Quantifying the Immediate Response of Soil to Wild Boar (Sus scrofa L.) Grubbing in Mediterranean Olive Orchards
Abstract
1. Introduction
2. Materials and Methods
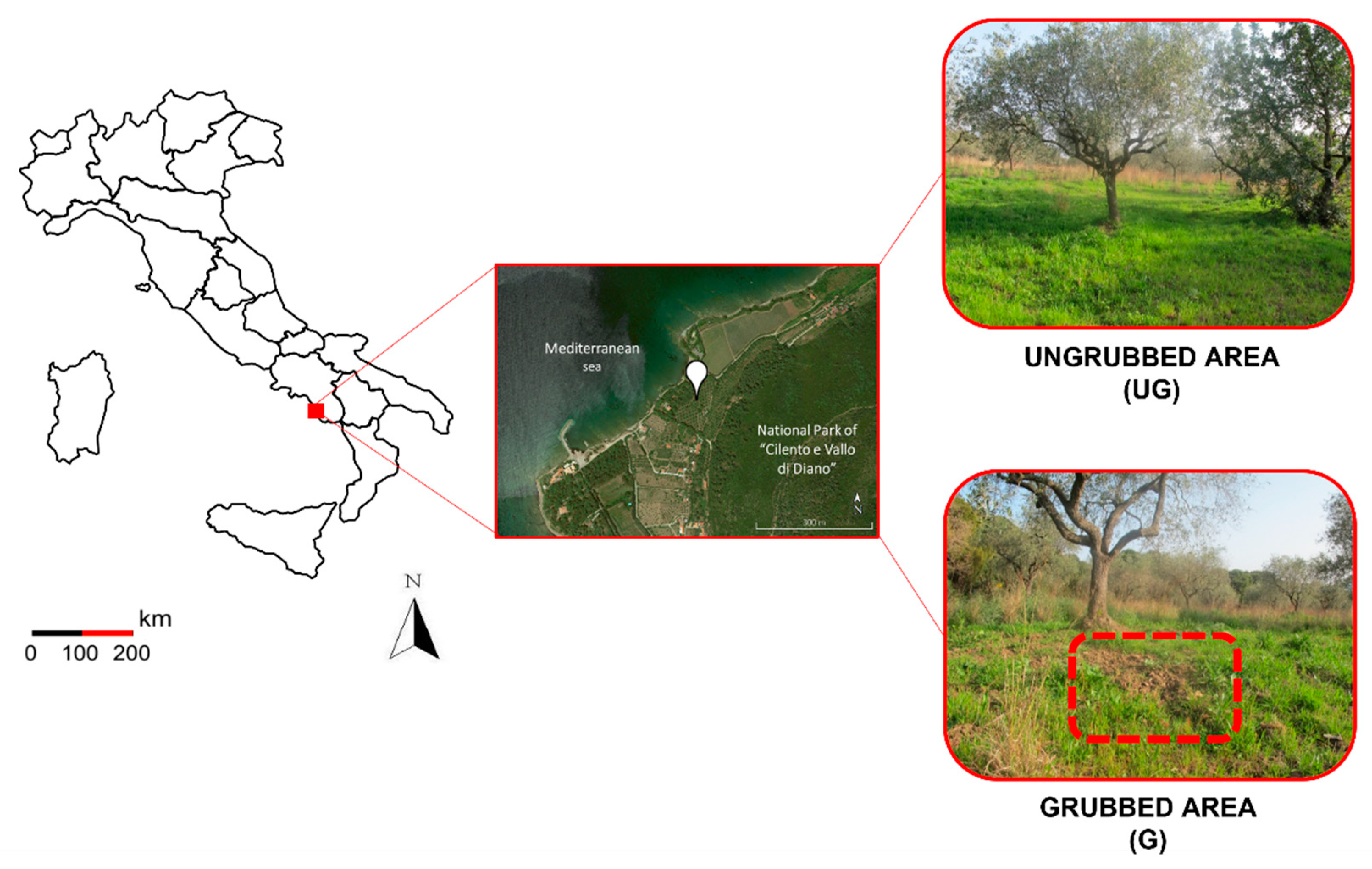
2.1. Study Area and Soil Sampling
2.2. Soil Physical and Chemical Analysis
2.3. Soil Biological Analysis
2.4. Soil Quality Index
2.5. Grubbing Impact Factor (IFG)
2.6. Leaf and Fruit Sampling and Analyses
2.7. Statistical Analysis
3. Results
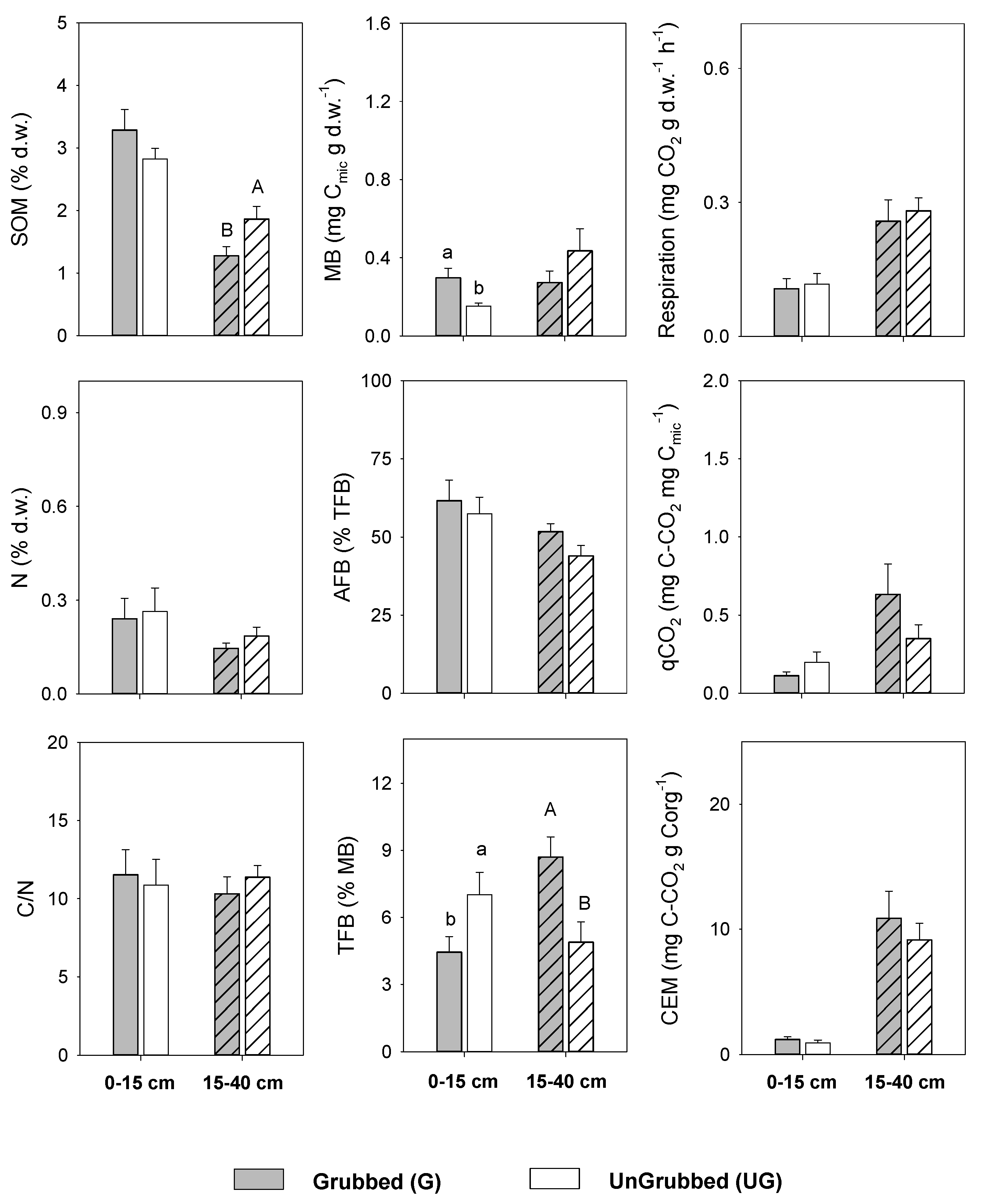
3.1. Effect of Wild Boar Grubbing on Soil Quality
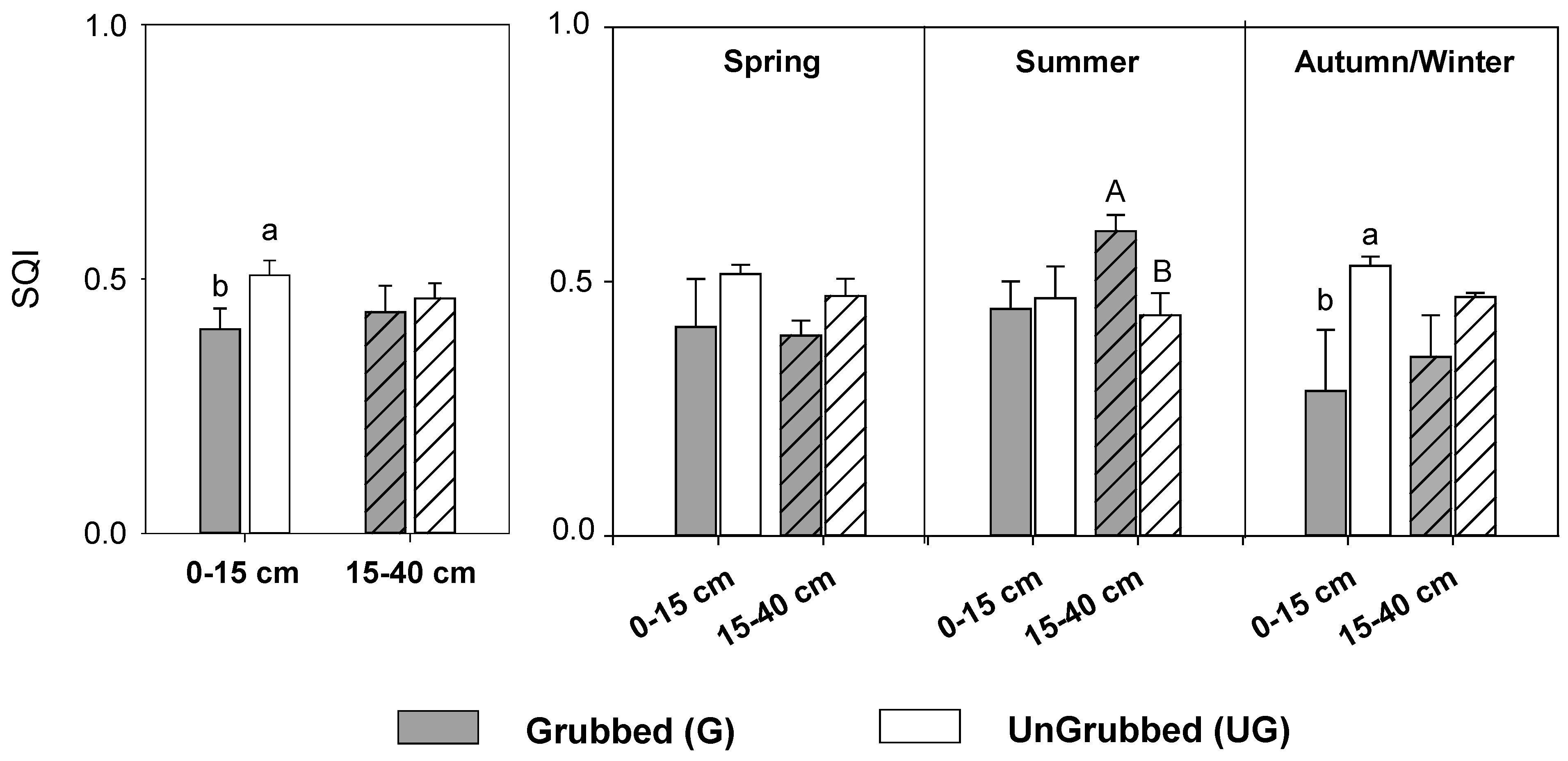
3.2. Seasonal Responses to Soil Grubbing
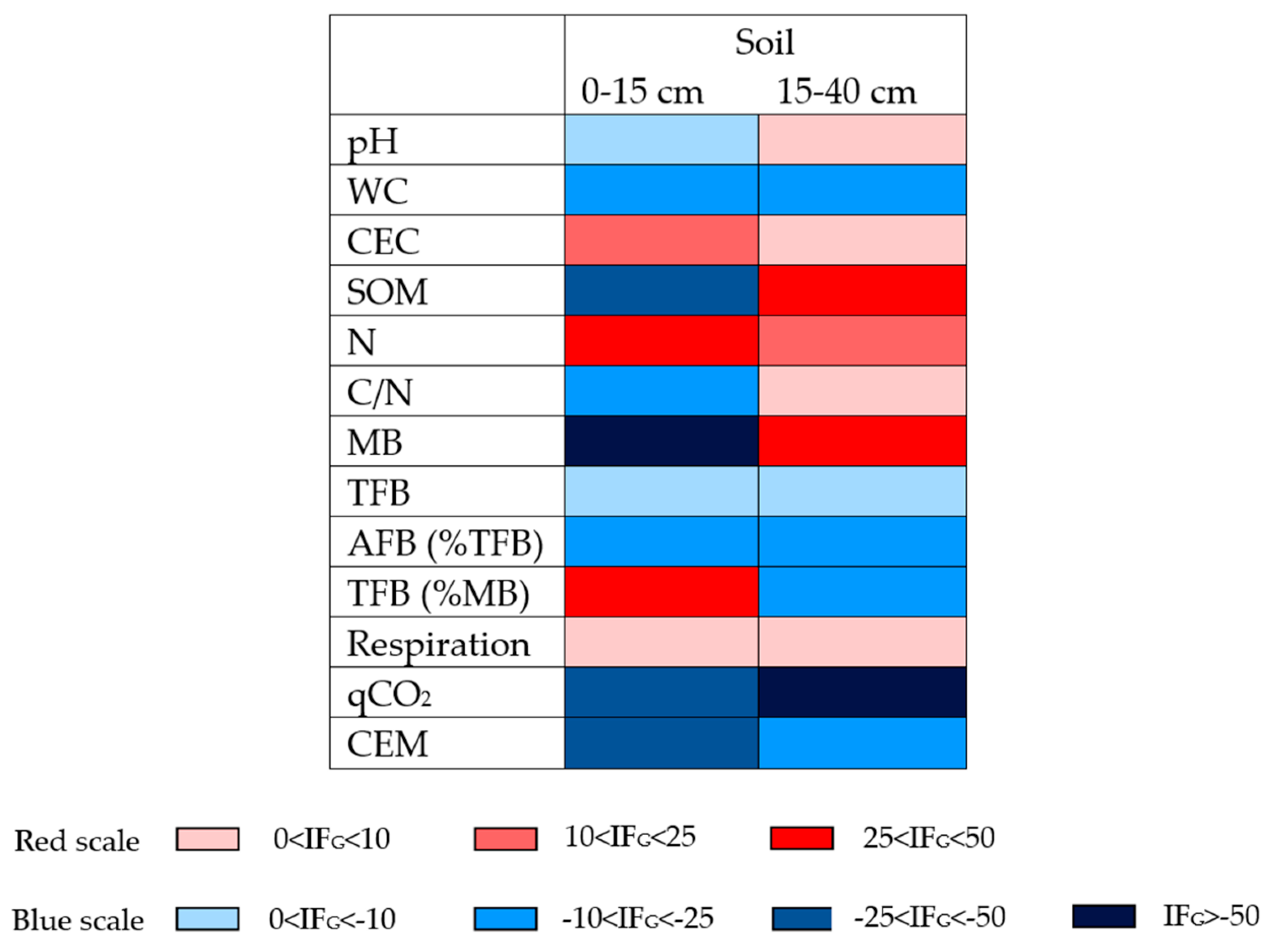
3.3. Grubbing Impact Factor
3.4. Leaf and Fruit Characteristics of Olive Trees
4. Discussion
4.1. Overall Assessment of Immediate Grubbing Effect on Olive Groves
4.2. Seasonal Variation of Grubbing Effects on Soil Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, C.; Papatheodorou, E.M.; Monokrousos, N.; Stamou, G.P. Spatial variability of soil organic C, inorganic C and extractable P in a Mediterranean grazed area. Land Degrad. Dev. 2012, 26, 103–109. [Google Scholar] [CrossRef]
- Troiano, C.; Buglione, M.; Petrelli, S.; Belardinelli, S.; De Natale, A.; Svenning, J.C.; Fulgione, D. Traditional free-ranging livestock farming as a management strategy for biological and cultural landscape diversity: A case from the southern apennines. Land 2021, 10, 957. [Google Scholar] [CrossRef]
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Napoletano, P.; Panico, S.C.; Memoli, V.; Santorufo, L.; Ruggiero, A.G.; Colombo, C.; Barile, R.; Maisto, G. Combined effect of black locust invasion and fire on soils of Mediterranean shrublands and pine forests. Catena 2023, 220, 106656. [Google Scholar] [CrossRef]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: Contexts, causes, monitoring and management recommendations. Mamm. Rev. 2021, 51, 95–108. [Google Scholar] [CrossRef]
- Toyota, K.; Young, I.M.; Ritz, K. Effects of soil matric potential and bulk density on the growth of Fusarium oxysporum f. sp. raphani. Soil Biol. Biochem. 1996, 28, 1139–1145. [Google Scholar] [CrossRef]
- Greenwood, K.L.; McKenzie, B.M. Grazing effects on soil physical properties and the consequences for pastures: A review. Aust. J. Exp. Agric. 2001, 41, 1231–1250. [Google Scholar] [CrossRef]
- Macci, C.; Doni, S.; Bondi, G.; Davini, D.; Masciandaro, G.; Pistoia, A. Effects of wild boar (Sus scrofa) grazing on soil properties in Mediterranean environment. Catena 2012, 98, 79–86. [Google Scholar] [CrossRef]
- Fulgione, D.; Rippa, D.; Buglione, M.; Trapanese, M.; Petrelli, S.; Maselli, V. Unexpected but welcome. Artificially selected traits may increase fitness in wild boar. Evol. Appl. 2016, 9, 769–776. [Google Scholar] [CrossRef]
- Maselli, V.; Rippa, D.; De Luca, A.; Larson, G.; Wilkens, B.; Linderholm, A.; Masseti, M.; Fulgione, D. Southern Italian wild boar population, hotspot of genetic diversity. Hystrix 2016, 27, 137–144. [Google Scholar] [CrossRef]
- Fulgione, D.; Buglione, M. The Boar War: Five Hot Factors Unleashing Boar Expansion and Related Emergency. Land 2022, 11, 887. [Google Scholar] [CrossRef]
- Fanelli, A.; Perrone, A.; Ferroglio, E. Spatial and temporal dynamics of wild boars Sus scrofa hunted in Alpine environment. Eur. J. Wildl. Res. 2021, 67, 47. [Google Scholar] [CrossRef]
- Petrelli, S.; Buglione, M.; Maselli, V.; Troiano, C.; Larson, G.; Frantz, L.; Manin, A.; Ricca, E.; Baccigalupi, L.; Wright, D.; et al. Population genomic, olfactory, dietary, and gut microbiota analyses demonstrate the unique evolutionary trajectory of feral pigs. Mol. Ecol. 2022, 31, 220–237. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Sallustio, L.; Tarolli, P. The geomorphologic forcing of wild boars. Earth Surf. Process. Landf. 2019, 44, 2085–2094. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Ballari, S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: A review. Biol. Invasions 2012, 14, 2283–2300. [Google Scholar] [CrossRef]
- Singer, F.J.; Swank, W.T.; Clebsch, E.E.C. Effects of Wild Pig Rooting in a Deciduous Forest. J. Wildl. Manag. 1984, 48, 464–473. [Google Scholar] [CrossRef]
- Siemann, E.; Carrillo, J.A.; Gabler, C.A.; Zipp, R.; Rogers, W.E. Experimental test of the impacts of feral hogs on forest dynamics and processes in the southeastern US. For. Ecol. Manag. 2009, 258, 546–553. [Google Scholar] [CrossRef]
- Tierney, T.A.; Cushman, J.H. Temporal changes in native and exotic vegetation and soil characteristics following disturbances by feral pigs in a California grassland. Biol. Invasions 2006, 8, 1073–1089. [Google Scholar] [CrossRef]
- Moody, A.; Jones, J.A. Soil response to canopy position and feral pig disturbance beneath Quercus agrifolia on Santa Cruz Island, California. Appl. Soil Ecol. 2000, 14, 269–281. [Google Scholar] [CrossRef]
- Mohr, D.; Cohnstaedt, L.W.; Topp, W. Wild boar and red deer affect soil nutrients and soil biota in steep oak stands of the Eifel. Soil Biol. Biochem. 2005, 37, 693–700. [Google Scholar] [CrossRef]
- Wirthner, S.; Frey, B.; Busse, M.D.; Schütz, M.; Risch, A.C. Effects of wild boar (Sus scrofa L.) rooting on the bacterial community structure in mixed-hardwood forest soils in Switzerland. Eur. J. Soil Biol. 2011, 47, 296–302. [Google Scholar] [CrossRef]
- Panico, S.C.; Memoli, V.; Esposito, F.; Maisto, G.; De Marco, A. Plant cover and management practices as drivers of soil quality. Appl. Soil Ecol. 2018, 129, 34–42. [Google Scholar] [CrossRef]
- Memoli, V.; De Marco, A.; Esposito, F.; Panico, S.C.; Barile, R.; Maisto, G. Seasonality, altitude and human activities control soil quality in a national park surrounded by an urban area. Geoderma 2019, 337, 1–10. [Google Scholar] [CrossRef]
- Welander, J. Spatial and temporal dynamics of wild boar (Sus scrofa) rooting in a mosaic landscape. J. Zool. 2000, 252, 263–271. [Google Scholar] [CrossRef]
- Schley, L.; Roper, T.J. Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mamm. Rev. 2003, 33, 43–56. [Google Scholar] [CrossRef]
- Bobek, B.; Furtek, J.; Bobek, J.; Merta, D.; Wojciuch-Ploskonka, M. Spatio-temporal characteristics of crop damage caused by wild boar in north-eastern Poland. Crop Prot. 2017, 93, 106–112. [Google Scholar] [CrossRef]
- Prodotti Tipici Della Campania. Available online: http://www.agricoltura.regione.campania.it/tipici/olio-cilento.html (accessed on 9 January 2023).
- Scandurra, A.; Magliozzi, L.; Fulgione, D.; Aria, M.; D’Aniello, B. Lepidoptera Papilionoidea communities as a sentinel of biodiversity threat: The case of wild boar rooting in a Mediterranean habitat. J. Insect Conserv. 2016, 20, 353–362. [Google Scholar] [CrossRef]
- Maselli, V.; Rippa, D.; Russo, G.; Ligrone, R.; Soppelsa, O.; D’Aniello, B.; Raia, P.; Fulgione, D. Wild boars’ social structure in the Mediterranean habitat. Ital. J. Zool. 2014, 81, 610–617. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Manes, F. Blasi Analysis of a pioneer community of Dittrichia viscosa (L.) greuter in a mediter ranean environment in southern italy. G. Bot. Ital. 1990, 124, 321–329. [Google Scholar] [CrossRef]
- IUSS. IUSS Working Group WRB: World Reference Base for Soil Resources 2014, Update 2015; World Soil Resources Report No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Colombo, C.; Miano, T. Metodi di Analisi Chimica del Suolo; Pubblicità & Stampa: Bari, Italy, 2015. [Google Scholar]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Duncan, L.C. Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biol. Biochem. 2001, 33, 1143–1153. [Google Scholar] [CrossRef]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; De Santo, A.V. C stocks in forest floor and mineral soil of two mediterranean beech forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef]
- Memoli, V.; Esposito, F.; De Marco, A.; Arena, C.; Vitale, L.; Tedeschi, A.; Magliulo, V.; Maisto, G. Metal compartmentalization in different biomass portions of Helianthus annuus L. and Sorghum bicolor L. grown in an agricultural field inside an urban fabric. Appl. Soil Ecol. 2017, 121, 118–126. [Google Scholar] [CrossRef]
- Napoletano, P.; Colombo, C.; Di Iorio, E.; Memoli, V.; Panico, S.C.; Ruggiero, A.G.; Santorufo, L.; Maisto, G.; De Marco, A. Integrated approach for quality assessment of technosols in experimental mesocosms. Sustainability 2021, 13, 9101. [Google Scholar] [CrossRef]
- Liebig, M.A.; Varvel, G.; Doran, J. A simple performance-based index for assessing multiple agroecosystem functions. Agron. J. 2001, 93, 313–318. [Google Scholar] [CrossRef]
- Fathizad, H.; Ardakani, M.A.H.; Heung, B.; Sodaiezadeh, H.; Rahmani, A.; Fathabadi, A.; Scholten, T.; Taghizadeh-Mehrjardi, R. Spatio-temporal dynamic of soil quality in the central Iranian desert modeled with machine learning and digital soil assessment techniques. Ecol. Indic. 2020, 118, 106736. [Google Scholar] [CrossRef]
- Andrews, S.S.; Flora, C.B.; Mitchell, J.P.; Karlen, D.L. Growers’ perceptions and acceptance of soil quality indices. Geoderma 2003, 114, 187–213. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- De Marco, A.; Arena, C.; Giordano, M.; Virzo De Santo, A. Impact of the invasive tree black locust on soil properties of Mediterranean stone pine-holm oak forests. Plant Soil 2013, 372, 473–486. [Google Scholar] [CrossRef]
- Nergiz, C.; Engez, Y. Compositional variation of olive fruit during ripening. Food Chem. 2000, 69, 55–59. [Google Scholar] [CrossRef]
- Doesouky, I.M.; Haggag, F.L.; Abdel-Migeed, M.M.; El-hady, E.S. Changes in some physical and chemical fruit properties during fruit development stage of some olive oil cultivars. Am. J. Agri. & Environ. Sci. 2010, 7, 12–17. [Google Scholar]
- Savarese, M.; De Marco, E.; Sacchi, R. Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry. Food Chem. 2007, 105, 761–770. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Sadeghi, N.; Jannat, B.; Oveisi, M.R.; Madani, S.; Kiayi, M.; Akrami, M.R.; Ranjbar, A.M. Antioxidant activity, reducing power and total phenolic content of Iranian olive cultivar. J. Biol. Sci. 2008, 8, 779–783. [Google Scholar] [CrossRef]
- Schrama, M.; Heijning, P.; Bakker, J.P.; van Wijnen, H.J.; Berg, M.P.; Olff, H. Herbivore trampling as an alternative pathway for explaining differences in nitrogen mineralization in moist grasslands. Oecologia 2013, 172, 231–243. [Google Scholar] [CrossRef]
- Sitters, J.; Andriuzzi, W.S. Impacts of browsing and grazing ungulates on soil biota and nutrient dynamics. In The Ecology of Browsing and Grazing II. Ecological Studies (Analysis and Synthesis); Gordon, I., Prins, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 215–236. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Ventorino, V.; De Marco, A.; Pepe, O.; Virzo De Santo, A.; Moschetti, G. Impact of innovative agriculturalpractices of carbon sequestration on soilmicrobial community. In Carbon Sequestration in Agricultural Soils. A multidisciplinary Approach to Innovative Methods; Piccolo, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–177. [Google Scholar]
- De Marco, A.; Esposito, F.; Berg, B.; Zarrelli, A.; De Santo, A.V. Litter inhibitory effects on soil microbial biomass, activity, and catabolic diversity in two paired stands of Robinia pseudoacacia L. and Pinus nigra Arn. Forests 2018, 9, 766. [Google Scholar] [CrossRef]
- Panico, S.C.; Memoli, V.; Napoletano, P.; Esposito, F.; Colombo, C.; Maisto, G.; De Marco, A. Variation of the chemical and biological properties of a Technosol during seven years after a single application of compost. Appl. Soil Ecol. 2019, 138, 156–159. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Jones, A.C.; Jones, D.L.; Kemmitt, S.J.; Cook, R.; Hobbs, P.J. Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biol. Biochem. 2001, 33, 1653–1664. [Google Scholar] [CrossRef]
- Fterich, A.; Mahdhi, M.; Mars, M. Impact of grazing on soil microbial communities along a chronosequence of Acacia tortilis subsp. raddiana in arid soils in Tunisia. Eur. J. Soil Biol. 2012, 50, 56–63. [Google Scholar] [CrossRef]
- Di Iorio, E.; Napoletano, P.; Circelli, L.; Memoli, V.; Santorufo, L.; De Marco, A.; Colombo, C. Comparison of natural and technogenic soils developed on volcanic ash by Vis-NIR spectroscopy. Catena 2022, 216, 106369. [Google Scholar] [CrossRef]
- De Marco, A.; Meola, A.; Esposito, F.; De Santo, A.V. Productivity and modifications of ecosystem processes in gaps of a low Macchia on southern Italy. Web Ecol. 2008, 8, 55–66. [Google Scholar] [CrossRef]
- Panico, S.C.; Esposito, F.; Memoli, V.; Vitale, L.; Polimeno, F.; Magliulo, V.; Maisto, G.; De Marco, A. Variations of agricultural soil quality during the growth stages of sorghum and sunflower. Appl. Soil Ecol. 2020, 152, 103569. [Google Scholar] [CrossRef]
- Di Iorio, E.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303, 135172. [Google Scholar] [CrossRef] [PubMed]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.A.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Santorufo, L.; Memoli, V.; Panico, S.C.; Esposito, F.; Vitale, L.; Di Natale, G.; Trifuoggi, M.; Barile, R.; De Marco, A.; Maisto, G. Impact of anthropic activities on soil quality under different land uses. Int. J. Environ. Res. Public Health 2021, 18, 8423. [Google Scholar] [CrossRef]
- Askari, M.S.; Holden, N.M. Quantitative soil quality indexing of temperate arable management systems. Soil Tillage Res. 2015, 150, 57–67. [Google Scholar] [CrossRef]
- Raiesi, F.; Kabiri, V. Identification of soil quality indicators for assessing the effect of different tillage practices through a soil quality index in a semi-arid environment. Ecol. Indic. 2016, 71, 198–207. [Google Scholar] [CrossRef]
- Napoletano, P.; Guezgouz, N.; Di Iorio, E.; Colombo, C.; Guerriero, G.; De Marco, A. Anthropic impact on soil heavy metal contamination in riparian ecosystems of northern Algeria. Chemosphere 2022, 313, 137522. [Google Scholar] [CrossRef]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef]
- Smith, E.A.; Holden, E.M.; Brown, C.; Cahill, J.F. Disturbance has lasting effects on functional traits and diversity of grassland plant communities. PeerJ 2022, 10, e13179. [Google Scholar] [CrossRef]
- Loughnan, D.; Gilbert, B. Trait-mediated community assembly: Distinguishing the signatures of biotic and abiotic filters. Oikos 2017, 126, 1112–1122. [Google Scholar] [CrossRef]
- Cramer, M.D.; Hawkins, H.J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Moodley, R.; Koorbanally, N.; Jonnalagadda, S.B. Elemental composition and fatty acid profile of the edible fruits of Amatungula (Carissa macrocarpa) and impact of soil quality on chemical characteristics. Anal. Chim. Acta 2012, 730, 33–41. [Google Scholar] [CrossRef]
- Doğan, B.; Gülser, C. Soil quality assessment for olive groves areas of menderes district, izmir-turkey. Eurasian J. Soil Sci. 2020, 9, 298–305. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Marin, L.; Sánchez-Zamora, M.A.; García-Novelo, J.M.; Molina-Soria, C.; Parra, M.A. Long-term effects of N fertilization on cropping and growth of olive trees and on N accumulation in soil profile. Eur. J. Agron. 2009, 31, 223–232. [Google Scholar] [CrossRef]
- Grattan, S.R.; Berenguer, M.J.; Connell, J.H.; Polito, V.S.; Vossen, P.M. Olive oil production as influenced by different quantities of applied water. Agric. Water Manag. 2006, 85, 133–140. [Google Scholar] [CrossRef]
- Jess Tovar, M.; Paz Romero, M.; Girona, J.; Jos Motilva, M. L-phenylalanine ammonia-lyase activity and concentration of phenolics in developing olive (Olea europaea L. cv Arbequina) fruit grown under different irrigation regimes. J. Sci. Food Agric. 2002, 82, 892–898. [Google Scholar] [CrossRef]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Chehab, H.; Tekaya, M.; Ouhibi, M.; Gouiaa, M.; Zakhama, H.; Mahjoub, Z.; Laamari, S.; Sfina, H.; Chihaoui, B.; Boujnah, D.; et al. Effects of compost, olive mill wastewater and legume cover cropson soil characteristics, tree performance and oil quality of olive trees cv. Chemlali grown under organic farming system. Sci. Hortic. 2019, 253, 163–171. [Google Scholar] [CrossRef]
- Bremer, D.J.; Auen, L.M.; Ham, J.M.; Owensby, C.E. Evapotranspiration in a prairie ecosystem: Effects of grazing by cattle. Agron. J. 2001, 93, 338–348. [Google Scholar] [CrossRef]
- Odriozola, I.; García-Baquero, G.; Laskurain, N.A.; Aldezabal, A. Livestock grazing modifies the effect of environmental factors on soil temperature and water content in a temperate grassland. Geoderma 2014, 235–236, 347–354. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Wall, D.H. Soil biological responses to, and feedbacks on, trophic rewilding. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 3857–3868. [Google Scholar] [CrossRef]
- Mikola, J.; Setälä, H.; Virkajärvi, P.; Saarijärvi, K.; Ilmarinen, K.; Voigt, W.; Vestberg, M. Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecol. Monogr. 2009, 79, 221–244. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Frank, D.A.; Hinchey, P.M.; Murray, T.R. Defoliation ind1uces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol. Biochem. 2008, 40, 2865–2873. [Google Scholar] [CrossRef]
- Sun, L.; Ataka, M.; Kominami, Y.; Yoshimura, K. Relationship between fine-root exudation and respiration of two Quercus species in a Japanese temperate forest. Tree Physiol. 2017, 37, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Pollierer, M.M.; Langel, R.; Körner, C.; Maraun, M.; Scheu, S. The underestimated importance of belowground carbon input for forest soil animal food webs. Ecol. Lett. 2007, 10, 729–736. [Google Scholar] [CrossRef]

| Soil | G | UG | G | UG |
|---|---|---|---|---|
| 0–15 cm | 15–40 cm | |||
| pH | 7.07 ± 0.16 a | 6.68 ± 0.08 b | 7.12 ± 0.04 b | 7.77 ± 0.06 a |
| SM (% d.w.) | 15.1 ± 1.6 a | 12.8 ± 1.7 b | 16.5 ± 1.7 a | 14.1 ± 1.8 b |
| CEC (cmol+ kg−1) | 9.18 ± 0.01 b | 11.5 ± 0.02 a | 8.76 ± 3.42 | 9.16 ± 0.01 |
| WHC (% d.w.) # | 27.6 ± 3.8 a | 20.3 ± 2.3 b | ||
| BD (g cm−3) # | 1.27 ± 0.04 b | 1.32 ± 0.02 a | ||
| Season | Grubbing | Depth | Season x Grubbing | Season x Depth | Grubbing x Depth | Season x Grubbing x Depth | |
|---|---|---|---|---|---|---|---|
| pH | <0.001 | <0.001 | <0.001 | 0.109 | <0.001 | <0.001 | <0.001 |
| SM | <0.001 | 0.006 | 0.044 | 0.407 | 0.048 | 0.778 | 0.995 |
| SOM | 0.058 | 0.621 | <0.001 | 0.622 | 0.007 | <0.001 | <0.001 |
| N | <0.001 | 0.028 | <0.001 | 0.032 | <0.001 | 0.415 | <0.001 |
| C/N | <0.001 | 0.454 | 0.007 | <0.001 | <0.001 | 0.005 | 0.005 |
| MB | <0.001 | 0.660 | 0.005 | 0.005 | 0.087 | <0.001 | <0.001 |
| TFB | <0.001 | 0.368 | 0.653 | <0.001 | <0.001 | 0.784 | 0.110 |
| AFB (% TFB) | 0.313 | 0.010 | <0.001 | <0.001 | 0.093 | 0.811 | <0.001 |
| TFB (% MB) | <0.001 | 0.186 | 0.228 | 0.007 | 0.395 | <0.001 | 0.004 |
| Respiration | <0.001 | 0.244 | <0.001 | 0.004 | <0.001 | 0.677 | <0.001 |
| qCO2 | <0.001 | 0.033 | <0.001 | 0.062 | <0.001 | <0.001 | <0.001 |
| CEM | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.734 | <0.001 |
| SQI | 0.154 | 0.038 | 0.322 | 0.050 | 0.056 | 0.065 | 0.044 |
| Leaf | G | UG |
|---|---|---|
| LA (cm2) | 6.42 ± 0.09 | 5.71 ± 0.35 |
| RWC (% d.w.) | 81.4 ± 2.6 | 76.4 ± 3.4 |
| N (% d.w.) | 1.58 ± 0.06 | 1.54 ± 0.04 |
| C/N | 30.3 ± 0.2 b | 37.2 ± 0.4 a |
| Fruit | ||
| P/S (g f.w.) | 4.02 ± 0.25 b | 4.40 ± 0.18 a |
| DM (%) | 32.4 ± 0.8 b | 36.2 ± 0.9 a |
| Tp (mg GAE 100 g d.w.−1) | 56.6 ± 1.6 b | 84.8 ± 2.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoletano, P.; Barbarisi, C.; Maselli, V.; Rippa, D.; Arena, C.; Volpe, M.G.; Colombo, C.; Fulgione, D.; De Marco, A. Quantifying the Immediate Response of Soil to Wild Boar (Sus scrofa L.) Grubbing in Mediterranean Olive Orchards. Soil Syst. 2023, 7, 38. https://doi.org/10.3390/soilsystems7020038
Napoletano P, Barbarisi C, Maselli V, Rippa D, Arena C, Volpe MG, Colombo C, Fulgione D, De Marco A. Quantifying the Immediate Response of Soil to Wild Boar (Sus scrofa L.) Grubbing in Mediterranean Olive Orchards. Soil Systems. 2023; 7(2):38. https://doi.org/10.3390/soilsystems7020038
Chicago/Turabian StyleNapoletano, Pasquale, Costantina Barbarisi, Valeria Maselli, Daniela Rippa, Carmen Arena, Maria Grazia Volpe, Claudio Colombo, Domenico Fulgione, and Anna De Marco. 2023. "Quantifying the Immediate Response of Soil to Wild Boar (Sus scrofa L.) Grubbing in Mediterranean Olive Orchards" Soil Systems 7, no. 2: 38. https://doi.org/10.3390/soilsystems7020038
APA StyleNapoletano, P., Barbarisi, C., Maselli, V., Rippa, D., Arena, C., Volpe, M. G., Colombo, C., Fulgione, D., & De Marco, A. (2023). Quantifying the Immediate Response of Soil to Wild Boar (Sus scrofa L.) Grubbing in Mediterranean Olive Orchards. Soil Systems, 7(2), 38. https://doi.org/10.3390/soilsystems7020038












