Hydrogen Is Promising for Medical Applications
Abstract
1. Introduction
2. Oxidative Stress as a Root of Many Diseases
2.1. Hydrogen Can Eliminate the Hydroxyl Radical
2.2. Chronic Inflammation as a Root of Many Disease
3. Methods of Hydrogen Ingestion
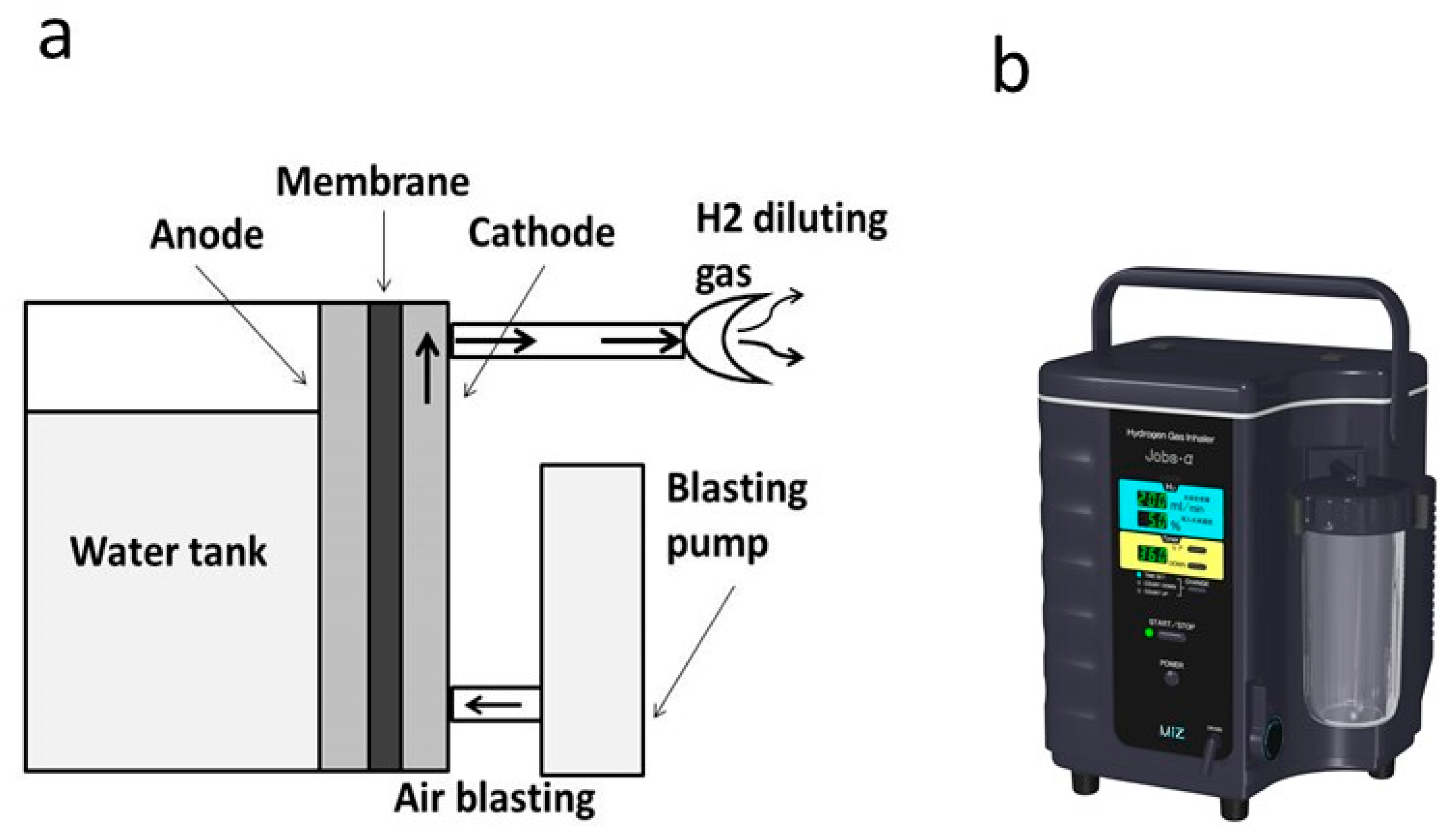
3.1. Hydrogen Gas Inhalation
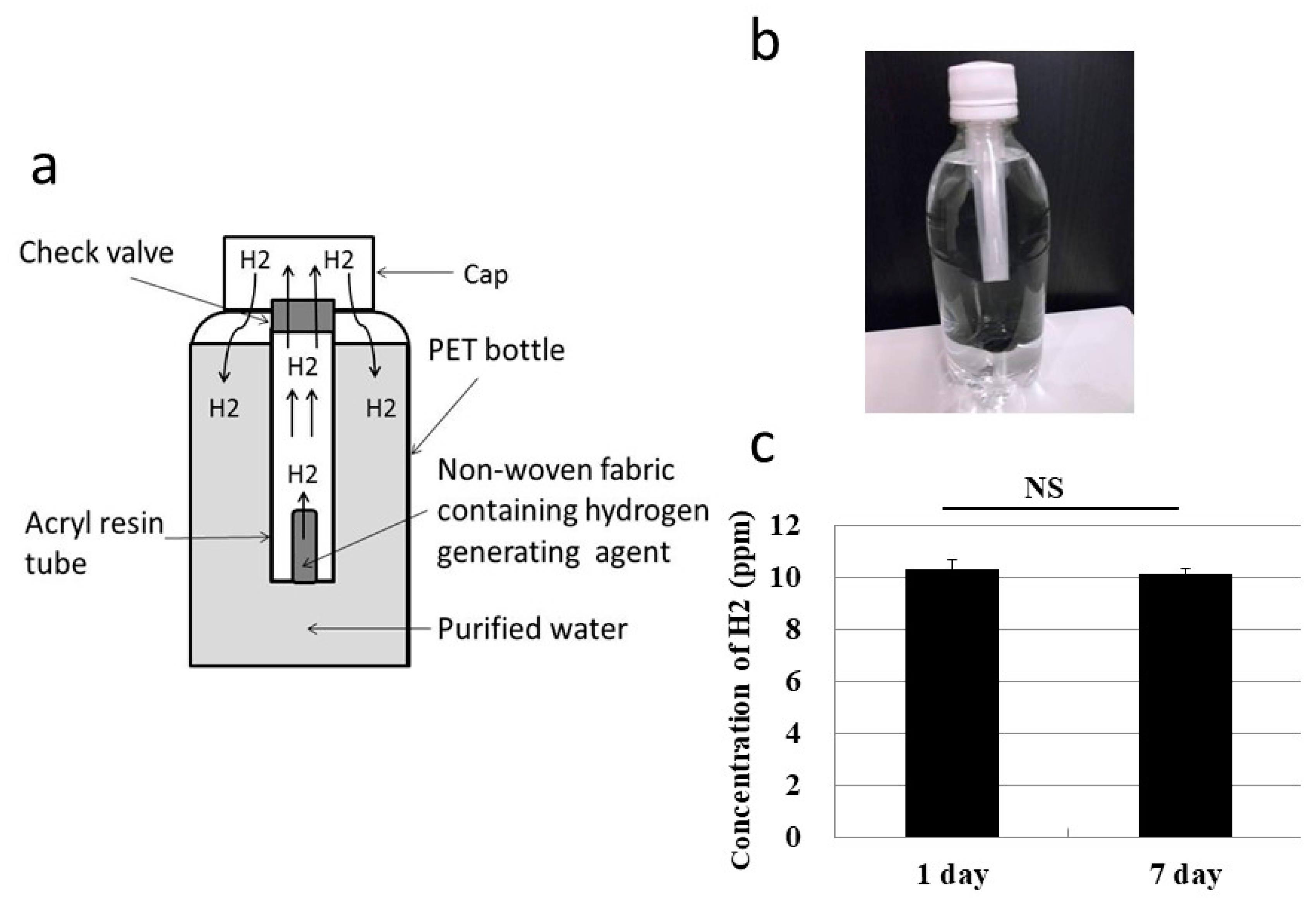
3.2. Oral Ingestion by Drinking Hydrogen Water
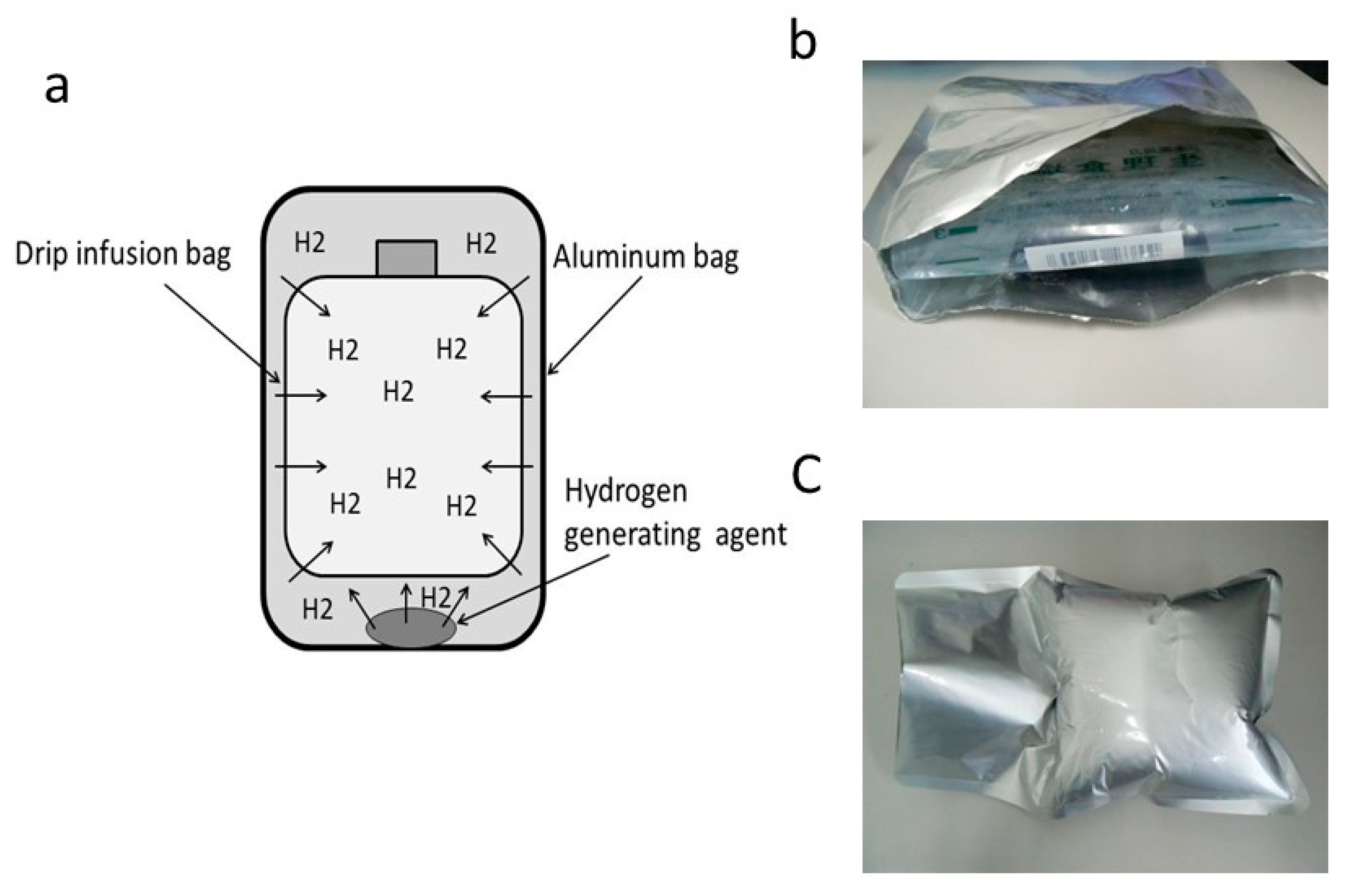
3.3. Injection of Hydrogen-Dissolved Saline
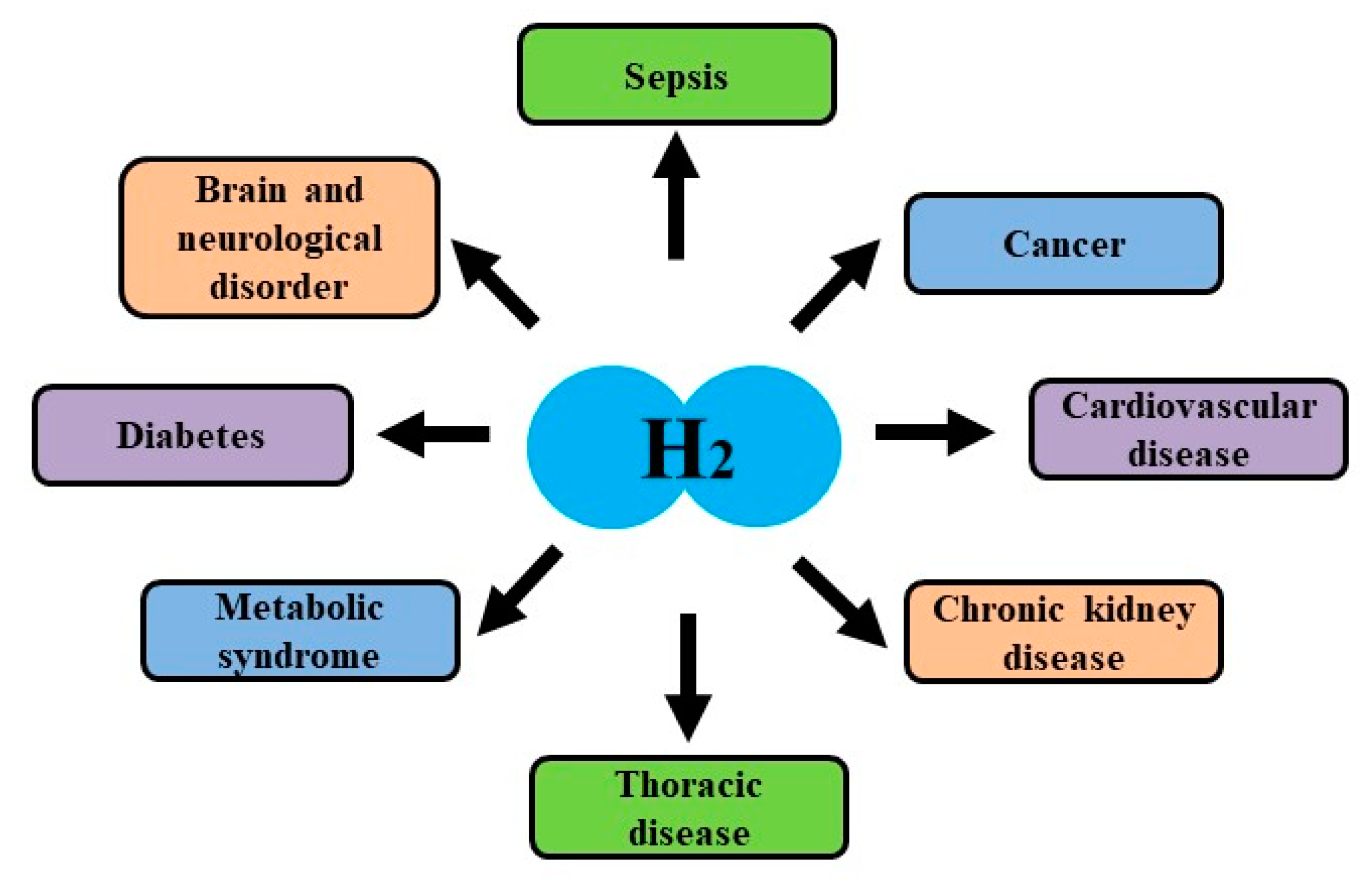
4. Effects of Hydrogen on Clinical Examinations
4.1. Effects on Brain and Neurological Disorders
4.2. Effects on Cardiovascular Disease
4.3. Effects on Thoracic Disease
4.4. Effects on Diabetes, Liver Disease and Metabolic Syndrome
4.5. Effects on Cancer and Side Effects by Cancer Therapies
5. Future Possibilities of Hydrogen Medicine
5.1. Problems in Modern Medicine
5.2. Prospective Medical Application of Hydrogen
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurokawa, R.; Seo, T.; Sato, B.; Hirano, S.I.; Sato, F. Convenient methods for ingestion of molecular hydrogen: Drinking, injection, and inhalation. Med. Gas Res. 2015, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, R.; Hirano, S.I.; Ichikawa, Y.; Matsuo, G.; Takefuji, Y. Preventing explosions of hydrogen gas inhalers. Med. Gas Res. 2019, 9, 160–162. [Google Scholar] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsuya, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of H2 therapy in Parkinson’s disease. A randomized double-blind placebo-controlled trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Ohta, S.; Sakamoto, M.; Kinone, K.; Horikoshi, T.; Tamaki, M.; Takeshita, H.; Futatuki, T.; Ohishi, W.; et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J. Stroke Cerebrovasc. 2017, 26, 2587–2594. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, T.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Shibata, S.; Sakai, T.; Hara, Y.; Naritomi, Y. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind placebo-controlled pilot study. Int. Immunopharmacol. 2014, 21, 468–473. [Google Scholar] [CrossRef]
- Nishimaki, K.; Asada, T.; Ohsawa, I.; Nakajima, E.; Ikejima, C.; Yokota, T.; Kamimura, N.; Ohta, S. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr. Alzheimer Res. 2017, 15, 482–492. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Yanagihara, T.; Arai, K.; Miyamae, K.; Sato, B.; Shudo, T.; Yamada, M.; Aoyama, M. Electrolyzed hydrogen-saturated water for drinking use elicits an antioxidative effect; a feeding test with rats. Biosci. Biotrechnol. Biochem. 2005, 69, 1985–1987. [Google Scholar] [CrossRef]
- Akagi, J.; Baba, H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol. Rep. 2018, 41, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Shimizu, K.; Ogura, H.; Kurokawa, T.; Umemoto, E.; Motooka, D.; Nakamura, S.; Ichimaru, N.; Takeda, K.; Takahara, S.; et al. Hydrogen-rich saline regulates intestinal barrier dysfunction, dysbiosis and bacterial translocation in a murine model of sepsis. Shock 2018, 50, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, Y.; Sano, F.; Abe, T.; Tamura, T.; Fujisawa, T.; Shiraishi, Y.; Khosaka, S.; Ueda, I.; Honmma, K.; Suzuki, M.; et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infraction. First pilot study in humans. Circ. J. 2017, 81, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Nagatani, K.; Otani, N.; Nawashiro, H.; Sugawara, T.; Wada, K.; Mori, K. Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Jin, K.; Xu, H.; Wang, C.; Zhang, Z. Subcutaneous injection of hydrogen gas is a novel effective treatment for type 2 diabetes. J. Diabetes Investig. 2018, 9, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome: An open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef]
- Setsukinai, K.I.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef]
- Hirano, S.I.; Ichikawa, Y.; Kurokawa, R.; Takefuji, Y.; Satoh, F. A “philosophical molecule”, hydrogen may overcome senescence and intractable diseases. Med. Gas Res. 2020, 10, 47–49. [Google Scholar] [CrossRef]
- Tschopp, J. Mitochondria: Sovereign of inflammation? Eur. J. Immunol. 2011, 41, 1196–1202. [Google Scholar] [CrossRef]
- Ismael, S.; Ahmed, H.A.; Adris, T.; Parveen, K.; Thakor, P.; Ishrat, T. The NLRP3 inflammasome: A potent therapeutic target for traumatic brain injury. Neural. Regen. Res. 2020, 16, 49–57. [Google Scholar]
- Hosseinian, N.; Cho, Y.; Lockey, R.F.; Kolliputi, N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther. Adv. Respir. Dis. 2015, 9, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [PubMed]
- Baldrighi, M.; Mallat, Z.; Li, X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 2017, 267, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tshopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavel, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Ren, J.D.; Wu, X.B.; Jiang, R.; Hao, D.P.; Liu, Y. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 50–55. [Google Scholar] [CrossRef]
- Ren, J.D.; Ma, J.; Hou, J.; Xiao, W.J.; Jin, W.H.; Wu, J.; Fan, K.H. Hydrogen-rich saline inhibits NLRP3 inflammasome activation and attenuates experimental acute pancreatitis in mice. Mediat. Inflamm. 2014, 2014, 930894. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Y.; Fan, X.; Chen, Y.; Yang, B.; Liu, K.X.; Zhou, J. Amelioration of coagulation disorders and inflammation by hydrogen-rich solution reduces intestinal ischemia/reperfusion injury in rats through NF-κB/NLRP3 pathway. Mediat. Inflamma. 2020, 2020, 4359305. [Google Scholar] [CrossRef]
- Zou, R.; Wang, M.H.; Chen, Y.; Fan, X.; Yang, B.; Du, J.; Wang, X.B.; Liu, K.H.; Zhou, J. Hydrogen-rich saline attenuates acute lung injury induced by limb ischemia/reperfusion via down-regulating chemerin and NLRP3 in rats. Shock 2018, 52, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, C.; Xie, K.; Meng, X.; Wang, Y.; Yu, Y. Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats. Neuroscience 2019, 421, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Wu, H.; Hong, Y.; Tu, S.; Sun, X.; Wu, Q.; Zhao, Q.; Zhang, J.; Sheng, J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: Possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol. Neurobiol. 2016, 53, 3462–3476. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Zuo, Y.C.; Scherchan, P.; Wang, J.K.; Yan, X.X.; Liu, F. Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front. Neurosci. 2020, 13, 1441. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, Y.; Wang, Y.; Meng, X.; Wang, Y.; Yu, Y.; Chen, H. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm. Res. 2015, 69, 697–710. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.; Meng, X.; Li, Y.; Feng, J.; Zhang, L.; Zhang, Y.; Wang, Y.; Yu, Y.; Xie, K. Hydrogen alleviates mitochondrial dysfunction and organ damage via autophagy-mediated NLRP3 inflammasome inactivation in sepsis. Int. J. Mol. Med. 2019, 44, 1309–1324. [Google Scholar] [CrossRef]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.I.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef]
- Yamamoto, R.; Homma, K.; Suzuki, S.; Sano, M.; Sasaki, J. Hydrogen gas distribution in organs after inhalation: Real-time monitoring of tissue hydrogen concentration in rat. Sci. Rep. 2019, 9, 1255. [Google Scholar] [CrossRef]
- Abe, T.; Li, X.K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Tsuda, H.; et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation 2012, 94, 14–21. [Google Scholar] [CrossRef]
- Tamaki, I.; Hata, K.; Okamura, Y.; Nigmet, Y.; Hirano, H.; Kubota, T.; Inamoto, O.; Kusakabe, J.; Goto, T.; Tajima, T.; et al. Hydrogen flush after cold storage as a new end-ischemic ex vivo treatment for liver graft against ischemia/reperfusion injury. Liver Transpl. 2018, 24, 1589–1602. [Google Scholar] [CrossRef]
- Igarashi, T.; Ohsawa, I.; Kobayashi, M.; Igarashi, T.; Suzuki, T.; Iketani, M.; Takahashi, H. Hydrogen prevents corneal endothelial damage in phacoemulsification cataract surgery. Sci. Rep. 2016, 6, 31190. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Ohsawa, I.; Kobayashi, M.; Umemoto, Y.; Arima, T.; Suzuki, T.; Igarashi, T.; Otsuka, T.; Takahashi, H. Effects of hydrogen in prevention of corneal endothelial damage during phacoemulsification: A prospective randomized clinical trial. Am. J. Ophthalmol. 2019, 207, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Adachi, N.; Tachibana, S.; Chitoku, S.; Mukaihara, S.; Sakamoto, M.; Kudo, Y.; Nakazawa, J.; Kaneko, K.; et al. Improved brain MRI indices in the acute brain stem infarct sires treated with hydroxy radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med. Gas Res. 2011, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishizima, Y.; Sakamoto, M.; Horikoshi, T.; Tamaki, M.; Oishi, W.; Naitoh, Y.; Futaki, T.; Ishii, S.; Suzuki, K.; et al. Pilot study on therapeutic inhalation of hydrogen gas for improving patients with Alzheimer’s disease assessed by cognitive subscale scores and magnetic resonance diffusion tensor imaging. Int. J. Alzheimers Neuro. Disord. 2018, 1, 1. [Google Scholar]
- Yang, L.; Li, D.; Chen, S. Hydrogen water reduces NSE, IL-6, and TNF-α levels in hypoxic-ischemic encephalopathy. Open Med. 2016, 11, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Hayashida, K.; Sano, M.; Suzuki, M.; Shibusawa, T.; Yoshizawa, J.; Kabayashi, Y.; Suzuki, T.; Ohta, S.; Morisaki, H.; et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome. Circ. J. 2016, 80, 1870–1873. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Suzuki, M.; Hayashida, K.; Kobayashi, Y.; Yoshizawa, J.; Shibusawa, T.; Sano, M.; Hori, S.; Sasaki, J. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J. Clin. Biochem. Nutr. 2020, 67, 214–221. [Google Scholar] [CrossRef]
- Guan, W.J.; Wei, C.H.; Chen, A.L.; Sun, X.C.; Guo, G.Y.; Zou, X.; Shi, J.D.; Lai, P.Z.; Zheng, Z.G.; Zhong, N.S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020, 12, 3448–3452. [Google Scholar] [CrossRef]
- Gong, Z.; Guan, J.; Ren, X.; Meng, D.; Zhang, H.; Wang, B.; Yan, X. Protective effect of hydrogen on the lung of sanitation workers exposed to haze. Chin. J. Tuberc. Respir. Dis. 2016, 39, 916–923. [Google Scholar]
- Wang, S.T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T.; Xu, K.F. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM Int. J. Med. 2020, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Zhong, C.H.; Su, Z.Q.; Li, X.Y.; Chen, Y.; Chen, X.B.; Tang, C.L.; Zhou, L.Q.; Li, S.Y. Breathing hydrogen-oxygen mixture decreases inspiratory effort in patients with tracheal stenosis. Respiration 2019, 97, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B. Clin. Trans. Sci. 2013, 6, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lin, Q.; Zhao, H.; Liu, M.; Ye, F.; Sun, Y.; Yu, Y.; Guo, S.; Jiao, P.; Wu, Y.; et al. Hydrogen activates ATP-binding cassette transporter A1-dependent ex vivo and improves high-density lipoprotein function in patients with hypercholesterolemia: A double-blinded, randomized, and placebo-controlled trial. J. Clin. Endocrinol. Metab. 2015, 100, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, M.; Sang, H.; Zhang, L.; Li, X.; Yao, S.; Yu, Y.; Zong, C.; Xue, Y.; Qin, S. Hydrogen-rich water decreases serum low-density lipoprotein cholesterol levels and improves high-density lipoprotein function in patients with potential metabolic syndrome. J. Lipid Res. 2013, 54, 1884–1893. [Google Scholar] [CrossRef]
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-rich water reduces liver fat accumulation and improves liver enzymes profiles in patients with non-alcoholic fatty liver disease: A randomized controlled pilot trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693. [Google Scholar] [CrossRef]
- Chen, J.B.; Kong, X.F.; Lv, Y.Y.; Qin, S.C.; Sun, X.J.; Mu, F.; Lu, T.Y.; Xu, K.C. “Real world survey” of hydrogen-controlled cancer: A follow-up report of 82 advanced cancer patients. Med. Gas Res. 2019, 9, 115–121. [Google Scholar]
- Chen, J.B.; Pan, Z.B.; Du, D.M.; Qian, W.; Ma, Y.Y.; Mu, F.; Xu, K.C. Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report. World J. Clin. Cases 2019, 6, 2065–2074. [Google Scholar] [CrossRef]
- Chen, J.; Mu, F.; Lu, T.; Du, D.; Xu, K. Brain metastases completely disappear in non-small cell lung cancer using hydrogen gas inhalation: A case report. Onco. Targets Ther. 2019, 12, 11145–11151. [Google Scholar] [CrossRef]
- Kang, K.M.; Kang, Y.M.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of hydrogen-rich water on treated with radiotherapy for liver tumors. Med. Gas Res. 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.I.; Aoki, Y.; Li, X.K.; Ichimaru, N.; Takahara, S.; Takefuji, Y. Protective effects of hydrogen gas inhalation on radiation-induced bone marrow damage in cancer patients: A retrospective observational study. Med. Gas Res. 2021, 11. in press. [Google Scholar]
- ICD-10 Version: 2016. Available online: http://apps.who.int/classifications/icd10/browse/2016/en#/U00-U49 (accessed on 24 November 2020).
- U.S. Pharmacopeia. Available online: http://www.usp.org/ (accessed on 24 November 2020).
- British Pharmacopeia. Available online: https://www.pharmacopoeia.com/ (accessed on 24 November 2020).
- Global Burden of Disease Health Financing Collaborator Network; Dieleman, J.L.; Campbell, M.; Chapin, A.; Eldrenkamp, E.; Fan, V.Y.; Haakenstad, A.; Kates, J.; Li, Z.; Matyasz, T.; et al. Future and potential spending on health 2015–40: Development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet 2017, 389, 2005–2030. [Google Scholar] [CrossRef]
- Seed, T.M.; Inal, C.E.; Singh, V.K. Radioprotection of hematopoietic progenitors by low dose amifostine prophylaxis. Int. J. Radiat. Biol. 2014, 90, 594–604. [Google Scholar] [CrossRef][Green Version]
- Zhou, C.; Coshi, E.; He, Q. Micro/nanomaterials-augmented hydrogen therapy. Adv. Healthc. Mater. 2019, 1900463. [Google Scholar] [CrossRef]
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Zhao, P.; Cheng, Q.; He, Q.; Ma, D.; Xue, W. NIR-laser-controlled hydrogen-releasing PdH nanohydride for synergistic hydrogen-photothermal antibacterial and wound-healing therapies. Adv. Funct. Mater. 2019, 29, 1905697. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, P.; Yue, C.; Jin, Z.; Liu, Q.; Du, X.; He, Q.; Du, X.; He, Q. Sustained release of bioactive hydrogen by Pd hydride nanoparticles overcome Alzheimer’s disease. Biomaterials 2019, 197, 393–404. [Google Scholar] [CrossRef]
- Ministry of Health, Labor and Welfare in Japan. Advanced Medical Technology Overview (in Japanese). Available online: https://www.mhlw.go.jp/topics/bukyoku/isei/sensiniryo/kikan03.html (accessed on 9 December 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, S.-i.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen Is Promising for Medical Applications. Clean Technol. 2020, 2, 529-541. https://doi.org/10.3390/cleantechnol2040033
Hirano S-i, Ichikawa Y, Sato B, Satoh F, Takefuji Y. Hydrogen Is Promising for Medical Applications. Clean Technologies. 2020; 2(4):529-541. https://doi.org/10.3390/cleantechnol2040033
Chicago/Turabian StyleHirano, Shin-ichi, Yusuke Ichikawa, Bunpei Sato, Fumitake Satoh, and Yoshiyasu Takefuji. 2020. "Hydrogen Is Promising for Medical Applications" Clean Technologies 2, no. 4: 529-541. https://doi.org/10.3390/cleantechnol2040033
APA StyleHirano, S.-i., Ichikawa, Y., Sato, B., Satoh, F., & Takefuji, Y. (2020). Hydrogen Is Promising for Medical Applications. Clean Technologies, 2(4), 529-541. https://doi.org/10.3390/cleantechnol2040033






