Abstract
Ternary and quaternary compounds offer vast potential for tailoring material properties through compositional adjustments and complex interactions among their constituent elements. However, many of their compositional possibilities still need to be investigated. Energy-dispersive X-ray spectroscopy (EDX) is crucial for determining elemental composition but is inadequate for identifying chemical bonds and physical properties. This work introduces a novel methodology using a stoichiometric deviation vector (SDV) to estimate the physical and compositional feature characteristics of Si, N, and O compounds by comparing actual molar ratios with ideal stoichiometric references. We validated this method by estimating Si-O bonds in silicon oxynitride samples, demonstrating strong agreement with FTIR and refractive index results. We also extended our proof of principle for SiAlON compounds and established an adaptable procedure to analyze compounds with more than three elements. This flexible methodology will significantly value the materials research community, providing valuable compositional features and physical insights by performing elemental EDX characterizations.
1. Introduction
A task of significant importance in materials science is the development of advanced techniques and tools that allow the compositional features characteristics of the materials produced to be controlled at will and in a precise manner []. Such control is critical because some material’s physical properties depend on their compositional features’ characteristics [,,,,,]. Evidently, to be able to control the compositional features characteristics of any compound, one must first be able to measure it.
Accurately determining the compositional features characteristics of a material or compound is not a simple task. In general, multiple techniques are required to provide complementary information. Among the most used are X-ray photoelectron spectroscopy (XPS) [], mass spectrometry (MS) [], X-ray diffraction (XRD) [], Fourier transform Infrared spectroscopy (FTIR) [], energy-dispersive X-ray spectroscopy (EDX) [], atomic absorption spectroscopy (AAS) [], inductively coupled plasma optical emission spectrometry (ICP-OES) [], and X-ray fluorescence spectroscopy (XRF) [].
Specifically, the EDX, AAS, ICP-OES, and XRF techniques focus on quantitative and semiquantitative elemental analysis. In other words, they are not designed to detect chemical bonds or provide information about the chemical structure or bonding states of elements in a sample. However, these techniques can indeed provide the percentage by weight (wt%) of the elements in a given compound. Regrettably, it is impossible to obtain conclusive information about the chemical bonds in the analyzed compound and, consequently, obtain conclusive information about its physical properties.
As for EDX, this analytical technique allows for the identification of elements present in a sample by measuring the quantity and energy of emitted X-rays. The presence of this technique in most modern scanning electron microscopes (SEMs) and transmission electron microscopes (TEMs) equipment makes it very useful as an initial screening method. However, confirming the presence of certain elements in a sample does not assure specific compositional features characteristics or physical behavior.
Therefore, further analysis of the results obtained from conventional EDX or other elemental characterizations could allow us to make educated guesses regarding the analyzed compounds’ physical and compositional feature characteristics. Unfortunately, to the best of our knowledge, there are no simple methodologies that allow for the practical analysis of weight percentages (wt%) obtained from techniques such as EDX, AAS, ICP-OES, and XRF which, in turn, would enable accurate inferences on certain physical and compositional feature characteristics of the compound analyzed.
In this work, we develop and present a simple methodology based on deviation vectors to evaluate the stoichiometry of compounds containing Si, N, and O, starting from the wt% values obtained by a conventional EDX analysis. Subsequently, we carried out a proof of principle demonstrating the applicability of our method in compounds of Si, Al, O, and N. These results suggest that our methodology can be easily adapted to analyze the stoichiometry of compounds formed by any combination of elements.
Our method compares the molar proportions of the elements present in the compound characterized by EDX against the molar proportions of ideal stoichiometric compounds. This comparison allows us to obtain deviation values with respect to the ideal stoichiometry that we quantify using a concept that we have called the total deviation index (TDI). In our methodology, the TDI represents the magnitude of the stoichiometric deviation vector (SDV); the direction of the SDV is determined by analyzing the change trends in molar proportions that are observed when progressing from an ideally stoichiometric compound to another with a different composition but that is also equally ideal in stoichiometric terms.
We have identified that our proof of principle effectively organizes the information obtained from conventional EDX analyses. Despite the sources of error inherent to the EDX technique and its elemental and semiquantitative nature, our methodology facilitates obtaining reliable insights about the physical and compositional feature characteristics of the material studied. This advantage is particularly useful when EDX is the only compositional characterization technique available or for guiding us in selecting subsequent characterization tests.
2. Experimental Details
Deposition and Characterization Equipment and Procedures
Silicon oxynitride (SiON) thin films were grown on crystalline silicon substrate using an RF sputtering system. This device uses a 13.56 MHz radio frequency source (model R301 from SEREN Industrial Power Systems, Inc., Vineland, NJ, USA), optimized through coupling with the MC2 Matching Network (also from SEREN). During the deposition processes, the cleaning vacuum, set at 2.0 ×10−5 Torr, and the distance between the objective and the substrate, 7.5 cm, were kept constant. The variation in the SiON composition was achieved by adjusting the working pressure and temperature of the substrate in ranges of 40 to 70 mTorr and 30 to 300 °C, respectively.
For the elemental analysis of our compounds, we used a scanning electron microscope with a field emission filament, (model SEM JSM-7800F from JEOL, Tokyo, Japan) equipped with an energy-dispersive X-ray spectroscopy (EDX) detector (model X-Max n from Oxford Instruments, Abingdon, Oxfordshire, UK), and we utilized AZtec 2.1 analysis software.
For the purpose of standardization, the evaluations were conducted using the following parameters: an acceleration voltage of 5 kV (a beam energy suitable for light materials []), a working distance of 10 mm, and an area analysis performed at a magnification of 150×.
For the purpose of characterization, we performed null ellipsometry using a helium-neon laser (632 nm, 70°). For the calculation of the FTIR absorbance spectra, we used a model Vertex 70v from Bruker, Billerica, MA, USA, operating in a range of 400 to 4000 cm−1 with a spectral resolution of 2 cm−1 and phase resolution of 32. Prior to each measurement cycle, a calibration was performed to correct any potential deviations in optical and electronic alignment, ensuring consistent and precise measurements. This process involves measuring the laser position at different points within the interferometer and adjusting the optical components to optimize alignment.
3. Results and Discussion
3.1. Fundamentals of Energy-Dispersive X-ray Spectroscopy (EDX)
As we will see in subsequent sections, our proof of principle rests on the basis of reliable results from a conventional EDX characterization. Therefore, it is timely to review both the operating principles of the technique and the minimum requirements necessary to achieve a correct characterization using EDX.
The basic principle of operation of EDX begins when the surface of a material is “illuminated” with a beam of electrons. Said illumination beam causes the expulsion of secondary electrons from the atoms that make up the sample. The ejected electrons leave vacancies in the lower energy levels of the atom. The vacancies are occupied by electrons from upper shells, following the selection rules of quantum mechanics () []. The energy difference between the upper level, from where the replacement electron descends, and the energy level that was vacated by irradiation with the microscope’s electron beam is precisely the energy of the X-ray emitted by the atom []. Because the differences between the energy levels of each element are unique and characteristic, it is possible to identify the presence of a specific element by detecting the energy of the X-rays it emits.
It is essential to note that EDX does not provide us with information about the chemical bonds of the sample studied; it only tells us what elements are present. In other words, EDX is an elemental analysis technique, not a chemical analysis technique []. Furthermore, we must consider that EDX is a semiquantitative technique because it is based on the premise that if an element is more abundant in the sample, more characteristic X-rays will be detected from that element. Consequently, the weight percentages (wt%) obtained from this technique are proportional to the number of X-rays produced by the elements that make up the sample, i.e., the greater abundance of a given element equals a greater amount of characteristic X-rays produced and, therefore, a greater wt%.
Although EDX cannot specify the existence of chemical compounds, if performed correctly, it provides a very reliable wt% []. Attention must be paid to multiple requirements to perform an EDX analysis, including the technical characteristics of the equipment, the type and location of the X-ray detectors, or the accelerating voltages. However, three thumb rules are standard for all equipment and all materials whose constituent elements have an atomic number equal to or greater than 5 [,], and these are:
- (a)
- The acceleration voltage for the “illumination” beam must be at least 2.5 times the K orbital energy of the heaviest element present in the sample.
- (b)
- The exposure time must be sufficient for all elements present in the sample to reach 3000 counts or more.
- (c)
- The wt% obtained for each element must be over three times its standard deviation. Otherwise, the element must be discarded, as its statistical representativeness is not sufficiently robust. The total wt% will only include those elements that meet the criterion that wt% > 3 * σwt%.
Furthermore, to improve the accuracy of quantitative results, the ZAF correction method has been developed. It accounts for three major effects that influence the intensity of characteristic X-rays emitted from a sample:
- Atomic number (Z) effect: differences in atomic number between the sample and the standard used for calibration can influence the generation and backscattering of X-rays.
- Absorption (A) effect: X-rays generated within the sample can be absorbed by the surrounding material before reaching the detector, affecting the measured intensity.
- Fluorescence (F) effect: high-energy X-rays from one element can excite secondary X-rays from another element, leading to an overestimation of the second element’s concentration.
The ZAF method involves dividing the measured intensities by the intensities obtained from a known standard of similar composition to obtain k-ratios. Corrections for the Z, A, and F effects are then iteratively applied to the k-ratios using theoretical models, and calculations based on the sample composition, X-ray energies, and detector geometry. These corrected k-ratios are used to calculate the weight or atomic percentages of the elements in the sample [].
Using the ZAF correction method ensures the more accurate quantification of elements by compensating for these physical effects that can otherwise skew the results. This method has also spurred the development of more advanced quantification algorithms, such as AZtec Oxford’s True-Q, which are more suitable for analyzing light elements.
3.2. Foundational Elements of the Analysis Methodology
This section defines the elements that form the foundation of our proposed analysis methodology. Likewise, in Section 3.3, we will apply this methodology to a set of thin films of silicon oxynitride (SiON), grown with different deposition conditions to modify their physical and compositional features characteristics.
Because we will start analyzing the stoichiometry of Si, N, and O compounds, let us start by selecting the compound we will use as a reference to evaluate stoichiometry. We have chosen stoichiometric silicon oxynitride, whose molecular formula is Si2N2O []. Based on this compound, we will determine the proximity or remoteness of any other Si, N, and O material in terms of stoichiometry.
One of the pivotal aspects of our methodology is the in-depth analysis of the stoichiometric relationships between the three elements that constitute SiON. This analysis will enable us to discern how many nitrogen or oxygen atoms silicon can chemically bond with. To accomplish this, we can start with the ideal stoichiometric silicon dioxide, whose molecular formula is SiO2 []. In this compound, we observe that each Si atom can chemically bond with two O atoms. On the other hand, to understand how silicon bonds with nitrogen, we analyze the ideal stoichiometric silicon nitride, Si3N4 []. In silicon nitride, three Si atoms bond with four N atoms. In other words, each Si atom is associated with 1.33 N atoms.
From the analysis of silicon dioxide and silicon nitride, we have determined the stoichiometric relationships between the elements of interest for SiON, concluding that Si can bond with 2 O or with 1.33 N. This relationship is critical because it indicates that when replacing oxygen with nitrogen in the SiO2 formula, we should do so in a proportion that incorporates 0.66 nitrogen for each oxygen removed. In our reference compound, Si2N2O, we found a ratio of two N atoms and one O atom for every two Si atoms, which confirms that the stoichiometric relationships observed in SiO2 and Si3N4 are maintained. Likewise, we can identify a stoichiometry line with SiO2 and Si3N4 at its ends and passing through Si2N2O. This line is represented by the equation Si3−yN4−2yOy for .
Once the stoichiometric relationships have been established, it is convenient to identify the molar proportions and of the compounds that we use to identify these relationships. These proportions are derived directly from their molecular formulas. We observe that in SiO2, there are no nitrogen atoms; therefore, , while the oxygen-to-silicon ratio is two to one, resulting in . In the case of Si3N4, we have and . Finally, the molar proportions of Si2N2O are and .
This molar ratio calculation is not limited to the molecular formulas of ideal stoichiometric compounds. It is also possible to calculate molar proportions from the weight percentages (wt%) of each constituent element of a compound, as obtained in EDX analyses. To calculate this, follow the “Interconversion between weight percentages (wt%) and empirical molecular formula” procedure in the Appendix A: Glossary of core concepts. This procedure allows us to elucidate the empirical molecular formula from the wt%, allowing us to directly determine the molar proportions of the compounds studied using EDX.
Up to this point, we have determined the stoichiometric relationships between the SiON elements. Likewise, we have identified the molar proportions of three ideal stoichiometric compounds formed by Si, N, and O. In addition, we know how to determine the molar proportions from the wt% obtained through an EDX analysis. Therefore, the strategy we will follow next will consist of comparing the molar proportions of the compounds characterized by EDX against the molar proportions of the three ideal stoichiometric compounds formed by Si, N, and O.
The comparison between molar proportions would only be useful if it could be quantified. To assign numerical values to our comparisons, we will define the N and O deviations as the absolute value of the difference between the molar ratio of the SiON compound under study and the molar ratio of the reference stoichiometric SiON (Si2N2O). The expressions for calculating the deviations of N () and O () are presented in Equations (1) and (2), respectively. In these equations, and represent the molar proportions in the analyzed SiON compound, while the values of one and one-half correspond to the proportions and of the reference compound Si2N2O.
Let us now introduce the concept of the total deviation index (TDI), which quantifies the magnitude of the deviation of a given SiON compound with respect to the Si2N2O that we are using as a reference. This index offers a numerical value that allows for a direct comparison of the compound under study against the reference compound. The TDI is calculated as the sum of plus and is presented in Equation (3).
Suppose that a certain SiON compound under analysis is identical to Si2N2O; in that scenario, we would have and , leading to both and being zero. Consequently, the TDI is also equal to zero. Due to the absolute values in the and expressions, the minimum possible value for the TDI is zero. As we have observed, this situation occurs when the studied compound has molar proportions identical to those of the reference compound. On the other hand, any TDI value different from zero will be positive, indicating that the higher this value, the more significant the discrepancy between the molar proportions of the analyzed compound and those of the reference compound. Based on the above, we can see that the TDI behaves analogously to the magnitude of a vector.
Now, let us calculate the TDIs of the compounds at the ends of the SiON stoichiometry line, i.e., Si3N4 and SiO2. If our analyzed compound is identical to Si3N4, then , , , and , and we obtain a first maximum value for the TDI, which will be 5/6 or approximately 0.83. At the other extreme, if the SiON compound is identical to SiO2, then , , , and , so we will obtain a second value maximum for the TDI, which is 5/2 or 2.5. The previous calculations allow us to affirm that the compound we are using as a reference (Si2N2O) is closer to Si3N4 than to SiO2.
Let us proceed one step further, analyzing what happens with the TDI of the SiON compounds that lie on the Si3−yN4−2yOy stoichiometry line for . We can immediately identify that when y = 0, we have Si3N4 with TDI = 0.83. For y = 1, we find our reference compound Si2N2O, whose TDI is 0; finally, when y = 2, the compound is SiO2 with a TDI of 2.5. Therefore, we identify that when starting at y = 0, we have a TDI of 0.83 that decreases progressively as the value of y increases until it reaches its minimum value of zero when y = 1. However, if we continue increasing the value of y from 1 to 2, the TDI increases again from 0 to 2.5. The behavior of the TDI on the stoichiometry line confirms its character as a vector magnitude.
At this point, we can safely refer to the TDI as the magnitude of a vector that correctly quantifies the differences between the molar proportions of the compounds under study and the reference compound. However, more than the TDI is needed to provide complete information about the studied compound. For example, in the SiON stoichiometry line, we find a pair of TDIs with a value equal to 0.5, the first occurring when y = 1/2 (Si5/2N3O1/2) and the second when y = 4/3 (Si5/3N4/3O4/3). The amount of oxygen in Si5/3N4/3O4/3 is more than double that in Si5/2N3O1/2, so both compounds’ compositional features and physical characteristics are considerably different; however, their TDI is the same. For this reason, it is crucial to not only know the TDI, which acts as the magnitude of a vector, but also determine its direction. We will call this vector the stoichiometric deviation vector (SDV).
To determine the deviation direction (DD) of the SDV, we will compare the molar proportions of nitrogen and oxygen with respect to the silicon ( and ) of the SiON compound for evaluation against the molar proportions of N and O for Si2N2O. For SiO2, the molar proportions are and , while in Si3N4, they are and . Therefore, to change from the reference Si2N2O towards Si3N4 or SiO2, the molar ratios must conform to the following sets of specific conditions:
| For Si3N4 | For SiO2 |
Consequently, any compound of Si, N, and O that meets the criteria and will have an SDV directed from Si2N2O towards Si3N4, with a magnitude defined by the TDI. Similarly, those compounds that comply with and will have an SDV pointing towards SiO2 with a magnitude equal to the TDI.
There are cases where there is no specific DD, such as when or . These compositions do not agree with the stoichiometric relationships observed in SiON composites, probably indicating the presence of a heterogeneous physical mixture of Si3N4 + SiO2 or reflecting limitations in the EDX characterization.
As a conclusion to the section, we have detailed that from the information obtained by the EDX technique, it is possible to determine a deviation magnitude (TDI) and a deviation direction (DD), which together form the stoichiometric deviation vector (SDV). The need to define an SDV arises from being able to represent whether the molar proportions of the compound under analysis make it similar to Si3N4 or SiO2. All those compounds that meet one or another set of specific deviation conditions will have a defined direction. On the other hand, compounds that do not meet pre-established sets of criteria will lack such a vector, probably indicating the presence of atypical phases or mixtures, which require deeper characterization to understand their structure, composition, and physical and compositional features characteristics traits.
3.3. Application of the Analysis Methodology to Silicon Oxynitrides: A Proof of Principle
To experimentally evaluate the compositions, ten silicon oxynitride (SiON) films were grown using RF sputtering equipment. The common deposition parameters are described in the section “Deposition and Characterization Equipment and Procedures”. The specific variations for each sample are presented in Table 1.

Table 1.
Deposition conditions of the SiON films grown by RF sputtering.
To generate an appropriate volume of interaction that provides significant X-ray signals, all thin films were grown to an estimated thickness of 2 μm, as shown in Figure 1, corresponding to sample 9.
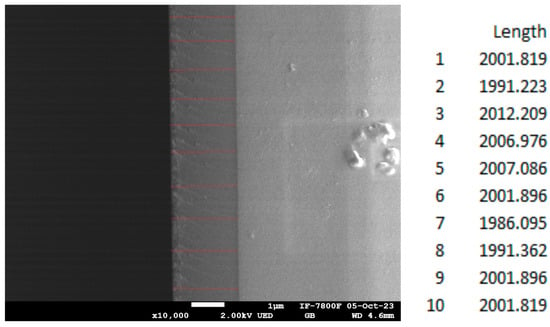
Figure 1.
Cross-sectional SEM micrograph of sample 9. The estimated thickness of the sample is approximately 2 μm (red lines indicate the measurements positions).
The resulting EDX spectra of samples 6, 7, 8, and 9 are shown in Figure 2 (inset depicts the evaluated area). The presence of nitrogen, oxygen, and silicon is listed in Table 2.
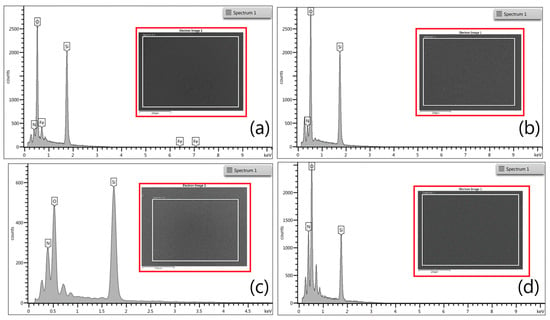
Figure 2.
EDX spectra of SiON films: (a) sample 6; (b) sample 7; (c) sample 8; (d) sample 9. The evaluated area is shown in the inset.

Table 2.
Atomic weight percentages and total deviation indices (TDIs) of silicon oxynitride samples by RF sputtering as determined by EDX processing.
Subsequently, our SiON films’ weight percentages were analyzed to calculate the total deviation indices (TDIs). The sets of deviation direction criteria discussed in the previous section were applied to determine the presence of a stoichiometric deviation vector (SDV) and, if applicable, its direction. Our analysis indicates the formation of four compounds with similarities to SiO2 (dioxide-like), six compounds without defined SDVs, and no compounds with SDVs directed toward Si3N4. These findings are summarized in Table 2. Meanwhile, Figure 3 illustrates samples with SDVs directed towards SiO2 and displays the magnitude and direction of the resulting vectors.
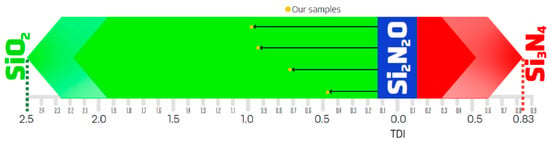
Figure 3.
Graphical representation of the stoichiometric deviation vectors (SDVs) for samples with SiO2-like characteristics. This diagram illustrates the variation of the total deviation indices (TDIs), ranging between 0 and 2.5, reflecting the stoichiometric affinity towards SiO2; and from 0 and 0.833, corresponding to the affinity towards Si3N4.
To highlight the regions that meet the criteria for presenting an SDV either towards Si3N4 or SiO2, a ternary diagram is presented in Figure 4. This diagram assesses the concentrations of nitrogen, oxygen, and silicon. It serves as a computational visualization tool designed to illustrate how the presence of a stoichiometric deviation vector (SDV) positions a compound within a specific region of the diagram, suggesting compositional similarity with stoichiometric compounds.
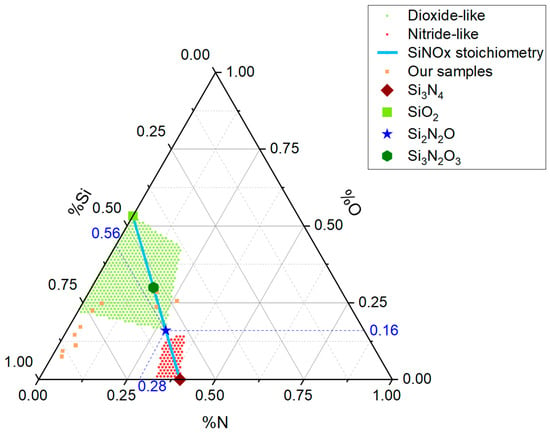
Figure 4.
Ternary diagram of the compositions of nitrogen, silicon, and oxygen in terms of wt%, highlighting the points corresponding to the key stoichiometric compounds (Si3N2O3, Si3N4, SiO2, and Si2N2O). The samples analyzed in our study are also located in the diagram. The green and red regions indicate oxide-like and nitride-like compositions, respectively, according to the established SDV criteria. The stoichiometric line corresponding to the formula Si3−yN4−2yOy that connects Si3N4 with SiO2 is also included.
This ternary diagram was generated by discretizing the possible concentration universe of nitrogen, oxygen, and silicon, starting at [0 0 100], where the first column represents the weight percentage of N, the second of O, and the third of Si. The result was 5185 distinct combinations. Upon applying the deviation criteria and calculating the TDIs for each of the 5185 combinations, it was identified that 672 (12.96%) of the discrete concentrations exhibited an SDV towards SiO2 (dioxide-like). On the other hand, only 84 concentrations (1.62%) were oriented towards Si3N4 (nitride-like).
In Figure 4, the position of the stoichiometric compound Si2N2O with percentage concentrations of 27.96% N, 15.97% O, and 56.07% Si is represented by a star. Dashed blue lines were drawn from the Si2N2O to each of the three concentration axes, indicating the corresponding values in blue. To determine the concentration of any component element, one must draw lines parallel to the grid and identify the position in each of the three linear axes.
Figure 4 also displays the ten samples referenced in Table 2 and the locations of the stoichiometric compounds Si3N2O3, Si3N4, and SiO2. The red and green areas represent concentrations with stoichiometric deviation vectors similar to nitride and dioxide, respectively. Furthermore, the stoichiometric line, described by the formula Si3−yN4−2yOy, is incorporated. The stoichiometric line connects the ideal stoichiometric compounds Si3N4 and SiO2. The analysis of our samples’ positions suggests that similarity to SiO2 does not require very strict alignment with the trajectory represented by the formula Si3−yN4−2yOy.
Figure 5 shows the TDIs with values less than or equal to 2.5. Within this representation, two regions are delimited for compounds with defined SDVs (regardless of their position with respect to the Si3−yN4−2yOy line). The area limited by the blue line groups the concentrations classified as dioxide-like, while the red line confines the nitride-like components.
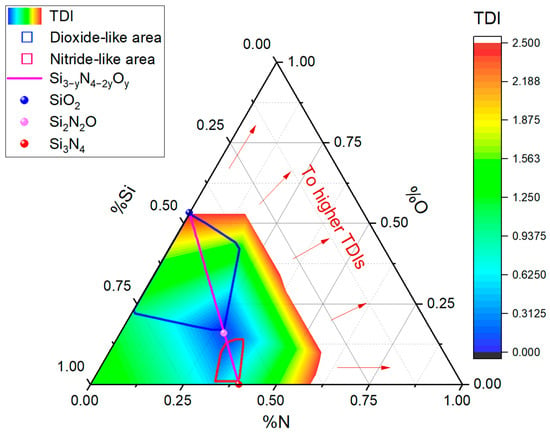
Figure 5.
Ternary diagram of the wt% of nitrogen, silicon, and oxygen, showing the positions corresponding to important stoichiometric compounds (Si3N2O3, Si3N4, SiO2, and Si2N2O). A stoichiometric line connecting Si3N4 to SiO2 is highlighted, and areas formed by dioxide- and nitride-like compounds are delineated according to our sets of SDV criteria. These areas are visualized using a chromatic scale ranging from 0 to 2.5. The blank region shows values exceeding 2.5, trending towards infinity as they approach the oxygen axis (%O).
The color gradation reflects the magnitude of the TDI, varying from cool colors for lower values to warm colors for higher values. Specifically, in the dioxide-like zone, the TDIs gradually increase from 0, close to Si2N2O, until reaching 2.5 when approaching SiO2. Compounds with values close to 2.5 exhibit characteristics distinctly similar to silicon dioxide.
On the other hand, the extensive white region reflects TDI values that exceed the threshold of 2.5 with a tendency to infinity on the oxygen axis. Contrary to the compounds within the nitride- and dioxide-like zones, those located out from SiO2 or Si3N4 zones do not have a defined SDV as they do not meet the established criteria for deviation and magnitude. We estimate that Si, N, and O compounds without a defined SDV could indicate the presence of atypical structures or complex mixtures with physical and compositional feature characteristics that are substantially different from the compounds of the stoichiometric line.
It is essential to highlight that the SiON compounds found within the dioxide-like and nitride-like areas may not necessarily be ideal stoichiometric compounds or mixtures of SiO2 + Si3N4. Moreover, we know little about how the constituent elements might be bonded or whether the studied compounds might have even fallen into the regions due to the biases inherent in a conventional EDX analysis. However, our proposal is that despite not precisely knowing the compositional feature characteristics of the studied compound, it is possible to estimate whether the behavior, both compositional feature characteristics and physical characteristics, is more similar to Si2N2O, SiO2, or Si3N4 depending on the magnitude and direction of SDV for those SiON compounds that have fallen into the dioxide-like and nitride-like areas.
To prove the above, we performed a Fourier transform infrared spectroscopy characterization (FTIR) to validate the effectiveness of the SDV in predicting compositional features characteristics from the data obtained by EDX. Absorbance measurements were taken in three different areas for each sample, and the results were averaged to ensure a reliable representation. Figure 6 presents the FTIR absorbance spectrum of our ten samples presented in Table 2.
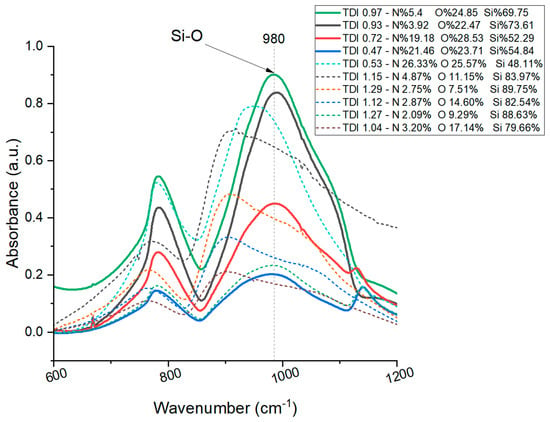
Figure 6.
Fourier transform infrared absorbance spectrum corresponding to our ten samples. The legends indicate the TDI (total deviation index) and the atomic weight percentages of nitrogen (N), oxygen (O), and silicon (Si) calculated from the formulas defined in this article and the data obtained by EDX. The presence of peaks at 980 cm−1 associated with the absorption band of silicon–oxygen (Si-O) bonds is highlighted.
From Figure 6, the spectra of compounds without a defined SDV (represented with dashed lines for differentiation) do not show peaks in the silicon–oxygen bond band. Conversely, for the four compounds with a determined SDV, the spectra reveal pronounced peaks at 980 cm−1, corresponding to the absorption band of silicon–oxygen (Si-O) bonds []. The observed trend is that as the TDI increases, the absorbance at 980 cm−1 also intensifies, indicating a higher proportion of Si-O bonds characteristic of SiO2. In contrast, as the TDI decreases, the absorbance at the peak diminishes, and the presence of nitrogen increases, suggesting a departure from the characteristics of SiO2 and an approach to the properties of Si2N2O. To quantify this, we calculated the area under the curve in the FTIR spectra for compounds with a defined SDV as shown on Table 3, which correlates with the number of bonds absorbing at that frequency.

Table 3.
Area under the curve of silicon oxynitride samples with a determined SDV.
Continuing with the evaluation of our methodology, we incorporated the calculation of the refractive index. These values are detailed in Table 2. The refractive index of silicon oxynitrides can range from 1.44 (SiO2) to 2.05 (Si3N4) []. Our methodology predicts that compounds with a well-defined SDV and greater similarity to SiO2 will have indices close to 1.44, while those with a lower oxygen proportion will approach 2.05. The refractive index listed in Table 2 confirms this trend, showing a non-systematic oscillation in compounds without a defined SDV.
These findings are consistent with one of our initial hypotheses: it is possible to obtain insights into the compound’s physical and compositional feature characteristics by comparing molar proportions and defining the directions of the vectors.
3.4. Beyond Three Elements: Application of the Methodology to Aluminum–Silicon–Oxynitride Compounds
This section aims to demonstrate that our proof of principle is scalable to more complex materials. For this purpose, we will use the previous methodology, adding a new element: aluminum. Firstly, we present the algorithm used in the previous section as a set of six steps that can be tailored to different compounds:
- Selecting a reference compound to evaluate the stoichiometry of the analyzed compounds.
- Determining the stoichiometric relationships between the elements present in the reference compound.
- Identifying the molar proportions and formulation of deviation equations.
- Analyzing the samples under study using EDX to determine their constituent elements’ weight percentages (wt%). From the EDX wt%, we will determine the empirical molecular formula and the molar proportions of the compounds under study.
- Evaluating the molar proportions of the studied compounds in the deviation equations and calculating the TDI.
- Determining the SDV direction by indicating how the molar proportions change from the reference compound to the compounds at the stoichiometry lines’ ends.
By following these steps, we can easily tailor the algorithm to different compounds, making our methodology scalable and adaptable to a wide range of materials. Let us apply this algorithm now to compounds with more elements, such as aluminosilicate oxynitrides (SiAlON).
1. Selection of a reference compound.
As in the previous case, we start from a base ideal stoichiometric compound that will serve as a reference to assess the proximity or distance of the compounds to be evaluated. The ideal stoichiometry chosen as the basis for this proof of principle is Si4Al2O2N6, commonly abbreviated as SiAlON [].
2. Determination of stoichiometric relationships.
In order to determine the stoichiometric relationships in the four elements of SiAlON, we first need to identify other ideal stoichiometric compounds consisting of at least two SiAlON elements. We have identified six such compounds. Let us start by considering the two compounds from the previous case, SiO2 and Si3N4. We will also add Si2N2O, which was used as a reference, to this list, thus completing the possible deviations towards silicon oxynitrides. We also need to evaluate deviations towards aluminum oxynitrides. The stoichiometric formulas that will serve as aluminum-based ideal stoichiometric compounds are AlN [], Al23O27N5 [], and Al2O3 [].
For silicon oxynitrides, we can recall from the previous section that in SiO2, each Si atom can bond with two oxygen atoms. In Si3N4, the Si is associated with 1.33 N atoms; in Si2N2O, each silicon can be associated with 2 O or 1.33 N. Now, let us discuss the case of aluminum oxynitrides. In this case, aluminum is the element used to define the stoichiometric relationships. We observe that the aluminum nitrogen ratio for AlN is 1, whereas, for Al2O3, the aluminum oxygen ratio is 1.5. Finally, in Al23O27N5, 27 oxygen and 5 nitrogen atoms can be bonded for every 23 aluminum atoms, which confirms that the stoichiometric relationships observed in AlN and Al2O3 are maintained, that is, that aluminum can carry either 1 nitrogen or 1.5 oxygens.
These stoichiometric relations will help us construct stoichiometric lines originating from Si4Al2O2N6. It is important to note that we have extended beyond a one-dimensional stoichiometric deviation as in Figure 3 for the Si2N2O compound; now, the deviations extend across a plane. Six trajectories, where the proportions are conserved, are summarized in Table 4, along with the ranges in which they are valid.

Table 4.
Stoichiometric lines from Si4Al2O2N6 and their validity ranges.
3. Identification of Molar Proportions and Formulation of Deviation Equations.
The previous analysis has allowed us to elucidate that stoichiometric proportions can be formulated from either silicon or aluminum, depending on which destination we are heading toward. For instance, when the deviation is towards aluminum oxynitrides, the weight proportion of silicon wt%Si→0. Conversely, if the compound leans toward silicon oxynitrides, the weight proportion of aluminum (wt%Al) is reduced to zero. The latter leads to the formation of indeterminations.
To handle this issue, we adopted a convenient perspective that considers aluminum and silicon not as individual elements but as a combination of both, that is, the sum of the number of silicon atoms plus aluminum atoms. This approach helps us eliminate the indeterminacies that would occur depending on which compound we are heading. Therefore, in Table 5, we present the molar proportions of oxygen and nitrogen with respect to the sum of aluminum + silicon in Si4Al2O2N6 (our reference compound).

Table 5.
Molar proportion of N and O in the Si4Al2O2N6 stoichiometry. Used as a zero molar proportion or reference.
These proportions help us quantify the deviation from the initial stoichiometry. The magnitude of the deviation is defined as the absolute value of the difference in the proportions of nitrogen or oxygen with respect to the sum of aluminum and silicon. The nitrogen and oxygen deviation equations are presented as follows in Equations (4) and (5).
where:
represents the deviation of nitrogen relative to aluminum and silicon from the stoichiometry of Si4Al2O2N6 to the concentration under analysis.
represents the deviation of oxygen relative to aluminum and silicon from the stoichiometry of Si4Al2O2N6.
is the current molar proportions of nitrogen.
is the current molar proportions of oxygen.
As in the previous analysis, the total deviation index (TDI) is the sum of the deviations and is presented in Equation (6). TDI is the magnitude of the total deviation of the composite with respect to Si4Al2O2N6:
4. EDX Analysis
In Section 3.3, samples grown by RF sputtering were assessed to evaluate the effectiveness of the proposed methodology. The results obtained with this method showed good correspondence with those from the compositional feature characteristics characterization (FTIR). For SiAlON in this conceptual test phase, we are unable to perform multiple depositions; instead, we analyze the entire universe of compounds formed by Si, Al, O and N using the discretized values of the weight proportions of SiAlON elements that are calculated in Section 3.5.
Subsequently, we define the fundamental molar proportions, those corresponding to the stoichiometric formulas of each ideally stoichiometric compound. Using the stoichiometric formulas in Table 4, we determine the weight percentages. These calculations allow us to precisely know the amount of each element present in each sample. The detailed results are presented in Table 6.

Table 6.
Elemental composition of stoichiometric compounds of Si, Al, O, and N.
As shown in the data in Table 6, the silicon oxynitride compounds exhibit a complete absence of aluminum. Similarly, the compounds AlN, Al23O27N5, and Al2O3 contain no silicon, reinforcing the validity of the approach that groups aluminum and silicon as a “single element” to reduce indeterminacies in our estimations. This methodology allows us to reduce the uncertainties inherent to the composition of complex materials.
5. Evaluation of Molar Proportions and Calculation of TDI.
Unlike SiON, we are now in a plane rather than a single dimension. Therefore, we must calculate different TDIs, one for each ideal stoichiometric compound. To obtain the magnitude of the SDV, the TDI is calculated using formulas 4 to 6. The results are shown in Table 7 and Figure 7.

Table 7.
TDIs of ideal stoichiometric compounds of Si, Al, O, and N with respect to Si4Al2O2N6.
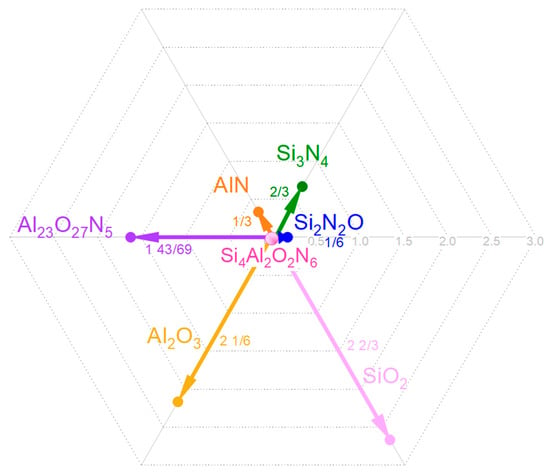
Figure 7.
Vector diagram of deviation from ideal stoichiometry from the reference composition (Si4Al2O2N6) to the stoichiometric compositions of Si2N2O, SiO2, Si3N4, AlN, Al23O27N5, and Al2O3. The vectors in the diagram represent the direction and magnitude of compositional changes required to achieve the ideal stoichiometry of the mentioned compounds.
Figure 7 provides a vector diagram that helps us understand how the compositions of the six compounds in the quaternary system differ from our reference composition. The vectors show the adjustments necessary to move from the reference to any of the six compounds, with the compounds containing a high weight concentration of oxygen (SiO2, Al23O27N5, and Al2O3) corresponding to the longest vectors, hence being further away from SiAlON’s physical and compositional feature characteristics.
6. Determination of the direction of the SDV.
To determine the direction of deviation from the reference compound to one of the six extremes, we first identify the molar proportions of all involved compounds, as summarized in Table 8. Using these molar proportions, we can identify six compounds towards which SiAlON can deviate: SiO2, Si2N2O, Si3N4, AlN, Al23O27N5, and Al2O3. By formulating the inequalities based on these molar proportions, we can predict and validate the direction of deviation for each compound tested. Let us take Si4Al2O2N6 to SiO2 as an example; to be considered an SDV, we must meet the entire set of deviation conditions.

Table 8.
Molar proportions of Si4Al2O2N6.
- : This condition suggests that the material favors a more silicon-rich composition, consistent with a shift toward SiO2, a silicon–oxygen compound without aluminum or nitrogen.
- < 3/2: This reflects that the nitrogen content decreases relative to silicon as we move towards a stoichiometry that favors SiO2. This condition ensures that nitrogen is present in a lower relative quantity, approaching SiO2 where N = 0.
- : This limitation refines the ratio of silicon to oxygen, ensuring that we are moving in that direction by reducing the silicon content but without exceeding the concentration in the SiO2 (where it acquires the value of one-half).
- : This restriction does not favor a stoichiometry rich in aluminum with respect to oxygen, which is consistent with a deviation towards SiO2, where aluminum does not play a predominant role.
- : Because SiO2 does not contain nitrogen, the relative presence of nitrogen relative to oxygen must also decrease, reflecting a reduction in nitrogen’s contribution to the composition.
These five constraints form the set of deviation conditions towards SiO2. The six sets of deviation conditions from Si4Al2O2N6 to the ideal stoichiometric compounds are presented in Table 9.

Table 9.
Set of deviation conditions towards stoichiometric components. Meeting these sets of conditions, along with the TDI restrictions, ensures the presence of SDV. Stoichiometric compounds are presented in bold.
The evaluation process classifies compounds that do not meet all of the requirements for a given set of conditions as “No stoichiometric similar”. However, compounds that satisfy any criteria set’s conditions are classified as “like” the compound towards which they are directed to. As we saw in the previous section, this process can predict compound properties with limited prior information.
After establishing and justifying the deviation conditions and formulas to measure TDI, programs were developed in Mathematica, Excel, and OriginLab software. These programs are not necessary to use our methodology. However, they enable the calculation of compliance percentages with the six conditions and the identification of the location of the stoichiometric SiAlON compounds in the universe of Si, Al, O, and N compositions, and they provide visual tools to facilitate the understanding of the utility of our tool. The results are detailed in the next section.
3.5. SiAlON Graphical Representation
In order to gain a better understanding of the percentage of compounds that meet the similarity conditions with stoichiometric compounds, we created quaternary diagrams. These diagrams are in the shape of a tetrahedron or a pyramid with four faces, with each vertex corresponding to one of the four components—Si, Al, O, and N. Any point within the tetrahedron displays the molecular weight distribution of the constituent elements. To generate these images, we used a method that discretizes the space of possible combinations of the distribution of molecular weights of the SiAlON elements.
Methodology
Initially, we ensured that the sum of the molecular weight percentages of these elements reached 100% in each possible combination. Using Mathematica, we organized the data into an array for each combination of percentages, arranged in four columns representing each element, N, O, Al, and Si, respectively.
The described script uses a “for” loop that iterates from 0 to 101. In each iteration, a row of four columns is formed. The first column contains each integer from 0 to n − 1 repeated n − i times; the second column contains a sequence from 0 to n − 1 − i for each integer i; the third column inverts this sequence; and the fourth column calculates the complement up to 100 for the sum of the first three columns. Subsequently, all rows are combined into a table. The total number of rows (combinations) generated was 176,851, varying from [100 0 0 0] (100% nitrogen) to [0 0 0 100] (pure silicon).
Next, we evaluate each combination using the formulas and sets of criteria discussed in steps 3 and 6 of the previous section. This comprehensive analysis allows us to explore the universe of discrete weight percentages within the SiAlON system, providing a detailed understanding of its potential molecular configurations.
Figure 8 shows a detailed representation of the relative position of the six stoichiometric components that have been analyzed, along with the stoichiometric lines that connect them to the reference compound (Si4Al2O2N6). The stoichiometric lines correspond to the formulas indicated in Table 4. The diagram has been presented from multiple perspectives to help visualize the position of the ideal stoichiometric compounds and the length of the stoichiometric lines. By analyzing Figure 8, it is possible to conclude that the silicon oxynitrides are much closer to Si4Al2O2N6, which means they will exhibit properties more similar to SiAlON than the aluminum oxynitrides.
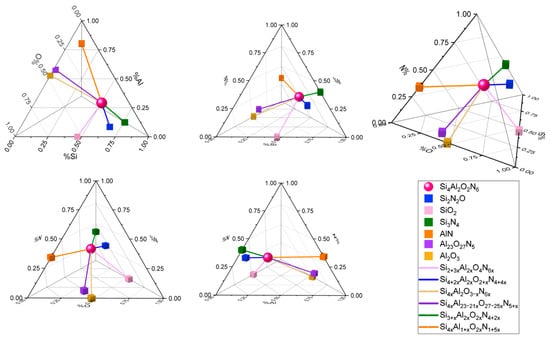
Figure 8.
Quaternary diagram of the molecular wt% of silicon, nitrogen, oxygen, and aluminum in 4 two-dimensional views and one isometric view of the resulting tetrahedron. Points of particular interest are highlighted as a sphere, showing the reference compound (Si4Al2O2N6); as cubes, which indicate the stoichiometric positions of Si2N2O, SiO2, Si3N4, AlN, Al23O27N5, and Al2O3; and as stoichiometric lines that connect them with the reference, corresponding to the formulas in Table 4.
In order to provide a clear understanding of the proportion of compounds with a defined stoichiometric deviation vector, i.e., stoichiometry-like compounds (Si2N2O, SiO2, Si3N4, AlN, Al23O27N5, and Al2O3) and those lacking an SDV, evaluations were carried out based on the criteria outlined in step 6 for determining the direction of the SDV.
The calculations indicate that 35,616 combinations, equivalent to 20.14%, fulfilled at least one of the conditions for stoichiometric similarity. The remaining 141,235 combinations, representing 79.86%, did not exhibit a defined SDV and were therefore classified as non-stoichiometric-like. These findings are detailed in Figure 9. The number of possible compounds directed toward each ideal stoichiometric compound is summarized in Table 10.
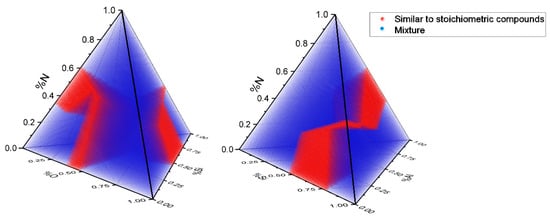
Figure 9.
Quaternary diagram of SiAlON elements. The volume in red represents the region occupied by compounds with characteristics that are like the stoichiometric compounds Si2N2O, SiO2, Si3N4, AlN, Al23O27N5, and Al2O3, according to the criteria discussed in this article. The combinations classified as non-stoichiometric-like are shown in blue.

Table 10.
Percentage of the presence of discrete combinations that meet the criteria concerning the universe of Si, Al, O, and N combinations.
From Table 10, the absence of combinations like Si2N2O stands out, attributed to the strict deviation criteria applied (mainly ), making it practically impossible to meet. Despite generating a percentage of combinations close to the real one, it should not be overlooked that this analysis is discrete, and an infinite number of potential combinations has not been considered. Those components that were adjusted to a set of conditions are shown in Figure 10.
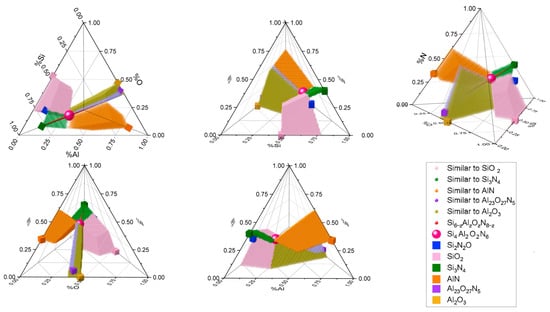
Figure 10.
Quaternary diagram of the molecular weight percentages of SiAlON elements in four planar views and one isometric view of the composition tetrahedron. Points of particular interest are shown, such as a sphere representing Si4Al2O2N6; cubes displaying the stoichiometric forms of Si2N2O, SiO2, Si3N4, AlN, Al23O27N5, and Al2O3; a straight line corresponding to the formula Si6−zAlzOzN8−z; and the volumes formed by small spheres that represent compounds that are “like” the stoichiometric according to the criteria discussed in this article.
The graph includes the ideal stoichiometric components to illustrate how the region of like compounds extends from the reference compound to them. The large volumes corresponding to compounds similar to SiO2, AlN, and Al2O3 are evident.
Lastly, Figure 11 presents a diagram with TDI values on a color scale, analogous to Figure 4 but adapted for four-element compounds. An increase in TDIs is observed as they diverge from the reference compound, consistent with the definition provided in the “Foundational Elements of the Analysis Methodology” (Section 3.2), where the TDI evaluates how much a component deviates from the reference stoichiometry.
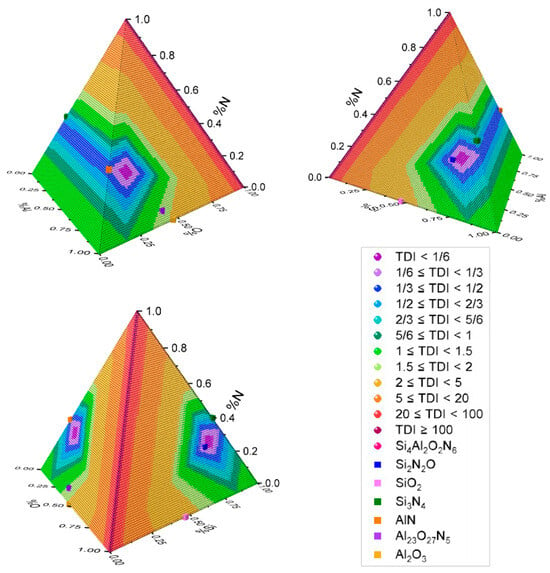
Figure 11.
A quaternary diagram of TDIs is calculated using the formulas established in this article. The stoichiometric compounds Si2N2O, SiO2, Si3N4, AlN, Al23O27N5, and Al2O3 are also displayed.
The TDI scale values are consistent with those in Table 7, highlighting Si2N2O as the closest compound with a TDI of one-sixth and SiO2 as the farthest with a TDI ≈ 2.667. These TDIs, combined with stoichiometric lines, illustrate the proximity between the reference compound and other compounds, which can be interpreted in terms of their physical and compositional feature characteristics. Additionally, the visual representation aids in identifying the percentage of stoichiometric compounds that meet the set of criteria for each ideal compound, thereby providing insights into their characteristics. Finally, a three-dimensional view emphasizes proportionality and placement within the universe of possible combinations.
The visualization of the behavior of SiAlON compounds under the studied conditions offers a comprehensible way to confirm the validity of our approach. It provides a practical tool for researchers in the field of materials. This multidimensional approach is a valuable tool for gaining insights into the physical and compositional feature characteristics of the compounds based on a standard EDX elemental analysis.
4. Conclusions
For those interested in consulting the methodology of our proof of principle without needing to read the full article, please refer to Appendix B: “Determining Stoichiometric Deviation and Directionality: A Six-Step Process.” If this summary is insufficient or if you have doubts about the validity or applicability of any aspect of the methodology, the results and discussion section provides additional detailed information.
This article presented a proof of principle for evaluating a compound’s stoichiometric character and estimating its physical and compositional feature characteristics through a conventional energy-dispersive X-ray spectroscopy (EDX) analysis, despite this technique’s semiquantitative and elemental nature. We introduced the stoichiometric deviation vector (SDV) concept, which facilitates identifying and classifying the compounds studied solely based on the percentages by weight (wt%) of the compound elements determined by EDX.
We showed that only a small percentage of compounds formed by Si, N, and O simultaneously meet the conditions and or and . The above indicates that most Si, N, and O compounds do not have a defined deviation direction and, therefore, lack an SDV. Consequently, it was impossible to estimate their physical and compositional feature characteristics. We estimate that compounds without a defined direction of deviation can be considered mixtures of their constituent elements, whose physical and compositional feature characteristics are difficult to estimate through a conventional EDX analysis. In contrast, for compounds where it is possible to completely determine the SDV (both in magnitude and direction), the vector provides reliable insights into some of their physical and compositional feature characteristics. For example, an SDV that points towards SiO2 with a magnitude of two reveals compositional features characteristics and physical features more similar to SiO2 than a compound whose SDV also points towards SiO2 but with a magnitude of one. In the same way, compounds with SDV oriented towards Si3N4 will present characteristics that combine the features of Si2N2O and Si3N4 but tend to be more similar to those of Si3N4 as the magnitude of the SDV increases.
From the above, our methodology offers a valuable tool for gaining insights into the physical and compositional feature characteristics of materials and compounds characterized by conventional EXD analyses. We implement the method as a proof of principle in compounds with more than three elements, such as SiAlON, identifying six different deviation directions. These directions allow us to estimate whether a given compound’s stoichiometric character resembles those of AlN, Al23O27N5, Al2O3, SiO2, Si2N2O, or Si3N4.
Author Contributions
Conceptualization, Y.H.W. and A.R.-G.; Methodology, L.F.G.-G., A.L.P.-M., J.R.-G., M.d.P.A.-D.-V., Y.H.W. and A.R.-G.; Software, L.F.G.-G., Y.H.W. and A.R.-G.; Validation, L.F.G.-G., A.L.P.-M., J.R.-G., M.d.P.A.-D.-V., Y.H.W. and A.R.-G.; Formal analysis, L.F.G.-G., A.L.P.-M., J.R.-G., M.d.P.A.-D.-V., Y.H.W. and A.R.-G.; Investigation, L.F.G.-G., A.L.P.-M., J.R.-G., M.d.P.A.-D.-V., Y.H.W. and A.R.-G.; Resources, A.R.-G.; Data curation, L.F.G.-G., J.R.-G., Y.H.W. and A.R.-G.; Writing—original draft, L.F.G.-G., Y.H.W. and A.R.-G.; Writing—review and editing, J.R.-G., M.d.P.A.-D.-V. and A.R.-G.; Visualization, L.F.G.-G., A.L.P.-M., J.R.-G., Y.H.W. and A.R.-G.; Supervision, J.R.-G., Y.H.W. and A.R.-G.; Project administration, Y.H.W. and A.R.-G.; Funding acquisition, A.R.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PAPIIT-UNAM grant number IN111723 and CONACYT-Apoyos para Adquisición y Mantenimiento de Infraestructura en Instituciones y Laboratorios de Investigación Especializada 2019, grant number: 299881. The APC was funded by PAPIIT-UNAM project number: IN111723.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The first author wants to acknowledge the Consejo Nacional de Humanidades, Ciencias y Tecnologías, México, for the doctoral scholarship, CVU No. 244745. The authors would like to acknowledge the support provided by Roberto Hernández Reyes, Samuel Tehuacanero Cuapa, Juan Gabriel Morales Morales, Carlos Magaña, and Diego Quiterio for technical assistance in TEM, SEM, and sample preparation, respectively. All funding for the realization and publication of this research work came from the following projects: (a) PAPIIT-UNAM project number: IN111723; and (c) CONACYT-Apoyos para Adquisición y Mantenimiento de Infraestructura en Instituciones y Laboratorios de Investigación Especializada 2019, project number: 299881, which are under the technical administration of Arturo Rodríguez-Gómez.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Appendix A. Glossary of Core Concepts
This section defines the concepts we used to develop our proof of principle. To preserve the clarity and accessibility of a glossary, we will use bullet points for each concept.
- Stoichiometry: Studies how chemical elements combine to form compounds. Stoichiometry specializes in quantifying proportions and how elements react with each other. It is used to know how much reactant is needed to react with other reactants and how much product will be generated given the available amount of reactants. The term was coined by Karl Friedrich Wenzel and Jeremias Benjamin Richter [].
- Ideal stoichiometric compound: It is a compound that maintains the exact mass proportions dictated by its chemical formula. The above implies that the reactants are mixed according to the specific proportions indicated by the reaction equation, allowing the reactants to be consumed entirely without leaving residues [].
- Molecular formula: It is the written representation of a compound. The types of atoms are specified through their chemical symbols, while the quantity of each is indicated with subscripts to the right of the corresponding symbol. For example, the molecular formulas of ideally stoichiometric silicon nitride and silicon dioxide are Si3N4 and SiO2, respectively [].
- Molar proportion: It is defined as the relationship between the amounts of the different elements of a chemical compound expressed in moles []. For example, in the reaction to form silicon nitride (Si3N4) from nitrogen and silicon, the molar ratio between nitrogen and silicon () is 4:3 or , indicating that four moles of nitrogen are required for every three moles of silicon to complete the reaction.
- Stoichiometry line: It is a line that represents a family of ideal stoichiometric compounds whose composition varies, maintaining stoichiometric relationships between the elements of the compound []. An example is the stoichiometric line of silicon oxynitride, represented by the formula Si3−yN4−2yOy for 0 ≤ y ≤ 2. The variable “y” represents one element’s substitution degree for another. In the case of silicon oxynitride, it indicates that, for each mole of oxygen introduced into the system, two moles of nitrogen and one mole of silicon are removed. This variation adjusts the composition of the compound within a specific range, creating a “line” that contains all possible ideal stoichiometric compositions. The ends of the Si3−yN4−2yOy line are identified when y = 0, having ideal stoichiometric silicon nitride (Si3N4), and when y = 2, where ideal stoichiometric silicon dioxide (SiO2) is found.
- Interconversion between weight percentages (wt%) and the empirical molecular formula: If the wt% of the elements of a compound are known, it is possible to determine its empirical molecular formula by following three steps. First, divide the wt% of each element by its atomic weight to obtain the number of moles of each one. Second, identify the element with the smallest number of moles and normalize the numbers of moles of the other elements to this value, i.e., by dividing each number of moles by this minimum value. Third, use the normalized values to write the empirical molecular formula.
For example, if a compound has Si = 58 wt%, O = 12 wt%, and N = 30 wt%, with the wt% of silicon, oxygen, and nitrogen being 28.0855 g/mol, 16.00 g/mol, and 14.01 g/mol, respectively, the process would be as follows: (1) for silicon, we calculate , oxygen , and nitrogen ; (2) the element with the smallest number of moles is oxygen with 0.75, so we normalize to this value as follows: , , ; (3) therefore, the empirical molecular formula results in Si2.75N2.85O.
The inverse process allows us to determine the percentage composition of each element in a known compound from its molecular formula by following two steps. First, the total molar mass of the compound is calculated by adding the atomic weights of the elements, each multiplied by their respective number of atoms present in the formula. Second, the wt% of each element is calculated by multiplying its atomic weight by the number of atoms corresponding to it in the formula, dividing this result by the total molar mass of the compound, and multiplying by 100 to obtain the percentage [].
Taking the Si2N2O formula as an example: (1) We calculate the molar mass as , , . Therefore, the total molar mass of the compound is: ; (2) Consequently, the weight percentages are: , , .
Appendix B. Determining Stoichiometric Deviation and Directionality: A Six-Step Process
This section outlines the six-step process we used to determine stoichiometric deviation and directionality, a central aspect of our research. By explaining the detailed steps involved, from selecting a reference compound to calculating the total deviation index (TDI) and directionality, we provide a comprehensive guide for reproducing or critically evaluating our approach. This detailed explanation is crucial for those interested in applying similar methodologies to other systems or in understanding the nuances of our analysis.
Our methodology consists of six steps:
- Select a compound that serves as a reference to evaluate the stoichiometric character of the compounds studied by EDX. For example, we can use the ideal stoichiometric compound silicon oxynitride, whose molecular formula is Si2N2O [].
- Determine the stoichiometric relationships between the elements of the reference compound. For the proposed example of Si2N2O, in the relationship between Si and O, whose minimum formula is SiO2 [], it is observed that for each Si atom, there are two O atoms, while in Si3N4 [], each Si is associated with 1.33 N atoms. Meanwhile, in the reference compound Si2N2O, two N atoms and one O atom shared between two Si atoms are observed. The above indicates that the stoichiometric relationships present in SiO2 and Si3N4 are conserved in Si2N2O. Because these three compounds share the same stoichiometric relationships, we can identify a stoichiometry line with SiO2 and Si3N4 at its two ends, which also passes through Si2N2O. Said line is represented by the equation: Si3−yN4−2yOy for 0 ≤ y ≤ 2.
- Identify the molar proportions of the ideal stoichiometric compound used as a reference. These proportions are necessary to formulate the deviation equations that allow for the quantification of the difference between the molar proportions of the analyzed compound and those of the reference compound. For example, the molar proportions of Si2N2O are and . Consequently, the deviations we must analyze are nitrogen () and oxygen (). To determine and , the absolute value of the difference between the molar proportion observed in the compound studied and the molar proportion in the reference compound is used. Therefore, in our example, the formulas for and are and .
- Carry out the elemental characterization of the compounds to be studied using EDX to determine the percentages by weight (wt%) of their constituent elements. From the determined wt%, calculate the empirical molecular formula and the molar proportions of the compounds under study.
- Evaluate the molar proportions of the compound studied in the deviation equations for and formulated in point 3. The sum of + is called the total deviation index (TDI) and represents the magnitude of the SDV. Lower TDI values indicate a more significant similarity of the compound studied with the reference compound in our sample, Si2N2O. As the TDI values increase, there will be greater stoichiometric, physical, and chemical differences between the studied and reference compounds.
- Determine the direction of the SDV. To accomplish this, we must examine how the molar proportions change when going from the reference compound to one of the compounds located at the ends of the stoichiometry line. The molar proportions in SiO2 are and , while in Si3N4, they are and . Therefore, to change from Si2N2O to Si3N4, the molar proportions must be adjusted, meeting the following conditions: and . Meanwhile, changing from Si2N2O to SiO2 requires that the molar proportions be adjusted, meeting the following conditions: and . In this way, any compound of Si, N, and O that meets the criteria and will have an SDV directed from Si2N2O towards Si3N4, with a magnitude defined by the TDI determined in point 5. Likewise, compounds that comply with and will have an SDV that points towards SiO2 from Si2N2O and a magnitude equal to the TDI.
References
- Whitesides, G.M. Nanoscience, Nanotechnology, and Chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Komuraiah, A.; Kumar, N.S.; Prasad, B.D. Chemical Composition of Natural Fibers and Its Influence on Their Mechanical Properties. Mech. Compos. Mater. 2014, 50, 359–376. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mohanty, H.S.; Pradhan, D.K.; Kumar, A.; Shukla, V.K.; Singh, F.; Kulriya, P.K. Structural, Dielectric and Electrical Properties of Pyrochlore-Type Gd2Zr2O7 Ceramic. J. Mater. Sci. Mater. Electron. 2020, 31, 21959–21970. [Google Scholar] [CrossRef]
- Dunn, A.; Wang, Q.; Ganose, A.; Dopp, D.; Jain, A. Benchmarking Materials Property Prediction Methods: The Matbench Test Set and Automatminer Reference Algorithm. npj Comput. Mater. 2020, 6, 138. [Google Scholar] [CrossRef]
- Madhu, M.; Venkateswara Rao, A.; Parajuli, D.; Yonatan Mulushoa, S.; Murali, N. Cr3+ Substitution Influence on Structural, Magnetic and Electrical Properties of the Ni0.3Zn0.5Co0.2Fe2−XCrxO4 (0.00 ≤ x ≤ 0.20) Nanosized Spinel Ferrites. Inorg. Chem. Commun. 2022, 143, 109818. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, A.; Moreno-Rios, M.; García-García, R.; Pérez-Martínez, A.L.; Reyes-Gasga, J. Role of the Substrate on the Growth of Silicon Quantum Dots Embedded in Silicon Nitride Thin Films. Mater. Chem. Phys. 2018, 208, 61–67. [Google Scholar] [CrossRef]
- Muñoz-Rosas, A.; Rodríguez-Gómez, A.; Alonso-Huitrón, J. Enhanced Electroluminescence from Silicon Quantum Dots Embedded in Silicon Nitride Thin Films Coupled with Gold Nanoparticles in Light Emitting Devices. Nanomaterials 2018, 8, 182. [Google Scholar] [CrossRef]
- Mather, R.R. Surface Modification of Textiles by Plasma Treatments. In Surface Modification of Textiles; Elsevier: Amsterdam, The Netherlands, 2009; pp. 296–317. [Google Scholar]
- Weidner, S.M. Mass Spectrometry. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 93–109. [Google Scholar]
- Assad, H.; Sharma, S.; Kaya, S.; Sharma, P.K.; Kumar, A. Overview and Fundamentals of Polymer Nanocomposites. In Nanocomposites-Advanced Materials for Energy and Environmental Aspects; Elsevier: Amsterdam, The Netherlands, 2023; pp. 41–66. [Google Scholar]
- Khan, S.B.S.A.; Khan, S.B.S.A.; Khan, L.U.; Farooq, A.; Akhtar, K.; Asiri, A.M. Fourier Transform Infrared Spectroscopy: Fundamentals and Application in Functional Groups and Nanomaterials Characterization. In Handbook of Materials Characterization; Springer International Publishing: Cham, Switzerland, 2018; pp. 317–344. [Google Scholar]
- Schneider, R. Energy-Dispersive X-ray Spectroscopy (EDXS). In Surface and Thin Film Analysis; Wiley: Hoboken, NJ, USA, 2011; pp. 293–310. ISBN 9783527320479. [Google Scholar]
- Li, Y.; Legendre, B. Enthalpy of Formation of Al–Fe–Si Alloys II (Τ6, Τ2, Τ3, Τ8, Τ4). J. Alloys Compd. 2000, 302, 187–191. [Google Scholar] [CrossRef]
- Yang, Y.; Bahl, S.; Sisco, K.; Lance, M.; Shin, D.; Shyam, A.; Plotkowski, A.; Dehoff, R.R. Primary Solidification of Ternary Compounds in Al-Rich Al–Ce–Mn Alloys. J. Alloys Compd. 2020, 844, 156048. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, J.; Li, S. Study on the Analysis of Main Components of Aluminum Alloy by XRF (X-ray Fluorescence Analysis) Method. Open Access Libr. J. 2021, 8, e7845. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2003; ISBN 9781493966769. [Google Scholar]
- Williams, D.B.; Goldstein, J.I.; Fiori, C.E. Principles of X-Ray Energy-Dispersive Spectrometry in the Analytical Electron Microscope. In Principles of Analytical Electron Microscopy; Springer: Boston, MA, USA, 1986; pp. 123–153. [Google Scholar]
- Zhou, W.; Wang, Z.L. Scanning Microscopy for Nanotechnology Techniques and Applications, 1st ed.; Zhou, W., Wang, Z.L., Eds.; Springer: New York, NY, USA, 2007; ISBN 978-0-387-33325-0. [Google Scholar]
- Thomere, A.; Guillot-Deudon, C.; Caldes, M.T.; Bodeux, R.; Barreau, N.; Jobic, S.; Lafond, A. Chemical Crystallographic Investigation on Cu2S-In2S3-Ga2S3 Ternary System. Thin Solid Films 2018, 665, 46–50. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy: A Textbook for Materials Science, 2nd ed.; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-76500-6. [Google Scholar]
- Van Borm, W.A.; Adams, F.C. A Standardless ZAF Correction for Semi-quantitative Electron Probe Microanalysis of Microscopical Particles. X-Ray Spectrom. 1991, 20, 51–62. [Google Scholar] [CrossRef]
- Andersen, C.A.; Keil, K.; Mason, B. Silicon Oxynitride: A Meteoritic Mineral. Science 1964, 146, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, A.; Escobar-Alarcón, L.; Serna, R.; Cabello, F.; Haro-Poniatowski, E.; García-Valenzuela, A.; Alonso, J.C. Modeling of the Refractive Index and Composition of Luminescent Nanometric Chlorinated-Silicon Nitride Films with Embedded Si-Quantum Dots. J. Appl. Phys. 2016, 120, 145305. [Google Scholar] [CrossRef]
- Muñoz-Rosas, A.L.; Rodríguez-Gómez, A.; Arenas-Alatorre, J.A.; Alonso-Huitrón, J.C. Photoluminescence Enhancement from Silicon Quantum Dots Located in the Vicinity of a Monolayer of Gold Nanoparticles. RSC Adv. 2015, 5, 92923–92931. [Google Scholar] [CrossRef]
- Liu, W.T.; Shen, Y.R. Surface Vibrational Modes of α-Quartz(0001) Probed by Sum-Frequency Spectroscopy. Phys. Rev. Lett. 2008, 101, 016101. [Google Scholar] [CrossRef]
- Mutilin, S.V.; Khasanov, T. The Refractive Index of Homogeneous SiO2 Thin Films. Opt. Spectrosc. 2008, 105, 461–465. [Google Scholar] [CrossRef]
- El-Amir, A.A.M.; El-Maddah, A.A.; Ewais, E.M.M.; El-Sheikh, S.M.; Bayoumi, I.M.I.; Ahmed, Y.M.Z. Sialon from Synthesis to Applications: An Overview. J. Asian Ceram. Soc. 2021, 9, 1390–1418. [Google Scholar] [CrossRef]
- Gupta, N.; Pandey, A.; Vanjari, S.R.K.; Dutta, S. Influence of Residual Stress on Performance of AlN Thin Film Based Piezoelectric MEMS Accelerometer Structure. Microsyst. Technol. 2019, 25, 3959–3967. [Google Scholar] [CrossRef]
- Prosvirnin, D.V.; Prutskov, M.E.; Larionov, M.D.; Kolmakov, A.G. Evaluation of the Method of Measuring Crack Resistance by the Introduction of the Vickers Indentor for Aluminum Oxynitride Ceramics. J. Phys. Conf. Ser. 2020, 1431, 012047. [Google Scholar] [CrossRef]
- Hegedüs, N.; Balázsi, K.; Balázsi, C. Silicon Nitride and Hydrogenated Silicon Nitride Thin Films: A Review of Fabrication Methods and Applications. Materials 2021, 14, 5658. [Google Scholar] [CrossRef] [PubMed]
- Shaviv, G. Order in the Chemical Elements. In The Synthesis of the Elements: The Astrophysical Quest for Nucleosynthesis and What It Can Tell Us about the Universe; Springer: Berlin, Heidelberg, 2012; pp. 1–59. ISBN 978-3-642-28385-7. [Google Scholar]
- Uda, S. Stoichiometry of Oxide Crystals. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 175–214. ISBN 9780444593764. [Google Scholar]
- Barrera-Mendivelso, E.S.; Rodríguez-Gómez, A. Thin Films of Silicon Nitride Deposited at Room Temperature by Non-Reactive Magnetron Sputtering: Radiofrequency Power and Deposition Time Influence on the Formation of α-Si3N4 and Its Optical Properties. Front. Phys. 2023, 11. [Google Scholar] [CrossRef]
- Ault, A. How to Say How Much: Amounts and Stoichiometry. J. Chem. Educ. 2001, 78, 1347. [Google Scholar] [CrossRef]
- Gilbert, G.L. Percent Composition and Empirical Formula—A New View. J. Chem. Educ. 1998, 75, 851. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).