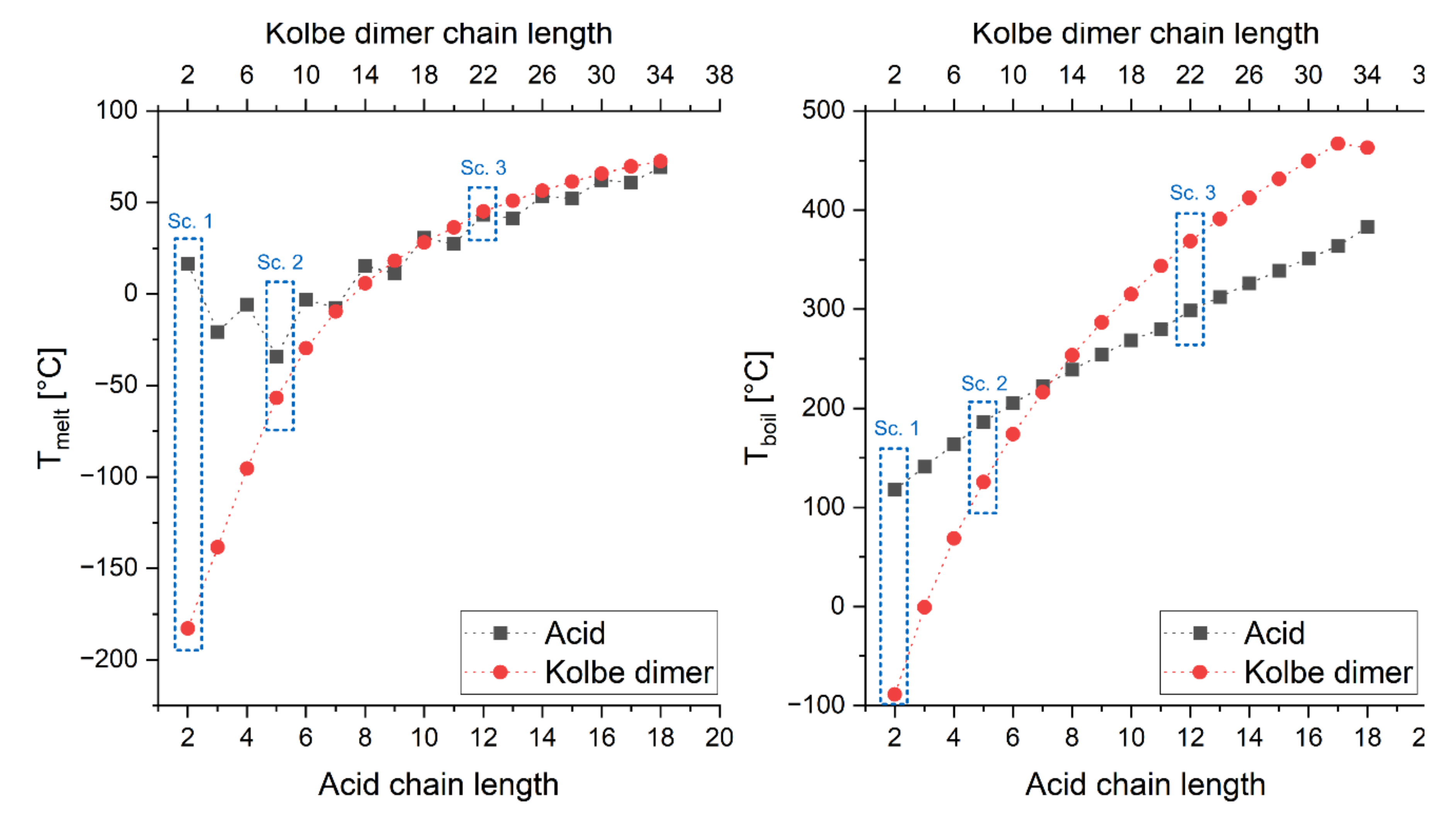


| FA Chain Length | Solubility | lgKOW | ||||
|---|---|---|---|---|---|---|
| [-] | g/L | [-] | ||||
| FA [a,b] | NaFA [a,c,d] | R2 [b] | FA [a] | NaFA [a] | R2 [a] | |
| 2 | soluble | soluble | 0.68 | −0.17 | 1.8 | |
| 3 | soluble | soluble | 0.12 | 0.33 | 2.9 | |
| 4 | 60 | 0.02 | 0.79 | −3.2 | 3.9 | |
| 5 | 24 | soluble | 0.00 | 1.39 | 5.2 | |
| 6 | 10.3 | 0.00 | 1.92 | 5.0 | ||
| 7 | 2.82 | soluble | 0.00 | 2.42 | 6.1 | |
| 8 | 0.72 | 0.00 | 3.05 | −1.38 | 7.2 | |
| 9 | 0.21 | 70.4 [e] | 0.00 | 3.42 | 8.2 | |
| 10 | 0.06 | 0.00 | 4.09 | 8.4 | ||
| 11 | 0.01 | 22.4 [f] | 0.00 | 4.42 | 10.2 | |
| 12 | 0.00 | 50 | 0.00 | 4.60 | 11.2 | |
| 13 | 0.00 | 1.86 | 0.00 | 12.1 | ||
| 14 | 0.00 | <10 | 0.00 | 6.11 | 13.1 | |
| 15 | 0.00 | 0.58 | 0.00 | 14.1 | ||
| 16 | 0.00 | insoluble | 0.00 | 7.17 | 15.0 | |
| 17 | 0.00 | 0.00 | 16.1 | |||
| 18 | 0.00 | insoluble | 8.23 | |||
| FA Chain Length | TKrafft [a,b] | CMC [c,d] |
|---|---|---|
| [-] | °C | g/L |
| 4 | 385.3 [e] | |
| 5 | 291.7 [e] | |
| 6 | 100.8 [e] | |
| 7 | 144.5 [e] | |
| 8 | 17.1 | 58.2 |
| 9 | 10.5 | 28.7 |
| 10 | 31.1 | 18.3 |
| 12 | 44.1 | 5.1 |
| 13 | 39.8 | |
| 14 | 53.2 | 1.7 |
| 15 | 52.8 | |
| 16 | 62.3 | 0.7 |
| 18 | 71 | 0.1 |
| Compound | Boiling Point |
|---|---|
| Water | 100 °C |
| 1-butanol | 117 °C |
| N-octane | 126 °C |
| Butyl valerate | 186 °C |
| Valeric acid | 186 °C |
| Feedstock | Acetic Acid | Valeric Acid | Lauric Acid |
|---|---|---|---|
| Product | Ethane | n-octane | Docosane |
| Side products | Hydrogen, carbon dioxide, methane | Hydrogen, carbon dioxide, 1-butanol, butyl valerate | Hydrogen, carbon dioxide, 1-undecanol, undecyl laurate |
| Catalyst amount | Equimolar to feedstock | Equimolar to feedstock | Excess molar to feedstock, preferably KOH |
| Downstream processing | Liquid–gas separation | Liquid–liquid–gas separation and distillation | Liquid–liquid–gas separation |
| Product utilization | Natural gas substitute, specialty gas | Specialty chemicals, fuel | Lubricant, cosmetics |
| Max. carbon efficiency [a] | 50% | 80% | 92% |
| Required charge per product [a,b] | 6,417,359 C/kg | 1,691,681 C/kg | 622,343 C/kg |
| Maximum energetic efficiency | 27.9% | 54.6% | 75.9% |
| Operating temperature | ≈20 °C | ≈20 °C | >45.1 °C [c] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klüh, D.; Waldmüller, W.; Gaderer, M. Kolbe Electrolysis for the Conversion of Carboxylic Acids to Valuable Products—A Process Design Study. Clean Technol. 2021, 3, 1-18. https://doi.org/10.3390/cleantechnol3010001
Klüh D, Waldmüller W, Gaderer M. Kolbe Electrolysis for the Conversion of Carboxylic Acids to Valuable Products—A Process Design Study. Clean Technologies. 2021; 3(1):1-18. https://doi.org/10.3390/cleantechnol3010001
Chicago/Turabian StyleKlüh, Daniel, Wolfgang Waldmüller, and Matthias Gaderer. 2021. "Kolbe Electrolysis for the Conversion of Carboxylic Acids to Valuable Products—A Process Design Study" Clean Technologies 3, no. 1: 1-18. https://doi.org/10.3390/cleantechnol3010001
APA StyleKlüh, D., Waldmüller, W., & Gaderer, M. (2021). Kolbe Electrolysis for the Conversion of Carboxylic Acids to Valuable Products—A Process Design Study. Clean Technologies, 3(1), 1-18. https://doi.org/10.3390/cleantechnol3010001





