Improving Seed Germination by Cold Atmospheric Plasma
Abstract
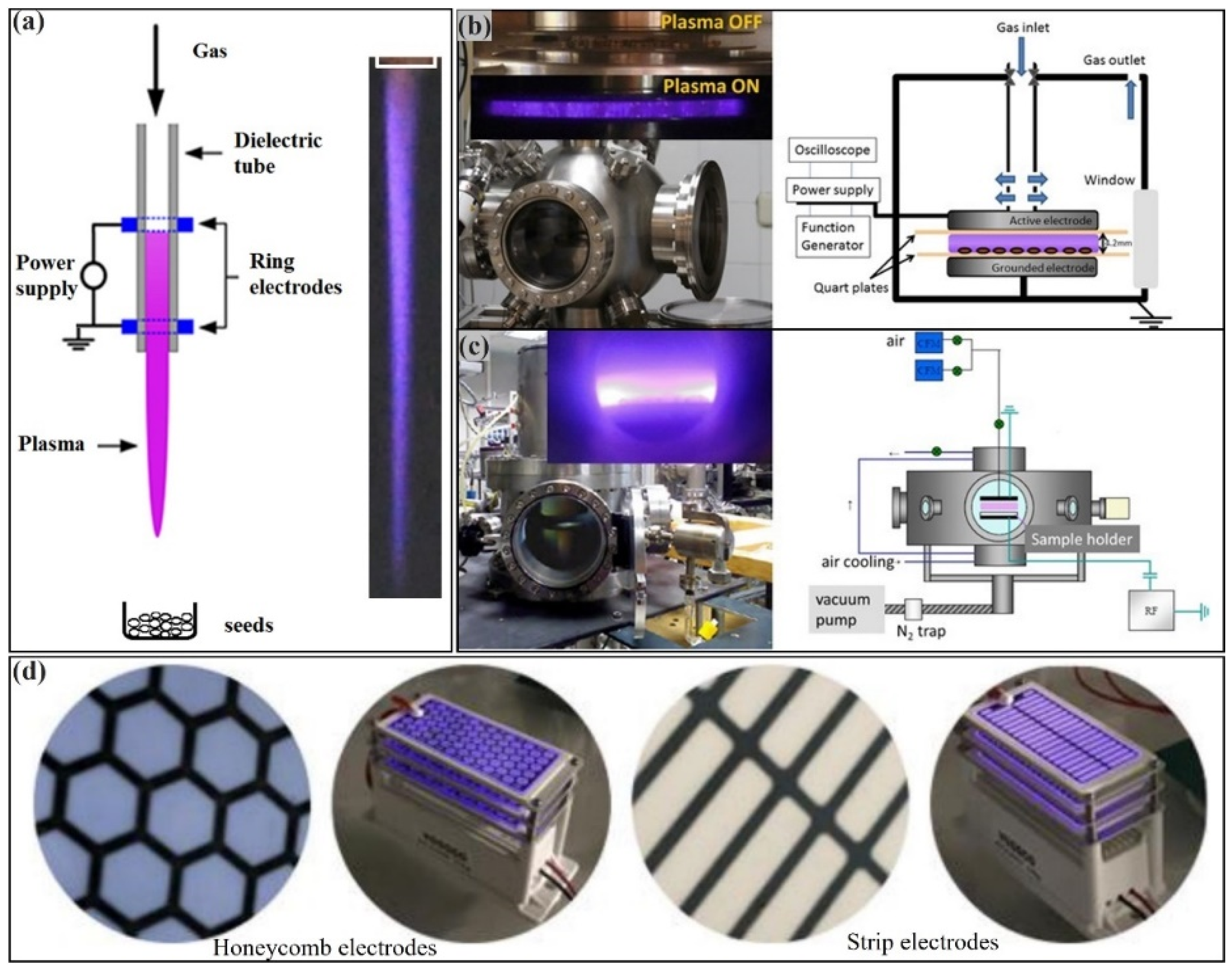
1. Cold Atmospheric Plasma (CAP): Sources and Physics
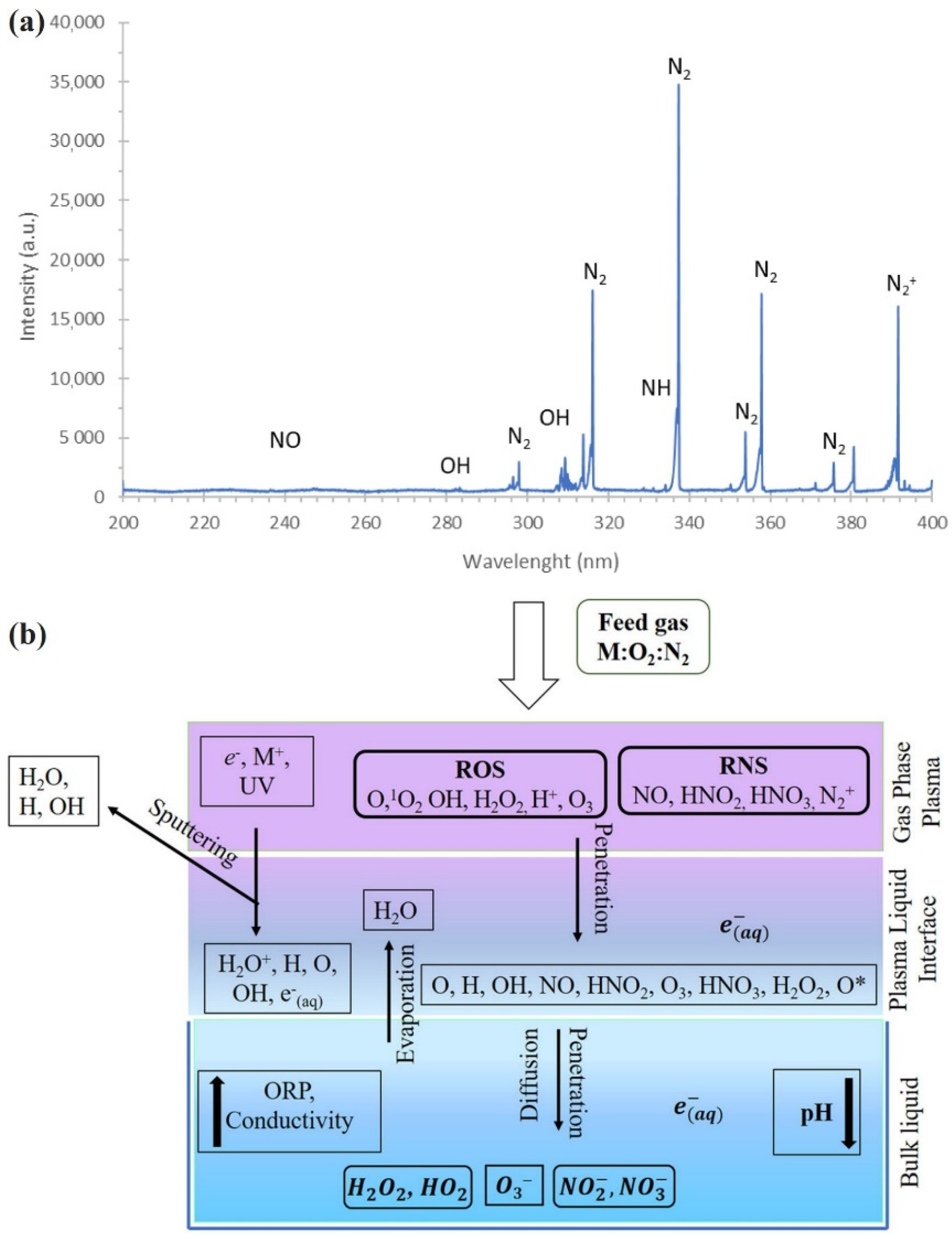
2. Reactive Species in CAP
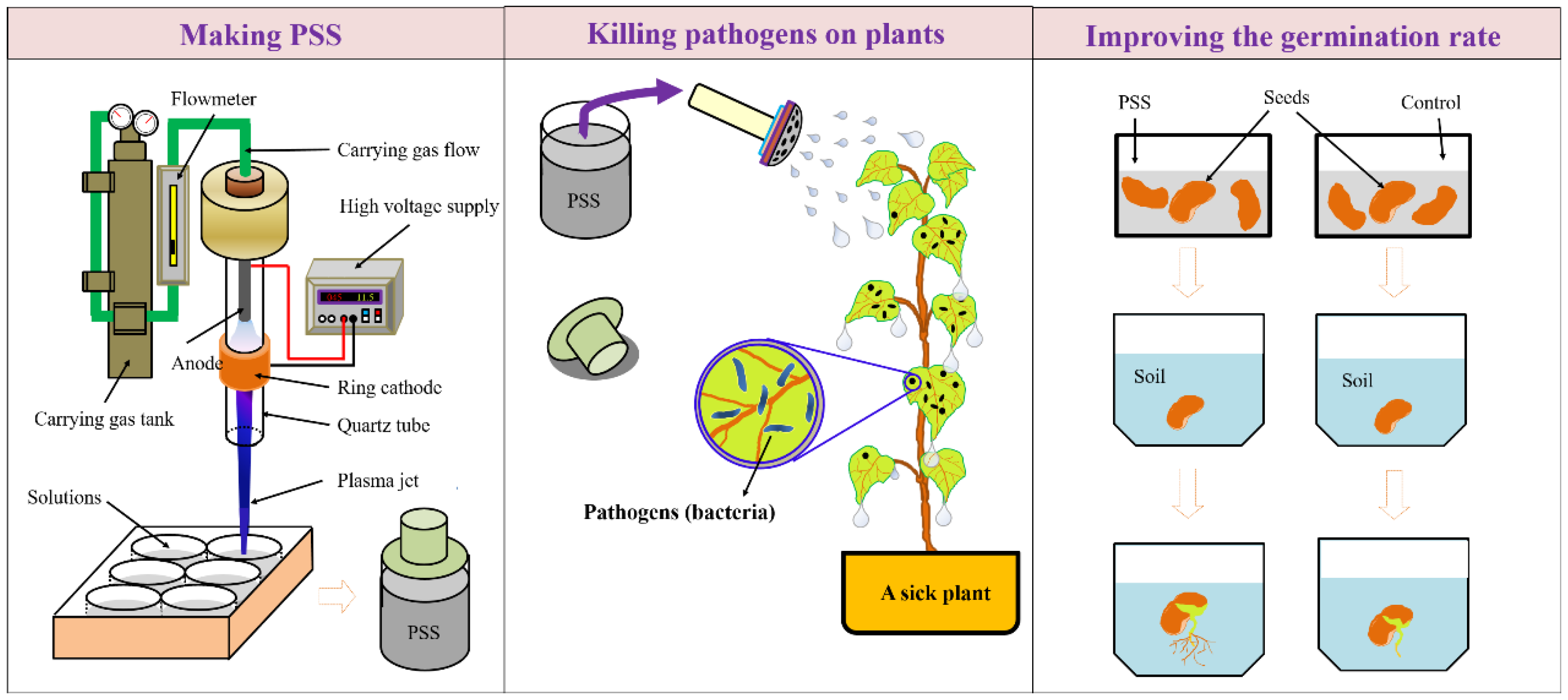
3. Plasma Agriculture, an Overview
4. Seeds Germination
4.1. Germination Mechanism
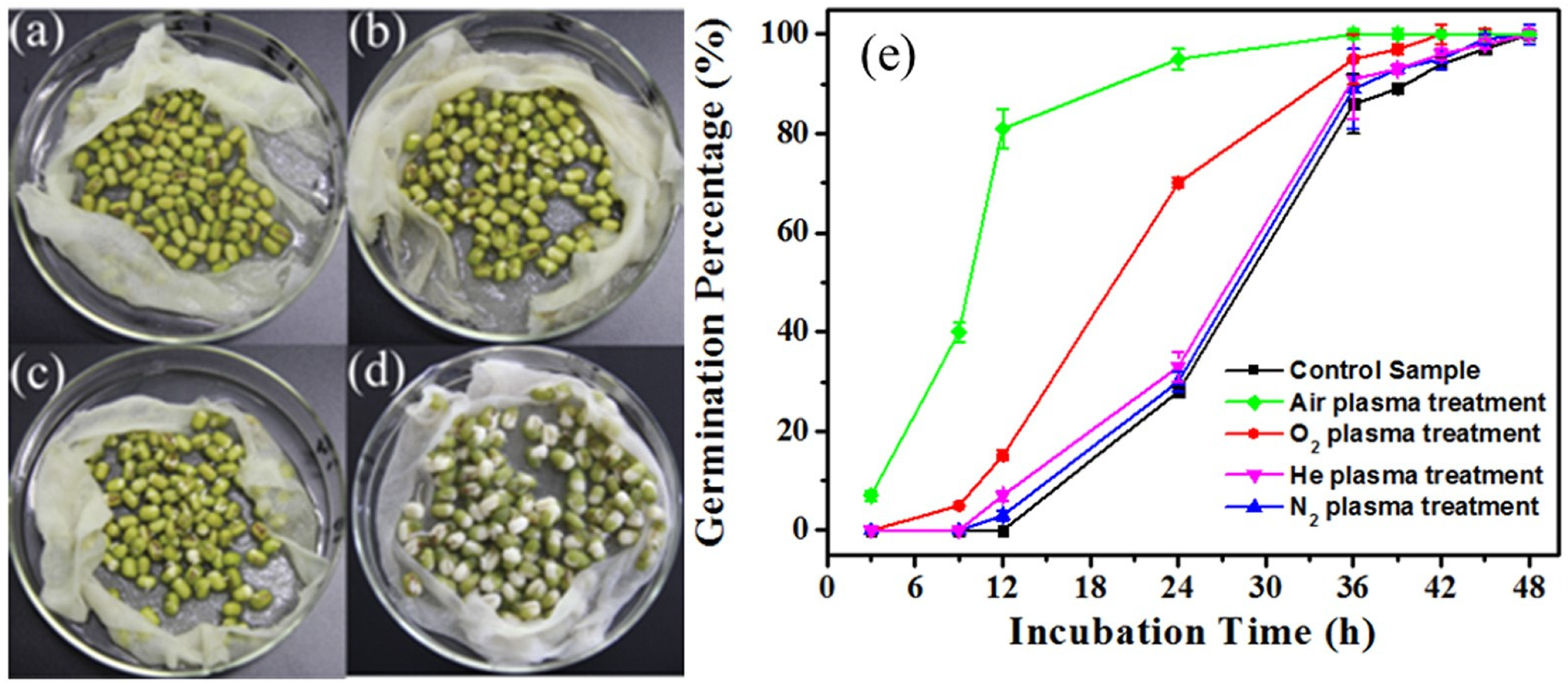
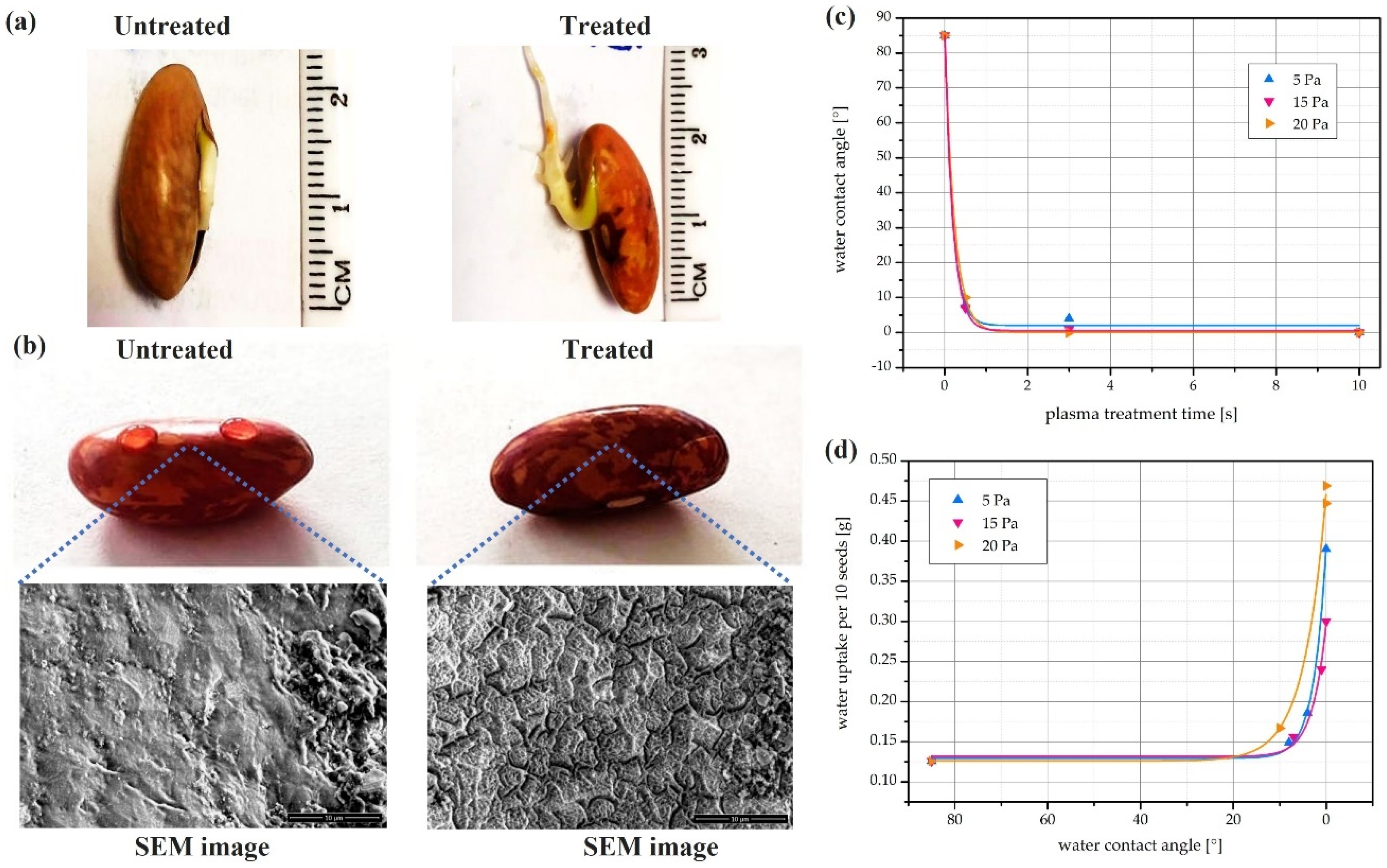
4.2. CAP’s Capability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gutsol, A.F.; Starikovskaia, S.; et al. The 2012 Plasma Roadmap. J. Phys. D Appl. Phys. 2012, 45, 253001. [Google Scholar] [CrossRef]
- Attri, P.; Arora, B.; Choi, E.H. Utility of Plasma: A New Road from Physics to Chemistry. RSC Adv. 2013, 3, 12540–12567. [Google Scholar] [CrossRef]
- Laroussi, M.; Akan, T. Arc-Free Atmospheric Pressure Cold Plasma Jets: A Review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-Thermal Atmospheric Pressure Discharges. J. Phys. D Appl. Phys. 2005, 38, R1. [Google Scholar] [CrossRef]
- Lin, L.; Keidar, M. A Map of Control for Cold Atmospheric Plasma Jets: From Physical Mechanisms to Optimizations. Appl. Phys. Rev. 2021, 8, 011306. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold Atmospheric Plasma, a Novel Promising Anti-Cancer Treatment Modality. Oncotarget 2017, 8, 15977. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M. Plasma for Cancer Treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Pawłat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds’ germination. PLoS ONE 2018, 13, 0194349. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface Chemistry and Germination Improvement of Quinoa Seeds Subjected to Plasma Activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, Z.; Xiong, Q.; Tang, Z.; Hu, X.; Pan, Y. An 11 Cm Long Atmospheric Pressure Cold Plasma Plume for Applications of Plasma Medicine. Appl. Phys. Lett. 2008, 92, 2006–2008. [Google Scholar]
- Waskow, A.; Howling, A.; Furno, I. Advantages and Limitations of Surface Analysis Techniques on Plasma-Treated Arabidopsis Thaliana Seeds. Front. Mater. Sci. 2021, 8, 123. [Google Scholar] [CrossRef]
- Lu, X.P.; Ostrikov, K.K. Guided Ionization Waves: The Physics of Repeatability. Appl. Phys. Rev. 2018, 5, 031102. [Google Scholar] [CrossRef]
- Lu, X.P.; Naidis, G.V.; Laroussi, M.; Ostrikov, K. Guided Ionization Waves: Theory and Experiments. Phys. Rep. 2014, 540, 123–166. [Google Scholar] [CrossRef]
- Yan, D.; Horkowitz, A.; Keidar, M. On the Selective Killing of Cold Atmospheric Plasma Cancer Treatment: Status and Beyond. Plasma Process Polym. 2021, 18, 2100020. [Google Scholar] [CrossRef]
- Graves, D.B. The Emerging Role of Reactive Oxygen and Nitrogen Species in Redox Biology and Some Implications for Plasma Applications to Medicine and Biology. J. Phys. D Appl. Phys. 2012, 45, 263001–263042. [Google Scholar] [CrossRef]
- Motyka-Pomagruk, A.; Dzimitrowicz, A.; Orlowski, J.; Babinska, W.; Terefinko, D.; Rychlowski, M.; Prusinski, M.; Pohl, P.; Lojkowska, E.; Jamroz, P.; et al. Implementation of a Non-Thermal Atmospheric Pressure Plasma for Eradication of Plant Pathogens from a Surface of Economically Important Seeds. Int. J. Mol. Sci. 2021, 22, 9256. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lyu, Y.; Trink, B.; Canady, J.; Keidar, M. Cold Atmospheric Helium Plasma Jet in Humid Air Environment. J. Appl. Phys. 2019, 125, 153301. [Google Scholar] [CrossRef]
- Lyu, Y.; Lin, L.; Gjika, E.; Lee, T.; Keidar, M. Mathematical Modeling and Control for Cancer Treatment with Cold Atmospheric Plasma Jet. J. Phys. D Appl. Phys. 2019, 52, 185202. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Raghavan, V. Nonthermal Plasma-Liquid Interactions in Food Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1985–2008. [Google Scholar] [CrossRef] [PubMed]
- Malyavko, A.; Yan, D.; Wang, Q.; Klein, A.L.; Patel, K.C. Cold Atmospheric Plasma Cancer Treatment, Direct versus Indirect Approaches. Mater. Adv. 2020, 1, 1494–1505. [Google Scholar] [CrossRef]
- Tanaka, H.; Bekeschus, S.; Yan, D.; Hori, M.; Keidar, M.; Laroussi, M. Plasma-Treated Solutions (PTS) in Cancer Therapy. Cancers 2021, 13, 1737. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical Modification of Amino Acids by Atmospheric-Pressure Cold Plasma in Aqueous Solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Panngom, K.; Baik, K.Y.; Nam, M.K.; Han, J.H.; Rhim, H.; Choi, E.H. Preferential Killing of Human Lung Cancer Cell Lines with Mitochondrial Dysfunction by Nonthermal Dielectric Barrier Discharge Plasma. Cell Death Dis. 2013, 4, e642. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Dielectric Barrier Discharge Plasma Efficiently Delivers an Apoptotic Response in Human Monocytic Lymphoma. Plasma Process. Polym. 2014, 11, 1175–1187. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Holubová, L.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-Thermal Plasma—A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef] [PubMed]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, D.; Song, Y.; Zhou, R.; Niu, J. Inactivation of the Tomato Pathogen Cladosporium Fulvum by an Atmospheric-Pressure Cold Plasma Jet. Plasma Process. Polym. 2014, 11, 1028–1036. [Google Scholar] [CrossRef]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Zhou, R.; Song, Y.; Sun, Y.; Zhang, Q.; Niu, J.; Fan, H.; Yang, S.Z. Atmospheric Cold Plasma Jet for Plant Disease Treatment. Appl. Phys. Lett. 2014, 104, 043702. [Google Scholar] [CrossRef]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.A.H. Inhibitory Effect of Double Atmospheric Pressure Argon Cold Plasma on Spores and Mycotoxin Production of Aspergillus Niger Contaminating Date Palm Fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Kim, J.; Mok, C. Effect of Corona Discharge Plasma Jet Treatment on Decontamination and Sprouting of Rapeseed (Brassica Napus L.) Seeds. Food Control 2017, 71, 376–382. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Hertwig, C.; Meneses, N.; Mathys, A. Cold Atmospheric Pressure Plasma and Low Energy Electron Beam as Alternative Nonthermal Decontamination Technologies for Dry Food Surfaces: A Review. Trends Food Sci. Technol. 2018, 77, 131–142. [Google Scholar] [CrossRef]
- Filipić, A.; Primc, G.; Zaplotnik, R.; Mehle, N.; Gutierrez-Aguirre, I.; Ravnikar, M.; Mozetič, M.; Žel, J.; Dobnik, D. Cold Atmospheric Plasma as a Novel Method for Inactivation of Potato Virus Y in Water Samples. Food Environ. Virol. 2019, 11, 220–228. [Google Scholar] [CrossRef]
- Fernández, A.; Noriega, E.; Thompson, A. Inactivation of Salmonella Enterica Serovar Typhimurium on Fresh Produce by Cold Atmospheric Gas Plasma Technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar] [CrossRef]
- Misra, N.N.; Moiseev, T.; Patil, S.; Pankaj, S.K.; Bourke, P.; Mosnier, J.P.; Keener, K.M.; Cullen, P.J. Cold Plasma in Modified Atmospheres for Post-Harvest Treatment of Strawberries. Food Bioproc. Technol. 2014, 7, 3045–3054. [Google Scholar] [CrossRef]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold Plasma Rapid Decontamination of Food Contact Surfaces Contaminated with Salmonella Biofilms. J. Food Sci. 2014, 79, M917–M922. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, Y. Effect of Seed Treatment by Cold Plasma on the Resistance of Tomato to Ralstonia Solanacearum (Bacterial Wilt). PLoS ONE 2014, 9, e97753. [Google Scholar]
- Wang, J.; Zhuang, H.; Zhang, J. Inactivation of Spoilage Bacteria in Package by Dielectric Barrier Discharge Atmospheric Cold Plasma Treatment Time Effects. Food Bioproc. Technol. 2016, 9, 1648–1652. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold Plasma Reduction of Salmonella and Escherichia Coli O157: H7 on Almonds Using Ambient Pressure Gases. J. Food Sci. 2012, 77, 171–175. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Zhang, C.; Dong, Y. Cold Plasma Treatment Enhances Oilseed Rape Seed Germination under Drought Stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of Cold Radiofrequency Plasma with Seeds of Beans (Phaseolus Vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef]
- Desikan, R.; Cheung, M.K.; Bright, J.; Henson, D.; Hancock, J.T.; Neill, S.J. ABA, Hydrogen Peroxide and Nitric Oxide Signalling in Stomatal Guard Cells. J. Exp. Bot. 2004, 55, 205–212. [Google Scholar] [CrossRef]
- Lum, H.K.; Butt, Y.K.C.; Lo, S.C.L. Hydrogen Peroxide Induces a Rapid Production of Nitric Oxide in Mung Bean (Phaseolus Aureus). Nitric Oxide Biol. Chem. 2002, 6, 205–213. [Google Scholar] [CrossRef]
- Wilson, I.D.; Neill, S.J.; Hancock, J.T. Nitric Oxide Synthesis and Signalling in Plants. Plant Cell Environ. 2008, 31, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Beligni Maria, V.; Lamattina, L. Nitric Oxide Counteracts Reactive Oxygen Species Actions in Plant Tissues. Planta 1999, 208, 337–344. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of Nitric Oxide in Tolerance of Plants to Abiotic Stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric Oxide Stimulates Seed Germination and Counteracts the Inhibitory Effect of Heavy Metals and Salinity on Root Growth of Lupinus Luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Shi, S.; Wang, G.; Wang, Y.; Zhang, L.; Zhang, L. Protective Effect of Nitric Oxide against Oxidative Stress under Ultraviolet-B Radiation. Nitric Oxide: Biol. Chem. 2005, 13, 1–9. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric Oxide Protects against Cellular Damage Produced by Methylviologen Herbicides in Potato Plants. Nitric Oxide Biol. Chem. 1999, 3, 199–208. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Cullen, P.; Bourke, P. Controlled Cytotoxicity of Plasma Treated Water Formulated by Open-Air Hybrid Mode Discharge. Appl. Phys. Lett. 2017, 110, 264102. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting Lentil Germination and Stem Growth by Plasma Activated Tap Water, Demineralized Water and Liquid Fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar] [CrossRef]
- Lotfy, K. Effects of Cold Atmospheric Plasma Jet Treatment on the Seed Germination and Enhancement Growth of Watermelon. Open J. Appl. Sci. 2017, 7, 705–719. [Google Scholar] [CrossRef][Green Version]
- Kostoláni, D.; Ndiffo Yemeli, G.B.; Švubová, R.; Kyzek, S.; Machala, Z. Physiological Responses of Young Pea and Barley Seedlings to Plasma-Activated Water. Plants 2021, 10, 1750. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene Expression in Tomato Seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [PubMed]
- Than, H.A.Q.; Pham, T.H.; Nguyen, D.K.V.; Pham, T.H.; Khacef, A. Non-Thermal Plasma Activated Water for Increasing Germination and Plant Growth of Lactuca Sativa L. Plasma Chem. Plasma Process. 2021; in press. [Google Scholar]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of Plasma-Activated Water Treatment on Seed Germination and Growth of Mung Bean Sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Chen, D.; Chen, P.; Cheng, Y.; Peng, P.; Liu, J.; Ma, Y.; Liu, Y.; Ruan, R. Deoxynivalenol Decontamination in Raw and Germinating Barley Treated by Plasma-Activated Water and Intense Pulsed Light. Food Bioproc. Technol. 2019, 12, 246–254. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced Seed Germination and Plant Growth by Atmospheric Pressure Cold Air Plasma: Combined Effect of Seed and Water Treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- El Shaer, M.; El Welily, H.; Zaki, A.; Arafa, H.; Elsebaei, A.; Eldaly, M.; Mobasher, M. Germination of Wheat Seeds Exposed to Cold Atmospheric Plasma in Dry and Wet Plasma-Activated Water and Mist. Plasma Med. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Terebun, P.; Kwiatkowski, M.; Hensel, K.; Kopacki, M.; Pawłat, J. Influence of Plasma Activated Water Generated in a Gliding Arc Discharge Reactor on Germination of Beetroot and Carrot Seeds. Appl. Sci. 2021, 11, 6164. [Google Scholar] [CrossRef]
- Herianto, S.; Shih, M.K.; Lin, C.M.; Hung, Y.C.; Hsieh, C.W.; Wu, J.S.; Chen, M.H.; Chen, H.L.; Hou, C.Y. The Effects of Glazing with Plasma-Activated Water Generated by a Piezoelectric Direct Discharge Plasma System on Whiteleg Shrimp (Litopenaeus Vannamei). LWT 2022, 154, 112547. [Google Scholar] [CrossRef]
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of PH on Bacterial Inactivation in Aqueous Solutions Due to Low-Temperature Atmospheric Pressure Plasma Application. Plasma Process. Polym. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Ochi, A.; Konishi, H.; Ando, S.; Sato, K.; Yokoyama, K.; Tsushima, S.; Yoshida, S.; Morikawa, T.; Kaneko, T.; Takahashi, H. Management of Bakanae and Bacterial Seedling Blight Diseases in Nurseries by Irradiating Rice Seeds with Atmospheric Plasma. Plant Pathol. 2017, 66, 67–76. [Google Scholar] [CrossRef]
- Ono, R.; Uchida, S.; Hayashi, N.; Kosaka, R.; Soeda, Y. Inactivation of Bacteria on Plant Seed Surface by Low-Pressure RF Plasma Using a Vibrating Stirring Device. Vaccum 2016, 136, 214–220. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Gangal, U.; Youssef, M.M.; Goyal, S.M.; Bruggeman, P.J. Inactivation of Virus in Solution by Cold Atmospheric Pressure Plasma: Identification of Chemical Inactivation Pathways. J. Phys. D Appl. Phys. 2016, 49, 204001. [Google Scholar] [CrossRef]
- Filipić, A.; Dobnik, D.; Tušek Žnidarič, M.; Žegura, B.; Štern, A.; Primc, G.; Mozetič, M.; Ravnikar, M.; Žel, J.; Gutierrez Aguirre, I. Inactivation of Pepper Mild Mottle Virus in Water by Cold Atmospheric Plasma. Front. Microbiol. 2021, 12, 618209. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Li, Y.F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of Surface-Borne Microorganisms and Increased Germination of Seed Specimen by Cold Atmospheric Plasma. Food Bioproc. Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the Mark: Early Seed Germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular Aspects of Seed Dormancy. Annu. Rev. Plant. Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Linkies, A.; Vreeburg, R.A.M.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In Vivo Cell Wall Loosening by Hydroxyl Radicals during Cress Seed Germination and Elongation Growth. Plant Physiol. 2009, 150, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Iwabuchi, M. A Mechanism for Promoting the Germination of Zinnia Elegans Seeds by Hydrogen Peroxide. Plant Cell Physiol. 2001, 42, 286–291. [Google Scholar] [CrossRef]
- Barba-Espin, G.; Diaz-Vivancos, P.; Clemente-Moreno, M.J.; Albacete, A.; Faize, L.; Faize, M.; Pérez-Alfocea, F.; Hernández, J.A. Interaction between Hydrogen Peroxide and Plant Hormones during Germination and the Early Growth of Pea Seedlings. Plant Cell Environ. 2010, 33, 981–994. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative Signaling in Seed Germination and Dormancy. Plant Signal Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Garcia, Q. Reactive Oxygen Species and Seed Germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, W.-B.; Xu, L.-L. Effects of Nitric Oxide on the Germination of Wheat Seeds and Its Reactive Oxygen Species Metabolisms under Osmotic Stress. Acta Bot. Sin. 2003, 45, 901–905. [Google Scholar]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Liu, W.; Jing, Q.; Cao, W. Exogenous Nitric Oxide Improves Seed Germination in Wheat against Mitochondrial Oxidative Damage Induced by High Salinity. Environ. Exp. Bot. 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Hu, K.D.; Hu, L.Y.; Li, Y.H.; Zhang, F.Q.; Zhang, H. Protective Roles of Nitric Oxide on Germination and Antioxidant Metabolism in Wheat Seeds under Copper Stress. Plant Growth Regul. 2007, 53, 173–183. [Google Scholar] [CrossRef]
- Sarath, G.; Bethke, P.C.; Jones, R.; Baird, L.M.; Hou, G.; Mitchell, R.B. Nitric Oxide Accelerates Seed Germination in Warm-Season Grasses. Planta 2006, 223, 1154–1164. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.L.; Jones, R.L. Nitric Oxide Reduces Seed Dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, S.B.; Taylorson, R.B. Promotion of Seed Germination by Nitrate, Nitrite, Hydroxylamine, and Ammonium Salts. Plant Physiol. 1974, 54, 304–309. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- de Groot, G.J.J.B.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold Plasma Treatment for Cotton Seed Germination Improvement. Sci. Rep. 2018, 8, 14372. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of Cold Plasma Treatment on Seed Germination and Seedling Growth of Soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Han, H.; Chia, H.; Kuo, Y.; Lun, C.; Yi, S.; Sheng, Y. An Improved Process for High Nutrition of Germinated Brown Rice Production: Low-Pressure Plasma. Food Chem. 2016, 191, 120–127. [Google Scholar]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effects of Atmospheric-Pressure N2, He, Air, and O2 Microplasmas on Mung Bean Seed Germination and Seedling Growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef]
- Junior, C.A.; de Oliveira Vitoriano, J.; da Silva, D.L.S.; de Lima Farias, M.; de Lima Dantas, N.B. Water Uptake Mechanism and Germination of Erythrina Velutina Seeds Treated with Atmospheric Plasma. Sci. Rep. 2016, 6, 33722. [Google Scholar] [CrossRef] [PubMed]
- Ambrico, P.F.; Šimek, M.; Morano, M.; de Miccolis Angelini, R.M.; Minafra, A.; Trotti, P.; Ambrico, M.; Prukner, V.; Faretra, F. Reduction of Microbial Contamination and Improvement of Germination of Sweet Basil (Ocimum Basilicum L.) Seeds via Surface Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2017, 50, 305401. [Google Scholar] [CrossRef]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of Germination and Seedling Growth of Wheat Seed Using Dielectric Barrier Discharge Plasma with Various Gas Sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Lotfy, K.; Al-Harbi, N.A.; Abd El-Raheem, H. Cold Atmospheric Pressure Nitrogen Plasma Jet for Enhancement Germination of Wheat Seeds. Plasma Chem. Plasma Process. 2019, 39, 897–912. [Google Scholar] [CrossRef]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.H.; et al. Effects of High Voltage Nanosecond Pulsed Plasma and Micro DBD Plasma on Seed Germination, Growth Development and Physiological Activities in Spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef]
- Sera, B.; Sery, M.; Gavril, B.; Gajdova, I. Seed Germination and Early Growth Responses to Seed Pre-Treatment by Non-Thermal Plasma in Hemp Cultivars (Cannabis Sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of Perennial Woody Plants to Seed Treatment by Electromagnetic Field and Low-Temperature Plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus Annus L.) Seeds with Radio-Frequency Electromagnetic Field and Cold Plasma Induces Changes in Seed Phytohormone Balance, Seedling Development and Leaf Protein Expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Ghaemi, M.; Majd, A.; Iranbakhsh, A. Transcriptional Responses Following Seed Priming with Cold Plasma and Electromagnetic Field in Salvia Nemorosa L. J. Theor. Appl. Phys. 2020, 14, 323–328. [Google Scholar] [CrossRef]
- Pet’ková, M.; Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, L.; Ševčovičová, A.; Gálová, E. The Effects of Cold Atmospheric Pressure Plasma on Germination Parameters, Enzyme Activities and Induction of Dna Damage in Barley. Int. J. Mol. Sci. 2021, 22, 2833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-Inducing Effects of Argon Plasma on Soybean Sprouts via the Regulation of Demethylation Levels of Energy Metabolism-Related Genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Švubová, R.; Slováková, Ľ.; Bokor, B.; Kročková, V.C.; Renčko, J.; Uhrin, F.; Medvecká, V.; Zahoranová, A.; Gálová, E. Cold Atmospheric Pressure Plasma Treatment of Maize Grains—Induction of Growth, Enzyme Activities and Heat Shock Proteins. Int. J. Mol. Sci. 2021, 22, 8509. [Google Scholar] [CrossRef]
- Attri, P.; Koga, K.; Okumura, T.; Shiratani, M. Impact of Atmospheric Pressure Plasma Treated Seeds on Germination, Morphology, Gene Expression and Biochemical Responses. Jpn. J. Appl. Phys. 2021, 60, 040502. [Google Scholar] [CrossRef]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability increase in plasma-treated agricultural seeds and its relation to germination improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Recek, N.; Holc, M.; Vesel, A.; Zaplotnik, R.; Gselman, P.; Mozetič, M.; Primc, G. Germination of Phaseolus Vulgaris l. Seeds after a Short Treatment with a Powerful Rf Plasma. Int. J. Mol. Sci. 2021, 22, 6672. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M.; Zahoranová, A.; Tomeková, J. Germination Improvement of Three Pine Species (Pinus) After Diffuse Coplanar Surface Barrier Discharge Plasma Treatment. Plasma Chem. Plasma Process. 2021, 41, 211–226. [Google Scholar] [CrossRef]
- Siddique, S.S.; Hardy, G.E.S.J.; Bayliss, K.L. Cold Plasma: A Potential New Method to Manage Postharvest Diseases Caused by Fungal Plant Pathogens. Plant Pathol. 2018, 67, 1011–1021. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Lin, L.; Zvansky, M.; Kohanzadeh, L.; Taban, S.; Chriqui, S.; Keidar, M. Improving Seed Germination by Cold Atmospheric Plasma. Plasma 2022, 5, 98-110. https://doi.org/10.3390/plasma5010008
Yan D, Lin L, Zvansky M, Kohanzadeh L, Taban S, Chriqui S, Keidar M. Improving Seed Germination by Cold Atmospheric Plasma. Plasma. 2022; 5(1):98-110. https://doi.org/10.3390/plasma5010008
Chicago/Turabian StyleYan, Dayun, Li Lin, Michelle Zvansky, Leat Kohanzadeh, Shannon Taban, Sabrina Chriqui, and Michael Keidar. 2022. "Improving Seed Germination by Cold Atmospheric Plasma" Plasma 5, no. 1: 98-110. https://doi.org/10.3390/plasma5010008
APA StyleYan, D., Lin, L., Zvansky, M., Kohanzadeh, L., Taban, S., Chriqui, S., & Keidar, M. (2022). Improving Seed Germination by Cold Atmospheric Plasma. Plasma, 5(1), 98-110. https://doi.org/10.3390/plasma5010008








