Hardening of Mortars from Blended Cement with Opoka Additive in CO2 Environment
Abstract
1. Introduction
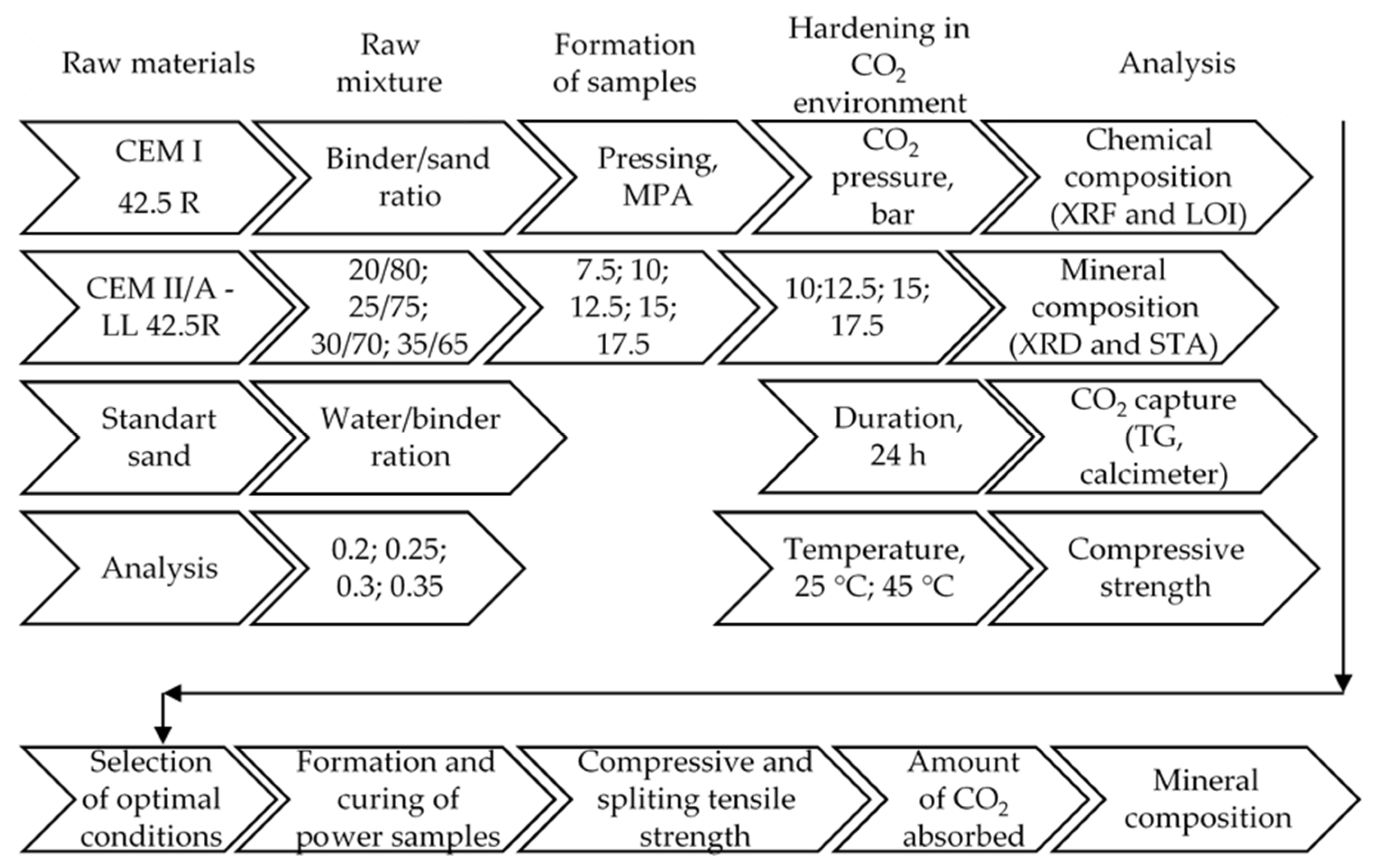
2. Materials and Methods
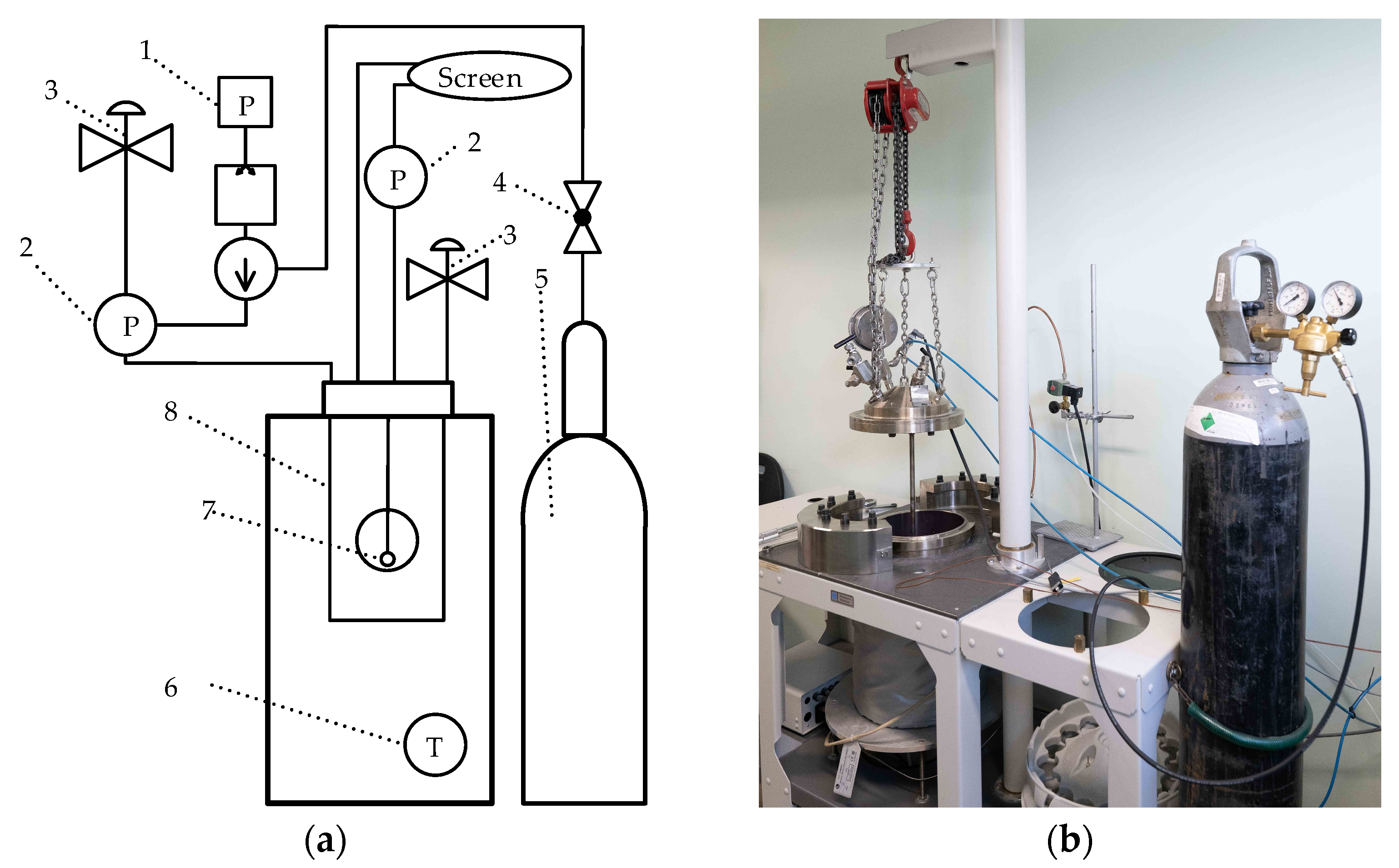
2.1. Raw Materials, Sample Preparation, and Hardening
2.2. Instrumental Analysis
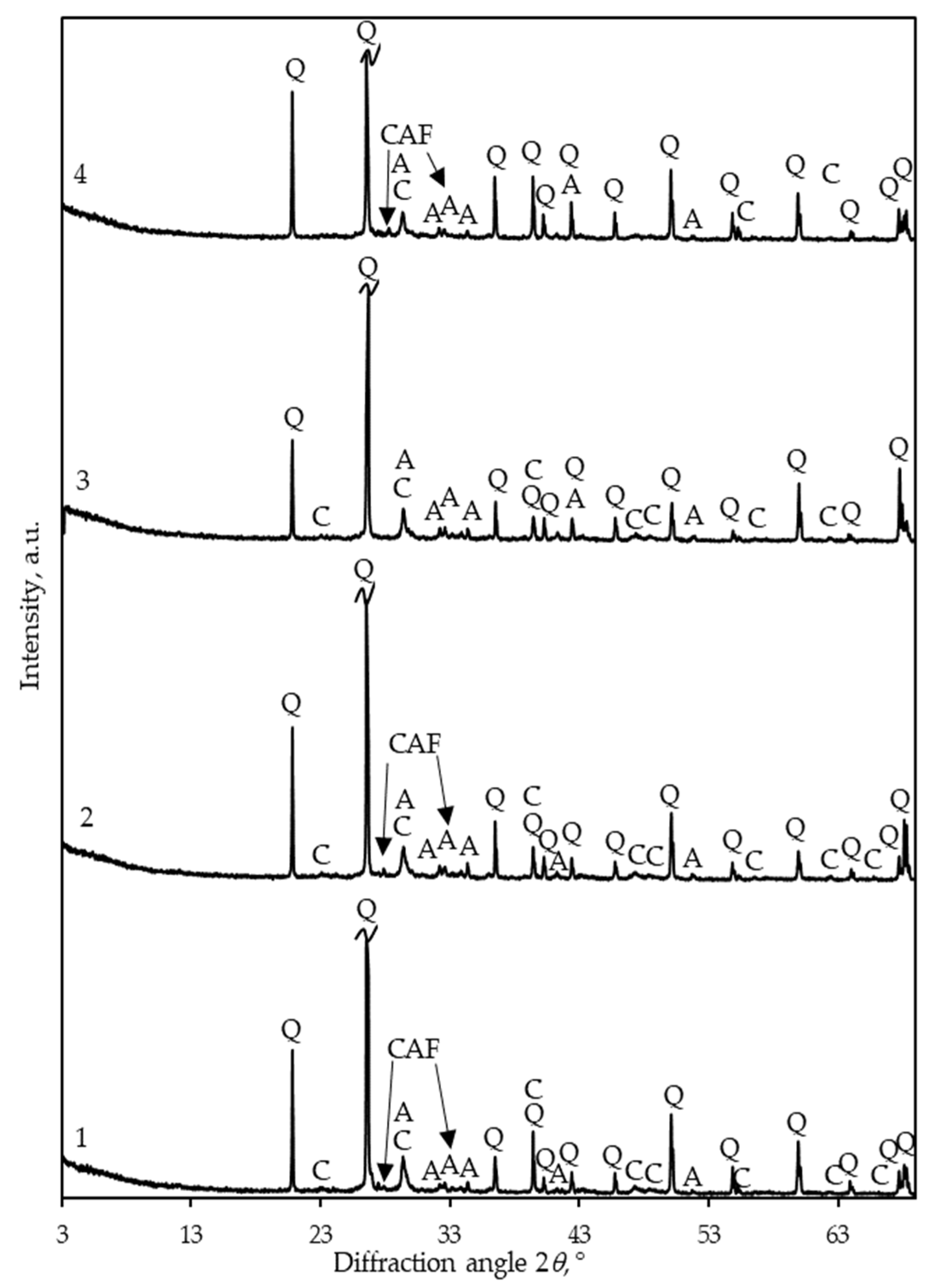
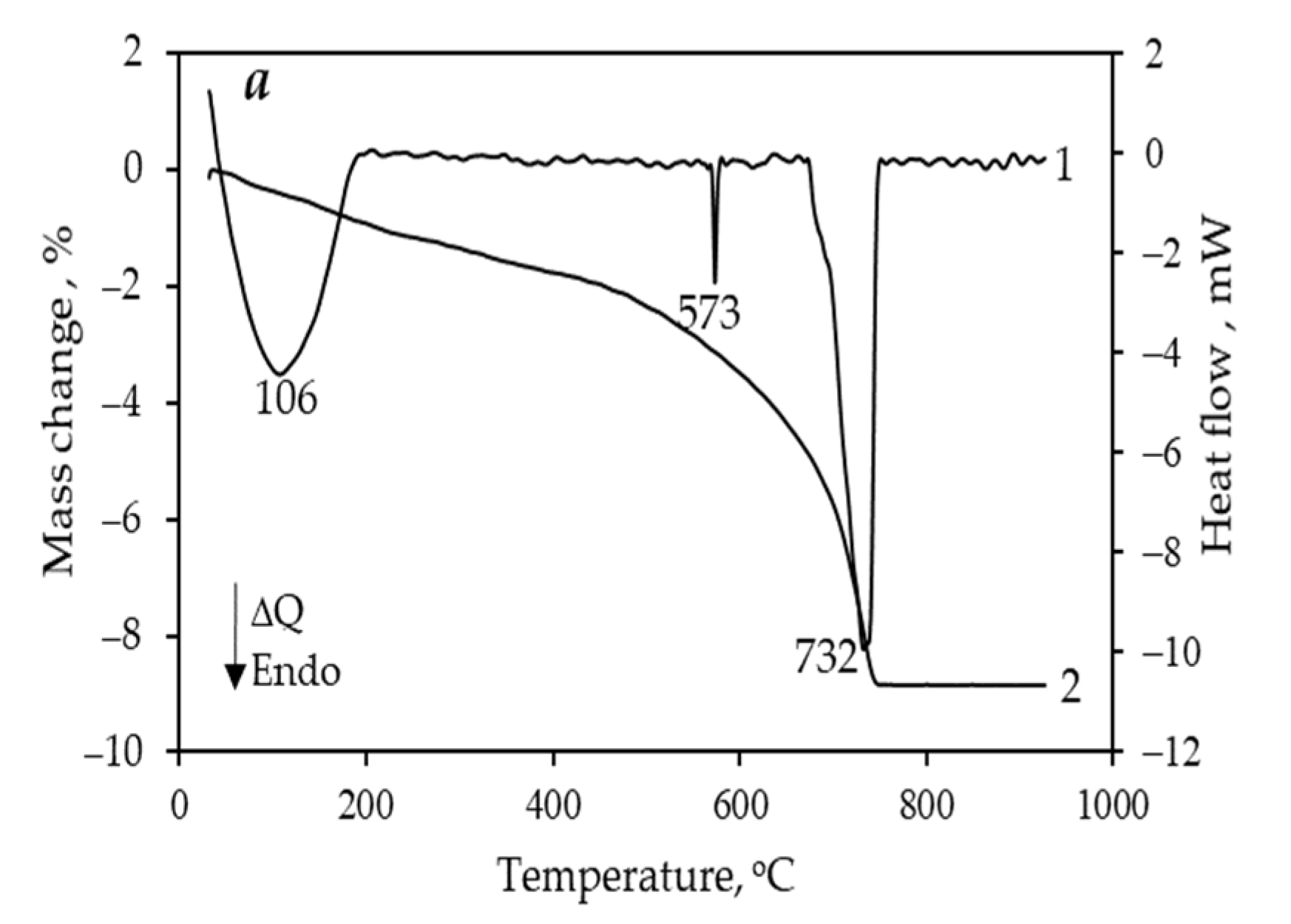
3. Results and Discussion
4. Conclusions
- It was determined that the blended cement with a 15 wt% opoka additive is a suitable cementitious material for high-strength carbonated mortar products, and it has a lower negative impact on the environment due to the reduced amount of OPC clinker, combined with carbonation curing and permanent CO2 sequestration.
- When curing mortar samples in a CO2 environment, the replacement of OPC clinker with 15% opoka has no effect on the qualitative composition of the crystalline phases of the products: in both cases, calcite forms, and small parts of unreacted alite and calcium aluminum iron silicate remain.
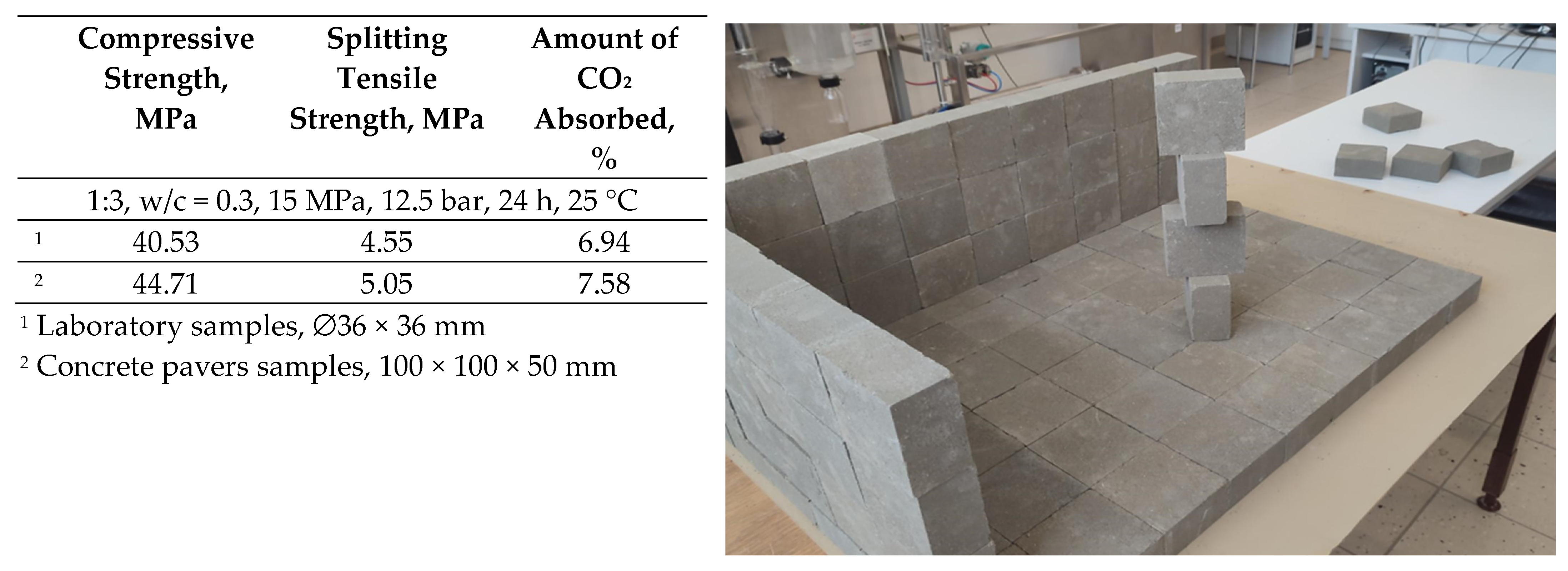
- Concrete pavers of industrial dimensions with a compressive strength of ~45 MPa can be produced from cement blended with opoka and sand mixtures when carbonation is performed at the following conditions: cement/sand ratio—1/3, water/cement ratio—0.3, compacting pressure—15 MPa, CO2 pressure—12.5 bar, duration—24 h, and temperature—25 °C. The strength properties have been found to be similar or even better than those of samples based on OPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Economic Forum, Nature Positive: Role of the Cement and Concrete Sector. 2023. Available online: https://www3.weforum.org/docs/WEF_Nature_Positive_Role_of_the_Cement_and_Concrete_Sector_2023.pdf (accessed on 15 July 2024).
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Mahmoodi, O.; Şahmaran, M. Geopolymer binders containing construction and demolition waste. In Handbook of Sustainable Concrete and Industrial Waste Management; Woodhead Publishing in Civil and Structural Engineering: Cambridge, UK, 2022; pp. 437–474. [Google Scholar] [CrossRef]
- Beguedou, E.; Narra, S.; Armoo, E.A.; Agboka, K.; Damgou, M.K. Alternative fuels substitution in cement industries for improved energy efficiency and sustainability. Energies 2023, 16, 3533. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A.; Hashim, M.H. Environmental impact of cement production and solutions: A review. Matter. Today Proc. 2022, 48, 741–746. [Google Scholar] [CrossRef]
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Song, O.; Guo, M.Z.; Gu, Y.; Ling, T.C. CO2 curing of SCMs blended cement blocks subject to elevated temperatures. Constr. Build. Mater. 2023, 374, 130907. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and Ordinary Portland Cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Sahu, S.; DeCristofaro, N. Solidia Cement. Solidia Technologies. 2013. Available online: https://www.semanticscholar.org/paper/Solidia-Cement-TM-Part-One-of-a-Two-Part-Series-the-Sahu-Decristofaro/691a16464fff1a9379e23f30f487cc0e9c846f5e (accessed on 7 August 2023).
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Carbon storage through concrete block carbonation. J. Clean Energy Technol. 2014, 2, 287–291. [Google Scholar] [CrossRef]
- Savija, B.; Lukovic, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Winnefeld, F.; Leemann, A.; German, A.; Lothenbach, B. CO2 storage in cement and concrete by mineral carbonation. Curr. Opin. Green Sustain. Chem. 2022, 38, 100672. [Google Scholar] [CrossRef]
- Blended Cement—Green, Durable & Sustainable. Global Cement and Concrete Association, India. 2022. Available online: https://gccassociation.org/wp-content/uploads/2022/04/Report_Blended-Cement-Green-Duratable-Sustainable_13Apr2022.pdf (accessed on 15 July 2024).
- Rhaouti, Y.; Taha, Y.; Benzaazoua, M. Assessment of the Environmental Performance of Blended Cements from a Life Cycle Perspective: A Systematic Review. Sustain. Prod. Consum. 2023, 36, 32–48. [Google Scholar] [CrossRef]
- Boscaro, F.; Palacios, M.; Robert, J. Flatt Formulation of low clinker blended cements and concrete with enhanced fresh and hardened properties. Cem. Concr. Res. 2021, 150, 106605. [Google Scholar] [CrossRef]
- Bizley, D. Thyssenkrupp Polysius Presents New Meca-Clay Clinker Reduction Solution. World Cement. 2023. Available online: https://www.worldcement.com/product-news/12122023/thyssenkrupp-polysius-presents-new-meca-clay-clinker-reduction-solution/ (accessed on 15 July 2024).
- Reddy, S.S.; Reddy, M.A.K.M. Lime calcined clay cement (LC3): A review. IOP Conf. Ser. Earth Environ Sci. 2021, 796, 012037. [Google Scholar] [CrossRef]
- Wang, L.; Rehman, N.U.; Curosu, I.; Zhu, Z.; Beigh, M.A.B.; Liebscher, M.; Chen, L.; Tsang, D.C.W.; Hempel, S.; Mechtcherine, V. On the use of limestone calcined clay cement (LC3) in high-strength strain hardening cement-based composites (HS-SHCC). Cem. Concr. Res. 2021, 144, 106421. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.L.; Mishra, D.K.; Li, G.; Leung, C.K.Y. Compressive strength and environmental impact of sustainable blended cement with high-dosage limestone and calcined clay (LC2). J. Clean. Prod. 2021, 278, 123616. [Google Scholar] [CrossRef]
- García-Segura, T.; Yepes, V.; Alcalá, J. Life cycle greenhouse gas emissions of blended cement concrete including carbonation and durability. Int. J. Life Cycle Assess. 2014, 19, 3–12. [Google Scholar] [CrossRef]
- Seo, J.H.; Amr, I.T.; Park, S.M.; Bamagain, R.A.; Fadhel, B.A.; Kim, G.M.; Hunaidy, A.S.; Lee, H.K. CO2 Uptake of Carbonation-Cured Cement Blended with Ground Volcanic Ash. Materials 2018, 11, 2187. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lin, S.; Li, Y.; Long, W.; Dong, Z.; Tang, L. Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products. Materials 2020, 13, 3404. [Google Scholar] [CrossRef]
- Kuzielová, E.; Slaný, M.; Žemlička, M.; Másilko, J. Accelerated carbonation of oil-well cement blended with pozzolans and latent hydraulic materials. J. Therm. Anal. Calorim. 2023, 148, 9963–9977. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. The European Committee for Standardization: Brussels, Belgium, 2016.
- Gasiūnienė, V.E. Lietuvos Kietosios Naudingosios Iškasenos = Lithuanian Solid Mineral Resources; Lietuvos Geologijos Tarnyba: Vilnius, Lithuania, 1998; pp. 5–14, 19–20, 30–31. (In Lithuanian) [Google Scholar]
- Siauciunas, R.; Mikaliunaite, J.; Urbonas, L.; Baltakys, K. Tribochemical and thermal activation of α-C2S hydrate as precursor for cementitious binders. J. Therm. Anal. Calorim. 2014, 118, 817–823. [Google Scholar] [CrossRef]
- Smigelskyte, A.; Siauciunas, R.; Hilbig, H.; Decker, M.; Urbonas, L.; Skripkiunas, G. Carbonated rankinite binder: Effect of curing parameters on microstructure, strength development and durability performance. Sci. Rep. 2020, 10, 14462. [Google Scholar] [CrossRef] [PubMed]
- Siauciunas, R.; Prichockiene, E.; Valancius, Z. The influence of Mg-impurities in raw materials on the synthesis of rankinite clinker and the strength of mortar hardening in CO2 environment. Materials 2023, 16, 2930. [Google Scholar] [CrossRef] [PubMed]
- Naik, T.N.; Kumar, R.; Kraus, N.R. Carbon Dioxide Sequestration in Cementitious Products. In Report Submitted to the Electric Power Research Institute, Palo Alto, California. [Interactive]. 2009. Available online: https://typeset.io/papers/carbon-dioxide-sequestration-in-cementitious-products-117rn0mb58 (accessed on 6 June 2024).
- Fernandez Bertos, M.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Wang, T.; Huang, H.; Hu, X.; Fang, M.; Luo, Z.; Guo, R. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- Klemm, W.A.; Berger, R.L. Accelerated curing of cementitious systems by carbon dioxide: Part I. Portland cement. Cem. Concr. Res. 1972, 2, 567–576. [Google Scholar] [CrossRef]
- Kumar, R.; Bhattacharjee, B. Porosity, pore size distribution and in situ strength of concrete. Cem. Concr. Res. 2003, 33, 155–164. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J.; Cao, M. Influence of compaction pressure on the accelerated carbonation of calcium hydroxide. J. Wuhan Univ. Technol. 2016, 31, 1187–1192. [Google Scholar] [CrossRef]
- EN 12390-3:2019; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. The European Committee for Standardization: Brussels, Belgium, 2019.
- EN 12390-6:2023; Testing Hardened Concrete—Part 6: Tensile splitting Strength of Test Specimens. The European Committee for Standardization: Brussels, Belgium, 2023.


| Material | CaO | SiO2 | Al2O3 | K2O | Na2O | MgO | Fe2O3 | SO3 | Other | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| CEM 1 42.5 R | 64.4 | 20.4 | 5.4 | 0.77 | 0.22 | 1.4 | 2.6 | 2.7 | 0.5 | 1.87 |
| CEM II/A-LL 42.5R | 62.1 | 21.9 | 5.1 | 0.74 | 0.18 | 1.53 | 2.45 | 2.6 | 0.32 | 3.08 |
| Opoka | 26.1 | 50.6 | 2.53 | 0.83 | 0.09 | 0.55 | 1.66 | 0.58 | 0.74 | 16.41 |
| No. | CO2 Pressure in a Reactor, Bar | Compacting Pressure, MPa | Compressive Strength, MPa | Mass Increase, g | Adsorbed CO2, wt% |
|---|---|---|---|---|---|
| 1 | 10 | 15 | 44.67 | 3.26 | 6.90 |
| 2 | 12.5 | 15 | 50.32 | 4.16 | 7.69 |
| 3 | 15 | 12.5 | 52.71 | 3.95 | 7.40 |
| 4 | 15 | 15 | 53.72 | 3.90 | 6.95 |
| 5 | 17.5 | 15 | 51.20 | 4.02 | 7.05 |
| No. | Composition of the Mixture | w/c Ratio | Compressive Strength, MPa | Mass Increase, g | Adsorbed CO2, wt% |
|---|---|---|---|---|---|
| 1 | 25 wt% of CEM II/A-LL 42.5R and 75 wt% of sand | 0.2 | 31.13 | 3.58 | 6.63 |
| 2 | 0.25 | 38.53 | 3.31 | 6.94 | |
| 3 | 0.3 | 32.23 | 3.07 | 6.30 | |
| 4 | 0.35 | 24.17 | 2.97 | 6.07 | |
| 5 | 25 wt% of CEM I 42.5 R and 75 wt% of sand | 0.25 | 35.55 | – | – |
| 6 | 0.3 | 38.12 | – | – | |
| 7 | 0.35 | 41.62 | – | – | |
| 8 | 30 wt% of CEM II/A-LL 42.5R and 70 wt% of sand | 0.2 | 33.45 | 4.35 | – |
| 9 | 0.25 | 52.71 | 3.95 | 7.40 | |
| 10 | 0.3 | 44.66 | 2.89 | – | |
| 11 | 0.35 | 37.55 | 2.41 | – | |
| 12 | 35 wt% of CEM II/A-LL 42.5R and 65 wt% of sand | 0.2 | 65.63 | 4.80 | – |
| 13 | 0.25 | 53.86 | 4.19 | 7.9 | |
| 14 | 0.3 | 39.43 | 3.23 | – |
| No. | Composition of the Mixture | Compacting Pressure, MPa | Compressive Strength, MPa | Mass Increase, g | Adsorbed CO2, wt% |
|---|---|---|---|---|---|
| 1 | 25 wt% of CEM II/A-LL 42.5R and 75 wt% of sand | 7.5 | 32.31 | 3.13 | 6.82 |
| 2 | 10 | 34.21 | 3.30 | 7.11 | |
| 3 | 12.5 | 38.53 | 3.35 | 6.94 | |
| 4 | 15 | 40.38 | 3.64 | 6.75 | |
| 5 | 25 wt% of CEM I 42.5 R and 75 wt% of sand | 12.5 | 35.55 | – | – |
| 6 | 15 | 40.88 | – | – | |
| 7 | 17.5 | 32.55 | – | – | |
| 8 | 30 wt% of CEM II/A-LL 42.5R and 70 wt% of sand | 7.5 | 38.53 | 4.51 | 7.96 |
| 9 | 10 | 45.67 | 4.12 | 7.78 | |
| 10 | 12.5 | 52.71 | 3.92 | 7.402 | |
| 11 | 15 | 53.72 | 4.05 | 6.95 |
| No. | CEM II/A-LL 42.5R Content in the Mixture | Compacting Pressure | Compressive Strength, MPa | Mass Increase, g | Adsorbed CO2, wt% |
|---|---|---|---|---|---|
| 1 | 20 | 12.5 | 25.07 | 1.86 | 5.29 |
| 2 | 25 | 12.5 | 36.69 | 2.22 | 6.25 |
| 3 * | 25 | 12.5 | 35.85 | – | – |
| 4 | 25 | 15 | 38.42 | 2.64 | 6.64 |
| 5 | 30 | 12.5 | 46.09 | 3.37 | 7.24 |
| 6 | 35 | 12.5 | 52.08 | 3.72 | 7.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siauciunas, R.; Prichockiene, E.; Valancius, Z.; Elsteris, A. Hardening of Mortars from Blended Cement with Opoka Additive in CO2 Environment. Ceramics 2024, 7, 1301-1315. https://doi.org/10.3390/ceramics7040086
Siauciunas R, Prichockiene E, Valancius Z, Elsteris A. Hardening of Mortars from Blended Cement with Opoka Additive in CO2 Environment. Ceramics. 2024; 7(4):1301-1315. https://doi.org/10.3390/ceramics7040086
Chicago/Turabian StyleSiauciunas, Raimundas, Edita Prichockiene, Zenonas Valancius, and Arunas Elsteris. 2024. "Hardening of Mortars from Blended Cement with Opoka Additive in CO2 Environment" Ceramics 7, no. 4: 1301-1315. https://doi.org/10.3390/ceramics7040086
APA StyleSiauciunas, R., Prichockiene, E., Valancius, Z., & Elsteris, A. (2024). Hardening of Mortars from Blended Cement with Opoka Additive in CO2 Environment. Ceramics, 7(4), 1301-1315. https://doi.org/10.3390/ceramics7040086






