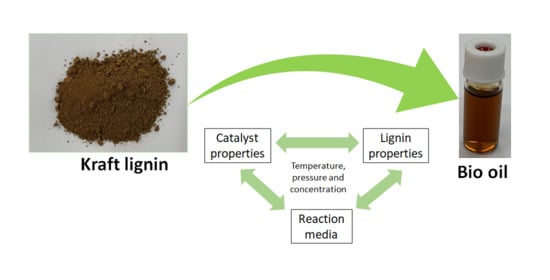
Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review
Abstract
1. Introduction
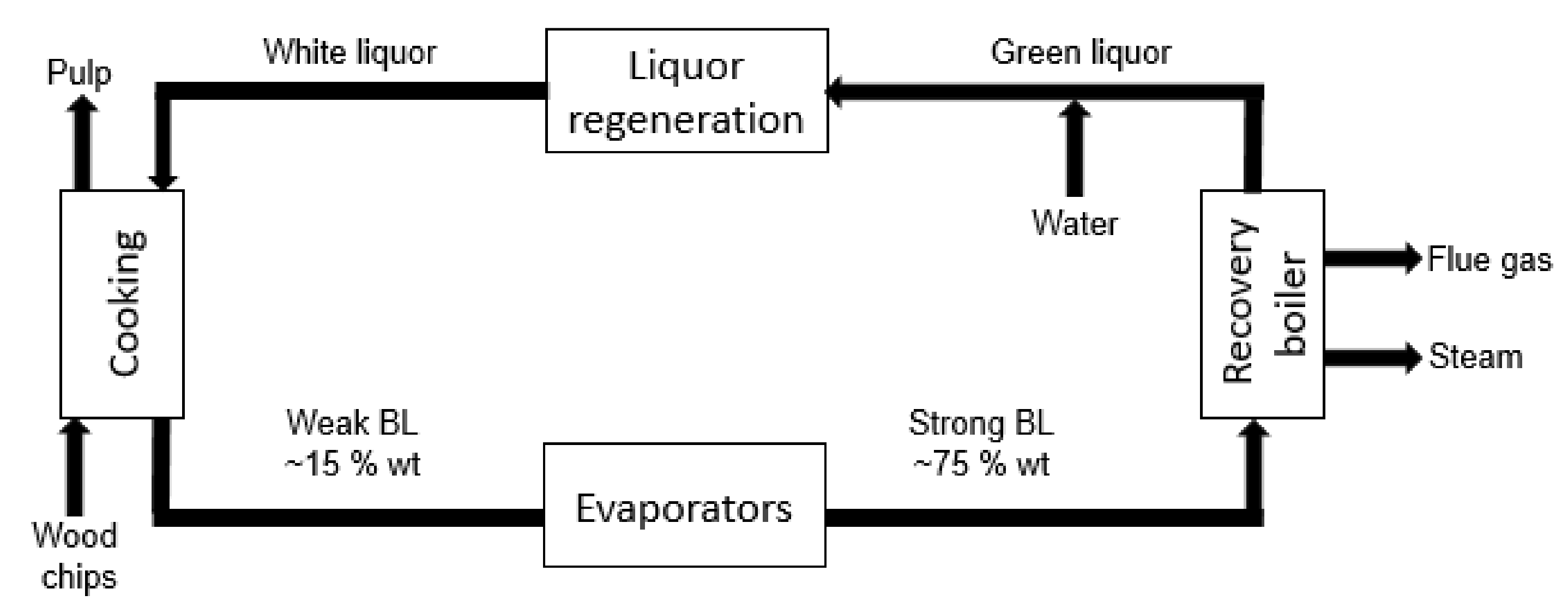
2. Availability and Economic Importance of Kraft Lignin
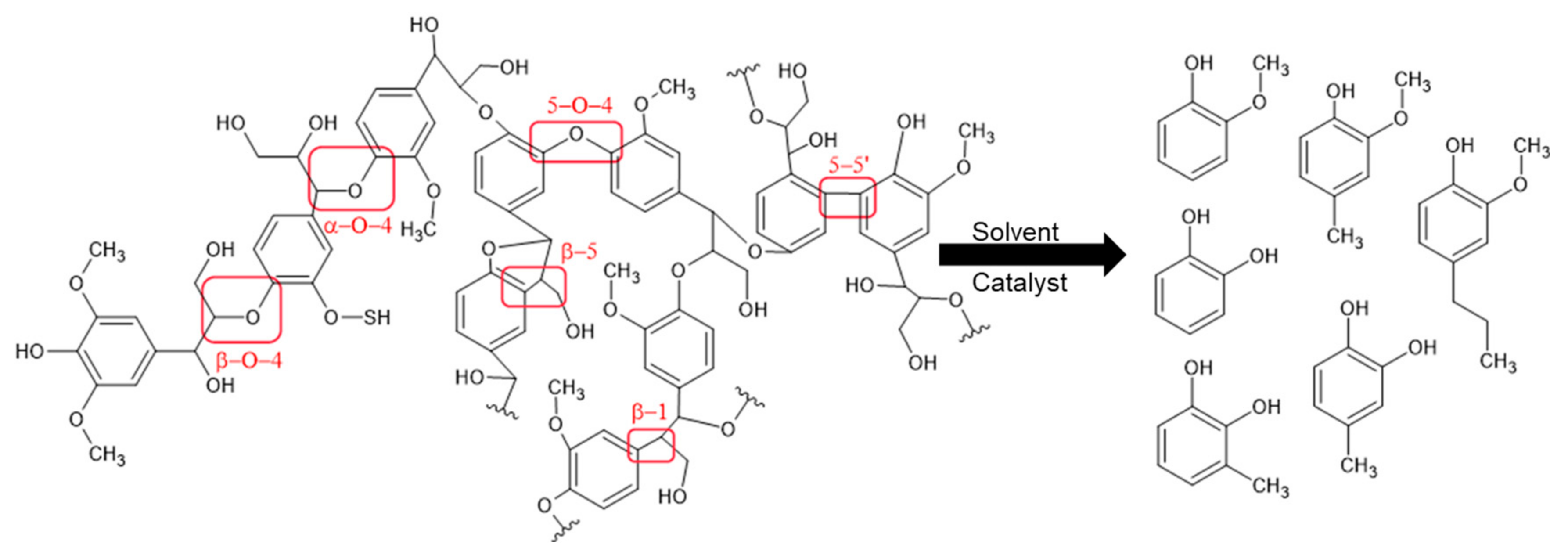
3. Lignin Solvolysis
4. The Role of Reaction Media on Kraft Lignin Depolymerization
4.1. Sub- and Supercritical Water as Reaction Medium
4.2. Short-Chain Alcohols as Reaction Media
4.3. Dioxane and Other Solvents as Reaction Media
4.4. Solvent–Water Mixtures
5. Catalysts in Kraft Lignin Depolymerization
5.1. Noble Metal Catalysts
5.2. Non-Noble Metal Catalysts
5.3. Role of Support Materials in Catalysts for Kraft Lignin Depolymerization
5.3.1. Acidic Support Materials
5.3.2. Neutral Support Materials
6. Conclusions and Recommendations
6.1. Conclusions
6.2. Recommendations
Funding
Acknowledgments
Conflicts of Interest
References
- Moriarty, P.; Honnery, D. Can renewable energy power the future? Energy Policy 2016, 93, 3–7. [Google Scholar] [CrossRef]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Novaes, E.; Kirst, M.; Chiang, V.; Winter-Sederoff, H.; Sederoff, R. Lignin and Biomass: A Negative Correlation for Wood Formation and Lignin Content in Trees. Plant Physiol. 2010, 154, 555–561. [Google Scholar] [CrossRef]
- Cherif, M.F.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Dorrestijn, E.; Laarhoven, L.J.; Arends, I.W.; Mulder, P. The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal. J. Anal. Appl. Pyrolysis 2000, 54, 153–192. [Google Scholar] [CrossRef]
- Al-Nuaimi, I.A.; Bohra, M.; Selam, M.; Choudhury, H.A.; El-Halwagi, M.M.; Elbashir, N.O. Optimization of the Aromatic/Paraffinic Composition of Synthetic Jet Fuels. Chem. Eng. Technol. 2016, 39, 2217–2228. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2010, 34, 29–41. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Liu, Y.; Ruan, X.; Tsang, D.C.; Hunt, A.J.; Zhang, S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef]
- Dorrestijn, E.; Kranenburg, M.; Poinsot, D.; Mulder, P. Lignin Depolymerization in Hydrogen-Donor Solvents. Holzforschung 1999, 53, 611–616. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013. [Google Scholar] [CrossRef]
- Sterba, M.J.; Haensel, V. Catalytic Reforming. Ind. Eng. Chem. Prod. Res. Dev. 1976, 15, 2–17. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Konnerth, J.; Herwijnen, H.W.; Zinovyev, G.; Budjav, E.; Silva, A.R.; Liebner, F. Commercial lignosulfonates from different sulfite processes as partial phenol replacement in PF resole resins. J. Appl. Polym. Sci. 2017, 135, 45893. [Google Scholar] [CrossRef]
- Huang, C.; Ma, J.; Zhang, W.; Huang, G.; Yong, Q. Preparation of Lignosulfonates from Biorefinery Lignins by Sulfomethylation and Their Application as a Water Reducer for Concrete. Polymers 2018, 10, 841. [Google Scholar] [CrossRef]
- Windeisen, E.; Wegener, G. Lignin as Building Unit for Polymers. Polym. Sci. A Compr. Ref. 2012, 10, 255–265. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Sewring, T.; Durruty, J.; Schneider, L.; Schneider, H.; Mattsson, T.; Theliander, H. Acid Precipitation of Kraft Lignin from Aqueous Solutions: The Influence of pH, Temperature, and Xylan. J. Wood Chem. Technol. 2019, 39, 1–13. [Google Scholar] [CrossRef]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Lundberg, V.; Bood, J.; Nilsson, L.; Axelsson, E.; Berntsson, T.; Svensson, E. Converting a kraft pulp mill into a multi-product biorefinery: Techno-economic analysis of a case mill. Clean Technol. Environ. Policy 2014, 16, 1411–1422. [Google Scholar] [CrossRef]
- Tomani, P.; Axegard, P.; Berglin, N.; Lovell, A.; Nordgren, D. Integration of lignin removal into a kraft pulp mill and use of lignin as a biofuel. Cellul. Chem. Technol. 2011, 45, 533–540. [Google Scholar]
- Insights, F. 13 August 2020. Pulp and Paper Market Size to Reach USD 368.10 Billion by 2027; Rising Usage of Smartphones & Internet to Boost Growth, Says Fortune Business Insights™. Available online: https://www.globenewswire.com/news-release/2020/08/13/2078087/0/en/Pulp-and-Paper-Market-Size-to-Reach-USD-368-10-Billion-by-2027-Rising-Usage-of-Smartphones-Internet-to-Boost-Growth-Says-Fortune-Business-Insights.html (accessed on 7 November 2020).
- Svensson, I.; Jönsson, J.; Berntsson, T.; Moshfegh, B. Excess heat from kraft pulp mills: Trade-offs between internal and external use in the case of Sweden—Part 1: Methodology. Energy Policy 2008, 36, 4178–4185. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaushik, N.; Biswas, S. Derivatives and Applications of Lignin—An Insight. SciTech J. 2014, 1, 30–36. [Google Scholar]
- Stephen, J.D.; Mabee, W.E.; Saddler, J.N. Biomass logistics as a determinant of second-generation biofuel facility scale, location and technology selection. Biofuels Bioprod. Biorefining 2010, 4, 503–518. [Google Scholar] [CrossRef]
- Miao, Z.; Shastri, Y.; Grift, T.E.; Hansen, A.C.; Ting, K. Lignocellulosic biomass feedstock transportation alternatives, logistics, equipment configurations, and modeling. Biofuels Bioprod. Biorefining 2012, 6, 351–362. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. (Eds.) Chapter 2—Refinery Feedstocks and Products. In Fundamentals of Petroleum Refining; Elsevier: Amsterdam, The Netherlands, 2010; pp. 11–31. ISBN 9780444527851. [Google Scholar]
- Bajwa, D.; Pourhashem, G.; Ullah, A.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Mattsson, C.; Andersson, S.-I.; Belkheiri, T.; Åmand, L.-E.; Olausson, L.; Vamling, L.; Theliander, H. Using 2D NMR to characterize the structure of the low and high molecular weight fractions of bio-oil obtained from LignoBoostTM kraft lignin depolymerized in subcritical water. Biomass Bioenergy 2016, 95, 364–377. [Google Scholar] [CrossRef]
- Zhang, J. Catalytic transfer hydrogenolysis as an efficient route in cleavage of lignin and model compounds. Green Energy Environ. 2018, 3, 328–334. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem. 2015, 17, 4941–4950. [Google Scholar] [CrossRef]
- Raj, K.J.; Malar, E.P.; Vijayaraghavan, V. Shape-selective reactions with AEL and AFI type molecular sieves alkylation of benzene, toluene and ethylbenzene with ethanol, 2-propanol, methanol and t-butanol. J. Mol. Catal. A Chem. 2006, 243, 99–105. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Perspective on Technical Lignin Fractionation. ACS Sustain. Chem. Eng. 2020, 8, 8086–8101. [Google Scholar] [CrossRef]
- Aouad, S.; Labaki, M.; Ojala, S.; Seelam, P.; Turpeinen, E.; Gennequin, C.; Aad, E.A. A Review on the Dry Reforming Processes for Hydrogen Production: Catalytic Materials and Technologies. Catal. Mater. Hydrog. Prod. Electro-Oxid. React. Front. Ceram. Sci. 2018, 2, 60–128. [Google Scholar] [CrossRef]
- Pal, D.; Chand, R.; Upadhyay, S.; Mishra, P. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Qi, S.; Hayashi, J.; Kudo, S.; Zhang, L. Catalytic hydrogenolysis of kraft lignin to monomers at high yield in alkaline water. Green Chem. 2017, 19, 2636–2645. [Google Scholar] [CrossRef]
- Rana, M.; Islam, M.N.; Agarwal, A.; Taki, G.; Park, S.; Dong, S.; Park, J. Production of Phenol-Rich Monomers from Kraft Lignin Hydrothermolysates in Basic-Subcritical Water over MoO3/SBA-15 Catalyst. Energy Fuels 2018, 32, 11564–11575. [Google Scholar] [CrossRef]
- Tang, K.; Zhou, X. The degradation of kraft lignin during hydrothermal treatment for phenolics. Pol. J. Chem. Technol. 2015, 17, 24–28. [Google Scholar] [CrossRef]
- Arturi, K.R.; Strandgaard, M.; Nielsen, R.P.; Søgaard, E.G.; Maschietti, M. Hydrothermal liquefaction of lignin in near-critical water in a new batch reactor: Influence of phenol and temperature. J. Supercrit. Fluids 2017, 123, 28–39. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Riaz, A.; Park, J.; Insyani, R.; Verma, D.; Kim, J. Depolymerization of concentrated sulfuric acid hydrolysis lignin to high-yield aromatic monomers in basic sub- and supercritical fluids. Chem. Eng. J. 2017, 317, 9–19. [Google Scholar] [CrossRef]
- Islam, M.N.; Taki, G.; Rana, M.; Park, J. Yield of Phenolic Monomers from Lignin Hydrothermolysis in Subcritical Water System. Ind. Eng. Chem. Res. 2018, 57, 4779–4784. [Google Scholar] [CrossRef]
- Kim, K.H.; Brown, R.C.; Kieffer, M.; Bai, X. Hydrogen-Donor-Assisted Solvent Liquefaction of Lignin to Short-Chain Alkylphenols Using a Micro Reactor/Gas Chromatography System. Energy Fuels 2014, 28, 6429–6437. [Google Scholar] [CrossRef]
- Molinari, V.; Clavel, G.; Graglia, M.; Antonietti, M.; Esposito, D. Mild Continuous Hydrogenolysis of Kraft Lignin over Titanium Nitride–Nickel Catalyst. ACS Catal. 2016, 6, 1663–1670. [Google Scholar] [CrossRef]
- Yan, F.; Ma, R.; Ma, X.; Cui, K.; Wu, K.; Chen, M.; Li, Y. Ethanolysis of Kraft lignin to platform chemicals on a MoC1-x/Cu-MgAlOz catalyst. Appl. Catal. B Environ. 2017, 202, 305–313. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Dou, X.; Wang, J.; Jin, L.; Ogunbiyi, A.T.; Li, X. Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts. Sustain. Energy Fuels 2020, 4, 1332–1339. [Google Scholar] [CrossRef]
- Narani, A.; Chowdari, R.K.; Cannilla, C.; Bonura, G.; Frusteri, F.; Heeres, H.J.; Barta, K. Efficient catalytic hydrotreatment of Kraft lignin to alkylphenolics using supported NiW and NiMo catalysts in supercritical methanol. Green Chem. 2015, 17, 5046–5057. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Liu, S.; Wang, Y.; Dai, L. High-quality bio-oil from one-pot catalytic hydrocracking of kraft lignin over supported noble metal catalysts in isopropanol system. Bioresour. Technol. 2016, 212, 302–310. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and controllable alcoholysis of Kraft lignin catalyzed by porous zeolite-supported nickel-copper catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef]
- Jin, L.; Li, W.; Liu, Q.; Wang, J.; Zhu, Y.; Xu, Z.; Zhang, Q. Liquefaction of kraft lignin over the composite catalyst HTaMoO6 and Rh/C in dioxane-water system. Fuel Process. Technol. 2018, 178, 62–70. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Zhang, J.; Li, X.; Zhang, Y.; Wang, F. Ethanol/1,4-dioxane/formic acid as synergistic solvents for the conversion of lignin into high-value added phenolic monomers. Bioresour. Technol. 2019, 278, 187–194. [Google Scholar] [CrossRef]
- Li, W.; Dou, X.; Zhu, C.; Wang, J.; Chang, H.; Jameel, H.; Li, X. Production of liquefied fuel from depolymerization of kraft lignin over a novel modified nickel/H-beta catalyst. Bioresour. Technol. 2018, 269, 346–354. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Wang, H.; Ma, Q.; Li, S.; Chang, H.; Jameel, H. Liquefaction of kraft lignin by hydrocracking with simultaneous use of a novel dual acid-base catalyst and a hydrogenation catalyst. Bioresour. Technol. 2017, 243, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Jiang, X.; Li, W.; Zhu, C.; Liu, Q.; Lu, Q.; Jameel, H. Highly efficient conversion of Kraft lignin into liquid fuels with a Co-Zn-beta zeolite catalyst. Appl. Catal. B Environ. 2020, 268, 118429. [Google Scholar] [CrossRef]
- Zhu, C.; Dou, X.; Li, W.; Liu, X.; Li, Q.; Ma, J.; Ma, L. Efficient depolymerization of Kraft lignin to liquid fuels over an amorphous titanium-zirconium mixed oxide supported partially reduced nickel-cobalt catalyst. Bioresour. Technol. 2019, 284, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tymchyshyn, M.; Xu, C.C. Reductive Depolymerization of Kraft and Organosolv Lignin in Supercritical Acetone for Chemicals and Materials. ChemCatChem 2016, 8, 1968–1976. [Google Scholar] [CrossRef]
- Dong, L.; Lin, L.; Han, X.; Si, X.; Liu, X.; Guo, Y.; Wang, Y. Breaking the Limit of Lignin Monomer Production via Cleavage of Interunit Carbon–Carbon Linkages. Chem 2019, 5, 1521–1536. [Google Scholar] [CrossRef]
- Mukundan, S.; Atanda, L.; Beltramini, J. Thermocatalytic cleavage of C–C and C–O bonds in model compounds and kraft lignin by NiMoS2/C nanocatalysts. Sustain. Energy Fuels 2019, 3, 1317–1328. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A. Aqueous acetone fractionation of kraft, organosolv and soda lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Ajao, O.; Jeaidi, J.; Benali, M.; Abdelaziz, O.Y.; Hulteberg, C.P. Green solvents-based fractionation process for kraft lignin with controlled dispersity and molecular weight. Bioresour. Technol. 2019, 291, 121799. [Google Scholar] [CrossRef]
- Fragoso, D.M.; Bouxin, F.P.; Montgomery, J.R.; Westwood, N.J.; Jackson, S.D. Catalytic depolymerisation of isolated lignin to fine chemicals: Depolymerisation of Kraft lignin. Bioresour. Technol. Rep. 2020, 9, 100400. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.; Weckhuysen, B.M. Catalytic Lignin Valorization Process for the Production of Aromatic Chemicals and Hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef]
- Lee, H.; Jae, J.; Ha, J.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mahmood, N.; Tymchyshyn, M.; Yuan, Z.; Xu, C. Reductive de-polymerization of kraft lignin for chemicals and fuels using formic acid as an in-situ hydrogen source. Bioresour. Technol. 2014, 171, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mahmood, N.; Zhang, Y.; Tymchyshyn, M.; Yuan, Z.; Xu, C. Reductive de-polymerization of kraft lignin with formic acid at low temperatures using inexpensive supported Ni-based catalysts. Fuel 2017, 209, 579–586. [Google Scholar] [CrossRef]
- Singh, S.K.; Ekhe, J.D. Solvent effect on HZSM-5 catalyzed solvolytic depolymerization of industrial waste lignin to phenols: Superiority of the water–methanol system over methanol. RSC Adv. 2014, 4, 53220–53228. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Producing jet fuel from biomass lignin: Potential pathways to alkyl-benzenes and cycloalkanes. Renew. Sustain. Energy Rev. 2017, 72, 673–722. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Maschietti, M.; Åmand, L.; Vamling, L.; Olausson, L.; Andersson, S.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Liu, C.; Wang, Y.; Dai, L. Catalytic ethanolysis and gasification of kraft lignin into aromatic alcohols and H2-rich gas over Rh supported on La2O3/CeO2–ZrO2. Bioresour. Technol. 2016, 218, 926–933. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Yao, G.; Jin, F. Controlling the selectivity to chemicals from catalytic depolymerization of kraft lignin with in-situ H2. Bioresour. Technol. 2018, 264, 1–6. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Liu, C.; Wang, Y.; Dai, L. Correlation between Liquid Product Distribution and H2 Selectivity in Lignin Depolymerization. Chem. Eng. Technol. 2018, 41, 867–874. [Google Scholar] [CrossRef]
- Angelici, R.J. An overview of modeling studies in HDS, HDN and HDO catalysis. Polyhedron 1997, 16, 3073–3088. [Google Scholar] [CrossRef]
- Sun, Y.; Prins, R. Friedel-Crafts alkylations over hierarchical zeolite catalysts. Appl. Catal. A Gen. 2008, 336, 11–16. [Google Scholar] [CrossRef]
- Tagusagawa, C.; Takagaki, A.; Takanabe, K.; Ebitani, K.; Hayashi, S.; Domen, K. Layered and nanosheet tantalum molybdate as strong solid acid catalysts. J. Catal. 2010, 270, 206–212. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Wu, X.; Betancourt, L.E.; Tu, C.; Liao, M.; Li, R. Ni/hierarchical ZSM-5 zeolites as promising systems for phenolic bio-oil upgrading: Guaiacol hydrodeoxygenation. Fuel 2020, 274, 117859. [Google Scholar] [CrossRef]
- Qiu, S.; Li, M.; Huang, Y.; Fang, Y. Catalytic Hydrotreatment of Kraft Lignin over NiW/SiC: Effective Depolymerization and Catalyst Regeneration. Ind. Eng. Chem. Res. 2018, 57, 2023–2030. [Google Scholar] [CrossRef]

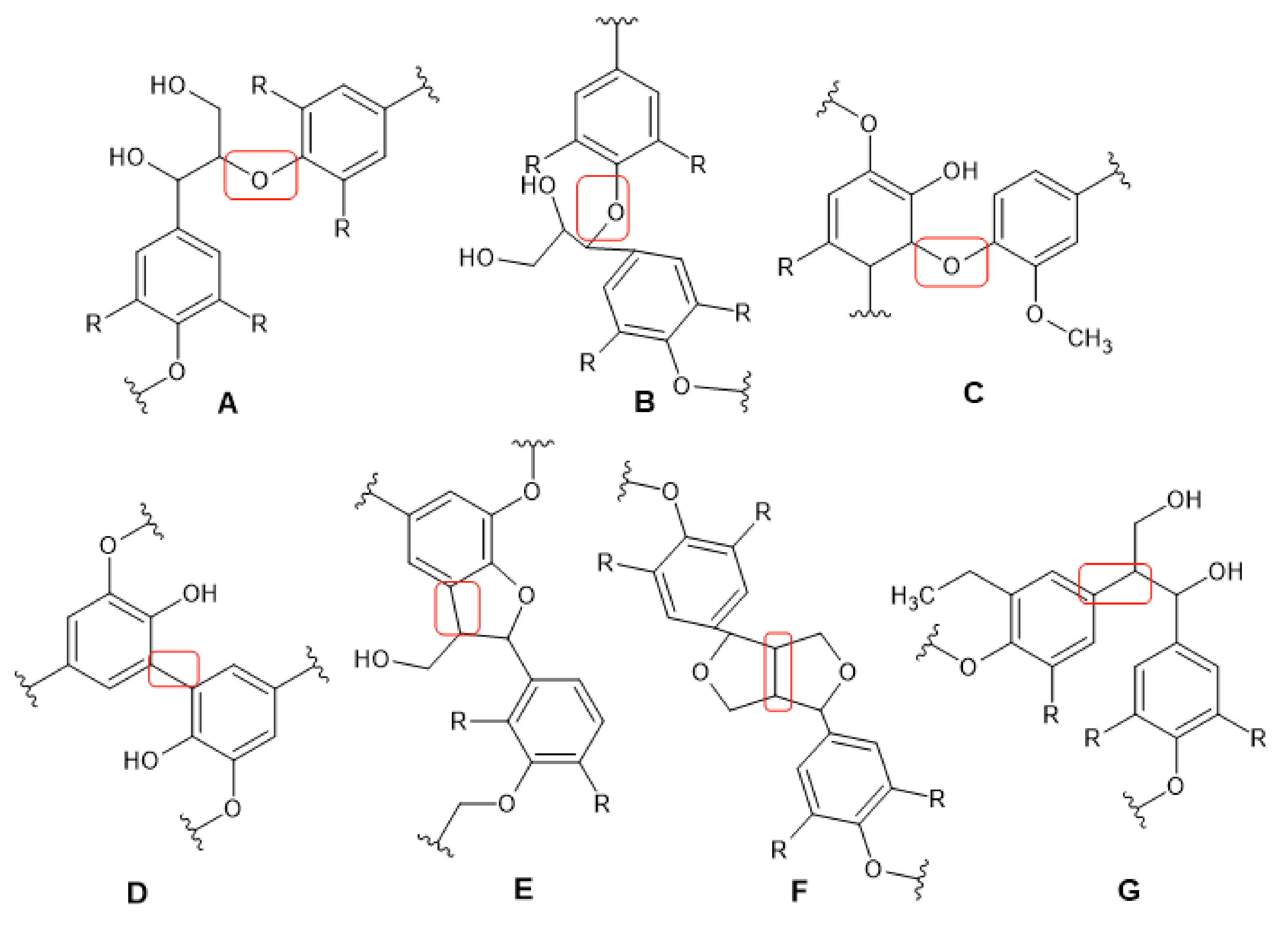
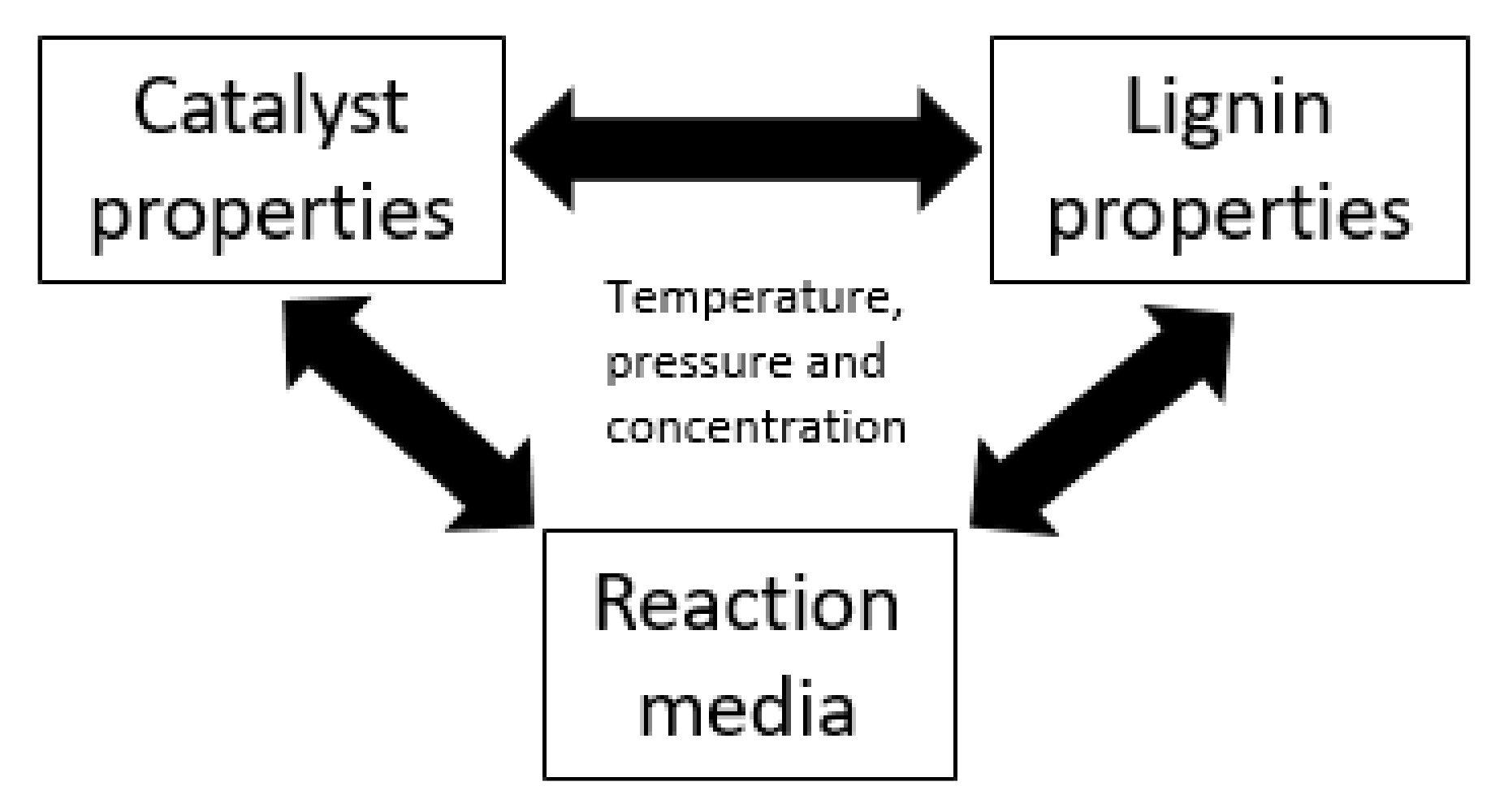
| Method | Temperature | Primary Products | References |
|---|---|---|---|
| Gasification | 700–1000 °C | Syngas | [11] |
| Pyrolysis | 300–600 °C | Gaseous hydrocarbons, bio-oil and char | [8] |
| Solvolysis | 200–350 °C | Bio-oil and char | [8] |
| Reaction Media | Catalyst | Reaction Conditions | Results | Ref. |
|---|---|---|---|---|
| Water | Ni/ZSM-5, Na2CO3 | 200 °C, 4 h, 4 MPa H2 | 83.4% oil yield | [37] |
| Methanol | NiW/NiMo/CoMo on alumina, ZSM-5 or AC | 320 °C and 35 bar H2 | 80% methanol soluble oil | [47] |
| Isopropanol | Ni-Cu on H-Beta, HZSM-5, MAS-7, MCM-41 and SAPO-11 | 330 °C for 3 h, purged with N2 | Bio-oil yield of 98.80 wt.% and monomer yield of 50.83 wt.% | [49] |
| Isopropanol | Pd/C, Pt/C and Ru/C, Rh/C | 270–350 °C, 1–5 h | Over 100% bio-oil yield | [48] |
| Dioxane-ethanol | None | 300 °C for 2 h | 55.2% bio-oil, monomers yield of 22.4% | [51] |
| Dioxane-methanol | Ru/C | 320 °C for 6 h | 93.44% bio-oil yield | [53] |
| Acetone | Ni, Ru, Mo, W on C | 100 bar H2, 250–350 °C | 93% bio-oil yield | [56] |
| Dodecane | Ru/NbOPO4 | 310 °C, 0.5 MPa H2, 40 h | 68% selectivity to arenes, liquid yield not specified | [57] |
| Ethanol–water | None | 200–350 °C, 1–2 h, 2 MPa with N2 | 90 wt.% oil yield | [64] |
| Ethanol–water | Ni10%/Zeolite and FHUDS-2) | 200–300 °C for 1–3 h | 93.5% oil yield | [65] |
| Dioxane–water | HTaMoO6 and Rh/C | 290–320 °C, 2–24 h, 2 MPa H2 | 95.6% bio-oil yield | [50] |
| Catalyst | Reaction Media | Reaction Conditions | Results | Ref. |
|---|---|---|---|---|
| Ru/C | Acetone | 100 bar H2, 250–350 °C | 95.2% liquid fraction | [56] |
| Pd, Pt, Ru and Rh on carbon | Isopropanol | 270–350 °C, 1–5 h | Total liquid yield over 100% | [48] |
| Ru/NbOPO4 | Dodecane | 310 °C, 0.5 MPa H2, 40 h of reaction | 68% selectivity to arenes | [57] |
| Rh supported on La2O3/CeO2–ZrO2 | Ethanol | 350 °C for 4 h. | Total liquid yield over 100% | [69] |
| Rh/La2O3/CeO2-ZrO2, Fe as a reductant | Isopropanol–water mixture | 373 °C for 2 h | Over 100% bio-oil rate | [70] |
| Rh/C and HTaMoO6 | Dioxane–water mixture | 290–320 °C, 2–24 h, 2 MPa H2 | 95.6% bio-oil yield | [50] |
| Ni 10%/Zeolite and FHUDS-2) | Water–ethanol mixture | 200–300 °C for 1–3 h | 93.5% KL liquid | [65] |
| Ni/C | Acetone | 100 bar H2 250–350 °C | 95.0% liquid fraction | [56] |
| Ni-Cu supported on H-Beta, HZSM-5, MAS-7, MCM-41 and SAPO-11 | Isopropanol | 330 °C for 3 h | 98.03% bio-oil yield | [49] |
| Ni–Sn metal oxide | Methanol or dioxane | 280 °C, 2 MPa H2, 1–24 h | 90%+ liquid yield at 24 h | [46] |
| NiW/SiC | Methanol | 3 MPa H2, 320 °C for 8 h | Max 74% methanol soluble liquid, 35.1% monomers | [76] |
| NiW/NiMo/CoMo on alumina, ZSM-5 or carbon | Methanol | 320 °C and 35 bar H2 | 80% methanol soluble oil | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.C.; Cheng, S.; Cross, J.S. Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review. Clean Technol. 2020, 2, 513-528. https://doi.org/10.3390/cleantechnol2040032
Garcia AC, Cheng S, Cross JS. Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review. Clean Technologies. 2020; 2(4):513-528. https://doi.org/10.3390/cleantechnol2040032
Chicago/Turabian StyleGarcia, Abraham Castro, Shuo Cheng, and Jeffrey S. Cross. 2020. "Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review" Clean Technologies 2, no. 4: 513-528. https://doi.org/10.3390/cleantechnol2040032
APA StyleGarcia, A. C., Cheng, S., & Cross, J. S. (2020). Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review. Clean Technologies, 2(4), 513-528. https://doi.org/10.3390/cleantechnol2040032